Advances in Electrocatalytic Hydrogen Sulfide Splitting for Sulfur Recovery: From Reaction Mechanisms to Application
Abstract
1. Introduction
2. The Significance of Electrochemical Recovery of Sulfur
2.1. Environmental Significance
2.2. Economic Benefits
3. SOR Mechanism
3.1. Direct Oxidation Pathway
3.2. Indirect Oxidation Pathway
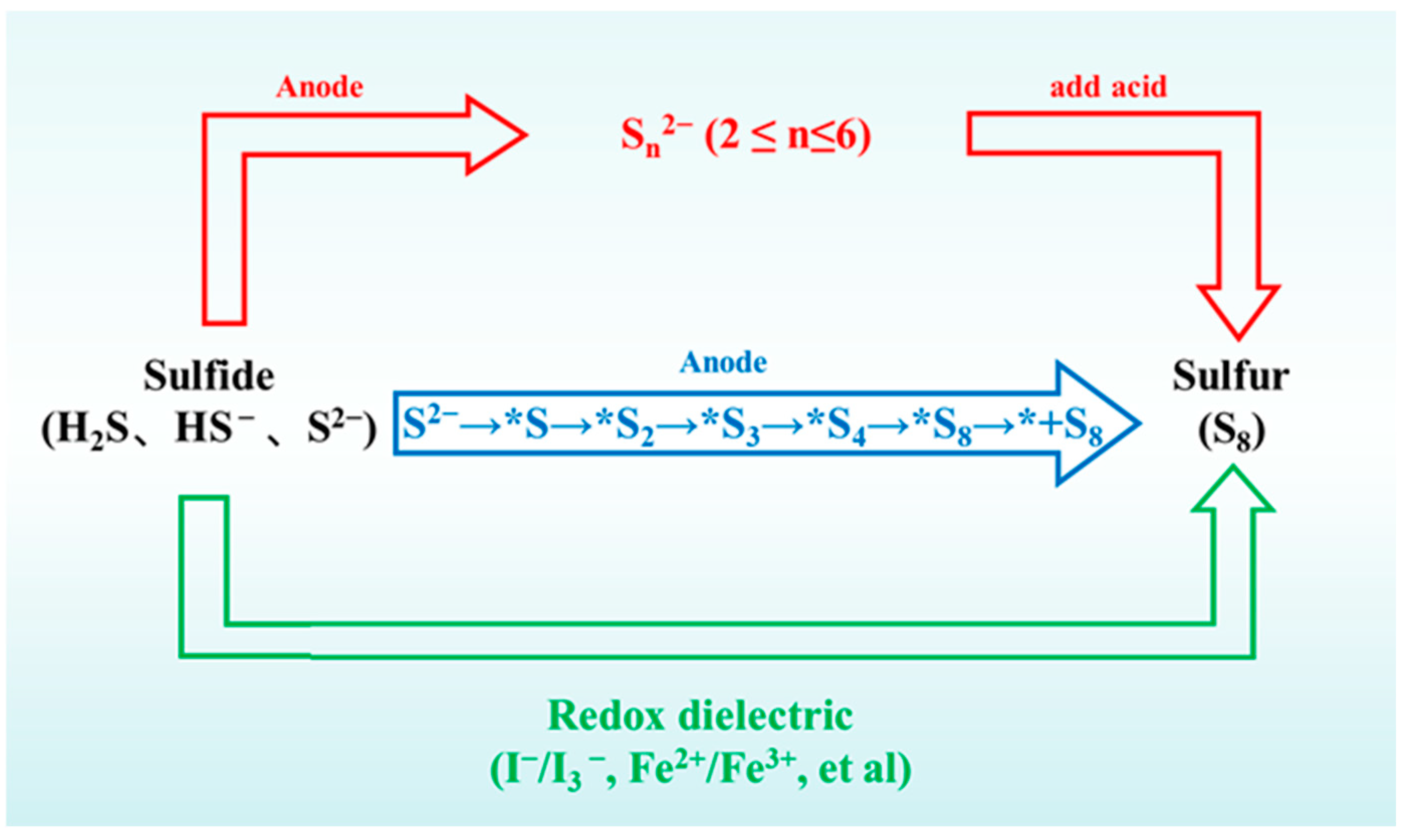
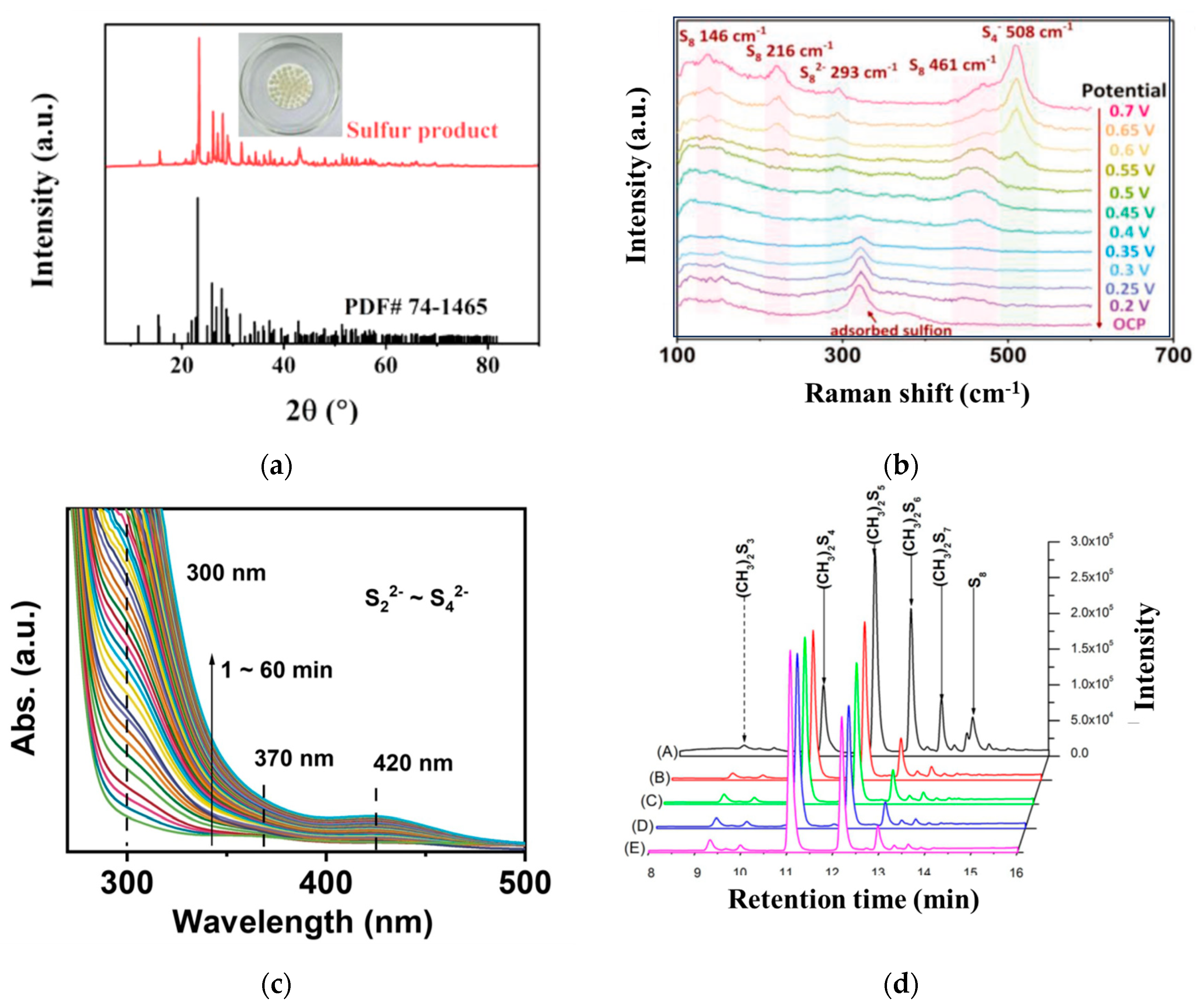
4. Analysis of Sulfur Oxidation Products
5. Electrodes for Recovering Sulfur
5.1. Direct Oxidation Electrodes
5.2. Controllable Sn2− Producing Electrodes
5.3. Medium Circulation-Driven Indirect Oxidation Electrodes
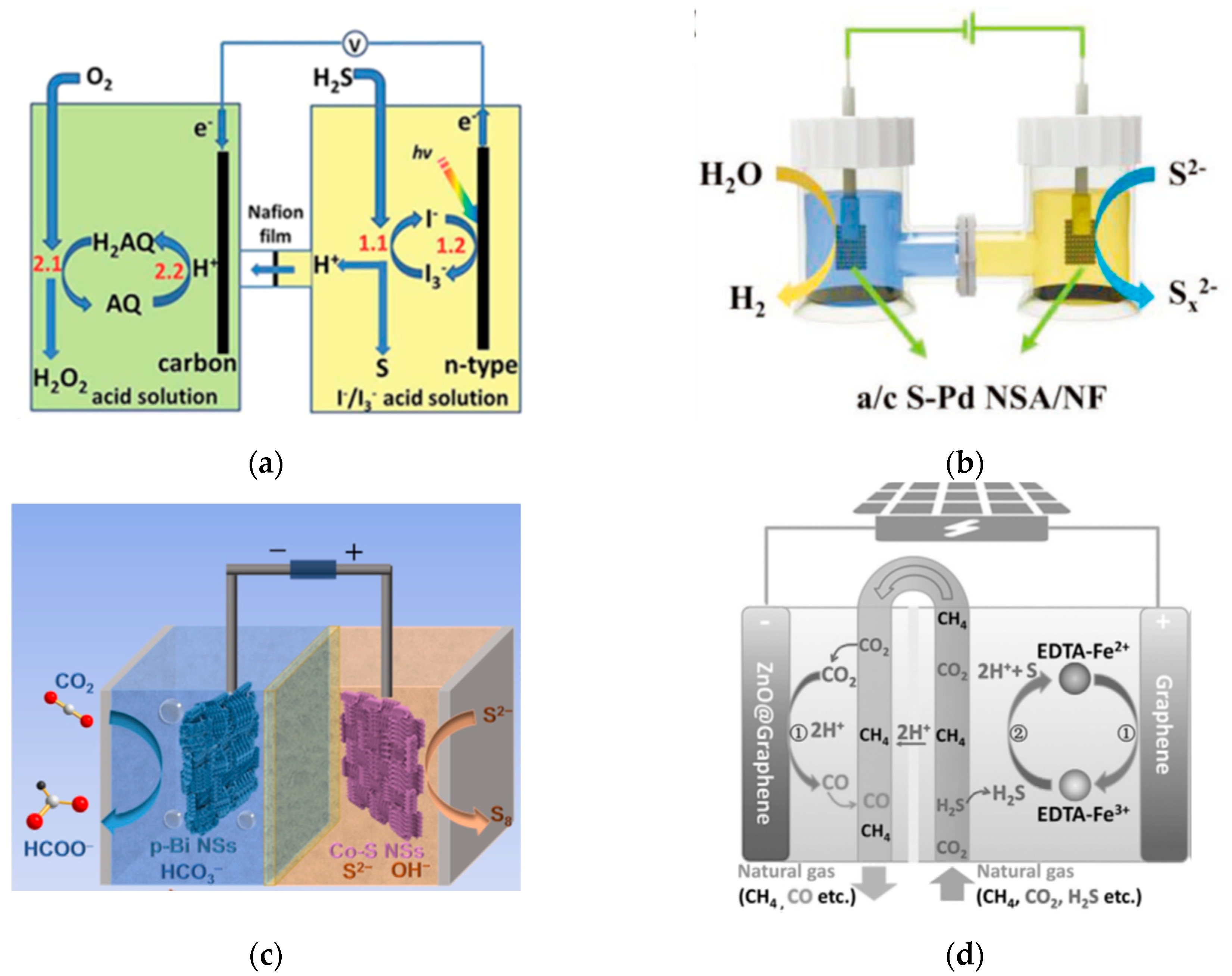
6. Electrochemical Coupling System for SOR
6.1. SOR Coupled with the Production of Energy Substances
6.1.1. SOR Coupled with ORR
6.1.2. SOR Coupled with HER
6.1.3. SOR Coupled with CO2RR
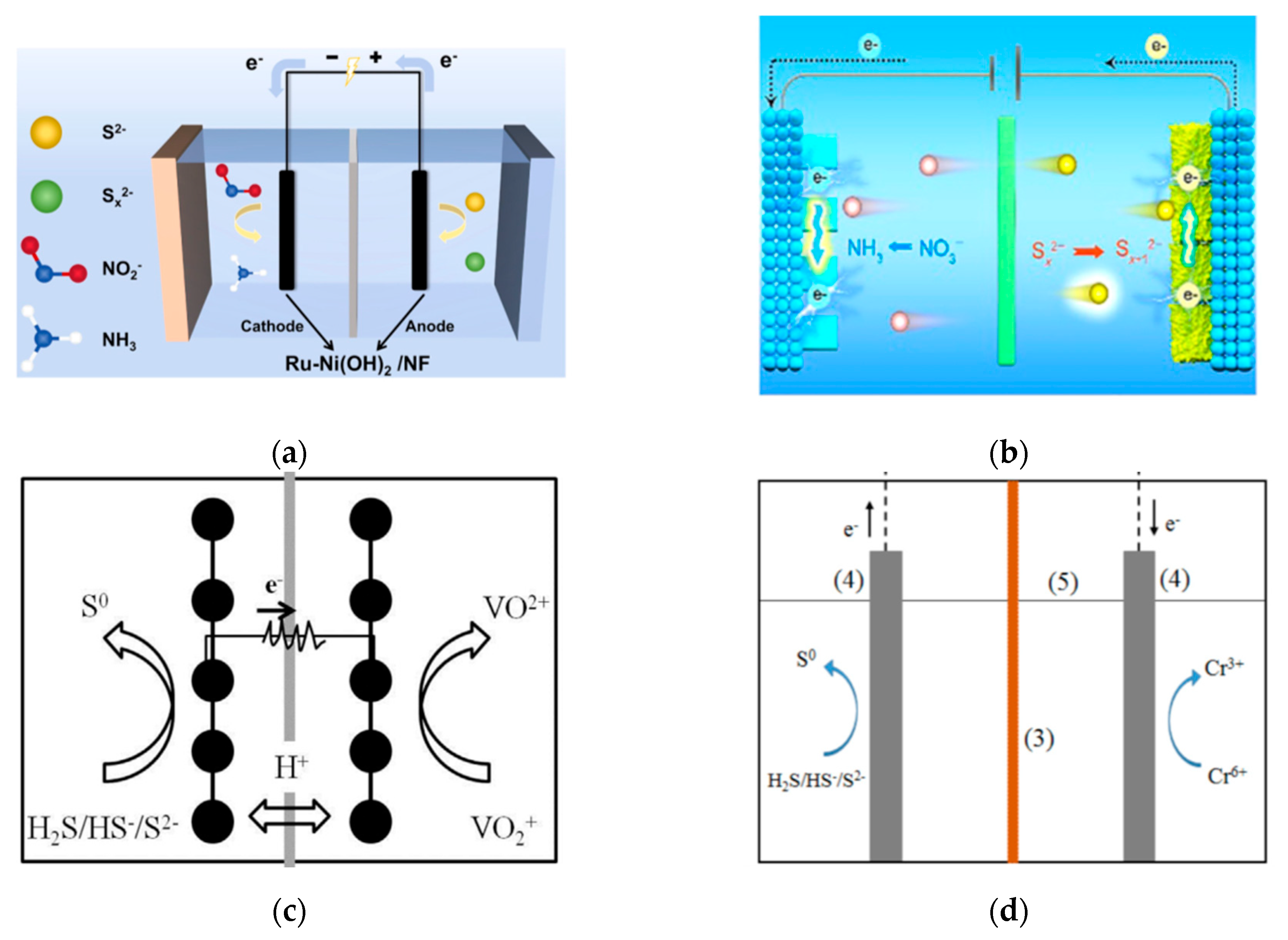
6.2. SOR Coupled with Oxidizing Pollutants Treatment
7. Conclusions and Perspectives
7.1. Conclusions
7.2. Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qiao, L.; Bai, J.; Luo, T.; Li, J.H.; Zhang, Y.; Xia, L.G.; Zhou, T.S.; Xu, Q.J.; Zhou, B.X. High yield of H2O2 and efficient S recovery from toxic H2S splitting through a self-driven photoelectrocatalytic system with a microporous GDE cathode. Appl. Catal. B 2018, 238, 491–497. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Lu, C.; Wang, J.; Qian, Y.Y.; Wang, B.W.; Zhang, Q.; Tang, A.D.; Yang, H.M. Bifunctional Co3S4 nanowires for robust sulfion oxidation and hydrogen generation with low power consumption. Adv. Funct. Mater. 2023, 33, 2212183. [Google Scholar] [CrossRef]
- Yu, Z.; Deng, Z.P.; Li, Y.; Wang, X.L. Advances in electrocatalyst design and mechanism for sulfide oxidation reaction in hydrogen sulfide splitting. Adv. Funct. Mater. 2024, 34, 2403435. [Google Scholar] [CrossRef]
- National Minerals Information Center. Sulfur Statistics and Information. Available online: https://www.usgs.gov/centers/national-minerals-information-center/sulfur-statistics-and-information (accessed on 29 October 2025).
- Wang, H.Q.; Song, S.; Zhang, Z.B.; Xin, L.Q.; Wang, T.Y.; Wang, L. A novel process of low-temperature fractionation combined with extractive distillation for H2S removal from natural gas. Sep. Purif. Technol. 2022, 302, 122102. [Google Scholar] [CrossRef]
- Song, Z.P.; Luo, T.F.; Ke, J.R.; Hu, S.S.; Yang, W.Q.; Ni, J.C.; Chen, X.P.; Chen, Z.H. A new-style pohotoelectrochemical sensing device based on NH2-UiO-66@Bi2O3 for the sensitive detection of hydrogen sulfide. Microchem. J. 2024, 206, 111669. [Google Scholar] [CrossRef]
- Sergienko, N.; Radjenovic, J. Manganese oxide-based porous electrodes for rapid and selective (electro)catalytic removal and recovery of sulfide from wastewater. Appl. Catal. B 2020, 267, 118608. [Google Scholar] [CrossRef]
- Pokorna, D.; Zabranska, J. Sulfur-oxidizing bacteria in environmental technology. Biotechnol. Adv. 2015, 33, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, V.B.; Dinamarca, C. Electrochemical and bioelectrochemical sulphide removal: A review. Rev. Environ. Sci. Biotechnol. 2024, 23, 989–1014. [Google Scholar] [CrossRef]
- Sergienko, N.; Lumbaque, E.C.; Radjenovic, J. (Electro)catalytic oxidation of sulfide and recovery of elemental sulfur from sulfide-laden streams. Water Res. 2023, 245, 120651. [Google Scholar] [CrossRef]
- Shao, X.H.; Huang, Y.X.; Wood, R.M.; Tarpeh, W.A. Electrochemical sulfate production from sulfide-containing wastewaters and integration with electrochemical nitrogen recovery. J. Hazard. Mater. 2024, 466, 133527. [Google Scholar] [CrossRef]
- Sun, X.M.; Huang, W.J.; Xu, H.M.; Qu, Z.; Yan, N.Q. Recovery of elemental sulfur direct from nonferrous flue gas by a low-temperature Claus process with H2S as the interim reducer. Fuel 2024, 359, 130352. [Google Scholar] [CrossRef]
- Teng, X.; Shi, K.; Chen, L.S.; Shi, J.L. Coupling electrochemical sulfion oxidation with CO2 reduction over highly dispersed p-Bi nanosheets and CO2-assisted sulfur extraction. Angew. Chem. 2024, 136, e202318585. [Google Scholar] [CrossRef]
- Zhou, Q.W.; Shen, Z.H.; Zhu, C.; Li, J.C.; Ding, Z.Y.; Wang, P.; Pan, F.; Zhang, Z.Y.; Ma, H.; Wang, S.Y.; et al. Nitrogen-doped CoP electrocatalysts for coupled hydrogen evolution and sulfur generation with low energy consumption. Adv. Mater. 2018, 30, 1800140. [Google Scholar] [CrossRef]
- Spedding, J.; Vujcich, M. Exchange of H2S between air and water. J. Geophys. Res. Ocean. 1982, 87, 8853–8856. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Z.X.; Wang, X.Y.; Xie, J. Hydrogen sulfide in seafood: Formation, hazards, and control. Trends Food Sci. Technol. 2024, 148, 104512. [Google Scholar] [CrossRef]
- Hedlund, F.H. Confined space hazards: Plain seawater, an insidious source of hydrogen sulfide. J. Occup. Environ. Hyg. 2023, 20, 322–328. [Google Scholar] [CrossRef]
- Lebrun, M.N.; Dorber, M.; Verones, F.; Henderson, A.D. Novel endpoint characterization factors for Life cycle impact assessment of terrestrial acidification. Ecol. Indic. 2025, 171, 113241. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, R.; Yu, W. The effects of PM2.5 concentrations and relative humidity on atmospheric visibility in beijing. J. Geophys. Res. Atmos. 2019, 124, 2235–2259. [Google Scholar] [CrossRef]
- Ma, Q.X.; Zhang, C.Y.; Liu, C.; He, G.Z.; Zhang, P.; Li, H.; Chu, B.W.; He, H. A review on the heterogeneous oxidation of SO2 on solid atmospheric particles: Implications for sulfate formation in haze chemistry. Crit. Rev. Environ. Sci. Technol. 2023, 53, 1888–1911. [Google Scholar] [CrossRef]
- Fedorov, Y.A.; Mikhailenko, A.V.; Dotsenko, I.V. Sulfide sulfur in water objects with different mineralization. Water Resour. 2019, 46, S59–S64. [Google Scholar] [CrossRef]
- Abdul-Kadhim, R.M.; Oleiwi, M.S. Effect of agricultural sulfur and humic acid on some growth indicators of potato. IOP Conf. Ser. Earth Environ. Sci. 2025, 1487. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Kesselmeier, J.; Schnug, E. Sulfur fertilization and fungal infections affect the exchange of H2S and COS from agricultural crops. J. Agric. Food Chem. 2012, 60, 7588–7596. [Google Scholar] [CrossRef]
- Pang, H.Y.; Gao, T.; Zhao, W.K.; Liu, L.; Liu, E.Z.; Wen, H.Y.; Sun, T. Construction of MoSe2/NixSey/NF Schottky heterojunction as electrocatalyst for water splitting and electrochemical oxidation for sulfur recovery. Fuel 2024, 374, 132532. [Google Scholar] [CrossRef]
- PiÉPlu, A.; Saur, O.; Lavalley, J.C.; Legendre, O.; NÉDez, C. Claus catalysis and H2S selective oxidation. Catal. Rev. 1998, 40, 409–450. [Google Scholar] [CrossRef]
- Dutta, P.K.; Rabaey, K.; Yuan, Z.G.; Rozendal, R.A.; Keller, J. Electrochemical sulfide removal and recovery from paper mill anaerobic treatment effluent. Water Res. 2010, 44, 2563–2571. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.B.; Kim, S.S.; Wie, J.J. Value-addition of wastes from petroleum refining process: Sulfur-rich polymers for sustainable and high-performanceoptical and energy applications. Acc. Mater. Res. 2024, 5, 625–639. [Google Scholar] [CrossRef]
- Zhang, J.; You, C.Y.; Lin, H.Z.; Wang, J. Electrochemical kinetic modulators in lithium–sulfur batteries: From defect-rich catalysts to single atomic catalysts. Energy Environ. Mater. 2022, 5, 731–750. [Google Scholar] [CrossRef]
- Yang, H.; Bai, J.; Zhou, T.S.; Zhou, C.H.; Xie, C.Y.; Zhang, Y.; Li, J.H.; Simchi, A.; Zhou, B.X. Electrochemical coupling conversion of sulfur-containing gaseous waste to treasure: A key review. Appl. Catal. A 2023, 654, 119085. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Gong, X.B.; Wang, J.L. Recovery of sulfur, generation of electricity and hydrogen peroxide from sulfion-rich wastewater using a novel self-driving photocatalytic fuel cell. Water Res. 2025, 275, 123232. [Google Scholar] [CrossRef]
- Huo, J.Y.; Jin, L.J.; Chen, C.C.; Chen, D.Y.; Xu, Z.C.; Wilfred, C.D.; Xu, Q.F.; Lu, J.M. Improving the sulfurophobicity of the NiS-doping CoS electrocatalyst boosts the low-energy-consumption sulfide oxidation reaction process. ACS Appl. Mater. Interfaces 2023, 15, 43976–43984. [Google Scholar] [CrossRef]
- Huo, J.Y.; Liu, Q.; Liu, X.F.; Cheng, X.F.; Chen, D.Y.; Li, N.J.; Liao, K.; Xu, Q.F.; Lu, J.M. Sulfur recovery assisted electrochemical water splitting for H2 production using CoMo-based nanorod arrays catalysts. ACS Mater. Lett. 2024, 6, 2633–2641. [Google Scholar] [CrossRef]
- Pei, Y.H.; Li, D.; Qiu, C.T.; Yan, L.; Li, Z.M.; Yu, Z.X.; Fang, W.Z.; Lu, Y.Y.; Zhang, B. High-entropy sulfide catalyst boosts energy-saving electrochemical sulfion upgrading to thiosulfate coupled with hydrogen production. Angew. Chem. Int. Ed. 2024, 63, e202411977. [Google Scholar] [CrossRef]
- Sergienko, N.; Irtem, E.; Gutierrez, O.; Radjenovic, J. Electrochemical removal of sulfide on porous carbon-based flow-through electrodes. J. Hazard. Mater. 2019, 375, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhou, Q.W.; Shen, Z.H.; Jin, X.; Zhang, Y.C.; Shi, M.; Zhou, J.; Liu, J.G.; Lu, Z.D.; Zhou, Y.N.; et al. Sulfophobic and vacancy design enables self-cleaning electrodes for efficient desulfurization and concurrent hydrogen evolution with low energy consumption. Adv. Funct. Mater. 2021, 31, 2101922. [Google Scholar] [CrossRef]
- Huang, C.; Yu, J.; Zhang, C.Y.; Cui, Z.B.; Chen, J.K.; Lai, W.H.; Lei, Y.J.; Nan, B.F.; Lu, X.; He, R.; et al. Electronic spin alignment within homologous NiS2/NiSe2 heterostructures to promote sulfur redox kinetics in lithium-sulfur batteries. Adv. Mater. 2024, 36, 2400810. [Google Scholar] [CrossRef]
- Xia, M.; Chen, R.; Zhu, X.; Liao, Q.; An, L.; Wang, Z.B.; He, X.F.; Jiao, L. A micro photocatalytic fuel cell with an air-breathing, membraneless and monolithic design. Sci. Bull. 2016, 61, 1699–1710. [Google Scholar] [CrossRef]
- Zong, X.; Chen, H.J.; Seger, B.; Pedersen, T.; Dargusch, M.S.; McFarland, E.W.; Li, C.; Wang, L.Z. Selective production of hydrogen peroxide and oxidation of hydrogen sulfide in an unbiased solar photoelectrochemical cell. Energy Environ. Sci. 2014, 7, 3347–3351. [Google Scholar] [CrossRef]
- Bai, J.; Zhang, B.; Li, J.H.; Zhou, B.X. Photoelectrocatalytic generation of H2 and S from toxic H2S by using a novel BiOI/WO3 nanoflake array photoanode. Front. Energy 2021, 15, 744–751. [Google Scholar] [CrossRef]
- Luo, T.; Bai, J.; Li, J.H.; Zeng, Q.Y.; Ji, Y.Z.; Qiao, L.; Li, X.Y.; Zhou, B.X. Self-driven photoelectrochemical splitting of H2S for S and H2 recovery and simultaneous electricity generation. Environ. Sci. Technol. 2017, 51, 12965–12971. [Google Scholar] [CrossRef] [PubMed]
- Zong, X.; Han, J.F.; Seger, B.; Chen, H.J.; Lu, G.Q.; Li, C.; Wang, L.Z. An integrated photoelectrochemical-chemical loop for solar-driven overall splitting of hydrogen sulfide. Angew. Chem. Int. Ed. 2014, 53, 4399–4403. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, J.L. Multivalent metal catalysts in Fenton/Fenton-like oxidation system: A critical review. Chem. Eng. J. 2023, 466, 143147. [Google Scholar] [CrossRef]
- Zheng, D.; Yang, X.Q.; Qu, D.Y. Reaction between lithium anode and polysulfide ions in a lithium-sulfur battery. ChemSusChem 2016, 9, 2348–2350. [Google Scholar] [CrossRef]
- He, D.T.; Yang, P.J.; Yang, K.Z.; Qiu, J.S.; Wang, Z.Y. Long-lasting hybrid seawater electrolysis enabled by anodic mass transport intensification for energy-saving hydrogen production. Adv. Funct. Mater. 2024, 34, 2407601. [Google Scholar] [CrossRef]
- Avetisyan, K.; Zweig, I.; Luther, G.W.; Kamyshny, A. Kinetics and mechanism of polysulfides and elemental sulfur formation by a reaction between hydrogen sulfide and δ-MnO2. Geochim. Cosmochim. Acta 2021, 313, 21–37. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Wang, J.W.; Wang, J.L. Photo-generated hydrated electrons for selective reduction of nitrate to dinitrogen: Selectivity and mechanism. Chem. Eng. J. 2024, 497, 154860. [Google Scholar] [CrossRef]
- Zhang, M.; Guan, J.; Tu, Y.C.; Chen, S.M.; Wang, Y.; Wang, S.H.; Yu, L.; Ma, C.; Deng, D.H.; Bao, X.H. Highly efficient H2 production from H2S via a robust graphene-encapsulated metal catalyst. Energy Environ. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Huang, Q.Q.; Chang, Q.Y.; Luo, Z.Y.; Xie, M.F.; Jiang, N.B.; Zhang, X.N.; Zhou, M.; Zhang, Y.H.; Xiao, P. Superimposing effect of electrocatalytic activity and photocatalytic activity for amorphous NiFe-sulfides photoanode in sulfion oxidation reaction. Surf. Interfaces 2024, 54, 105163. [Google Scholar] [CrossRef]
- Kim, K.Y.; Han, J.I. Carbon supported bimetallic Pd–Co catalysts for alkaline sulfide oxidation in direct alkaline sulfide fuel cell. Int. J. Hydrogen Energy 2015, 40, 4567–4572. [Google Scholar] [CrossRef]
- Haner, J.; Bejan, D.; Bunce, N.J. Electrochemical oxidation of sulfide ion at a Ti/IrO2-Ta2O5 anode in the presence and absence of naphthenic acids. J. Appl. Electrochem. 2009, 39, 1733–1738. [Google Scholar] [CrossRef]
- Pikaar, I.; Rozendal, R.A.; Yuan, Z.G.; Keller, J.; Rabaey, K. Electrochemical sulfide oxidation from domestic wastewater using mixed metal-coated titanium electrodes. Water Res. 2011, 45, 5381–5388. [Google Scholar] [CrossRef]
- Pei, Y.H.; Cheng, J.; Zhong, H.; Pi, Z.F.; Zhao, Y.; Jin, F.M. Sulfide-oxidation-assisted electrochemical water splitting for H2 production on a bifunctional Cu2S/nickel foam catalyst. Green Chem. 2021, 23, 6975–6983. [Google Scholar] [CrossRef]
- Bedoya Lora, F.; Hankin, A.; Kelsall, G.H. Photo-electrochemical hydrogen sulfide splitting using SnIV-doped hematite photo-anodes. Electrochem. Commun. 2016, 68, 19–22. [Google Scholar] [CrossRef]
- Bessekhouad, Y.; Mohammedi, M.; Trari, M. Hydrogen photoproduction from hydrogen sulfide on Bi2S3 catalyst. Sol. Energy Mater. Sol. Cells 2002, 73, 339–350. [Google Scholar] [CrossRef]
- Ren, J.T.; Chen, L.; Wang, H.Y.; Tian, W.W.; Wang, L.; Sun, M.L.; Feng, Y.; Zhai, S.X.; Yuan, Z.Y. Self-powered hydrogen production with improved energy efficiency via polysulfides redox. ACS Nano 2023, 17, 25707–25720. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; Ng, W.K.; Qiao, W.; Yang, Y.T.; Li, X.G.; Xi, B.J.; Yu, Y.; Fang, J.Y.; Li, P.; Xiong, S.L. Intercalation chemistry awakens transition metal hydroxide for boosted and sustained electrocatalytic sulfion oxidation. Angew. Chem. 2025, 137, e202511402. [Google Scholar] [CrossRef]
- Zhang, B.; Bai, J.; Zhang, Y.; Zhou, C.; Wang, P.B.; Zha, L.N.; Li, J.H.; Simchi, A.; Zhou, B.X. High yield of CO and synchronous S recovery from the conversion of CO2 and H2S in natural gas based on a novel electrochemical reactor. Environ. Sci. Technol. 2021, 55, 14854–14862. [Google Scholar] [CrossRef]
- Ma, W.G.; Wang, H.; Yu, W.; Wang, X.M.; Xu, Z.Q.; Zong, X.; Li, C. Achieving simultaneous CO2 and H2S conversion via a coupled solar-driven electrochemical approach on non-precious-metal catalysts. Angew. Chem. Int. Ed. 2018, 57, 3473–3477. [Google Scholar] [CrossRef]
- Liu, X.D.; Wang, X.Y.; Long, J.Q.; Xie, X.F.; Wu, L.; Wang, Z.J.; Fu, Y.X.; Chen, H.; Xiang, K.S.; Liu, H. Research on the selective electrocatalytic reduction of SO2 to recover S0 by Pb electrode. Metals 2023, 13, 569. [Google Scholar] [CrossRef]
- Jiao, S.L.; Fu, X.W.; Huang, H.W. Descriptors for the evaluation of electrocatalytic reactions: D-band theory and beyond. Adv. Funct. Mater. 2022, 32, 2107651. [Google Scholar] [CrossRef]
- Hua, W.X.; Shang, T.X.; Li, H.; Sun, Y.F.; Guo, Y.; Xia, J.Y.; Geng, C.N.; Hu, Z.H.; Peng, L.K.; Han, Z.Y.; et al. Optimizing the p charge of S in p-block metal sulfides for sulfur reduction electrocatalysis. Nat. Catal. 2023, 6, 174–184. [Google Scholar] [CrossRef]
- Sun, Y.F.; Wang, J.Y.; Shang, T.X.; Li, Z.J.; Li, K.H.; Wang, X.W.; Luo, H.R.; Lv, W.; Jiang, L.L.; Wan, Y. Counting d-orbital vacancies of transition-metal catalysts for the sulfur reduction reaction. Angew. Chem. Int. Ed. 2023, 62, e202306791. [Google Scholar] [CrossRef]
- He, R.Z.; Huang, X.Y.; Feng, L.G. Recent progress in transition-metal sulfide catalyst Regulation for improved oxygen evolution Reaction. Energy Fuels 2022, 36, 6675–6694. [Google Scholar] [CrossRef]
- Xia, H.; Shi, Z.D.; Gong, C.S.; He, Y.M. Recent strategies for activating the basal planes of transition metal dichalcogenides towards hydrogen production. J. Mater. Chem. A 2022, 10, 19067–19089. [Google Scholar] [CrossRef]
- Li, Y.F.; Duan, Y.X.; Zhang, K.; Yu, W.Z. Efficient anodic chemical conversion to boost hydrogen evolution with low energy consumption over cobalt-doped nickel sulfide electrocatalyst. Chem. Eng. J. 2022, 433, 134472. [Google Scholar] [CrossRef]
- Luo, M.; Yang, J.T.; Li, X.G.; Eguchi, M.; Yamauchi, Y.; Wang, Z.L. Insights into alloy/oxide or hydroxide interfaces in Ni–Mo-based electrocatalysts for hydrogen evolution under alkaline conditions. Chem. Sci. 2023, 14, 3400–3414. [Google Scholar] [CrossRef]
- Wang, Y.; Mi, J.X.; Wu, Z.S. Recent status and challenging perspective of high entropy oxides for chemical catalysis. Chem Catal. 2022, 2, 1624–1656. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, L.; Xu, W.; Xiong, C.; Chen, W.; Xiang, X.; Zhang, B.; Shang, H. Carbon-supported high-entropy Co-Zn-Cd-Cu-Mn sulfide nanoarrays promise high-performance overall water splitting. Nano Res. 2022, 15, 6054–6061. [Google Scholar] [CrossRef]
- Buckingham, M.A.; Ward-O’Brien, B.; Xiao, W.C.; Li, Y.; Qu, J.; Lewis, D.J. High entropy metal chalcogenides: Synthesis, properties, applications and future directions. Chem. Commun. 2022, 58, 8025–8037. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Li, J.Q.; Li, Y.J.; Shao, G.M.; Jia, Z.; Shen, B.L. Non-noble metal-based amorphous high-entropy oxides as efficient and reliable electrocatalysts for oxygen evolution reaction. Nano Res. 2022, 15, 8751–8759. [Google Scholar] [CrossRef]
- Guan, S.X.; Liang, H.; Wang, Q.M.; Tan, L.J.; Peng, F. Synthesis and phase stability of the high-entropy carbide (Ti0.2Zr0.2Nb0.2Ta0.2Mo0.2)C under extreme conditions. Inorg. Chem. Front. 2021, 60, 3807–3813. [Google Scholar] [CrossRef]
- Kim, K.; Han, J.-I. Carbon-supported bimetallic Pd-Ir catalysts for alkaline sulfide oxidation in direct alkaline sulfide fuel cell. J. Appl. Electrochem. 2015, 45, 533–539. [Google Scholar] [CrossRef]
- Li, J.C.; Hou, P.X.; Zhao, S.Y.; Liu, C.; Tang, D.M.; Cheng, M.; Zhang, F.; Cheng, H.M. A 3D bi-functional porous N-doped carbon microtube sponge electrocatalyst for oxygen reduction and oxygen evolution reactions. Energy Environ. Sci. 2016, 9, 3079–3084. [Google Scholar] [CrossRef]
- Deng, Z.P.; Gong, M.X.; Gong, Z.; Wang, X.L. Mesoscale mass transport enhancement on well-defined porous carbon platform for electrochemical H2O2 synthesis. Nano Lett. 2022, 22, 9551–9558. [Google Scholar] [CrossRef]
- Singh, R.; Chaudhary, S.; Yadav, S.; Patil, S.A. Bioelectrocatalytic sulfide oxidation by a haloalkaliphilic electroactive microbial community dominated by Desulfobulbaceae. Electrochim. Acta 2022, 423, 140576. [Google Scholar] [CrossRef]
- Słowiński, D.; Świerczyńska, M.; Grzelakowska, A.; Szala, M.; Kolińska, J.; Romański, J.; Podsiadły, R. Hymecromone naphthoquinone ethers as probes for hydrogen sulfide detection. Dye. Pigm. 2021, 196, 109765. [Google Scholar] [CrossRef]
- Xu, H.; Yan, W. Studies on the preparation of high efficient Ti/PbO2 electrode and degradation of acid red G. China Environ. Sci. 2017, 37, 2591–2598. [Google Scholar]
- Qi, F.L.; Wang, X.M.; Zhu, P.P.; Li, Q.D.; Wang, S.K.; Zhao, J.J. Asymmetric Fe2+/Fe3+-mediated flow-electrode capacitive deionization for the removal of chloride ions in reclaimed water. ACS Sustain. Chem. Eng. 2024, 12, 8609–8619. [Google Scholar] [CrossRef]
- Xu, N.; Wang, T.L.; Li, W.J.; Wang, Y.; Chen, J.J.; Liu, J. Tuning redox potential of anthraquinone-2-sulfonate (AQS) by chemical modification to facilitate electron transfer from electrodes in shewanella oneidensis. Front. Bioeng. Biotechnol. 2021, 9, 705414. [Google Scholar] [CrossRef]
- Blázquez, E.; Gabriel, D.; Baeza, J.A.; Guisasola, A.; Freguia, S.; Ledezma, P. Recovery of elemental sulfur with a novel integrated bioelectrochemical system with an electrochemical cell. Sci. Total Environ. 2019, 677, 175–183. [Google Scholar] [CrossRef]
- Rabaey, K.; Van de Sompel, K.; Maignien, L.; Boon, N.; Aelterman, P.; Clauwaert, P.; De Schamphelaire, L.; Pham, H.T.; Vermeulen, J.; Verhaege, M.; et al. Microbial Fuel Cells for Sulfide Removal. Environ. Sci. Technol. 2006, 40, 5218–5224. [Google Scholar] [CrossRef]
- Cao, B.C.; Zhao, Z.P.; Peng, L.L.; Shiu, H.Y.; Ding, M.N.; Song, F.; Guan, X.; Lee, C.K.; Huang, J.; Zhu, D.; et al. Silver nanoparticles boost charge-extraction efficiency in Shewanella microbial fuel cells. Science 2021, 373, 1336–1340. [Google Scholar] [CrossRef]
- Wu, J.; Cao, J.p.; Bi, H.l.; Zhang, J.; Cao, Q. Liquid-solid contact electrification and its effect on the formation of electric double layer: An atomic-level investigation. Nano Energy 2023, 111, 108442. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.M.; Ma, C.B.; Duan, X.G.; Li, S.; Li, N.; Liu, W.; Li, Y.; Fan, X.B.; Peng, W.C. Asymmetric S heteroatom coordinated dual-atom catalysts and coupled anodic sulfion oxidation to boost electrocatalysis oxygen reduction. Adv. Funct. Mater. 2025, 35, 2420157. [Google Scholar] [CrossRef]
- Mishra, P.; Saravanan, P.; Packirisamy, G.; Jang, M.; Wang, C.Y. A subtle review on the challenges of photocatalytic fuel cell for sustainable power production. Int. J. Hydrogen Energy 2021, 46, 22877–22906. [Google Scholar] [CrossRef]
- Wang, W.X.; Mao, Q.Q.; Deng, K.; Yu, H.J.; Wang, Z.Q.; Xu, Y.; Li, X.N.; Wang, L.; Wang, H.J. Sulfur-induced low crystallization of ultrathin Pd nanosheet arrays for sulfur Ion degradation-assisted energy-efficient H2 production. Small 2023, 19, 2207852. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.; Xie, Y.J.; Bai, X.; Fan, G.Y.; Yu, X.J. Ruthenium nanocluster-regulated electronic behaviors of nickel hydroxides for boosted coupled-upgrading of nitrite and sulfide pollutants while outputting energy through zinc-nitrite battery. J. Alloys Compd. 2025, 1039, 183232. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, Y.Y.; Zhao, D.L.; Fan, G.Y.; Long, Y. Synergy electrocatalysis between palladium and cobalt aligned on carbon nanosheet architectures for nitrate-to-ammonia conversion coupled with sulfur ion oxidation. J. Colloid Interface Sci. 2026, 701, 138688. [Google Scholar] [CrossRef]
- Baek, K.; Li, T.L.; Lee, C.; Li, W.Z.; Kim, K. MOF-derived CoSx as a bifunctional electrocatalyst for efficient sulfide oxidation and coupled ammonia synthesis. Green Chem. 2025, 27, 8637–8648. [Google Scholar] [CrossRef]
- Yang, M.; Hou, X.; Fu, R.; Wang, Z.; Hu, C.; Hu, G.; Zhuo, L.; Liu, X. Cation doping driven performance optimization of MoS2 nanoarrays for nitrate and sulfide co-electrolysis. Nano Res. 2025, 18, 94907778. [Google Scholar] [CrossRef]
- Yang, M.S.; Wei, T.R.; Zeng, C.H.; Zhang, J.W.; Liu, Y.F.; Luo, J.; Hu, G.Z.; Liu, X.J. CoNiOOH nanosheets array enables highly effective value-added chemicals production via nitrite and sulfide electrolysis. Chem. Eng. J. 2024, 498, 155799. [Google Scholar] [CrossRef]
- Zhang, Z.N.; Wang, X.H.; Du, Q.Y.; Hong, Q.L.; Ai, X.; Chen, Y.; Li, S.N. Defect-rich AuCu/CuS nanowires heterojunction for light-enhanced sulfur Ion electrooxidation coupled nitrite electroreduction. Adv. Energy Mater. 2025, 15, 2500176. [Google Scholar] [CrossRef]
- Wang, X.H.; Hong, Q.L.; Shao, L.Y.; Zhai, Q.G.; Jiang, Y.C.; Ai, X.; Chen, Y.; Li, S.N. Copper-nickel oxide nanosheets with atomic thickness for high-efficiency sulfur Ion electrooxidation assisted nitrate electroreduction to ammonia. Adv. Funct. Mater. 2024, 34, 2408834. [Google Scholar] [CrossRef]
- Kijjanapanich, P.; Kijjanapanich, P.; Annachhatre, A.P.; Esposito, G.; Lens, P.N.L. Spontaneous electrochemical treatment for sulfur recovery by a sulfide oxidation/vanadium(V) reduction galvanic cell. J. Environ. Manag. 2015, 149, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.G.; Zewail, T.M.; Zatout, A.A.; El-Ashtoukhy, E.S.Z.; Abdel-Aziz, M.H. Electrochemical removal of sulfide ions and recovery of sulfur from sulfide ions containing wastes. J. Ind. Eng. Chem. 2021, 94, 390–396. [Google Scholar] [CrossRef]
- Xiong, Y.R.; Wang, L.L.; Ning, P.; Luo, J.F.; Li, X.; Yuan, L.; Xie, Y.B.; Ma, Y.X.; Wang, X.Q. Constructing oxygen vacancy-enriched Fe3O4@MnO2 core-shell nanoplates for highly efficient catalytic oxidation of H2S in blast furnace gas. Sep. Purif. Technol. 2024, 336, 126234. [Google Scholar] [CrossRef]
- Peng, W.; Tan, H.T.; Liu, X.T.; Hou, F.; Liang, J. Perspectives on carbon-based catalysts for the two-electron oxygen reduction reaction for electrochemical synthesis of hydrogen peroxide: A minireview. Energy Fuels 2023, 37, 17863–17874. [Google Scholar] [CrossRef]
- Wu, Q.Y.; Cao, J.J.; Wang, X.; Liu, Y.; Zhao, Y.J.; Wang, H.; Liu, Y.; Huang, H.; Liao, F.; Shao, M.W.; et al. A metal-free photocatalyst for highly efficient hydrogen peroxide photoproduction in real seawater. Nat. Commun. 2021, 12, 483. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y.; Gong, X.B.; Wang, J.L. Photocatalytic degradation of chlorinated organic pollutants by ZnS@ZIF-8 composite through hydrogen peroxide generation by activating dioxygen under simulated sunlight irradiation. J. Colloid Interface Sci. 2024, 654, 1417–1430. [Google Scholar] [CrossRef]
- Liu, L.j.; Gao, M.Y.; Yang, H.f.; Wang, X.y.; Li, X.b.; Cooper, A.I. Linear conjugated polymers for solar-driven hydrogen peroxide production: The importance of catalyst stability. J. Am. Chem. Soc. 2021, 143, 19287–19293. [Google Scholar] [CrossRef]
- Tan, N.; Yang, Z.; Gong, X.B.; Wang, Z.R.; Fu, T.; Liu, Y. In situ generation of H2O2 using MWCNT-Al/O2 system and possible application for glyphosate degradation. Sci. Total Environ. 2019, 650, 2567–2576. [Google Scholar] [CrossRef]
- Gong, X.B.; Yang, Z.; Peng, L.; Zhou, A.L.; Liu, Y.L.; Liu, Y. In-situ synthesis of hydrogen peroxide in a novel Zn-CNTs-O2 system. J. Power Sources 2018, 378, 190–197. [Google Scholar] [CrossRef]
- Bo, X.Y.; Yang, C.J.; Li, B.L.; Fan, Y.K.; Li, J.Y.; Huang, H.M.; Kou, J.H.; Lu, C.H. Reverse quantum wells in gradient-doped CdS for photocatalytic H2O2 production. Inorg. Chem. Front. 2025, 12, 5177–5188. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Zheng, X.; Su, H.; Ling, Y.; Guo, R.; Zhang, M.S.; Wang, Q.X.; Niu, L. Regulating the bubble-water/catalyst interface microenvironment for accelerated electrosynthesis of H2O2 via optimizing oxygen functional groups on carbon black. Green Chem. 2025, 27, 3315–3325. [Google Scholar] [CrossRef]
- Garg, K.; Kumar, M.; Kaur, S.; Nagaiah, T.C. Electrochemical production of hydrogen from hydrogen sulfide using cobalt cadmium sulfide. ACS Appl. Mater. Interfaces 2023, 15, 27845–27852. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wang, S.; Chen, Y.X.; Zhang, Y.Y.; Feng, Y.F.; Zhang, G.Q. Screened d-p orbital hybridization in turing structure of confined nickel for sulfion oxidation accelerated hydrogen production. Angew. Chem. Int. Ed. 2025, 64, e202419572. [Google Scholar] [CrossRef] [PubMed]
| Electrodes Type | Anodes | Active Species of SOR | Products of SOR | References |
|---|---|---|---|---|
| Direct oxidation electrodes | CoNi@NGs | Co3+/Co2+, Ni3+/Ni2+ | S8 | [47] |
| NiFeS/ZnIn2S4 | Ni3+/Ni2+ | S8 | [48] | |
| Pd8Co2/C | Pd2+/Pd0, Co3+/Co2+, OHads | S8 | [49] | |
| Ti/IrO2-Ta2O5 | Ir4+/Ir3+ | S8 | [50] | |
| MMO/Ti | O2, OH | S0, S2O32−, SO42−, SO32− | [51] | |
| Ir/Ta/Ti | Ir4+/Ir3+, OH/O2 | S0, SO42−, S2O32− | [11] | |
| Controllable Sn2− producing electrodes | CuCoNiMnCrSx/NF | Cu2+/Cu+, Mn3+/Cr3+ | Sn2− | [33] |
| Cu2S/NF | Cu2+/Cu+ | Sn2− | [52] | |
| Sn4+-doped α-Fe2O3 | Fe3+/Fe2+ | Sn2− | [53] | |
| Bi2S3 | Bi3+/Bi2+ | Sn2− | [54] | |
| HEA-Mo2C/HPC | Sx2−/Sx+12− | Sn2− | [55] | |
| NF/Mo-Co(OH)2 | Co(OH)2 | Sn2− | [56] | |
| Medium circulation-driven indirect oxidation electrodes | Graphite felt | I−/I3− | S8 | [57] |
| WO3/SiPVC | I−/I3− | S8 | [1] | |
| WO3/FTO | I−/I3− | S0 | [40] | |
| n-Si@PEDOT/p-Si@Pt | Fe3+/Fe2+ | S0 | [41] | |
| G/GCS | EDTA-Fe3+/Fe2+ | S0 | [58] |
| Electrochemical Coupling Systems | Anodes | Products of SOR | Cathodes | Products in Cathodes | References |
|---|---|---|---|---|---|
| SOR coupled with the production of energy substances | Sn4+-doped α-Fe2O3 | Sn2− | Pt | H2 | [53] |
| Bi2S3 | Sn2− | Pt | H2 | [54] | |
| WO3/SiPVC | Sn2− | GDE | H2O2 | [1] | |
| p-Si | S | TiO2/Ti/n+p-Si | H2O2 | [38] | |
| WO3/FTO | S0 | Pt/SiPVC | H2 | [40] | |
| Cu2S/NF | Sn2− | Pt/Gr | H2 | [52] | |
| HEA-Mo2C/HPC | Sn2− | HEA-Mo2C/HPC | H2 | [55] | |
| CoS-NF | Sn2− | CoGa-NS-C | H2O2 | [84] | |
| CoNiS2/NF | S0/S2O32− | Pt | H2 | [85] | |
| a/c S-Pd NSA/NF | Sn2− | a/c S-Pd NSA/NF | H2 | [86] | |
| NiS2 | S0 | NiS2 | H2 | [35] | |
| Co-S NSs | Sn2− | p-Bi NSs | HCOOH | [13] | |
| n-Si@PEDOT/p-Si@Pt | S0 | p-Si@Pt | H2 | [41] | |
| NiS–CoS/CNF | S0 | Pt | H2 | [31] | |
| G/GCS | S0 | ZnO@Gr | CO | [58] | |
| SOR coupled with oxidizing pollutants treatment | Ru-Ni(OH)2/NF | S0 | Ru-Ni(OH)2/NF | NH3 | [87] |
| Pd-Co@NC/CC | S0 | Pd–Co@NC/CC | NH3 | [88] | |
| MOF-derived CoSx | S0 | MOF-derived CoSx | NH3 | [89] | |
| Ni-MoS2@ACF | S0 | Ni-MoS2@ACF | NH3 | [90] | |
| CoNiOOH/CC | S0 | CoNiOOH/CC | NH3 | [91] | |
| AuCu/CuS NWs | S0 | AuCu/CuS NWs | NH3 | [92] | |
| Cu-NiO UTNSs | S0 | Cu–NiO UTNSs | NH3 | [93] | |
| Gr | S0 | Gr | VO2+ | [94] | |
| Gr | S0 | Gr | Cr3+ | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, C.; Geng, X.; Liu, H.; Chen, Y.; Deng, X. Advances in Electrocatalytic Hydrogen Sulfide Splitting for Sulfur Recovery: From Reaction Mechanisms to Application. Catalysts 2025, 15, 1019. https://doi.org/10.3390/catal15111019
Chen C, Geng X, Liu H, Chen Y, Deng X. Advances in Electrocatalytic Hydrogen Sulfide Splitting for Sulfur Recovery: From Reaction Mechanisms to Application. Catalysts. 2025; 15(11):1019. https://doi.org/10.3390/catal15111019
Chicago/Turabian StyleChen, Chuntan, Xiangyong Geng, Hepei Liu, Yong Chen, and Xinshuang Deng. 2025. "Advances in Electrocatalytic Hydrogen Sulfide Splitting for Sulfur Recovery: From Reaction Mechanisms to Application" Catalysts 15, no. 11: 1019. https://doi.org/10.3390/catal15111019
APA StyleChen, C., Geng, X., Liu, H., Chen, Y., & Deng, X. (2025). Advances in Electrocatalytic Hydrogen Sulfide Splitting for Sulfur Recovery: From Reaction Mechanisms to Application. Catalysts, 15(11), 1019. https://doi.org/10.3390/catal15111019





