Abstract
The urgent global issue of climate change caused by rising carbon dioxide (CO2) levels has led to the widespread use of gas separation processes. Among the available processes, chemical absorption has received more attention due to its maturity and higher efficiency compared to others. However, the high energy consumption during the desorption step poses several technical challenges, limiting its industrial applications. To overcome those challenges, several research studies have been conducted to improve the performance of the desorption process. In particular, various types of catalysts have been tested to improve the performance of the CO2 desorption process. Among the available catalysts, Titanium Oxyhydrate (TiO(OH)2) has shown remarkable characteristics for replacing conventional catalysts, mainly due to its stability and the potential for increasing the CO2 desorption rate. However, limited studies have been conducted to evaluate the performance of the CO2 desorption process, especially by utilizing commercial solvents such as piperazine (PZ) promoted methyldiethanolamine (MDEA). Hence, this study aims to evaluate the stability of TiO(OH)2 as a catalyst during the CO2 desorption process using various characterization techniques. The CO2 desorption performance is also assessed under different operating conditions. Moreover, the regeneration energy is determined and reported as the sensible heat duty per released CO2. The results show no significant difference between fresh and cycled TiO(OH)2, indicating its substantial thermal stability. Furthermore, a notable rise of 19.58% is observed in desorption rate while utilizing TiO(OH)2 with a mass concentration of 5 wt%, reflecting less energy consumption. These findings suggest that TiO(OH)2 could serve as a transformative catalyst in industrial-scale CO2 desorption processes, potentially paving the way for more sustainable CO2 capture technologies.
1. Introduction
Excessive carbon dioxide (CO2) emissions from industrial and energy sectors are a major driver of global warming in developing countries [1]. While natural sources of CO2, emissions, such as biomass decomposition or respiration, are largely balanced by natural sinks, human-induced emissions from fossil fuel combustion have caused a persistent rise in atmospheric CO2 since the Industrial Revolution [2,3]. This increase has intensified the need for effective CO2 capture and utilization technologies to mitigate climate change [4,5,6,7].
Among the available separation processes, particularly chemical absorption, is regarded as the most promising technology due to its maturity, cost-effectiveness, and effectiveness in diluting CO2 streams [8,9]. To evaluate the performance of the absorption process, various factors must be considered. Notably, the solvent plays a crucial role, as key aspects such as CO2 loading capacity, reaction kinetics, regeneration energy requirements, and mass transfer characteristics are directly linked to the solvent’s properties [10,11]. Despite being the most advanced CO2 separation process, the performance of the conventional absorption columns is still restricted by an excessive footprint, operating/maintenance issues, and energy-intensive regeneration steps [12,13,14]. Several research studies have been conducted to eliminate these drawbacks and to improve the CO2 absorption process in terms of reducing operating and capital expenses (OPEX and CAPEX) by focusing on three different approaches:
- Utilization of alternative solvents.
- Development of alternative intensification technologies.
- Energy integration and thermal optimization of the process.
Among the above-mentioned approaches, the last one involves reducing energy usage and loss during the CO2 absorption process. It has been reported that between 50 and 80% of the energy requirements of the whole CO2 chemical absorption process are associated with the regeneration step. To reduce such high energy consumption during the regeneration process, absorbents that need less heat to regenerate should be used. Among the available absorbents, alkanolamines are considered to be the most extensively used absorbents in the CO2 absorption process [15]. Special attention is given to MDEA due to its low vapor pressure, high concentration capabilities, and resistance to degradation and corrosion [16]. Despite its various advantages, MDEA still suffers from a slow reaction rate with CO2. The slow reaction kinetics result in significant energy consumption during the desorption step. To address this issue, blending different chemical solvents with MDEA has been proposed [17]. Such blends can combine the beneficial characteristics of each solvent in the mixture while suppressing their undesirable properties. Thus, the performance of the resulting blends can exceed the expected performance of the individual solvents.
PZ has received significant attention for mixing with MDEA because of its high CO2 absorption rate and capacity. However, at higher PZ concentrations, increased viscosity could reduce the rate enhancement effect during both absorption and desorption steps. Therefore, there is a need to address this issue by preserving the rate-enhancement effect of MDEA/PZ blends while reducing the negative impact. In recent years, catalytic-aided CO2 desorption has been widely researched as it promises more efficient progress in CO2 capture. Various catalysts have been studied to improve the performance of MDEA/PZ solvent systems in CO2 absorption and desorption. Common catalysts include transition metal oxides, sulfated metal oxides, zeolites, mesoporous silica, and hybrid catalysts. However, these catalysts often face challenges such as limited thermal stability, high cost, and reduced efficiency under industrial conditions, shifting the attention toward TiO(OH)2 as a superior alternative.
The use of TiO(OH)2 in CO2 separation was first studied by Dutcher et al. [18], where nanoporous TiO(OH)2 was used as a catalyst for CO2 desorption in Na2CO3. The obtained results indicated that TiO(OH)2 has the potential to improve the desorption process by reducing the activation energy. Moreover, the catalyst demonstrated remarkable stability up to 300 °C. TiO(OH)2 stability was also reported in subsequent research conducted by Toan et al. [19]. According to the obtained results, the amount of CO2 desorbed increased by up to 1200%, and the catalyst remained unaffected after 50 cycles. This enhanced performance brings multiple advantages, as the fast desorption process allows for smaller reactors and lower capital costs, the low desorption temperature reduces energy requirements, and the use of an inorganic sorbent results in fewer toxic emissions. Later, the catalytic performance of TiO(OH)2 during the CO2 desorption process was evaluated in the presence of tetraethylenepentamine (TEPA). The results showed that the desorption kinetics required less regeneration energy compared to the non-catalytic system [20]. In addition to high stability and low regeneration energy demand, the regeneration temperature was also investigated by Lai et al. [21]. The results revealed that the presence of TiO(OH)2 has the potential to lower the regeneration temperature during the CO2 desorption process in MEA. This was attributed to the accelerated reactions originating from the ability of the hydroxyl group on TiO(OH)2 to donate and accept protons in proton-involved reactions.
Based on the above studies, TiO(OH)2 offers several advantages over other catalysts used in MDEA/PZ solvent systems. Unlike many conventional catalysts, TiO(OH)2 exhibits excellent thermal stability and resistance to degradation, ensuring consistent performance under industrial conditions. Its unique structure facilitates a higher CO2 desorption rate while requiring less regeneration energy, making it more energy efficient. Additionally, TiO(OH)2 is cost-effective compared to complex catalysts such as hybrid systems. It also avoids the operational challenges associated with high-viscosity systems or catalyst deactivation. Consequently, TiO(OH)2 proves to be a potential catalyst for improving the sustainability and efficiency of CO2 capture processes. Previous studies have shown that TiO(OH)2 exhibits desirable solid catalyst properties, such as high CO2 absorption capacity, long-term stability, and good regenerability with minimal degradation [20,21,22].
Nevertheless, further investigation on the application of TiO(OH)2 as a catalyst to enhance desorption performance is still needed, particularly in the context of its integration with commercial solvent systems like MDEA/PZ. This lack of comprehensive investigation creates a gap in understanding its practical effectiveness, and thus exploring the potential of TiO(OH)2 in combination with MDEA/PZ to improve desorption performance is crucial. Therefore, the main goal of the current study is the synthesis, characterization, and stability check of the TiO(OH)2 catalyst for CO2 desorption in the MDEA/PZ system. Subsequently, the potential of TiO(OH)2 in combination with MDEA/PZ solvents to improve CO2 desorption efficiency and reduce energy consumption during solvent regeneration is investigated. Key parameters for optimizing desorption kinetics and catalytic performance, including temperature, PZ concentration, and TiO(OH)2 concentration, are evaluated. Additionally, regeneration energy is analyzed to assess the efficiency and feasibility of integrating TiO(OH)2 into CO2 capture processes.
2. Results
In the following subsections, the obtained results are presented and critically analyzed.
2.1. Characterization of TiO(OH)2 and Cyclic Stability Evaluation
The stability of TiO(OH)2 was evaluated over five absorption-desorption cycles. As mentioned earlier to assess the stability, several characterization techniques were employed to provide substantial data about the stability and the performance of TiO(OH)2. For each test two samples, the fresh and cycled TiO(OH)2, were characterized.
Figure 1 shows the FTIR characterization results of those samples. According to the obtained results four distinct peaks were identified for each sample. A broad peak (1) was observed for both testing samples at 2500–3600 cm−1, which corresponds to O-H stretching vibrations, suggesting the presence of surface hydroxyl groups. This observation was attributed to adsorbed moisture on the TiO(OH)2 surface, consistent with previous studies investigating TiO(OH)2 [18,22,23]. The sharp peak (2), attributed to the H-O-H bending vibration, was also detected for both samples at 1630–1640 cm−1. TiO(OH)2, a hydrated form of titanium oxide, contains water molecules chemically bound to Ti-O-Ti bridges within its crystal structure. This bending vibration is known to be highly sensitive to hydrogen bonding and the interaction with neighboring water molecules. The position and intensity of this peak provided valuable insights into the nature of hydrogen bonding and the structural arrangement of water molecules within the sample. The small peak (3) at 1062 cm−1 was observed for both samples. Additionally, two smaller peaks were detected in the cycled sample at 1086 cm−1 and 1133 cm−1, which were related to the Ti-O-H group. It was suggested that these peaks corresponded to the acceleration of CO2 desorption [24]. This suggestion was based on the fact that TiO(OH)2 had been synthesized directly through the hydrolysis of Ti(OCH(CH3)2)4 [24,25].. A wide peak (4) was also observed in both testing samples within the range of 600–800 cm−1, which represented Ti-O bends [24].
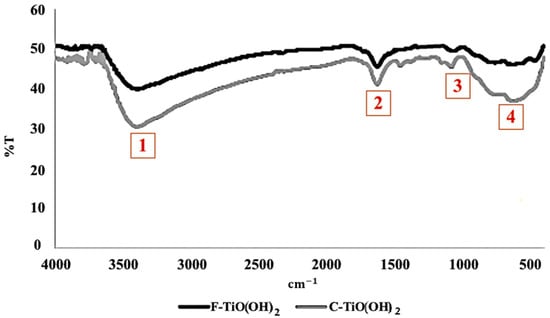
Figure 1.
FTIR spectra for Fresh and Cycled TiO(OH)2 in the range of 4000–400 cm−1.
The morphological features and particle size of the synthesized TiO(OH)2 were analyzed using FESEM at varying magnifications, including 5000× and 30,000×. Both the fresh and cycled TiO(OH)2 samples were characterized. Figure 2 presents the obtained results. Figure 2a,b show the FESEM images of the fresh sample at different magnifications, while Figure 2c,d show the cycled one at different magnifications. The comparison between the fresh and cycled TiO(OH)2 revealed that the basic structure of TiO(OH)2 was not affected by the heating process.
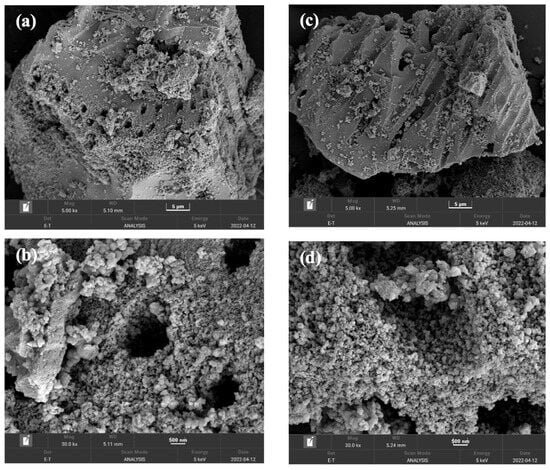
Figure 2.
FESEM imaging of (a) fresh TiO(OH)2 at 5 μm, (b) fresh TiO(OH)2 at 500 nm, (c) cycled TiO(OH)2 at 5 μm, and (d) cycled TiO(OH)2 at 500 nm.
In addition to FTIR and FESEM, the EDS technique was also employed to characterize both fresh and cycled TiO(OH)2 to provide detailed information on their elemental composition and to assess any changes in the material’s surface structure. The results are shown in Figure 3. The fresh TiO(OH)2 was found to contain 61.5 wt% Ti, 31.6 wt% O, and 6.9 wt% C. In contrast, the cycled TiO(OH)2 was found to contain 49.6 wt% Ti, 39.5 wt% O, and 10.9 wt% C. The Ti and O peaks were clearly observed in both EDS spectra, which confirmed their presence along with the corresponding weight percentages. Moreover, the presence of C in the spectra was likely attributed to the fact that TiO(OH)2 had been synthesized directly from the hydrolysis of Ti(OCH(CH3)2)4, which is an organometallic compound containing titanium, isopropyl groups, and oxygen atoms. Ti(OCH(CH3)2)4 is commonly used as a precursor in the synthesis of various titanium-containing materials through processes like hydrolysis or condensation reactions. It is also known to be reactive with moisture and air, which can lead to the formation of carbon-containing species on the surface of the synthesized catalyst, as evidenced by the results.
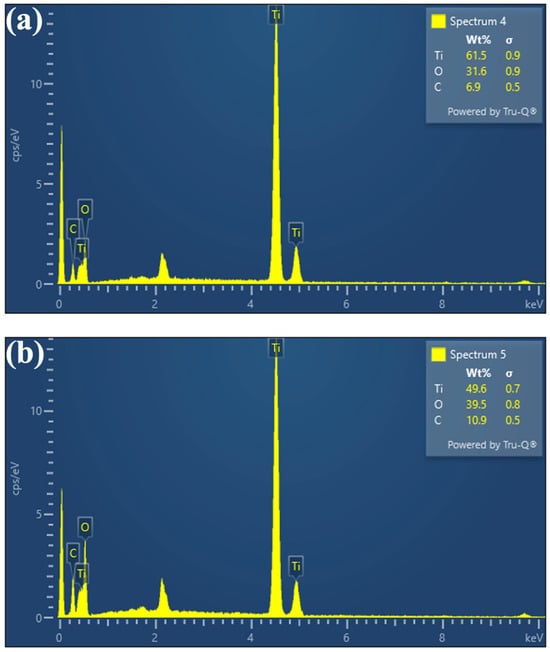
Figure 3.
EDS peaks for (a) Fresh and (b) Cycled TiO(OH)2.
The XRD results for fresh and cycled TiO(OH)2 are shown in Figure 4. Generally, the TiO(OH)2 was found to exhibit an amorphous nature. Nevertheless, minor traces of the anatase phase of TiO2 were observed to have developed. This was evident from the XRD pattern, which displayed three peaks centered around 2θ values of 27, 46, and 61 [26]. The XRD patterns for the fresh and cycled TiO(OH)2 were found to be identical, but with different intensities. This suggested that the crystal structure and phase composition of the catalyst had not changed significantly, but there may have been changes in other properties such as the particle size or preferred orientation of the crystal lattice.
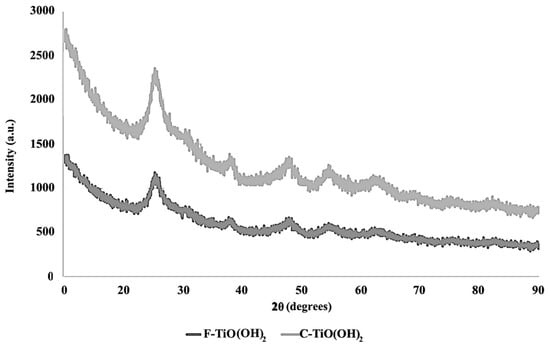
Figure 4.
XRD image of fresh and cycled TiO(OH)2 ranging from 10° to 80°, a scan step size of 0.5, and a scan speed of 0.2 s/step.
As the final characterization technique, the TGA test was performed to investigate the thermal stability and weight loss behavior of TiO(OH)2 under controlled heating conditions. Overall, no significant changes were observed in the TGA curves of the fresh and cycled TiO(OH)2, as can be seen in Figure 5. This suggested that TiO(OH)2 had maintained its thermal stability after the absorption-desorption cycle. This could indicate that the absorption-desorption process had not affected the thermal stability of TiO(OH)2, and it remained stable under the tested conditions. Initial weight loss of approximately 20% was observed in the temperature range of 50–200 °C which can be attributed to the transformation of TiO(OH)2 into a more stable form of titanium oxide, such as TiO2. This suggest that the conversion TiO2 occurs predominantly below 200 °C. A smaller additional weight loss of around 5% was detected between 200 and 500 °C, likely due to residual hydroxyl group or other volatile species on the oxide surface. Beyond 500 °C, the mass remained largely stable, implying no further significant decomposition under tested conditions.
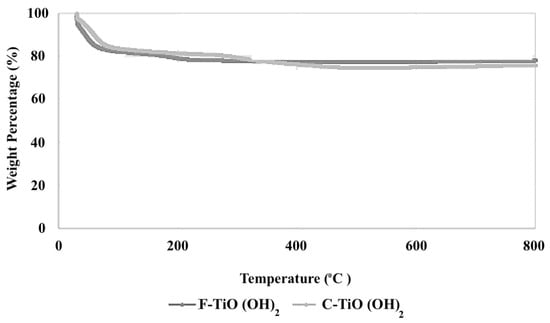
Figure 5.
TGA data of fresh and Cycled TiO(OH)2 at a heating rate of 10 °C/min.
2.2. CO2 Desorption Performance
The CO2 absorption performance of the TiO(OH)2-enhanced solvent is presented in the following subsections. The CO2 desorption rate of the solvent mixture was evaluated under various operating conditions, including TiO(OH)2 and PZ concentration ranges of 0–5 wt% and a temperature range of 80–110 °C.
2.2.1. Effect of TiO(OH)2 Concentration
Figure 6 illustrates the effect of various mass concentrations of TiO(OH)2 on the desorption rate and desorption capacity of a 45 wt% MDEA solvent blended with 2.5 wt% PZ at a constant temperature of 95 °C. The results indicated a slight increase in the desorption rate with increasing TiO(OH)2 concentration. The desorption rate at 0 wt% TiO(OH)2 was approximately 1.43 mol/hr, which increased to 1.62 mol/hr at 2.5 wt% TiO(OH)2, and further reached 1.71 mol/hr at 5 wt% TiO(OH)2. Similarly, the desorption capacity exhibited an increasing trend. At 0 wt% TiO(OH)2, the desorption capacity was 3.72 mol, which increased to 4.69 mol at 2.5 wt% TiO(OH)2, and showed a slight increase to 4.97 mol at 5 wt% TiO(OH)2. The overall percentage increases of 19.58% and 33.63% were observed for the desorption rate and desorption capacity, respectively. These values indicated that the presence of TiO(OH)2 had a positive impact on the desorption performance, leading to a significant improvement in both the rate and capacity of CO2 desorption. The observed percentage increase in desorption rate and capacity with the addition of TiO(OH)2 can be attributed to its catalytic properties. It has been reported that TiO(OH)2 can facilitate the interaction between CO2 and MDEA. Furthermore, it can enhance CO2 desorption from MDEA by accelerating proton-involved reactions, promoting the formation of bicarbonates [21] and facilitating the release of CO2 by breaking C-O bonds in the bicarbonate species [27].
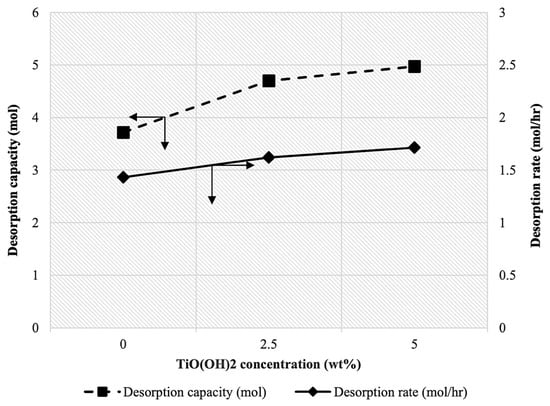
Figure 6.
Effect of TiO(OH)2 concentration on CO2 desorption rate and capacity at [MDEA] = 45 wt%, [PZ] = 2.5 wt%, and T= 95 °C.
At the microscopic level, the catalytic effect of TiO(OH)2 can be attributed to several structural and electronic features. The surface hydroxyl groups act as proton-transfer sites, which accelerate the decomposition of bicarbonate into CO2 and H2O. In addition, the amorphous nature of TiO(OH)2, as confirmed by our XRD results, provides a high density of unsaturated coordination sites that function as active sites for reaction. The Ti–O bonds also modifies the local electronic structure of the catalyst, lowering the activation energy barrier for proton-related reactions and thereby promoting faster CO2 release. These interpretations are consistent with previous reported results on catalytic desorption mechanisms [21,24,28] which demonstrate that TiO(OH)2 lowers the energy barrier for bicarbonate decomposition and accelerates proton-transfer kinetics. It should be noted that, in the CO2 desorption process, bicarbonate is heated to decompose and release CO2, therefore these combined effects lead to an enhanced CO2 desorption performance.
2.2.2. Effect of Temperature
Figure 7 shows the influence of temperature on the CO2 desorption rate. The absorbent used was a mixture containing 45 wt% of MDEA, 2.5 wt% of PZ, and 2.5 wt% of TiO(OH)2. The temperature is an important factor for evaluating the CO2 desorption performance, as it can significantly affect both the reaction kinetics and the equilibrium of the desorption process [29]. The obtained results revealed a clear trend in the desorption rate with increasing temperature. At 80 °C, the desorption rate was 1.05 mol/hr, with a desorption capacity of 3.06 mol. At 95 °C, both the CO2 desorption rate and capacity significantly increased to 1.62 mol/hr and 4.69 mol, respectively. The upward trend continued, with the desorption rate further rising to 2.04 mol/hr and the desorption capacity reaching 5.90 mol at 110 °C. Therefore, as evidenced by the 25.93% increase in desorption rate (from 1.62 mol/hr at 95 °C to 2.04 mol/hr at 110 °C) and a 25.83% rise in desorption capacity (from 4.69 mol at 95 °C to 5.90 mol at 110 °C), temperature plays a crucial role in CO2 desorption performance. The improved performance can be explained by the fact that the CO2 desorption process is endothermic, meaning that external energy must be supplied to drive the process [30].
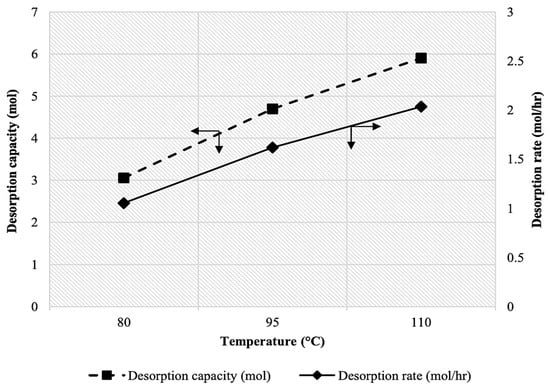
Figure 7.
Effect of temperature on CO2 desorption rate and capacity at [MDEA] = 45 wt%, [PZ] = 2.5 wt%, and [TiO(OH)2] = 2.5 wt%.
Typically, amine-based solutions are heated within the range of 100–150 °C to ensure that the thermodynamics of the CO2 desorption reaction are favorable [30,31]. This means that an increase in the operating temperature of the solvent would substantially result in a higher percentage of desorption and enhanced mass transfer rates [32]. Apart from that, the kinetic energy of the molecules in the solvent increases with an increase in the reaction temperature. This higher molecular energy facilitates more frequent and energetic collisions between the CO2 molecules and the catalyst. As a result of these collisions, the dissolved CO2 bubbles in the solvent are discharged more rapidly, leading to an increased desorption rate [28]. Furthermore, it should be noted that while increasing the temperature enhances the CO2 desorption rate, it also increases energy consumption. The optimal temperature for CO2 desorption depends on the specific system and operating conditions. By carefully controlling and monitoring the temperature, it is possible to achieve an optimal balance between desorption performance and energy efficiency, ultimately resulting in a more sustainable and cost-effective process.
2.2.3. Effect of PZ Concentration
Figure 8 illustrates the effect of PZ concentration on the CO2 desorption rate and capacity. The absorbent consisted of a blend containing 45 wt% MDEA, 2.5 wt% TiO(OH)2, and varying mass concentrations of PZ. The temperature was maintained at 95 °C. The results indicated a slight decrease in both the CO2 desorption rate and capacity was observed as the mass concentration of PZ increased from 0 to 5 wt%. Specifically, the desorption rate was reduced from 1.70 mol/hr at 0 wt% PZ to 1.62 mol/hr at 2.5 wt% PZ. A further reduction in desorption rate to 1.43 mol/hr was recorded at 5 wt% PZ. These values corresponded to an overall decrease of 15.88% in the CO2 desorption rate. A similar trend was observed for CO2 desorption capacity, with reductions noted as PZ concentration increased.
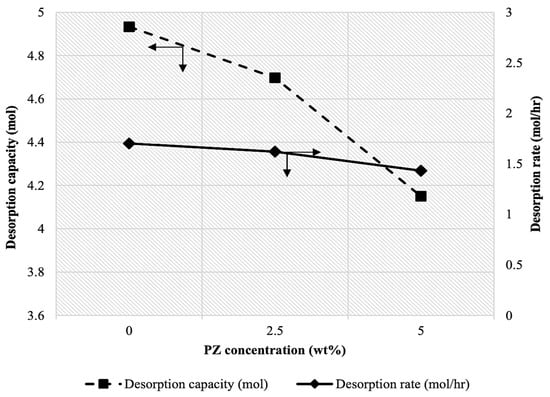
Figure 8.
Effect of PZ concentration on CO2 desorption rate and capacity at [MDEA] = 45 wt%, [TiO(OH)2] = 2.5 wt%, and T= 95 °C.
The decreasing trend of the CO2 desorption rate indicated that the addition of a promoter, such as PZ, significantly influenced both the equilibrium capacity of the solvent and its kinetic performance. While promoters can enhance CO2 absorption capacity and selectivity [33], they may also form stronger chemical bonds between CO2 and the solvent, making desorption more challenging.
PZ is commonly used as a promoter in CO2 absorption processes to enhance the absorption rate. When blended with MDEA, PZ accelerates the reaction kinetics, leading to improved CO2 capture efficiency. However, during the desorption phase, differences in the stability of the formed compounds influence the regeneration performance:
- In unpromoted MDEA systems, CO2 primarily forms bicarbonate ions, which are relatively unstable and decompose readily upon heating, facilitating efficient desorption.
- In PZ-promoted MDEA systems, PZ promotes the formation of carbamate species, which are more stable and decompose at a slower rate during desorption. This slower decomposition rate results in a reduced desorption rate for PZ-promoted MDEA compared to unpromoted MDEA.
These findings align with the research conducted by Kang et al. (2021) [33], which reported a similar correlation between PZ presence and CO2 desorption performance. Although PZ improves CO2 absorption in MDEA-based systems, it introduces stable carbamate formations that require higher energy for regeneration. This trade-off can potentially affect the overall energy efficiency of the CO2 capture process. Furthermore, the effect of PZ on CO2 desorption rate is complex and depends on various factors, such as temperature and the presence of other chemicals in the solvent. The findings presented here highlight the need for further investigation to optimize PZ concentration and operating conditions for enhanced performance.
2.3. Regeneration Energy
Regeneration energy is a critical factor in determining the efficiency and economic viability of the CO2 absorption-desorption process. It is often quantified as the sensible heat required per unit of CO2 released during the desorption phase. This value reflects the energy efficiency of the solvent regeneration process by measuring the thermal energy consumed in relation to the amount of CO2 desorbed. Evaluating this parameter helps optimize CO2 capture systems for reduced energy consumption and enhanced performance.
In this study, experiments were performed to examine the regeneration energy of various solvent blends and to assess the overall impact of TiO(OH)2 and PZ on the sensible heat requirements during the CO2 desorption process at operating temperatures of 80, 95, and 110 °C. The tested absorbents were single-component MDEA, MDEA/PZ blends, MDEA/TiO(OH)2 mixtures, and MDEA/PZ/TiO(OH)2 combinations. The results are shown in Figure 9.
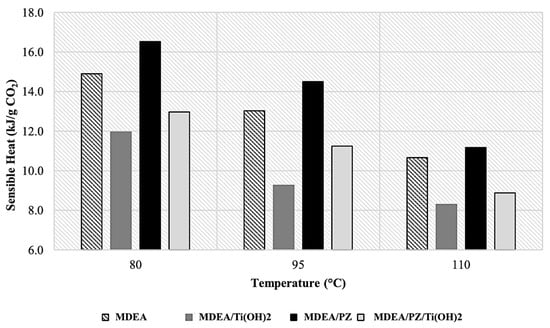
Figure 9.
Sensible heat evaluated per g of CO2 release.
Comparative studies of CO2 desorption performance indicate that, regardless of operating temperature, MDEA/PZ blends consistently exhibited the highest sensible heat values, whereas the MDEA/PZ/TiO(OH)2 combinations demonstrated the lowest energy demand. These differences can be attributed to the distinct properties and chemical mechanisms of promoters and catalysts during the CO2 absorption-desorption process.
PZ, acting as a promoter, enhances the CO2 absorption capacity of MDEA but forms stable carbamate species with CO2, which require additional energy for regeneration. This increased energy requirement is reflected in the higher sensible heat values for MDEA/PZ blends. Conversely, TiO(OH)2, used as a catalyst, has been reported to facilitate CO2 desorption by promoting reactions that assist carbamate decomposition, potentially lowering the energy barrier for CO2 release. While direct mechanistic evidence is not provided in this study, the previous literature supports this catalytic effect [21,34]. These findings suggest that while PZ acts as a promoter, enhancing absorption efficiency, the addition of TiO(OH)2 serves as a catalyst, improving desorption energy efficiency, highlighting the importance of customized solvent formulations in optimizing CO2 capture processes. Additionally, as illustrated in Figure 9, the low desorption rate observed at 80 °C contributed to a comparatively higher heat duty. Conversely, operating at a desorption temperature of 110 °C led to an increased reaction rate and a reduced energy demand. This also highlights the critical importance of temperature optimization in reducing energy consumption during the CO2 desorption process.
2.4. Comparative Analysis of TiO(OH)2
To clarify the potential of TiO(OH)2, a comparative evaluation with other catalyst reported in the literature is presented in Table 1. This evaluation further highlights the differences in desorption performance as well as the mechanistic features that contribute to the improved desorption performance of TiO(OH)2.

Table 1.
Comparative desorption performance of different catalysts in amine-based CO2 capture systems.
3. Materials and Methods
3.1. Materials
The chemicals required for the synthesis of TiO(OH)2 were Titanium Isopropoxide (Ti(OCH(CH3)2)4), deionized water (DI H2O), and ethanol (C2H5OH). The detailed procedure for preparing TiO(OH)2 is provided in Section 3.2. The Ti(OCH(CH3)2)4 was purchased from Sigma Aldrich with a purity of 97%. The ethanol solution, with a 99.9% purity, was purchased from VWR Chemicals BDH. The MDEA (CH3N(C2H4OH)2) and PZ (C4H10N2), both with a purity above 99%, were supplied by R&M Chemicals and Sigma-Aldrich, Malaysia, respectively. The DI H2O required to prepare aqueous solutions was produced in the lab.
3.2. Preparation of TiO(OH)2
Initially, 25 mL of Ti(OCH(CH3)2)4 was added to 350 mL of DI H2O at room temperature, followed by continuous stirring at 400 rpm for 4 h [21]. During this process, the Ti–O bonds reacted with water (hydrolysis), helped by the electron-withdrawing nature of the titanium center, which made it easier for water molecules to attack. This led to the formation of Ti–OH groups, which then linked together to form Ti–O–Ti bonds, reorganizing the molecules into a three-dimensional TiO(OH)2 network [38,39]. The resulting white precipitate was separated using vacuum filtration and rinsed three times consecutively with DI H2O and then by C2H5OH. It should be noted that C2H5OH was used as a rinsing agent to remove any impurities or residual reactants from the TiO(OH)2 precipitate [40]. Finally, the wet TiO(OH)2 was dried in an oven at 110 °C overnight (approximately 14 h) [21]. The overall process for synthesizing TiO(OH)2 is shown in Figure 10.
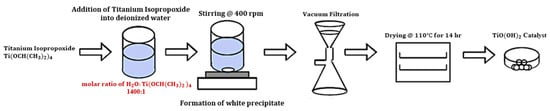
Figure 10.
Schematic overview of TiO(OH)2 synthesis from Ti(OCH(CH3)2)4.
3.3. Characterization of TiO(OH)2
Ti(OH)2 was characterized using four different techniques, including Fourier Transform Infrared Spectroscopy (FTIR), Field Emission Scanning Electron Microscopy (FESEM), Thermogravimetric Analysis (TGA), and X-ray Diffraction (XRD).
The FTIR spectra were recorded using a Frontier 01 FTIR spectrometer (Perkin Elmer, Shelton, CT, USA) in the range of 4000–400 cm−1. This technique was used to identify the functional groups present and evaluate its stability. The FTIR spectra of the fresh and cycled TiO(OH)2 were compared to identify any changes in the functional groups or chemical bonds present in the catalyst. Subsequently, a Supra 55 FESEM (Carl Zeiss, Oberkochen, Germany) operated at 15 kV was used to study the microscopic appearance of the TiO(OH)2 catalyst before and after the CO2 desorption test. It should be noted that, the FESEM images were used to evaluate any changes in particle size or morphology that may have occurred as a result of exposure to the high temperature of a CO2 desorption process. Moreover, in this study, TGA measurements were performed on both fresh and cycled TiO(OH)2 samples using an STA 6000 thermal analyzer (Perkin Elmer, USA) at a heating rate of 10 °C/min from room temperature up to 800 °C. The samples were heated in an alumina crucible, and their weight was continuously monitored as a function of temperature. It is worth noting that the TGA data were used to determine the weight loss and thermal stability of the samples. Finally, The XRD tests were performed using an Xpert3 Powder diffraction system (Malvern Panalytical, Malvern, UK) ranging from 10° to 80° and a scan step size of 0.5 and a scan speed of 0.2 s/step. The XRD analysis was used to compare the crystal structure and phase purity of fresh and cycled TiO(OH)2.
The information obtained from all of these characterization techniques provided sufficient details about the chemical and physical properties of TiO(OH)2, such as chemical composition, morphology, crystal structure, and phase purity. The results of fresh and cycled TiO(OH)2 were compared through these characterization techniques, providing valuable insights into the material’s properties and stable performance.
3.4. Process Description
The experiment was performed in an equilibrium cell where the changes in pressure inside the cell were observed and used to evaluate the absorption and desorption performance of CO2 in the presence of TiO(OH)2. Three key parameters—operating temperature, PZ concentration, and TiO(OH)2 concentration—were experimentally investigated.
The operating temperature was studied within the range of 80–110 °C. It is worth noting that the temperature range for the investigation was selected based on the standard operating conditions commonly employed for CO2 desorption with MDEA solvent. The concentrations of PZ and TiO(OH)2 were also assessed at 0, 2.5, and 5 wt%. The significance of including 0 wt% as a parameter was to establish a baseline for comparison, providing a reference point to evaluate the effects of other concentrations.
For each test, 100 mL of a freshly prepared 45 wt% MDEA aqueous solution was mixed with varying mass concentrations of PZ and TiO(OH)2. The 45 wt% MDEA concentration was selected because it is the most commonly used composition in commercial CO2 capture applications. This choice was supported by its proven efficiency and performance [41,42].
Initially, a vacuum condition was established to facilitate the CO2 absorption process. At each temperature setpoint, CO2 was introduced into the cell through a mixing vessel at a set operating pressure of 15 bar. A noticeable pressure decrease was recorded, and once the pressure stabilized at a fixed value, maximum absorption was considered achieved.
For the desorption process, the remaining CO2 gas pressure in the solubility cell was purged by opening the gas valve until the pressure inside the equilibrium cell reached 0 bar. The valve was then closed, and the stirrer was activated. The pressure increase was monitored and recorded until no further changes were observed, indicating that maximum desorption had occurred. The following Equation was used to determine the CO2 desorption rate:
where ṅCO2 is the CO2 desorption rate, VT is the total volume of the cell, Z is the compressibility factor, R is the ideal gas constant, T is the absolute temperature, and indicates the change in the system pressure over time. Moreover, for calculating the compressibility factor, the Peng–Robinson Equation of state was used. Figure 11 and Figure 12 illustrate the schematic representation and the actual experimental setup, respectively.
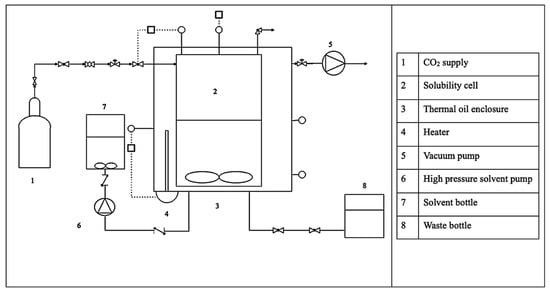
Figure 11.
Schematic diagram of the CO2 absorption/desorption system.
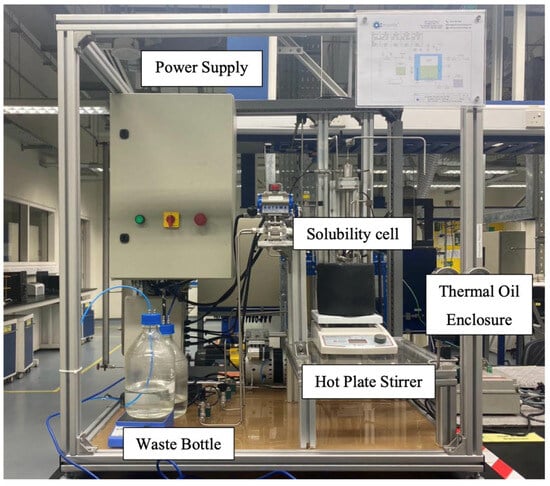
Figure 12.
Image of Solubility Cell Setup.
3.5. Estimation of Regeneration Energy
Oexmann et al. [43] proposed a simplified analysis of the regeneration energy during the CO2 absorption process. According to the proposed equation, the total heat duty for solvent regeneration (qreb) was expressed as the sum of three components, including sensible heat (qsens), heat of vaporization (), and heat of absorption () as shown in Equation (2).
represented the energy required to raise the temperature of the CO2-rich solvent to the desorption temperature within the regeneration column, which was calculated using Equation (3) [44]:
Here, was defined as the molar specific heat of the solvent (J/mol.K), was the desorption temperature (K), was the absorption temperature (K), was the molar weight of the solvent (g/mol), was the molar weight of CO2 (g/mol), was the molar fraction of the solvent in the solution, and was the cyclic CO2 absorption capacity (mol CO2/mol amine). The CO2 absorption capacity was determined using Equation (4):
To calculate a weighted average approach was used, based on the mass fractions of the components in the solvent mixture, as expressed in Equation (5):
where the masses of MDEA (), PZ (), TiO(OH)2 (), and H2O () in the solvent were expressed in gr, with the total solvent mass represented as (gr). The specific heat capacities in units of J/gr.K for each component (, , were calculated using established correlations, depending on temperature [45,46]. whereas as a function of temperature was readily available [47].
4. Conclusions
The significant potential of TiO(OH)2-enhanced MDEA as an effective approach for enhancing CO2 desorption efficiency was investigated in this study. MDEA and MDEA-PZ were evaluated with TiO(OH)2 introduced as a catalyst. Initially, through extensive analytical techniques, including FTIR, FESEM, XRD, and TGA, the thermal stability of TiO(OH)2 was assessed. The results indicated that TiO(OH)2 possesses remarkable thermal stability and resistance to degradation in both its fresh and cycled forms. Subsequently, the influence of operating temperature, TiO(OH)2 concentration, and PZ concentration on CO2 desorption performance was evaluated experimentally. The findings revealed that operating temperature plays a crucial role in the desorption process, as demonstrated by a 94.29% increase in the CO2 desorption rate when the temperature was raised from 80 to 110 °C. Finally, the regeneration energy analysis demonstrated significant improvements in energy efficiency when using the TiO(OH)2-enhanced MDEA/PZ absorbent. In conclusion, these findings highlight the potential of TiO(OH)2 in advancing CO2 capture technologies by addressing a key challenge in chemical absorption processes, which is the high energy demand associated with solvent regeneration. Future research should focus on optimizing TiO(OH)2 under varying operational conditions and explore its integration with different solvent systems to enhance its performance for large-scale CO2 capture applications.
Author Contributions
Conceptualization, K.K.L. and S.A.M.R.; writing—original draft preparation, K.K.L. and S.A.M.R.; writing—review and editing, K.K.L. and F.S.; supervision, K.K.L.; funding acquisition, K.K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This work was supported by YUTP-FRG (015LC0-509) and Universiti Teknologi PETRONAS.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CAPEX | Capital Expenses |
| Heat capacity | |
| FESEM | Field Emission Scanning Electron Microscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| GHG | Greenhouse Gas |
| MCM-41 | Mobil Composition of Matter No. 41 |
| MDEA | Methyldiethanolamine |
| OPEX | Operating Expenses |
| PZ | Piperazine |
| q | Heat Duty |
| R | Ideal Gas Constant |
| T | Temperature |
| TGA | Thermogravimetric Analysis |
| XRD | X-ray Diffraction |
| Z | Compressibility Factor |
References
- Huang, S.; Shen, J.; Wu, Y.; Li, X.; Ma, Y.; Xie, Y.; Yu, C.; Zhang, Y.; Zhang, J. Bi2O2CO3 Co-Catalyst Modification BiOBr Driving Efficient Photoreduction CO2. Colloids Surf. A Physicochem. Eng. Asp. 2025, 725, 137731. [Google Scholar] [CrossRef]
- Filonchyk, M.; Peterson, M.P.; Zhang, L.; Hurynovich, V.; He, Y. Greenhouse Gases Emissions and Global Climate Change: Examining the Influence of CO2, CH4, and N2O. Sci. Total Environ. 2024, 935, 173359. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Keong Lau, K.; Partoon, B.; Sze Lai, L. Parametric Quantification of Sonochemical Effect during the High Frequency Ultrasonic-Assisted Absorption of Bulk CO2. Sep. Purif. Technol. 2024, 331, 125723. [Google Scholar] [CrossRef]
- Machale, J.; Majumder, S.K.; Ghosh, P.; Sen, T.K. Development of a Novel Biosurfactant for Enhanced Oil Recovery and Its Influence on the Rheological Properties of Polymer. Fuel 2019, 257, 116067. [Google Scholar] [CrossRef]
- Zou, C.; Zhao, Q.; Zhang, G.; Xiong, B. Energy Revolution: From a Fossil Energy Era to a New Energy Era. Nat. Gas Ind. B 2016, 3, 1–11. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and Trends in CO2 Capture/Separation Technologies: A Review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Yang, H.; Xu, Z.; Fan, M.; Gupta, R.; Slimane, R.B.; Bland, A.E.; Wright, I. Progress in Carbon Dioxide Separation and Capture: A Review. J. Environ. Sci. 2008, 20, 14–27. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Lau, K.K.; Partoon, B. Experimental Evaluation of Chemical Reactions Involved in Ultrasonic-Assisted Absorption of Bulk CO2. Processes 2023, 11, 3266. [Google Scholar] [CrossRef]
- Cai, W.; Singham, D.I. A Principal–Agent Problem with Heterogeneous Demand Distributions for a Carbon Capture and Storage System. Eur. J. Oper. Res. 2018, 264, 239–256. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Lau, K.K.; Partoon, B.; Smith, A.M. A Review on the Selection Criteria for Slow and Medium Kinetic Solvents Used in CO2 Absorption for Natural Gas Purification. J. Nat. Gas Sci. Eng. 2022, 98, 104390. [Google Scholar] [CrossRef]
- Wang, M.; Lawal, A.; Stephenson, P.; Sidders, J.; Ramshaw, C. Post-Combustion CO2 Capture with Chemical Absorption: A State-of-the-Art Review. Chem. Eng. Res. Des. 2011, 89, 1609–1624. [Google Scholar] [CrossRef]
- Resnik, K.P.; Yeh, J.T.; Pennline, H.W. Aqua Ammonia Process for Simultaneous Removal of CO2, SO2 and NOx. Int. J. Environ. Technol. Manag. 2004, 4, 89–104. [Google Scholar] [CrossRef]
- Stuart Haszeldine, R. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Lau, K.K.; Tay, W.H. Performance Comparison of Ultrasonic-Assisted and Magnetic Stirred Absorption Methods for CO2 Separation. SN Appl. Sci. 2020, 2, 1217. [Google Scholar] [CrossRef]
- Puxty, G.; Rowland, R.; Allport, A.; Yang, Q.; Bown, M.; Burns, R.; Maeder, M.; Attalla, M. Carbon Dioxide Postcombustion Capture: A Novel Screening Study of the Carbon Dioxide Absorption Performance of 76 Amines. Environ. Sci. Technol. 2009, 43, 6427–6433. [Google Scholar] [CrossRef] [PubMed]
- Feyzi, V.; Beheshti, M.; Gharibi Kharaji, A. Exergy Analysis: A CO2 Removal Plant Using a-MDEA as the Solvent. Energy 2017, 118, 77–84. [Google Scholar] [CrossRef]
- Shokrollahi, F.; Lau, K.K.; Partoon, B.; Lai, L.S. Elucidation of Operating Parameters Influencing the Ultrasonic-Assisted Absorption of Bulk CO2 Using Unpromoted and Promoted Methyldiethanolamine. Ind. Eng. Chem. Res. 2023, 62, 2843–2865. [Google Scholar] [CrossRef]
- Dutcher, B.; Fan, M.; Leonard, B. Use of Multifunctional Nanoporous TiO(OH)2 for Catalytic NaHCO3 Decomposition-Eventually for Na2CO3/NaHCO3 Based CO2 Separation Technology. Sep. Purif. Technol. 2011, 80, 364–374. [Google Scholar] [CrossRef]
- Toan, S.; Lai, Q.; O’Dell, W.; Sun, Z.; Song, H.; Zhao, Y.; Radosz, M.; Adidharma, H.; Russell, C.; Yao, H.; et al. Green, safe, fast, and inexpensive removal of CO2 from aqueous KHCO3 solutions using a nanostructured catalyst TiO(OH)2: A milestone toward truly low-cost CO2 capture that can ease implementation of the Paris Agreement. Nano Energy 2018, 53, 508–512. [Google Scholar]
- Irani, M.; Gasem, K.A.M.; Dutcher, B.; Fan, M. CO2 Capture Using Nanoporous TiO(OH)2/Tetraethylenepentamine. Fuel 2016, 183, 601–608. [Google Scholar] [CrossRef]
- Lai, Q.; Toan, S.; Assiri, M.A.; Cheng, H.; Russell, A.G.; Adidharma, H.; Radosz, M.; Fan, M. Catalyst-TiO(OH)2 Could Drastically Reduce the Energy Consumption of CO2 Capture. Nat. Commun. 2018, 9, 2672. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Y.; Cai, T.; Chen, X.; Liu, D. Use of Nanoparticles Cu/TiO(OH)2 for CO2 Removal with K2CO3/KHCO3 Based Solution: Enhanced Thermal Conductivity and Reaction Kinetics Enhancing the CO2 Sorption/Desorption Performance of K2CO3/KHCO3. Greenh. Gases Sci. Technol. 2019, 9, 10–18. [Google Scholar] [CrossRef]
- Ataeivarjovi, E.; Liu, Y.; Tang, Z.; Ding, H.; Guo, D.; Song, X.; Ben, G.; Zhou, M. TiO(OH)2 Nanoparticle Composite Membranes for Enhancing the Hot Potash Process. ACS Appl. Nano Mater. 2020, 3, 3223–3232. [Google Scholar] [CrossRef]
- Yao, H.; Toan, S.; Huang, L.; Fan, M.; Wang, Y.; Russell, A.G.; Luo, G.; Fei, W. TiO(OH)2—Highly Effective Catalysts for Optimizing CO2 Desorption Kinetics Reducing CO2 Capture Cost: A New Pathway. Sci. Rep. 2017, 7, 2943. [Google Scholar] [CrossRef]
- Sui, R.; Rizkalla, A.S.; Charpentier, P.A. FTIR Study on the Formation of TiO2 Nanostructures in Supercritical CO2. J. Phys. Chem. B 2006, 110, 16212–16218. [Google Scholar] [CrossRef]
- Derks, P.W.J.; Kleingeld, T.; van Aken, C.; Hogendoorn, J.A.; Versteeg, G.F. Kinetics of Absorption of Carbon Dioxide in Aqueous Piperazine Solutions. Chem. Eng. Sci. 2006, 61, 6837–6854. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Cheng, Y.; Yu, Q.; Luo, X.; Li, C.; Li, J.; Zeng, Z.; Liu, Y.; Jiang, X.; et al. New Approach with Universal Applicability for Evaluating the Heat Requirements in the Solvent Regeneration Process for Postcombustion CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 3261–3268. [Google Scholar] [CrossRef]
- Li, T.; Yu, Q.; Barzagli, F.; Li, C.; Che, M.; Zhang, Z.; Zhang, R. Energy Efficient Catalytic CO2 Desorption: Mechanism, Technological Progress and Perspective. Carbon Capture Sci. Technol. 2023, 6, 100099. [Google Scholar] [CrossRef]
- Li, Y.; Liu, C.; Parnas, R.; Liu, Y.; Liang, B.; Lu, H. The CO2 Absorption and Desorption Performance of the Triethylenetetramine + N,N-Diethylethanolamine + H2O System. Chin. J. Chem. Eng. 2018, 26, 2351–2360. [Google Scholar] [CrossRef]
- de Meyer, F.; Bignaud, C. The Use of Catalysis for Faster CO2 Absorption and Energy-Efficient Solvent Regeneration: An Industry-Focused Critical Review. Chem. Eng. J. 2022, 428, 131264. [Google Scholar] [CrossRef]
- Feng, B.; Du, M.; Dennis, T.J.; Anthony, K.; Perumal, M.J. Reduction of Energy Requirement of CO2 Desorption by Adding Acid into CO2-Loaded Solvent. Energy Fuels 2009, 24, 213–219. [Google Scholar] [CrossRef]
- Aghel, B.; Sahraie, S.; Heidaryan, E. Comparison of Aqueous and Non-Aqueous Alkanolamines Solutions for Carbon Dioxide Desorption in a Microreactor. Energy 2020, 201, 117618. [Google Scholar] [CrossRef]
- Kang, S.; Shen, Z.; Shen, X.; Fang, L.; Xiang, L.; Yang, W. Experimental Investigation on CO2 Desorption Kinetics from MDEA + PZ and Comparison with MDEA/MDEA + DEA Aqueous Solutions with Thermo-Gravimetric Analysis Method. Greenh. Gases Sci. Technol. 2021, 11, 974–987. [Google Scholar] [CrossRef]
- Rozaiddin, S.A.M.; Lau, K.K. A Review on Enhancing Solvent Regeneration in CO2 Absorption Process Using Nanoparticles. Sustain. 2022, 14, 4750. [Google Scholar] [CrossRef]
- Shi, H.; Lv, J.; Feng, Y.; Zhang, H.; Xiong, Z.; Lu, S.; Jin, J.; Tontiwachwuthikul, P. Catalytic CO2 Desorption from MEA Solution of Al-FeOOH Composite Catalysts’ Desorption Performance, Structure–Activity Relationship, and New Mechanism. Catalysts 2024, 14, 779. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, Y.; Yang, J.; Gao, H.; Huang, Y.; Luo, X.; Liang, Z.; Tontiwachwuthikul, P. Amine-Based CO2 Capture Aided by Acid-Basic Bifunctional Catalyst: Advancement of Amine Regeneration Using Metal Modified MCM-41. Chem. Eng. J. 2020, 383, 123077. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, J.; Liu, H.; Luo, X.; Olson, W.; Tontiwachwuthikul, P.; Liang, Z. SO42−/ZrO2 Supported on γ-Al2O3 as a Catalyst for CO2 Desorption from CO2-Loaded Monoethanolamine Solutions. AIChE J. 2018, 64, 3988–4001. [Google Scholar] [CrossRef]
- Nawaratna, G.; Lacey, R.; Fernando, S.D. Effect of Hydrocarbon Tail-Groups of Transition Metal Alkoxide Based Amphiphilic Catalysts on Transesterification. Catal. Sci. Technol. 2012, 2, 364–372. [Google Scholar] [CrossRef]
- Aso, K.; Higashimine, K.; Miyata, M.; Kamio, H.; Oshima, Y. Three-Dimensional Atomic-Scale Characterization of Titanium Oxyhydroxide Nanoparticles by Data-Driven Lattice Correlation Analysis. Commun. Chem. 2025, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Mahshid, S.; Askari, M.; Ghamsari, M.S. Synthesis of TiO2 Nanoparticles by Hydrolysis and Peptization of Titanium Isopropoxide Solution. J. Mater. Process. Technol. 2007, 189, 296–300. [Google Scholar] [CrossRef]
- Bishnoi, S.; Rochelle, G.T. Absorption of Carbon Dioxide in Aqueous Piperazine/Methyldiethanolamine. AIChE J. 2002, 48, 2788–2799. [Google Scholar] [CrossRef]
- Mudhasakul, S.; Ku, H.M.; Douglas, P.L. A Simulation Model of a CO2 Absorption Process with Methyldiethanolamine Solvent and Piperazine as an Activator. Int. J. Greenh. Gas Control 2013, 15, 134–141. [Google Scholar] [CrossRef]
- Oexmann, J.; Kather, A. Minimising the Regeneration Heat Duty of Post-Combustion CO2 Capture by Wet Chemical Absorption: The Misguided Focus on Low Heat of Absorption Solvents. Int. J. Greenh. Gas Control 2010, 4, 36–43. [Google Scholar] [CrossRef]
- Srisang, W.; Osei, P.A.; Decardi-Nelson, B.; Tontiwachwuthikul, A.A.P.; Idem, R. Effect of Acid Catalysts on CO2 Absorption Process by Mixed Amines. Energy Procedia 2017, 114, 1514–1522. [Google Scholar] [CrossRef]
- Nguyen, Q.N.; Bauschlicher, C.W.; Myers, D.L.; Jacobson, N.S.; Opila, E.J. Computational and Experimental Study of Thermodynamics of the Reaction of Titania and Water at High Temperatures. J. Phys. Chem. A 2017, 121, 9508–9517. [Google Scholar] [CrossRef] [PubMed]
- Agbonghae, E.O.; Hughes, K.J.; Ingham, D.B.; Ma, L.; Pourkashanian, M. A Semi-Empirical Model for Estimating the Heat Capacity of Aqueous Solutions of Alkanolamines for CO2 Capture. Ind. Eng. Chem. Res. 2014, 53, 8291–8301. [Google Scholar] [CrossRef]
- Manyà, J.J.; Antal, M.J.; Kinoshita, C.K.; Masutani, S.M. Specific Heat Capacity of Pure Water at 4.0 MPa between 298.15 and 465.65 K. Ind. Eng. Chem. Res. 2011, 50, 6470–6484. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).