Photocatalytic Degradation of VOCs Using Ga2O3-Coated Mesh for Practical Applications
Abstract
1. Introduction
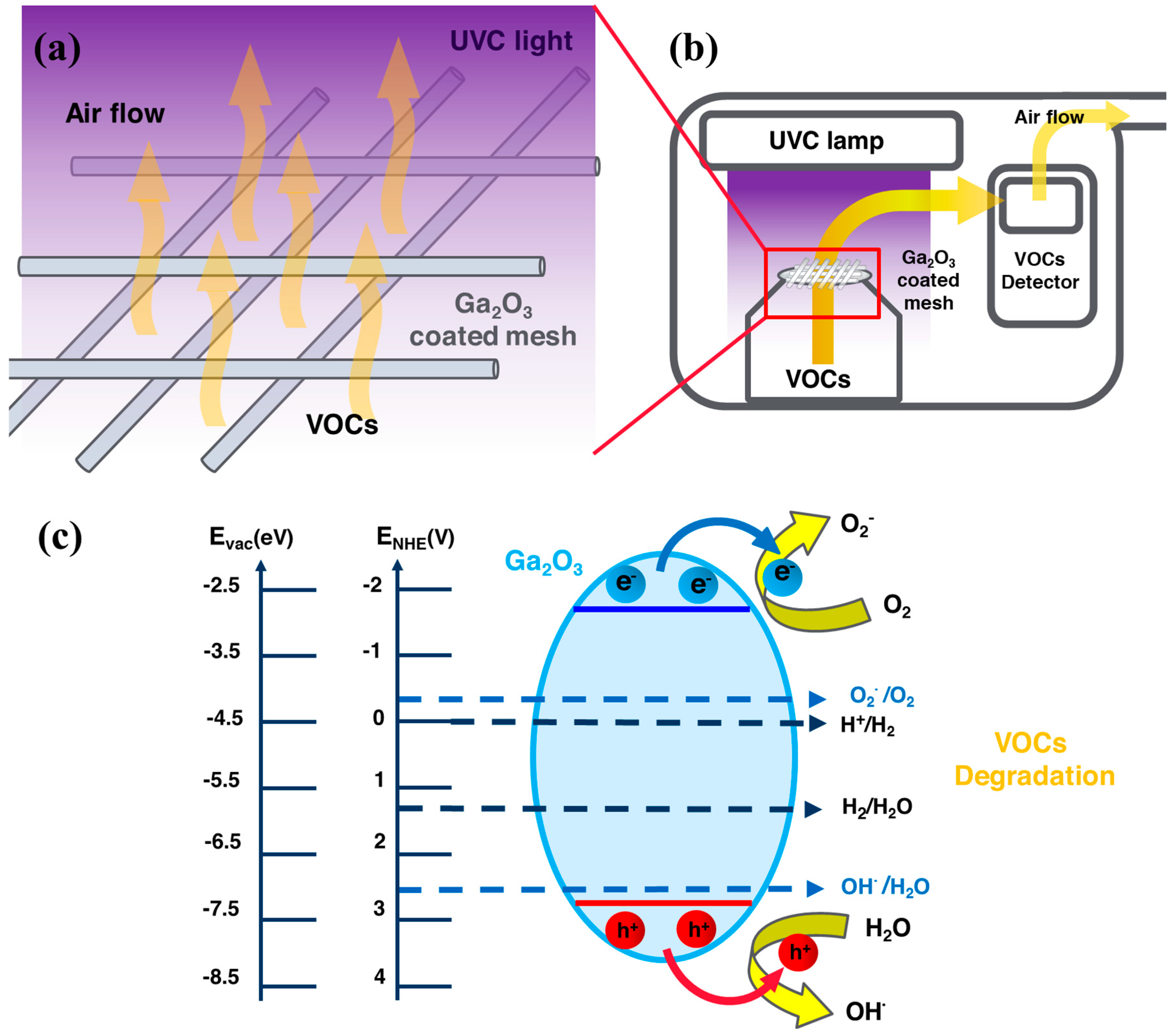
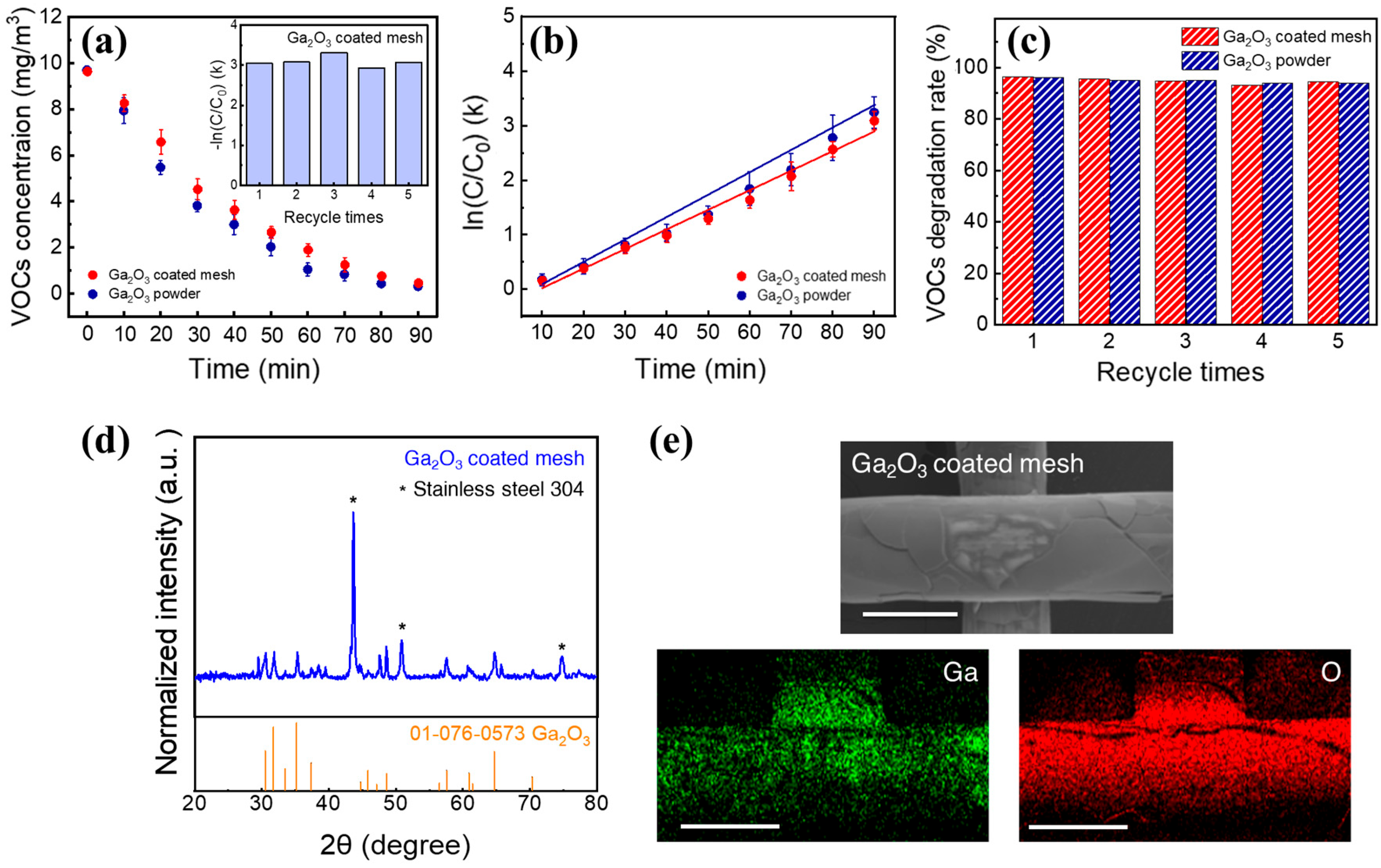
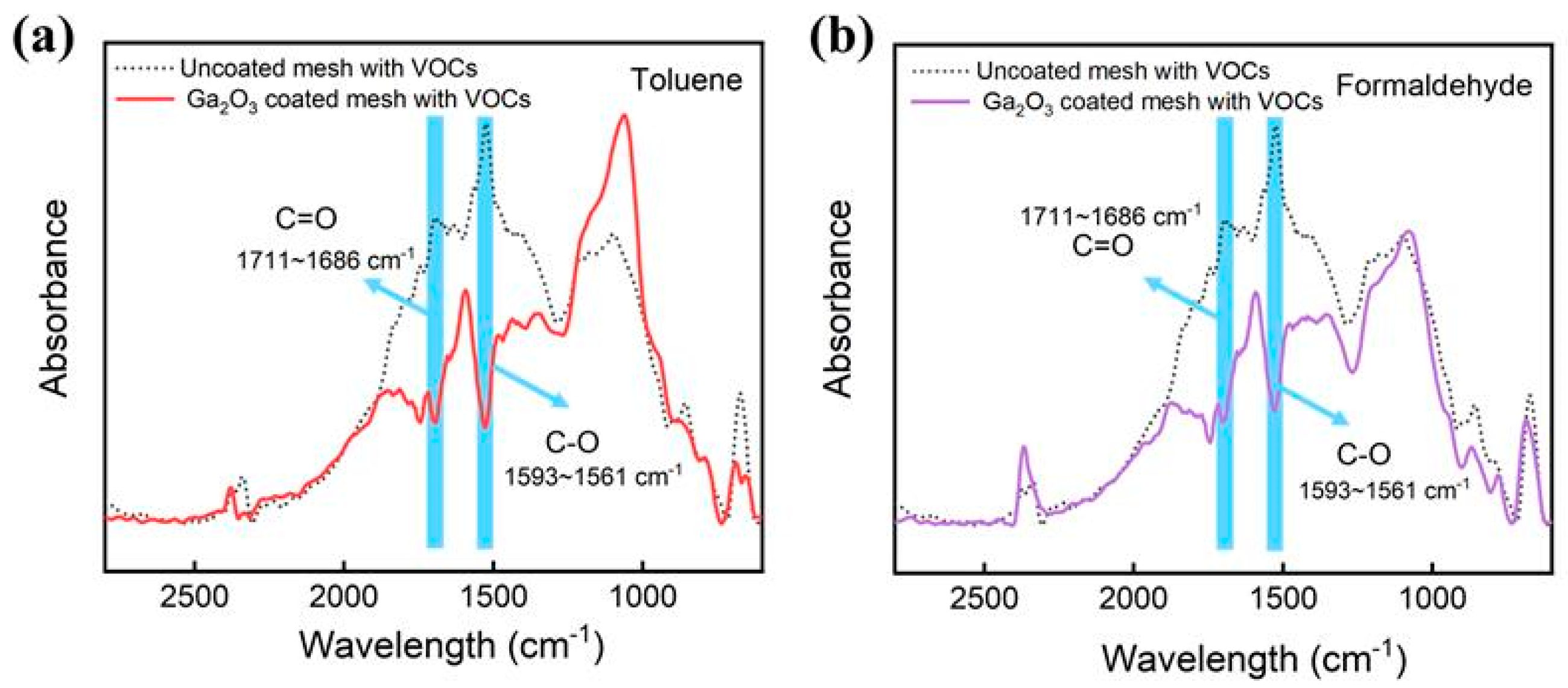
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, W.; Erickson, E.M.; Manthiram, A. High-nickel layered oxide cathodes for lithium-based automotive batteries. Nat. Energy 2020, 5, 26–34. [Google Scholar] [CrossRef]
- Li, S.; Lin, Y.; Liu, G.; Shi, C. Research status of volatile organic compound (VOC) removal technology and prospect of new strategies: A review. Environ. Sci. Process. Impacts 2023, 25, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Parmar, G.R.; Rao, N.N. Emerging control technologies for volatile organic compounds. Crit. Rev. Environ. Sci. Technol. 2008, 39, 41–78. [Google Scholar] [CrossRef]
- Gupta, A.K.; Modi, B.A. Selection of sustainable technology for VOC abatement in an industry: An integrated AHP–QFD approach. J. Inst. Eng. Ser. A 2018, 99, 565–578. [Google Scholar] [CrossRef]
- Chapuis, Y.; Klvana, D.; Guy, C.; Kirchnerova, J. Photocatalytic oxidation of volatile organic compounds using fluorescent visible light. J. Air Waste Manag. Assoc. 2002, 52, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Chai, Y.; Zhao, B.; Wei, W.; He, D.; He, B.; Tang, Q. Photocatalytic oxidation for degradation of VOCs. Open J. Inorg. Chem. 2013, 3, 14–25. [Google Scholar] [CrossRef]
- Masresha, G.; Jabasingh, S.A.; Kebede, S.; Doo-Arhin, D.; Assefa, M. A review of prospects and challenges of photocatalytic decomposition of volatile organic compounds (VOCs) under humid environment. Can. J. Chem. Eng. 2023, 101, 6905–6918. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Xie, R.; Huang, H.; Leung, M.K.H.; Li, J.; Leung, D.Y.C. Photocatalytic oxidation for volatile organic compounds elimination: From fundamental research to practical applications. Environ. Sci. Technol. 2022, 56, 16582–16601. [Google Scholar] [CrossRef]
- Debono, O.; Hequet, V.; Le Coq, L.; Locoge, N.; Thevenet, F. VOC ternary mixture effect on ppb level photocatalytic oxidation: Removal kinetic, reaction intermediates and mineralization. Appl. Catal. B Environ. 2017, 218, 359–369. [Google Scholar] [CrossRef]
- Sleiman, M.; Conchon, P.; Ferronato, C.; Chovelon, J.-M. Photocatalytic oxidation of toluene at indoor air levels (ppbv): Towards a better assessment of conversion, reaction intermediates and mineralization. Appl. Catal. B Environ. 2009, 86, 159–165. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; Van Langenhove, H. Heterogeneous photocatalysis as an advanced oxidation process for the abatement of chlorinated, monocyclic aromatic and sulfurous volatile organic compounds in air: State of the art. Crit. Rev. Environ. Sci. Technol. 2007, 37, 489–538. [Google Scholar] [CrossRef]
- Rao, Z.; Lu, G.; Chen, L.; Mahmood, A.; Shi, G.; Tang, Z.; Xie, X.; Sun, J. Photocatalytic oxidation mechanism of Gas-Phase VOCs: Unveiling the role of holes, •OH and •O2−. Chem. Eng. J. 2022, 430, 132766. [Google Scholar] [CrossRef]
- Yoo, T.H.; Ryou, H.; Lee, I.G.; Cho, J.; Cho, B.J.; Hwang, W.S. Comparison of Ga2O3 and TiO2 Nanostructures for Photocatalytic Degradation of Volatile Organic Compounds. Catalysts 2020, 10, 545. [Google Scholar] [CrossRef]
- Bae, H.J.; Yoo, T.H.; Kim, S.; Choi, W.; Song, Y.S.; Kwon, D.-K.; Cho, B.J.; Hwang, W.S. Enhanced photocatalytic degradation of 2-butanone using hybrid nanostructures of gallium oxide and reduced graphene oxide under ultraviolet-C irradiation. Catalysts 2019, 9, 449. [Google Scholar] [CrossRef]
- Lee, J.-H.; Doan, T.A.; Park, Y.J.; Hoa, H.T.M.; Phuong, P.H.; Le, D.T.; Hung, N.H.; Tran, Q.T.; Lee, H.-S.; Ryu, J.H.; et al. Synthesis and photocatalytic activity of β-Ga2O3 nanostructures for decomposition of formaldehyde under deep ultraviolet irradiation. Catalysts 2020, 10, 1105. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Wu, L.; Ding, Z.; Fu, X. Efficient decomposition of benzene over a β-Ga2O3 photocatalyst under ambient conditions. Environ. Sci. Technol. 2006, 40, 5799–5803. [Google Scholar] [CrossRef]
- Snetkov, P.P.; Morozkina, S.N.; Sosnin, I.M.; Bauman, D.A.; Hussainova, I.; Romanov, A.E. Electrospinning as a method for fabrication of nanofibrous photocatalysts based on gallium oxide. Phys. Status Solidi A 2025, 222, 2400669. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Dai, J.; Wu, F.; Wang, F.; Yu, J.; Liu, Y.-T.; Ding, B. Amorphous porous Ga2O3 nanofibers by iron modulation with intermediate energy level for enhanced degradation of azo dyes. J. Text. Inst. 2025, 1–11. [Google Scholar] [CrossRef]
- Orozco, S.; Rivero, M.; Montiel, E.; Valencia, J.E. Gallium oxides photocatalysts doped with Fe ions for discoloration of rhodamine under UV and visible light. Front. Environ. Sci. 2022, 10, 884758. [Google Scholar] [CrossRef]
- Orozco, S.; Martínez-Aguilar, E.; Belver, C.; Bedia, J.; Rivero, M. Simulation and experimentation of iron-doped liquid metal-based gallium oxide photocatalysts for environmental applications harnessing solar energy. Environ. Sci. Pollut. Res. 2025, 32, 12913–12944. [Google Scholar] [CrossRef] [PubMed]
- Fouad, K.; Bassyouni, M.; Alalm, M.G.; Saleh, M.Y. Recent developments in recalcitrant organic pollutants degradation using immobilized photocatalysts. Appl. Phys. A 2021, 127, 612. [Google Scholar] [CrossRef]
- Iervolino, G.; Zammit, I.; Vaiano, V.; Rizzo, L. Limitations and prospects for wastewater treatment by UV and visible-light-active heterogeneous photocatalysis: A critical review. In Heterogeneous Photocatalysis: Recent Advances; Springer: Cham, Switzerland, 2019; pp. 225–264. [Google Scholar] [CrossRef]
- Gjipalaj, J.; Alessandri, I. Easy recovery, mechanical stability, enhanced adsorption capacity and recyclability of alginate-based TiO2 macrobead photocatalysts for water treatment. J. Environ. Chem. Eng. 2017, 5, 1763–1770. [Google Scholar] [CrossRef]
- Ullah, S.; Ferreira-Neto, E.P.; Khan, A.A.; Medeiros, I.P.M.; Wender, H. Supported nanostructured photocatalysts: The role of support–photocatalyst interactions. Photochem. Photobiol. Sci. 2023, 22, 219–240. [Google Scholar] [CrossRef]
- Rana, A.; Sudhaik, A.; Raizada, P.; Khan, A.A.P.; Van Le, Q.; Singh, A.; Selvasembian, R.; Nadda, A.; Singh, P. An overview on cellulose-supported semiconductor photocatalysts for water purification. Nanotechnol. Environ. Eng. 2021, 6, 40. [Google Scholar] [CrossRef]
- Bai, B.; Qiu, L.; Mei, D.; Jin, Z.; Song, L.; Du, P. Firmly-supported porous fabric fiber photocatalysts: TiO2/porous carbon fiber cloth composites and their photocatalytic activity. Mater. Res. Bull. 2022, 148, 111672. [Google Scholar] [CrossRef]
- Lin, G.; Qiu, H. Diverse supports for immobilization of catalysts in continuous flow reactors. Chem.-A Eur. J. 2022, 28, e202200069. [Google Scholar] [CrossRef]
- Ali, R.; Sebastiani, M.; Bemporad, E. Influence of Ti–TiN multilayer PVD-coatings design on residual stresses and adhesion. Mater. Des. 2015, 75, 47–56. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Yang, R. Novel insight into VOC removal performance of photocatalytic oxidation reactors. Indoor Air 2005, 15, 291–300. [Google Scholar] [CrossRef]
- Escobedo, S.; De Lasa, H. Photocatalysis for air treatment processes: Current technologies and future applications for the removal of organic pollutants and viruses. Catalysts 2020, 10, 966. [Google Scholar] [CrossRef]
- El-Kalliny, A.S.; Ahmed, S.F.; Rietveld, L.C.; Appel, P.W. Immobilized photocatalyst on stainless steel woven meshes assuring efficient light distribution in a solar reactor. Drink. Water Eng. Sci. 2014, 7, 41–52. [Google Scholar] [CrossRef]
- Ramasundaram, S.; Seid, M.G.; Choe, J.W.; Kim, E.-J.; Chung, Y.C.; Cho, K.; Lee, C.; Hong, S.W. Highly reusable TiO2 nanoparticle photocatalyst by direct immobilization on steel mesh via PVDF coating, electrospraying, and thermal fixation. Chem. Eng. J. 2016, 306, 344–351. [Google Scholar] [CrossRef]
- Ryou, H.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Shin, M.; Cho, J.; Cho, B.J.; Hwang, W.S. Hydrothermal synthesis and photocatalytic property of Sn-doped β-Ga2O3 nanostructure. ECS J. Solid State Sci. Technol. 2020, 9, 045009. [Google Scholar] [CrossRef]
- Hou, Y.; Wu, L.; Wang, X.; Ding, Z.; Li, Z.; Fu, X. Photocatalytic performance of α-, β-, and γ-Ga2O3 for the destruction of volatile aromatic pollutants in air. J. Catal. 2007, 250, 12–18. [Google Scholar] [CrossRef]
- Kim, S.; Ryou, H.; Lee, I.G.; Shin, M.; Cho, B.J.; Hwang, W.S. Impact of Al doping on a hydrothermally synthesized β-Ga2O3 nanostructure for photocatalysis applications. RSC Adv. 2021, 11, 7338. [Google Scholar] [CrossRef]
- Kim, S.; Park, J.; Kim, H.W.; Jeon, D.; Hwang, W.S. Comparative study of photoinduced wettability and photocatalytic activity in different crystalline Ga2O3 phases. Mater. Sci. Semicond. Process. 2024, 175, 108289. [Google Scholar] [CrossRef]
- Ryou, H.; Kim, S.; Shin, M.; Cho, J.; Hwang, W.S. Fast-response colorimetric UVC sensor made of a Ga2O3 photocatalyst with a hole scavenger. Sensors 2021, 21, 387. [Google Scholar] [CrossRef]
- Service, R.J.; Hillier, W.; Debus, R.J. Evidence from FTIR difference spectroscopy of an extensive network of hydrogen bonds near the oxygen-evolving Mn4Ca cluster of photosystem II involving D1-Glu65, D2-Glu312, and D1-Glu329. Biochemistry 2010, 49, 6655. [Google Scholar] [CrossRef] [PubMed]
- Hauchecorne, B.; Terrens, D.; Verbruggen, S.; Martens, J.A.; Van Langenhove, H.; Demeestere, K.; Lenaerts, S. Elucidating the photocatalytic degradation pathway of acetaldehyde: An FTIR in situ study under atmospheric conditions. Appl. Catal. B Environ. 2011, 106, 630–638. [Google Scholar] [CrossRef]
- Xu, Z.; Chai, W.; Cao, J.; Huang, F.; Tong, T.; Dong, S.; Qiao, Q.; Shi, L.; Li, H.; Qian, X.; et al. Controlling the gas–water interface to enhance photocatalytic degradation of volatile organic compounds. ACS ES&T Eng. 2021, 1, 1140–1148. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cha, H.; Kim, S.; Jung, J.; Park, J.-H.; Hwang, W.S.; Jeon, D.-W.; Kim, H. Photocatalytic Degradation of VOCs Using Ga2O3-Coated Mesh for Practical Applications. Catalysts 2025, 15, 972. https://doi.org/10.3390/catal15100972
Cha H, Kim S, Jung J, Park J-H, Hwang WS, Jeon D-W, Kim H. Photocatalytic Degradation of VOCs Using Ga2O3-Coated Mesh for Practical Applications. Catalysts. 2025; 15(10):972. https://doi.org/10.3390/catal15100972
Chicago/Turabian StyleCha, Hyeongju, Sunjae Kim, Jinhan Jung, Ji-Hyeon Park, Wan Sik Hwang, Dae-Woo Jeon, and Hyunah Kim. 2025. "Photocatalytic Degradation of VOCs Using Ga2O3-Coated Mesh for Practical Applications" Catalysts 15, no. 10: 972. https://doi.org/10.3390/catal15100972
APA StyleCha, H., Kim, S., Jung, J., Park, J.-H., Hwang, W. S., Jeon, D.-W., & Kim, H. (2025). Photocatalytic Degradation of VOCs Using Ga2O3-Coated Mesh for Practical Applications. Catalysts, 15(10), 972. https://doi.org/10.3390/catal15100972







