2.1. Publication Trends of Nanomaterials in Soil and Groundwater Remediation Articles
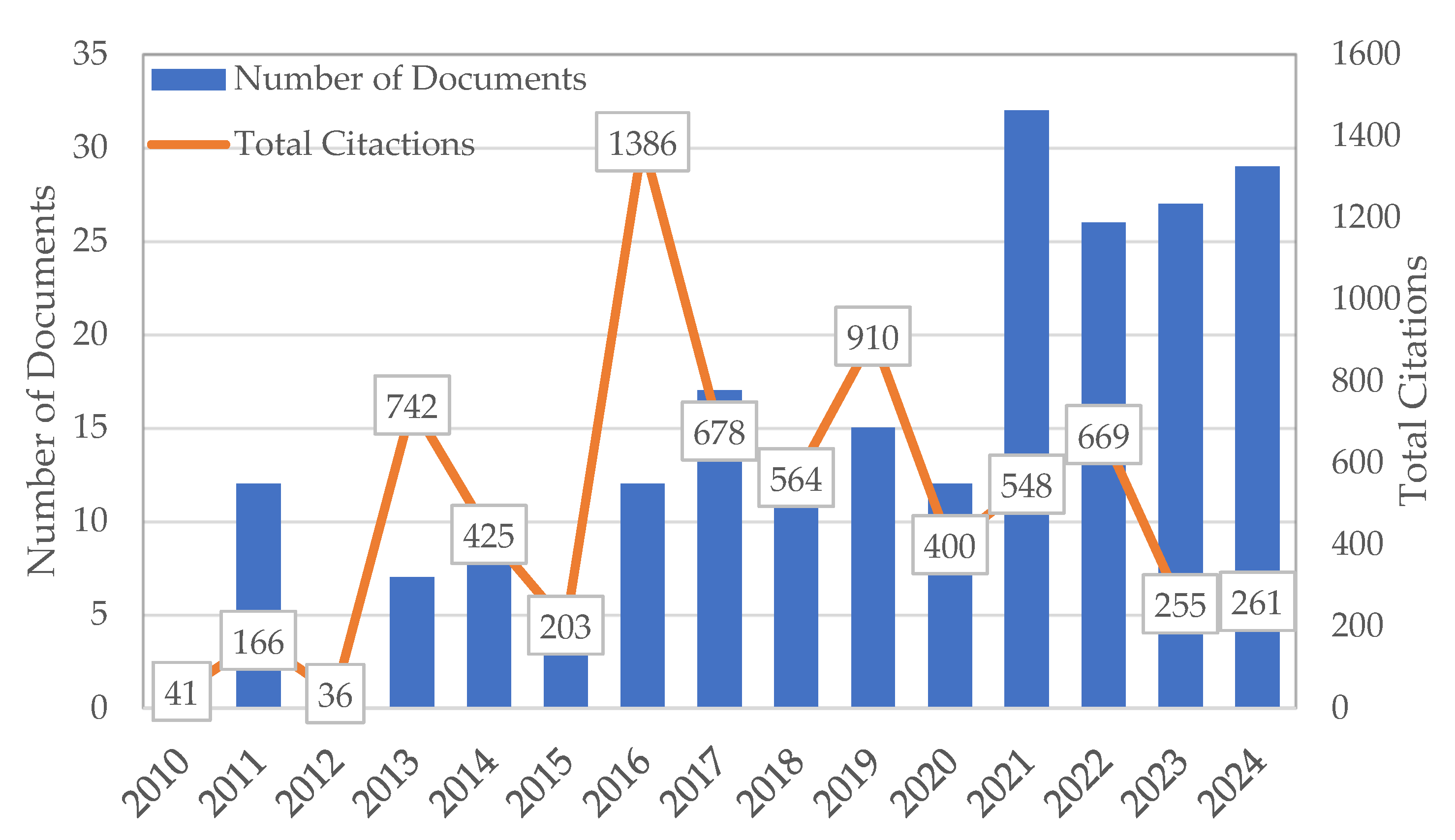
Figure 1 illustrates the publication trends and total citations for research on nanomaterials in soil and groundwater remediation from 2010 to 2024. This visual representation provides critical insights into the evolution and impact of research in this domain.
In 2010, the field began with 1 publication, which attracted 41 citations, indicating the nascent stage of research into the application of nanomaterials for environmental remediation. As the years progressed, a notable shift occurred; in 2011, the number of publications surged to 12, resulting in 166 citations. This increase signifies a growing interest and expanding activity within the field, indicating an early recognition of the potential benefits that nanomaterials could offer. However, the following year, 2012, witnessed a stark decline in research output, dropping to just 2 articles, which collectively attracted only 36 citations. This dip may suggest a temporary reduction in focus or funding, reflecting the natural ebb and flow of research priorities. Fortunately, 2013 marked a resurgence in the field, as 7 publications emerged, amassing 742 citations. This spike underscores the increasing acknowledgment of the significance of nanomaterials in remediation efforts, indicating a renewed enthusiasm among researchers. Continuing this upward trend, 2014 saw a slight increase in output with 8 articles, which accumulated 425 citations. This sustained interest in the subject matter reinforced the notion that nanomaterials were gaining traction as viable solutions for environmental challenges. However, in 2015, the number of publications dipped again to 6, resulting in 203 citations. This fluctuation in output highlights the ongoing engagement with research on nanomaterials, even amidst varying levels of publication activity.
The peak of research activity occurred in 2016, where 12 publications were released, generating an impressive 1386 citations. This peak year illustrates both the volume and the substantial impact of research during this period, showcasing the growing recognition of the importance of nanomaterials in remediation efforts. Nevertheless, the subsequent years demonstrated variability. In 2017, there was an increase to 17 publications, which resulted in 678 citations, while 2018 saw a decrease back to 12 articles with 564 citations. These fluctuations may reflect not only the dynamic nature of research interests but also the increasingly competitive landscape of scientific publishing.
From 2019 to 2024, a trend of stabilization in output emerged. In 2024, there were 29 publications, yielding 261 citations. While this indicates a significant volume of research output, it also highlights the challenge of citation accumulation for newer articles. The “citation lag” phenomenon is crucial to consider here; it emphasizes that recent publications may not achieve the same citation levels as older articles simply because they have had less time to be cited. This phenomenon reveals a methodological limitation in interpreting citation data, particularly for recent years [
28].
Overall, the analysis presents a complex narrative regarding publication trends in nanomaterials for soil and groundwater remediation. The data indicate a growing body of research, particularly from 2010 to 2016, followed by fluctuating output and citation dynamics in subsequent years. This variability suggests that while interest in the subject persists, external factors such as competition for research funding and publication opportunities may influence the consistency of output. The differences in citation counts for recent publications underscore the necessity for further investigation into the factors affecting the recognition of newer research. Future studies could focus on the dissemination strategies employed by researchers to enhance visibility and citation potential, which is critical for ensuring the practical impact of findings in environmental science.
In summary,
Figure 1 highlights a clear upward trend in research volume from 2010 to 2024, emphasizing the growing importance of nanomaterials in environmental remediation. However, the observed citation patterns warrant careful consideration, as they may not accurately reflect the quality of research due to the inherent dynamics of citation accumulation. This analysis advocates for continued research efforts and effective communication strategies to maximize the academic and practical significance of findings in this vital area.
Figure 2 illustrates the distribution of research publications across various scientific disciplines concerning nanomaterials for groundwater and soil remediation. This categorization highlights the multifaceted nature of research in this area and underscores the importance of interdisciplinary approaches to address environmental challenges. “Environmental Science” emerges as the leading category, comprising 23.5% of total publications. This significant share reflects the central role of environmental science in understanding contamination issues, particularly those related to nanomaterials. Research in this discipline is crucial for developing effective management and remediation strategies, aiming to mitigate the ecological impacts of nanomaterials on ecosystems. The emphasis on environmental science indicates the urgency of addressing the challenges posed by contaminants and the need for innovative solutions [
2].
“Water Quality and Treatment” accounts for 15.7% of the publications, underscoring the importance of ensuring safe and clean water resources. This field focuses on the application of nanomaterials in water treatment processes, which is vital for removing pollutants and enhancing water quality. The contributions from this discipline are essential for creating effective treatment solutions that can help manage waterborne contaminants [
29].
“Soil Science” represents 11.8% of the research, highlighting the significance of understanding how nanomaterials interact with soil properties and processes. This discipline is critical for assessing the implications of nanomaterials on soil health and fertility, as well as their potential for remediation of contaminated soils. The insights gained from soil science are vital for developing strategies that leverage nanomaterials for effective soil remediation [
17]. “Toxicology” comprises 21.6% of the publications, emphasizing the need to investigate the potential health impacts of nanomaterials. Understanding the toxicological effects is essential for evaluating risks associated with exposure to nanomaterials in various environmental contexts. This research is crucial for informing safety regulations and public health policies [
8].
“Pharmaceuticals and Personal Care Products (PPCPs)” contribute 9.8% to the total publications, reflecting the relevance of nanomaterials in addressing contaminants from these sources. Research in this area focuses on the detection, degradation, and remediation of PPCPs in environmental matrices, which is vital for maintaining ecosystem health [
30]. “Agricultural Biotechnology” accounts for 7.8% of the publications, emphasizing the implications of nanomaterials in agricultural practices. This field explores the use of nanomaterials in enhancing crop productivity and soil remediation, highlighting the potential benefits and risks associated with their application in agriculture [
8].
Overall,
Figure 2 illustrates a diverse array of research disciplines contributing to the understanding and application of nanomaterials for groundwater and soil remediation. The varied distribution of publications underscores the complexity of this field and the importance of interdisciplinary collaboration. Each discipline plays a unique role in addressing the multifaceted challenges posed by environmental contaminants, emphasizing the need for integrated approaches to develop comprehensive solutions for effective remediation and management strategies.
2.2. Leading Journals for Articles of Nanomaterials in Soil and Groundwater Remediation
Table 1 provides a detailed overview of the top 12 journals that publish articles related to nanomaterials in soil and groundwater remediation.
Table 1 includes significant metrics such as TLS, number of documents published, total citations, h-index, SCImago Journal Rank (SJR), and quartile ranking (Q). The data highlights the prominence of certain journals in disseminating critical research findings in this evolving field.
The journal “Science of the Total Environment” leads the list with a TLS of 243, publishing 9 articles that have collectively garnered 343 citations. Its h-index of 399 indicates that many of its articles are frequently cited, underscoring its influence in environmental research. Following closely is “Environmental Science and Technology”, which has published 4 articles that achieved a total of 437 citations, resulting in a TLS of 202. This journal’s high h-index of 504 signifies its strong impact in the field. The “Journal of Contaminant Hydrology” ranks third with a TLS of 198. It has published 6 articles that received 169 citations, reflecting a focused but significant contribution to the understanding of contaminant behavior in hydrological contexts.
“Environmental Science and Pollution Research” and “Chemosphere” also stand out, with TLS values of 153 and 149, respectively. Both journals have published multiple articles, with “Chemosphere” achieving a higher citation count of 453, indicating its substantial role in environmental science discourse. Other notable publications include “Journal of Nanoparticle Research” and “Environmental Science: Nano”, both of which, despite lower TLS values, contribute valuable insights into the nanomaterial’s domain. The h-index and SJR metrics across these journals illustrate their standing within the scientific community, with the majority residing in the first quartile (Q1), indicating their high impact and relevance in the field of nanomaterials research for soil and groundwater remediation. In summary,
Table 1 emphasizes the concentration of influential research in high-impact journals, which plays a crucial role in advancing the understanding of nanomaterials in environmental remediation efforts.
To analyze the network of international collaborations in nanomaterials research concerning soil and groundwater remediation, a network diagram was created using VOSviewer (version 1.6.18, Leiden University, Leiden, The Netherlands, 2022), as illustrated in
Figure 3. Each journal is represented as a node, and the connections (edges) between them signify collaborative relationships or thematic overlaps in published research. The size of each node reflects the number of publications related to nanomaterials, with larger nodes indicating journals that have made substantial contributions to the field [
27].
Prominent journals such as “Environmental Science and Technology” and “Chemosphere” stand out, highlighting their importance in the literature on nanomaterials. The lines connecting the nodes represent the strength of relationships between journals, with thicker lines indicating stronger collaborations or thematic similarities in research topics. For instance, “Environmental Science and Technology” exhibits strong connections with multiple other journals, suggesting that it plays a central role in disseminating research findings in this area.
The clustered arrangement of the nodes indicates that certain journals may focus on specific themes within nanomaterials research. Journals like “Journal of Contaminant Hydrology” and “Science of the Total Environment” likely cover similar environmental impacts of nanomaterials, showcasing a shared research focus. Moreover, the presence of diverse journals interconnected on the map highlights the interdisciplinary nature of this field of research. It encompasses environmental science, chemistry, and nanotechnology, suggesting a collaborative approach to addressing complex environmental challenges.
Figure 3 can serve as a valuable guide for researchers seeking potential collaborators and relevant publication venues. It emphasizes the importance of considering thematic connections when selecting journals, as closely linked publications may cater to similar audiences and research interests.
Overall, this co-occurrence map provides essential insights into the dynamics of research in nanomaterials related to soil and groundwater remediation. It illustrates the collaborative landscape of high-impact journals and underscores the critical role they play in advancing knowledge and fostering interdisciplinary research in this vital area.
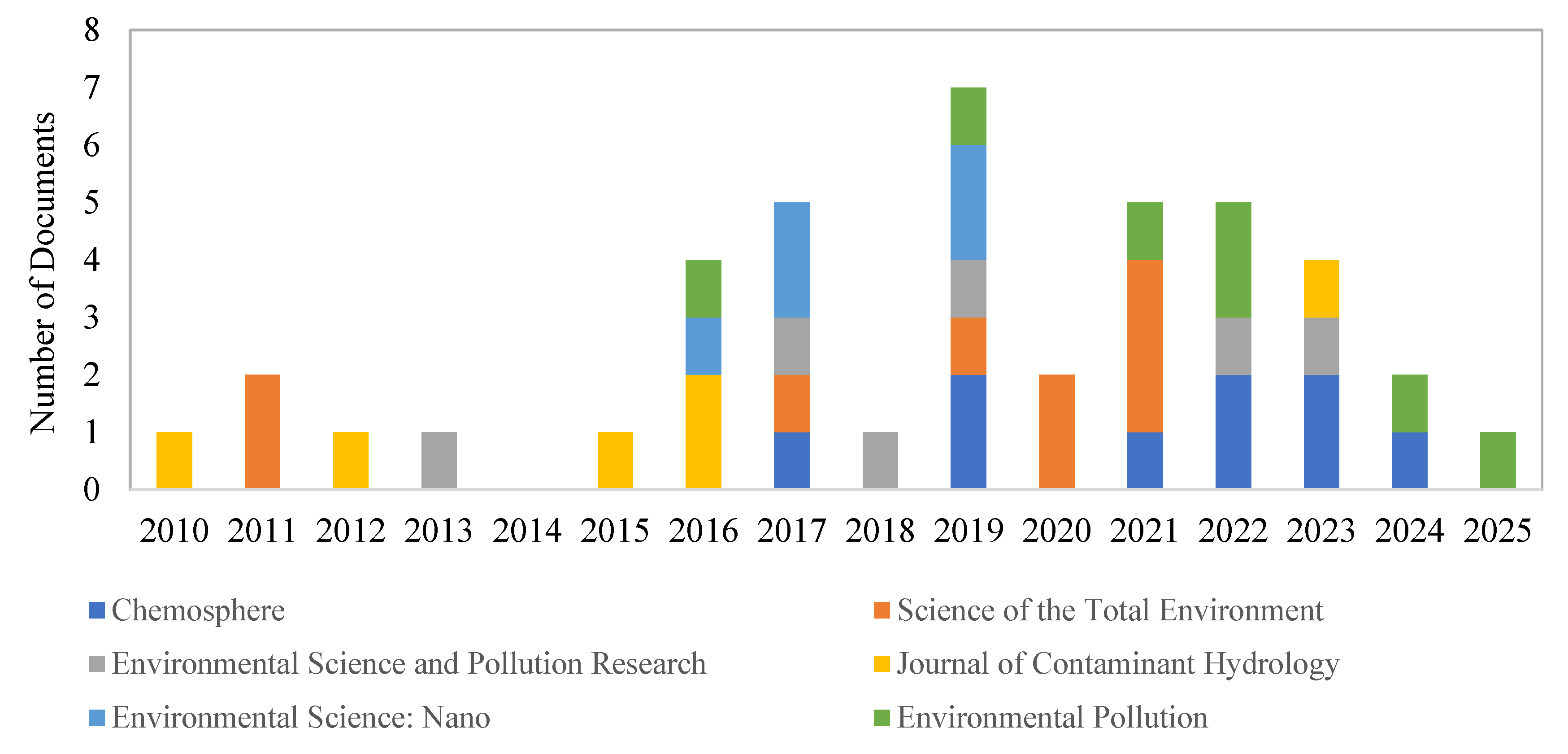
Figure 4 presents a comprehensive overview of the publication statistics concerning nanomaterials in soil and groundwater remediation. This analysis highlights the contributions of several key journals that have significantly shaped the discourse in this field. The journals included in the analysis are “Chemosphere”, “Science of the Total Environment”, “Environmental Science and Pollution Research”, “Journal of Contaminant Hydrology”, “Environmental Science: Nano”, and “Environmental Pollution”.
The overall trends indicate that both “Chemosphere” and “Science of the Total Environment” are the leading journals in this field, with each having published a total of 9 articles during the analyzed period. This significant output reflects a strong commitment from both journals to advancing research in this critical area, underscoring their importance within the environmental literature. Following closely, “Environmental Pollution” has published 7 articles, demonstrating a focused interest in pollution-related issues, including the implications of nanomaterials. The consistent output from this journal highlights its relevance in addressing environmental challenges.
In addition to these leading journals, “Environmental Science and Pollution Research” and “Journal of Contaminant Hydrology” each contributed 6 articles. Their outputs indicate an active engagement with the topic of nanomaterials, although they did not lead in publication counts. Meanwhile, “Environmental Science: Nano” published 5 articles, suggesting a growing interest in the applications of nanotechnology within environmental contexts, albeit with a lower output compared to the top-tier journals.
Examining the yearly trends reveals more nuanced insights into publication activity. The year 2016 marked a peak for “Chemosphere”, which published 2 articles, signaling a potential increase in research interest during that period. Similarly, 2018 saw a notable surge for both “Chemosphere” and “Environmental Pollution”, each contributing 2 articles. This indicates that 2018 was a significant year for advancements in research related to nanomaterials. In 2021, “Environmental Pollution” experienced another spike in activity, publishing 2 articles, reflecting heightened interest in pollution-related research at that time. However, the years from 2022 to 2024 show a decline in publication activity across most journals, suggesting a potential stabilization or shift in research focus.
In conclusion, the analysis of publication counts from 2010 to 2024 illustrates a competitive landscape among these key journals. Both “Chemosphere” and “Science of the Total Environment” emerge as leading sources of research on nanomaterials, while “Environmental Pollution”, “Environmental Science and Pollution Research”, and “Journal of Contaminant Hydrology” also make meaningful contributions to the discourse. The fluctuation in publication counts over the years reflect varying trends in research interest and urgency, highlighting the evolving nature of environmental research related to nanomaterials in soil and groundwater remediation. Collectively, these trends underscore the commitment of the academic community to address the challenges posed by nanomaterials in environmental contexts.
2.3. Country Distribution of Nanomaterials Research in Soil and Groundwater Remediation
The bibliometric analysis presented in
Table 2 offers a comprehensive overview of the leading countries involved in research on nanomaterials for soil and groundwater remediation. The data includes key metrics such as TLS, the number of publications, and total citations for each nation.
India stands out as the top contributor, boasting a TLS of 6281, 85 publications, and 1533 citations. This impressive output highlights India’s robust research capabilities and a growing focus on addressing soil and groundwater contamination challenges, underscoring the nation’s commitment to environmental remediation technologies. The high TLS indicates strong collaborative networks, suggesting that Indian research is influential in this field. China ranks second, with a TLS of 5646, 56 publications, and 1669 citations. This reflects China’s significant investment in environmental research and its proactive approach to tackling pollution issues. The notable number of citations indicates that Chinese research is impactful on a global scale, reinforcing its position as a key player in nanomaterials research.
One of the primary reasons for this engagement is the pressing environmental challenges faced by many countries. Rapid industrialization, agricultural runoff, and urbanization have led to severe soil and groundwater contamination issues, particularly in nations like India and China [
31]. As these countries grapple with the consequences of their growth, there is an urgent need to find effective solutions to mitigate pollution. This urgency drives governments and researchers to explore innovative technologies, such as nanomaterials, which offer promising approaches for environmental remediation [
31].
The United States follows closely in third place, contributing a TLS of 5460, 53 publications, and 1676 citations. The U.S. has a longstanding history of leadership in nanotechnology and environmental science, as evidenced by its publication output and citation impact. This strong presence suggests a well-established academic infrastructure and considerable funding dedicated to research in these areas. South Korea, with a TLS of 3100 and 16 publications, has also made significant contributions, accumulating 1425 citations. This indicates South Korea’s commitment to developing technologies for environmental remediation, despite its relatively lower publication count compared to leading nations. Australia’s contributions, with 12 publications and a TLS of 2406, reflect its focus on sustainable environmental practices, supported by its research institutions. Meanwhile, Saudi Arabia’s involvement, with 10 publications and a TLS of 2403, signifies its growing interest in addressing soil and groundwater challenges within the framework of its environmental policies.
Pakistan, with a TLS of 2246 and 11 publications, alongside Canada, which has 1605 TLS and 8 publications, shows that both countries are gradually increasing their participation in nanomaterials research. Their citation counts indicate that their findings are gaining recognition within the international scientific community. Spain and Mexico, each contributing 10 publications, demonstrate a commitment to environmental research, with citation counts of 298 and 218, respectively. Their involvement reflects a broader engagement across Europe and Latin America in addressing environmental challenges through innovative solutions.
Overall, the interest of these countries in nanomaterials research for soil and groundwater remediation is driven by a combination of environmental necessity, public health considerations, economic incentives, collaborative opportunities, regulatory pressures, and the scientific promise of nanotechnology. As global awareness of environmental issues continues to grow, these factors will likely sustain and enhance the commitment of nations to pursue innovative research in this vital field.
Figure 5 presents a network visualization that illustrates the primary nations engaged in nanomaterials research for groundwater and soil remediation. The analysis reveals a total of 11 countries, each represented by nodes of varying sizes and connecting lines of different thicknesses. The size of each node corresponds to the level of engagement in this research area, with larger nodes indicating a more prominent role and influence within the network. Among the countries represented, India and China emerge as central players, characterized by their larger nodes and thicker connecting lines. This positioning signifies their substantial contributions and the strong collaborative networks they have established in the field of nanomaterials research. The close connectivity between these two countries suggests a robust exchange of knowledge and resources, reinforcing their leadership roles in addressing environmental challenges associated with soil and groundwater.
The United States, while slightly smaller than India and China, also occupies a critical position in the network, reflecting its historical strength in nanotechnology and environmental science. The connections to other countries, such as Canada and Australia, further illustrate the collaborative nature of the research landscape, indicating active partnerships that enhance the dissemination of knowledge and technological advancements. South Korea and Saudi Arabia are also notable contributors, represented by medium-sized nodes that indicate a significant but comparatively lower level of engagement than the leading nations. Their connections within the network suggest that they are part of important collaborative efforts, although they may not yet have the same level of influence. The inclusion of countries like Spain, Mexico, and Pakistan highlights the global dimension of this research area. These nations, while having smaller nodes, still play a role in the network, contributing to the diversity of perspectives and approaches in nanomaterials research. Their participation underscores the importance of international collaboration in addressing complex environmental issues.
Overall, this network visualization provides valuable insights into the global research landscape surrounding nanomaterials for groundwater and soil remediation. By highlighting the major contributors and their interconnections, it emphasizes the collaborative dynamics that are essential for advancing understanding and management strategies in this vital area of environmental science. Recognizing these relationships is crucial for identifying key players and fostering future collaborations aimed at tackling the challenges associated with environmental remediation.
To highlight the primary universities engaged in nanomaterials research for soil and groundwater remediation, a comprehensive network visualization was constructed, as illustrated in
Figure 6. This figure maps the affiliations of key institutions based on their involvement in relevant research publications.
Table 3 presents a detailed analysis of the top eight universities according to author affiliations, showcasing their respective departments, locations, total citation metrics, and publication counts.
Among these leading institutions, the “Guangdong Provincial Academy of Environmental Science” in Guangzhou, China, emerges prominently with a total of 104 total citations derived from two publications. This indicates its foundational role in advancing research in this critical area. Similarly, the “Guangdong Provincial Research Center for Environment Control and Remediation Materials at Jinan University”, also in Guangzhou, records equivalent performance with two documents and 104 citations, reflecting its significant contributions to environmental remediation studies. The “National Institute of Technology” in Srinagar, India, has produced two publications with 32 citations, indicating a growing presence in this field. Notably, “Carnegie Mellon University” in Pittsburgh, USA, with its Department of Civil Engineering, has also contributed two documents, but with a higher citation count of 26, underscoring the impact of its research output.
Additionally, “Jiangsu Key Laboratory of Environmental Functional Materials at Suzhou University of Science and Technology” published three papers, accumulating 25 citations, which showcases its active engagement in this domain. The “Academy of Scientific and Innovative Research (ACSIR)” in Uttar Pradesh, India, has generated two publications with 13 citations, while “Tianjin Key Laboratory of Clean Energy and Pollution Control at Hebei University of Technology” has two documents but no citations, suggesting challenges in research dissemination or impact. Lastly, “Tecnologico De Monterrey” in Monterrey, Mexico, stands out with a striking citation count of 200 from only two publications, indicating a significant influence despite a relatively low output.
Overall, these institutions collectively reflect a rich tapestry of contributions to the study of nanomaterials in environmental remediation. The disparity in publication counts and citation metrics among these universities suggest varying research focuses and levels of impact, emphasizing the collaborative nature of advancements in this vital scientific field.
2.5. Top Cited Publications in Nanomaterials Research Related to Soil and Groundwater Remediation
Citation analysis is a crucial approach for understanding the intellectual connections among scholarly publications, especially when one work references another. This method allows researchers to pinpoint significant articles within a specific academic discipline and to analyze trends and patterns in citations [
33]. In this study, we conducted a citation analysis of papers focusing on nanomaterials in the context of soil and groundwater remediation.
Table 6 presents the top 30 publications ranked by citation counts, with the ranking determined by their adherence to the criteria of the Scopus database. This analysis offers important insights into the most impactful and influential works in this field. These key publications represent vital progress in our understanding of how nanomaterials can be utilized to remediate contaminated soil and groundwater, addressing their mechanisms of action, effectiveness, and environmental implications.
Nanomaterials have emerged as a transformative approach in addressing soil and groundwater contamination, presenting innovative solutions for remediation. These materials, which include nZVI, carbon nanotubes (CNTs), metal oxides, and various nanocomposites, possess unique attributes such as elevated surface area and reactivity. These properties make them exceptionally effective in the removal of pollutants. However, the implementation of nanotechnology in environmental remediation necessitates careful consideration of synthesis methodologies, potential ecological impacts, and sustained performance.
In particular, nZVI has garnered attention for its heightened reactivity and ability to transform contaminants into less harmful forms. While much of the existing literature focuses on adsorption and redox processes, it is crucial to highlight its catalytic potential in degrading hazardous chlorinated compounds, such as polychlorinated biphenyls (PCBs) and various herbicides. The catalytic activity of nZVI is primarily attributed to its capacity to facilitate electron transfer reactions. For example, it can break carbon-chlorine bonds through catalytic reductive dechlorination, resulting in the formation of less chlorinated species or complete dechlorination into benign products like ethylene or methane [
34].
Several investigations underscore the efficacy of nanomaterials in the remediation of diverse contaminants. Studies such as Stefaniuk et al. [
35] emphasize the cost-effectiveness and adaptability of nZVI, while Yan et al. [
36] and Galdames et al. [
17] highlight its potential in addressing a spectrum of subsurface contaminants. Conversely, Bae et al. [
37] address the challenges associated with the surface passivation of nZVI, underscoring the necessity for inventive strategies to uphold its reactivity. CNTs are also garnering attention for their distinctive adsorption characteristics, rendering them appropriate for remediating both organic and inorganic pollutants. Metal oxides and their modified configurations, as elucidated by Siddiqui et al. [
38], demonstrate promise as efficacious adsorbents for arsenic removal, effectively tackling challenges related to toxicity and separation.
To alleviate the ecological apprehensions linked to conventional nanomaterial synthesis, researchers are exploring environmentally conscious green synthesis methodologies. Hao et al. [
39] exemplify the utilization of green tea extract for synthesizing iron nanoparticles, thereby proffering a cost-efficient and sustainable approach for eliminating hexavalent chromium. Dutta et al. [
40] accentuate the imperative of sustainable practices in nanomaterial development, underscoring the potential of bio-inspired synthesis using waste materials and natural precursors. These green synthesis techniques not only curtail ecological impact but also augment the stability and performance of nanomaterials in remediation applications.
Comprehending the transport and fate of nanomaterials within the environment is paramount for evaluating their prospective risks and optimizing their application. Yin et al. [
41] explore the co-transport of graphene oxide and heavy metal ions in porous media, unveiling intricate interactions influenced by surface characteristics. Rahmatpour et al. [
42] scrutinize the transport of silver nanoparticles in calcareous soils, underscoring the significance of soil texture and flow conditions in influencing their behavior. Garner et al. [
43] address the ecological hazards linked to engineered nanomaterials, emphasizing the necessity for further research into their fate and toxicity across diverse environments.
Despite the encouraging potential of nanomaterials in environmental remediation, several challenges persist. These encompass the necessity for standardized reporting of toxicological data, the advancement of cost-efficient production methods, and the optimization of nanomaterial transport and mixing in subsurface environments. Zhang et al. [
44] underscore the challenges pertaining to nanomaterial mixing and transport in subsurface environments, emphasizing the exigency for enhancements in mixing techniques. Moreover, Al-Salim et al. [
45] accentuate the imperative of comprehending both the toxicity of nanomaterials to non-target organisms and their prospective exposure pathways.
Future research endeavors should concentrate on surmounting these challenges and fostering the integration of nanotechnology with other remediation methodologies to formulate sustainable and efficacious solutions for soil and groundwater contamination. The utilization of stabilized nanomaterials, such as polymer-coated nZVI, and the advancement of advanced filtration systems that selectively eliminate detrimental contaminants are also auspicious avenues for prospective research.
Table 6.
The top 30 most cited articles.
Table 6.
The top 30 most cited articles.
| First Author | Year | Document Title | Journal | Citations | Ref. |
|---|
| Stefaniuk, M. | 2016 | “Review on nano zerovalent iron (nZVI): From synthesis to environmental applications” | Chemical Engineering Journal | 805 | [35] |
| Tijani, J.O. | 2016 | “Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: a review” | Environmental Chemistry Letters | 396 | [30] |
| Yan, W. | 2013 | “Iron nanoparticles for environmental clean-up: Recent developments and future outlook” | Environmental Sciences: Processes and Impacts | 389 | [36] |
| Bae, S. | 2018 | “Advances in Surface Passivation of Nanoscale Zerovalent Iron: A Critical Review” | Environmental Science and Technology | 301 | [37] |
| Garner, K.L. | 2014 | “Emerging patterns for engineered nanomaterials in the environment: A review of fate and toxicity studies” | Journal of Nanoparticle Research | 293 | [43] |
| Siddiqui, S.I. | 2017 | “Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement” | Process Safety and Environmental Protection | 291 | [38] |
| Sarma, G.K. | 2019 | “Nanomaterials as versatile adsorbents for heavy metal ions in water: a review” | Environmental Science and Pollution Research | 243 | [46] |
| Mohmood, I. | 2013 | “Nanoscale materials and their use in water contaminants removal-a review” | Environmental Science and Pollution Research | 235 | [47] |
| Hussain, A. | 2022 | “In situ, Ex situ, and nano-remediation strategies to treat polluted soil, water, and air–A review” | Chemosphere | 193 | [2] |
| Galdames, A. | 2020 | “Zero-valent iron nanoparticles for soil and groundwater remediation” | International Journal of Environmental Research and Public Health | 153 | [17] |
| Shaji, E. | 2024 | “Fluoride contamination in groundwater: A global review of the status, processes, challenges, and remedial measures” | Geoscience Frontiers | 145 | [48] |
| Song, B. | 2019 | “Using nanomaterials to facilitate the phytoremediation of contaminated soil” | Critical Reviews in Environmental Science and Technology | 145 | [49] |
| Zhang, T. | 2019 | “In situ remediation of subsurface contamination: Opportunities and challenges for nanotechnology and advanced materials” | Environmental Science: Nano | 119 | [44] |
| Babaee, Y. | 2018 | “Removal of arsenic (III) and arsenic (V) from aqueous solutions through adsorption by Fe/Cu nanoparticles” | Journal of Chemical Technology and Biotechnology | 115 | [50] |
| Alazaiza, M.Y. | 2021 | “Recent advances of nanoremediation technologies for soil and groundwater remediation: A review” | Water (Switzerland) | 101 | [8] |
| Thomé, A. | 2015 | “Review of nanotechnology for soil and groundwater remediation: Brazilian perspectives” | Water, Air, and Soil Pollution | 100 | [51] |
| Al-Salim, N. | 2011 | “Quantum dot transport in soil, plants, and insects” | Science of the Total Environment | 92 | [45] |
| Taghipour, S. | 2019 | “Engineering nanomaterials for water and wastewater treatment: Review of classifications, properties and applications” | New Journal of Chemistry | 92 | [29] |
| Rathnayake, S. | 2014 | “Multitechnique investigation of the ph dependence of phosphate induced transformations of ZnO nanoparticles” | Environmental Science and Technology | 89 | [52] |
| Thakur, A.K. | 2020 | “A review on design, material selection, mechanism, and modelling of permeable reactive barrier for community-scale groundwater treatment” | Environmental Technology and Innovation | 87 | [53] |
| Raza, A. | 2022 | “Recent advances in carbonaceous sustainable nanomaterials for wastewater treatments” | Sustainable Materials and Technologies | 83 | [54] |
| Gomes, A.R. | 2017 | “Review of the ecotoxicological effects of emerging contaminants to soil biota” | Journal of Environmental Science and Health–Part A Toxic/Hazardous Substances and Environmental Engineering | 82 | [55] |
| Marcon, L. | 2021 | “In situ nanoremediation of soils and groundwater from the nanoparticle’s standpoint: A review” | Science of the Total Environment | 78 | [9] |
| Dutta, V. | 2022 | “Bio-inspired synthesis of carbon-based nanomaterials and their potential environmental applications: A state-of-the-art review” | Inorganics | 77 | [40] |
| Rahmatpour, S. | 2018 | “Transport of silver nanoparticles in intact columns of calcareous soils: The role of flow conditions and soil texture” | Geoderma | 65 | [42] |
| Mura, S. | 2013 | “Advanced of nanotechnology in agro-environmental studies” | Italian Journal of Agronomy | 65 | [56] |
| Boregowda, N. | 2022 | “Recent advances in nanoremediation: Carving sustainable solution to clean-up polluted agriculture soils” | Environmental Pollution | 63 | [57] |
| Yin, X. | 2019 | “Co-transport of graphene oxide and heavy metal ions in surface-modified porous media” | Chemosphere | 63 | [41] |
| Bossa, N. | 2017 | “Cellulose nanocrystal zero-valent iron nanocomposites for groundwater remediation” | Environmental Science: Nano | 62 | [58] |
| Hao, R. | 2021 | “Green synthesis of iron nanoparticles using green tea and its removal of hexavalent chromium” | Nanomaterials | 60 | [39] |
A significant publication in the field of nanomaterials for soil and groundwater remediation is the 2016 study by Stefaniuk, M., titled “Review on nano zerovalent iron (nZVI): From synthesis to environmental applications”, published in the “Chemical Engineering Journal”. This impactful paper has garnered 805 citations. This review focuses on nZVI, a widely utilized nanoparticle known for its effectiveness in removing contaminants in environmental remediation. While it discusses various nanoparticles, including zeolites and metal oxides, nZVI is highlighted for its cost efficiency and superior performance. Stefaniuk outlines both traditional and innovative methods for synthesizing nZVI, such as chemical reduction and green synthesis. These advancements are crucial for enhancing the application of nZVI in various environmental contexts. The review also addresses the complexities involved in modifying nZVI to enhance its stability and reactivity [
35].
The 2016 study by Tijani, J.O., titled “Pharmaceuticals, endocrine disruptors, personal care products, nanomaterials and perfluorinated pollutants: a review”, published in “Environmental Chemistry Letters”, is a highly cited work with 396 citations. This review examines the environmental risks posed by emerging micropollutants, including pharmaceuticals, endocrine disruptors, and perfluorinated substances. The paper highlights how these micropollutants enter the environment through human activities, being detected in surface water, groundwater, and drinking water at concentrations ranging from nanograms to micrograms per liter. The study emphasizes the need for increased public awareness and strong governmental policies to tackle these pollutants. It advocates for proactive measures, such as improved wastewater treatment technologies and enhanced monitoring efforts [
30].
In a related vein, Yan et al. (2013) [
36] delve into the chemistry and reactivity of nZVI, discussing methods to improve its stability and transport in porous media. Their work highlights the engineering challenges that need to be addressed to enhance the practical application of nZVI for environmental cleanup [
43]. This exploration of nZVI’s capabilities is vital for developing effective remediation strategies [
36]. Bae et al. (2017) [
37] further contribute to this discourse by exploring issues related to nZVI, particularly surface passivation and its detrimental effects on reactivity. Oxidation processes can lead to the formation of various iron-bearing phases, which ultimately compromise the material’s effectiveness in degrading contaminants. Bae explores the factors influencing this corrosion process, including the composition of nZVI, the types of aqueous species, reaction time, and environmental conditions. Interestingly, the presence of different iron oxidation states on nZVI surfaces may create unique reactive microenvironments that facilitate the adsorption and transformation of pollutants. The review aims to clarify the nature of passivation processes and byproducts formed in various environments, employing advanced techniques like electron microscopy and X-ray spectroscopy for identification. Additionally, it delves into the mechanisms of species in both oxic and anoxic conditions and examines how synthesis methods, along with the presence of inorganic and organic materials, affect passivation byproducts. Furthermore, Bae discusses potential depassivation strategies to enhance nZVI’s reactivity, thereby improving its effectiveness in contaminant degradation [
37].
Garner et al. (2014) [
43] offer a broader perspective on the environmental risks associated with engineered nanomaterials (ENMs). Their review synthesizes findings on the fate and transport of ENMs in various ecosystems, emphasizing the need for standardized toxicological data to assess risks effectively. In aquatic environments, ENMs demonstrate greater stability in freshwater and stormwater compared to seawater, suggesting a higher potential for transport in these settings. The behavior of ENMs in soil is influenced by factors such as aggregate size and soil chemistry, with certain materials like silver and nZVI raising toxicity concerns for aquatic organisms. Garner notes that while the overall risk from most ENMs appears low at current environmental concentrations, direct applications or accidental releases may have significant localized effects. This foundational understanding is critical for ensuring the safe application of nanomaterials in environmental contexts [
43].
In the 2017 study titled “Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement”, Siddiqui, S.I. addresses the critical issue of arsenic contamination, which poses severe health risks globally. Arsenic, found in dissolved forms in groundwater, has led to widespread health crises. The study highlights that arsenic exists in water as oxyacids in two oxidation states: As(III) and As(V), with As(III) being the more toxic form. While various techniques are employed for arsenic removal, many are ineffective against As(III). Among these, adsorption has emerged as the preferred method for removing both forms of arsenic, with nanomaterials demonstrating superior efficacy. However, some nanomaterials present challenges, including toxicity, difficulty in separating post-adsorption, and inefficacy under water constraints. Siddiqui emphasizes the advantages of iron oxides and their modified forms as adsorbents, which address these challenges effectively [
38].
Sarma et al. (2019) further explore the effectiveness of various nanomaterials in adsorbing heavy metals, detailing various types of nanomaterials such as clay-nanocomposites, metal oxides, carbon nanotubes, and polymeric nanocomposites, and their applicability for removing a range of heavy metals such as arsenic, cadmium, cobalt, chromium, copper, mercury, manganese, nickel, lead, tin, uranium, vanadium, and zinc from water [
46]. This research contributes to the growing body of literature demonstrating the utility of nanomaterials in environmental remediation [
46]. Mohmood et al. (2013) [
47] discuss the applications of nanoscale materials in water purification, emphasizing their advantages and potential toxicity. Their study raises critical concerns regarding the safe application of engineered nanomaterials in water treatment [
47].
Hussain et al. [
2] extends this discussion in “In-situ, Ex-situ, and nano-remediation strategies to treat polluted soil, water, and air”. The review discusses the role of nanostructured materials, such as titanium dioxide (TiO
2), dendrimers, iron-based nanoparticles, silica, carbon nanomaterials, graphene, nanotubes, and polymers, in mitigating environmental hazards. Hussain explores the roles of various nanostructured materials in innovative remediation methodologies, including in situ techniques like bioventing, bioslurping, biosparging, phytoremediation, and permeable reactive barriers, as well as ex situ methods such as biopiles, windows, bioreactors, and land farming. The study emphasizes the adaptability of nanoparticles for effective pollutant treatment, underscoring their small size and high surface area-to-volume ratio as critical advantages in various remediation contexts. The review highlights how the choice of nanomaterials and remediation methods depends on the specific properties of the contaminants and the characteristics of the pollution site [
2].
Galdames investigates the effectiveness of ZVI as a remediation agent for environmental contaminants. The study highlights the advantages of nZVI, particularly its high specific surface area, enhancing reactivity and efficiency in remediation processes. Galdames discusses the gap between laboratory findings and field applications. The review summarizes various nZVI systems and their remediation capacities. To address these challenges, the development of stabilized nZVI—particularly polymer-coated nZVI—has been proposed. These coatings improve the colloidal properties and mobility of the nanoparticles, making them more versatile for use in different contaminated sites. Among the coatings discussed, carboxymethyl cellulose (CMC) is highlighted for its excellent mobility and biodegradability, making it an environmentally friendly option. The study suggests that using biodegradable and natural polymer coatings can enhance the performance of nZVI while ensuring minimal environmental impact [
17].
In the 2024 study titled “Fluoride contamination in groundwater: A global review of the status, processes, challenges, and remedial measures”, Shaji, E. explores the critical issue of fluoride pollution in groundwater, emphasizing its toxic effects on human health. The primary sources of fluoride in drinking water are geogenic, along with contributions from agricultural products, dental products, and over-fluoridated water. Despite the existence of efficient fluoride removal techniques, including filters utilizing next-generation nanomaterials, no single method has emerged as a robust solution for practical fluoride removal from drinking water. The review advocates for the development of advanced filtration systems that can selectively remove harmful fluoride while retaining essential minerals [
48].
Song et al. (2019) [
49] examined the role of nanomaterials in phytoremediation, noting their potential to enhance plant growth and increase the uptake of pollutants. The review highlights the role of nanotechnology in enhancing the efficiency of phytoremediation. Nanomaterials can contribute to phytoremediation in several ways: they can directly remove contaminants, promote plant growth, and enhance the phytoavailability of pollutants. Among the various strategies for phytoremediation, phyto extraction is noted as the most effective, allowing plants to absorb and concentrate contaminants from the soil. The study focuses on nZVI. Additionally, fullerene nanoparticles are mentioned for their ability to increase the availability of pollutants to plants. While the use of nanomaterials in phytoremediation shows promise, the review notes that this approach is still in its exploratory phase [
49].
In the 2019 study, Zhang, T. outline the complexities of using nanotechnology for in situ remediation, discussing significant challenges related to the mixing and transport of nanomaterials in subsurface environments. Zhang discusses various nanomaterials that could enhance remediation efforts, including multifunctional nanocomposites that enable synergistic contaminant sequestration and degradation, selective adsorbents, catalysts, and nano-tracers for delineating subsurface contaminants. Additionally, stimuli-responsive nanomaterials can facilitate the slow release of reagents, improving remediation effectiveness. However, the study notes significant challenges related to the mixing and transport of nanomaterials in subsurface environments, particularly in laminar flow conditions. Enhancements in mixing techniques are needed to overcome these transport limitations, as well as strategies to generate reactive nanomaterials in situ for treating contamination, especially in low hydraulic conductivity zones [
44].
Babaee et al. (2018) [
50] investigate the effectiveness of iron/copper nanoparticles in removing arsenic from water, demonstrating their significant potential for practical applications in arsenic remediation. The study focused on the application of these nanoparticles in treating synthetic arsenic-laden solutions. The Fe/Cu nanoparticles demonstrated significant effectiveness in arsenic removal, achieving maximum sorption capacities of 19.68 mg/g for As(III) and 21.32 mg/g for As(V) at a pH of 7. The adsorption data fit well with the Langmuir isotherm model, indicating that adsorption occurs on a surface with a finite number of identical sites. The kinetics of sorption followed pseudo-second-order kinetics, suggesting that the rate of adsorption is influenced by the availability of active sites. Furthermore, the presence of coexisting carbonate, sulfate, and phosphate ions did not significantly affect the arsenic removal efficiency at the concentrations tested. The study found that removal efficiency was enhanced in acidic environments, while arsenic desorption could occur under basic conditions. This study contributes to the growing recognition of bimetallic nanoparticles in environmental cleanup efforts [
50].
In “Recent Advances of Nanoremediation Technologies for Soil and Groundwater Remediation”, Alazaiza explores the significant role of nanotechnology in addressing contamination. The review discusses various types of nanomaterials, including nZVI, carbon nanotubes (CNTs), and metallic and magnetic nanoparticles (MNPs), highlighting their applications in environmental cleanup. The use of metal and MNPs is advantageous due to their ease of magnetic separation and effective metal-ion adsorption capabilities. Additionally, the review addresses the environmental risks associated with the application of nanomaterials in soil remediation. It emphasizes that modified nZVI can exhibit reduced toxicity towards soil bacteria compared to unmodified versions, suggesting that coating or modifying these nanoparticles may mitigate their ecological impacts. The combination of nanoremediation with other remediation technologies is presented as a promising approach, as it can enhance the sustainability and effectiveness of the cleanup process [
8].
Thomé et al. (2015) [
51] examined the nascent field of soil remediation in Brazil. The review provides a bibliographic overview of nanotechnology applications in remediation efforts, assessing the potential for further research in Brazil. It begins by comparing the number of identified contaminated sites in the USA and Europe, setting the context for Brazil’s challenges. The paper explains fundamental concepts related to nanomaterials, including their classification, synthesis, and characterization, along with the types of contaminants that have been effectively addressed using this technology. Thomé discusses the chemical interactions between contaminants and nanoparticles, as well as the challenges associated with the delivery and migration of NPs in porous media [
51].
In the 2011 study by Al-Salim, the author investigates the environmental risk assessment of nanomaterials, specifically focusing on quantum dots (QDs). The study highlights the necessity of understanding both the toxicity of these nanomaterials to non-target organisms and their potential exposure pathways. The research examines the transport and fate of QDs, which are nanoparticles with an average size of 6.5 nm, across various environmental compartments. The study utilized breakthrough curves from experiments involving CdTe/mercaptopropionic acid QDs applied to soil columns from an organic apple orchard. The results indicated that preferential flow occurred through the soil’s macropores, but only 60% of the QDs were recovered in the effluent after several pore volumes. This suggests that approximately 40% of the QDs were filtered and retained by the soil, possibly through unknown exchange, adsorption, or sequestration mechanisms. Additionally, the study explored the uptake of QDs by plants, specifically ryegrass, onion, and chrysanthemum [
45].
In the 2019 review by Taghipour, the author discusses the rapid advancements in nanotechnology, which have led to the development of engineered nanomaterials (NMs) that offer innovative solutions for contaminant removal in water treatment. The review categorizes various nanomaterials and examines their properties and pollutant removal mechanisms. A novel categorization of these materials is presented, including carbonaceous nanostructures, nanoparticles, and nanocomposites. Among the various NMs, graphene and its derivatives, such as graphene oxide and reduced graphene oxide, were highlighted for their excellent environmental compatibility, large surface area, and high purity. These characteristics contribute to their remarkable adsorption capacity by trapping electrons and preventing their recombination. Metal oxides, known for their unique electronic structure and high porosity, are primarily utilized as catalysts in photocatalytic reactions due to their stability and efficient light absorption. The review also discusses the discovery of oxyacids, which have shown higher photoactivity and surface areas than traditional metal oxides, often at a lower cost. This has led to the development of binary or ternary composites combining metals, metal oxides, and oxy-acids, enhancing the surface area and reducing the band gap for improved contaminant cleanup [
29].
In the 2014 study by Rathnayake, the author emphasizes the importance of understanding the ecological and human health risks associated with zinc oxide manufactured nanomaterials (ZnO MNMs) released into the environment. The study investigates the transformation of 30 nm ZnO MNMs in the presence of varying concentrations of phosphate, examining the effects of time and pH using multiple analytical techniques. The composition of phosphorus species was found to be pH dependent. At pH 6, 82% of the phosphorus was present as hopeite-like phosphate, while this proportion dropped to only 15% at pH 8. These results underscore the influence of environmental variables, particularly pH, on the reactions between ZnO MNMs and phosphate, suggesting that such interactions can lead to structurally and morphologically heterogeneous end products [
52].
In the 2022 review by Raza titled “Recent Advances in Carbonaceous Sustainable Nanomaterials for Wastewater Treatments”, the review focuses on various carbonaceous nanomaterials, such as activated carbon, multi-walled and single-walled carbon nanotubes, and carbon quantum dots, emphasizing their roles as effective adsorbents in wastewater treatment and purification. It also discusses the significant contributions of graphene (GR) and graphene oxide (GO) derivatives, along with other carbon-based nanomaterials like graphitic carbon nitrides and carbon sponges/aerogels. These materials have shown promise in addressing wastewater treatment and remediation. The review encompasses various developed technologies and methodologies, as well as surface modifications of carbonaceous nanomaterials aimed at pollutant removal, including ionic metals in aqueous environments [
54].
In the 2017 review by Gomes, the author discusses the growing concern surrounding emerging contaminants, which include pesticides and their metabolites, pharmaceuticals, personal care products, lifestyle compounds, food additives, industrial products, and nanomaterials. The review highlights the limited data available on the toxicity and potential bioaccumulation of these contaminants in soil biota. When data do exist, they often pertain only to select representatives of major chemical groups and a restricted number of species, typically following non-standard testing protocols. This scarcity of information complicates the assessment of predicted no effect concentrations (PNEC) and hinders the establishment of regulatory limits for their environmental release [
55].
In the 2021 review by Marcon, this review focuses on recent advancements in the application of engineered NPs for in situ remediation of soil and groundwater. Key areas of emphasis include the successful applications of nanomaterials for environmental cleanup, the potential safety implications arising from the need for high reactivity toward pollutants while maintaining low reactivity toward biota, and the challenges faced in transport and evolution within environmental matrices. The review also discusses scientific and regulatory challenges associated with the use of nanomaterials. Promising nanomaterials highlighted in the review include nanoscale zero-valent iron, nano-oxides, and carbonaceous materials [
9].
In the 2022 review by Dutta, the author discusses the increasing global challenge of providing safe drinking water and clean water. Despite ongoing critical issues, the implementation of ecologically sustainable nanomaterials (NMs) with unique characteristics—such as high efficiency, selectivity, earth abundance, renewability, low-cost manufacturing, and stability—has become a priority. The review highlights the promise of carbon nanoparticles (NPs) in energy and environmental sectors. The review focuses on research surrounding these carbon-based NPs, particularly in their application for water treatment and purification, especially concerning industrial and pharmaceutical waste. Enhanced carbonaceous NMs, novel nano-sorbents, and methods for treating wastewater, drinking water, and groundwater, as well as removing ionic metals from aqueous environments, are examined. The review also discusses the latest advancements and challenges related to environmentally friendly carbon and graphene quantum dot NMs. It emphasizes the urgent need for organic compounds and heavy hydrocarbons to meet the growing demand for carbon-based nanostructures in emerging fields such as sensors, biomedicine, supercapacitors, and gas storage. Carbon-based NPs may offer advantages over other types of NPs regarding infrastructure, resource use, and safety concerns. The review suggests that using carbon nanotubes (CNTs), carbon quantum dots (CQDs), graphene quantum dots (GQDs), and graphene-based NPs could be more effective than traditional treatment methods [
40].
In the 2018 study by Rahmatpour, the author investigates the potential contamination of groundwater and soils by manufactured nanomaterials, specifically focusing on silver nanoparticles (AgNPs). The study examines the transport and retention of polyvinylpyrrolidone (PVP) stabilized AgNPs (with a diameter of 40 nm) under both saturated and unsaturated conditions in intact columns of two types of calcareous soils: sandy loam (TR) and loam (ZR). To provide a comparison, similar experiments were conducted using quartz sand as a reference medium. A pulse of AgNP suspension with an initial concentration (C0) of 50 mg/L was injected into the columns for three pore volumes, while the transport of bromide (Br) as a non-reactive inert tracer was also monitored. The results indicated high mobility of AgNPs through the sand columns due to unfavorable conditions for nanoparticle deposition on the quartz surfaces. In contrast, nearly all AgNPs introduced into the columns of both calcareous soils were retained, with less than 1% of the total injected mass leaching out. The retention profiles were hyperexponential, with maximum concentrations of 100–130 mg/kg occurring near the inlet of the columns. Notably, the ZR soil exhibited slightly stronger retention and higher maximum concentrations of AgNPs compared to the TR soil, likely due to its smaller grain size [
42].
In the 2013 paper by Mura titled “Advanced of Nanotechnology in Agro-Environmental Studies”. The paper reviews recent applications of nanotechnology in agro-environmental studies, focusing on the fate of nanomaterials in water and soil, their advantages, and potential toxicological effects. The findings suggest that nanomaterials can enhance environmental quality and aid in the detection and remediation of pollution. However, only a limited number of these materials have shown potential toxic effects, which are explored in detail. The intelligent use of nanotechnology can enhance environmental quality, contribute to the development of detection techniques for biological and chemical toxins, remediate contaminants, and foster green industrial processes that minimize energy consumption. Mura notes that while some nanomaterials pose hazards and potential toxic effects, not all exhibit toxicity. For instance, certain materials like carbon black and titanium dioxide (TiO
2) have been used for an extended period and demonstrate low toxicity [
56].
Boregowda et al. (2022) [
57] emphasized the ecological harms associated with excessive use of chemical fertilizers and pesticides. The review emphasizes the potential of engineered nanomaterials as effective solutions for the remediation of contaminated soils. Nanomaterial-enabled technologies can help prevent the uncontrolled release of harmful substances into the environment and are equipped to address soil and groundwater pollutants. Boregowda discusses recent advancements in agricultural nanobiotechnology and the tools developed to tackle soil pollution [
57].
In the 2019 study by Yin, the author explores the transport of heavy metal ions in porous media with varying surface characteristics. The research focuses on the effects of graphene oxide (GO) on the co-transport and remobilization of lead (Pb
2+) and cadmium (Cd
2+) in different coated sand media, including humic acid (HA), smectite, kaolinite, and ferrihydrite. Laboratory packed-column experiments were conducted to evaluate these interactions. Scanning electron microscopy and energy dispersive X-ray analysis revealed significant differences in the surface morphology of the coated sands, with approximately 56.7% to 89.9% of the surface covered by the coatings. The major elemental components identified were carbon (C), oxygen (O), silicon (Si), aluminum (Al), and iron (Fe). The findings indicated that GO exhibited high mobility in HA, kaolinite, and smectite-coated sand but showed considerable retention in ferrihydrite-coated sand. Interestingly, while GO reduced the transport of Pb
2+ and Cd
2+, the presence of these metal ions also decreased the mobility of GO within the coated sand columns. Elution experiments demonstrated that GO facilitated the remobilization and release of previously adsorbed Pb
2+ and Cd
2+ from the coated sand. However, it was noted that GO could not release these metal ions from smectite-coated sand columns, likely due to smectite’s stronger adsorption affinity for heavy metals compared to GO [
41].
Bossa et al. (2017) [
58] examines the use of nZVI for the in-situ remediation of groundwater and other environmental matrices. The study focuses on a novel approach where nZVI are stabilized on cellulose nanocrystal (CNC) supports, which enhances their contact with contaminants, reduces the electron transfer path to the target, and increases reactivity. The researchers synthesized CNC-nano-ZVI composites with a weight ratio of Fe/CNC = 1, utilizing a classic sodium borohydride synthesis method. The final nanocomposites were characterized, and their reactivity was assessed through methyl orange (MO) dye degradation tests. Flow-through transport column experiments were conducted to evaluate the mobility of the CNC-nano-ZVIs in porous media (sand/glass bead). The results indicated that the synthesized CNC-nano-ZVI formed a stable colloidal suspension and demonstrated high mobility in the porous media, with an attachment efficiency (α) value of less than 0.23. Notably, the reactivity of the CNC-nano-ZVIs towards MO increased by up to 25% compared to bare ZVI. The findings suggest that using CNC as a delivery vehicle significantly enhances the capability and applicability of nZVI for in situ groundwater remediation [
58].
Hao et al. (2021) [
39] explore a green synthesis method for iron nanoparticles using green tea extract, achieving high removal efficiency for hexavalent chromium (Cr(VI)). The research investigates the effects of various synthetic conditions on the remediation performance of the resulting nFe. The study revealed that the nFe particles had a core–shell structure, consisting of a core made of ZVI and a shell composed of iron oxide. A proposed mechanism for the synthesis of nFe using green tea extract was presented, highlighting how biomolecules in the extract acted as reducing and capping agents. These biomolecules, such as 1,2,3-benzenetriol, caffeine, and bis (2-ethylhexyl) phthalate, facilitated the reduction of Fe
2+ to ZVI and helped stabilize the nFe surface, preventing oxidation and enhancing atmospheric stability. The results demonstrated that the green tea extract effectively enabled the synthesis of nFe with a removal efficiency of 91.6% for Cr(VI) in aqueous solutions. This optimized synthesis approach allows for the practical application of nFe in water treatment, potentially offering advantages over existing methods [
39].
2.7. Primary Research Topics of the Nanomaterials in Soil and Groundwater Remediation
These studies illustrate a multifaceted approach to addressing environmental contamination through nanomaterials. Key themes highlight the effectiveness of nZVI, the importance of addressing emerging pollutants, and the potential for combining various remediation strategies. The studies emphasize the significance of in situ versus ex situ methodologies, with many authors advocating advanced treatment technologies that utilize nanomaterials to enhance traditional methods. The exploration of diverse synthesis methods, including green synthesis, demonstrates a commitment to minimizing environmental impact while maximizing effectiveness. Additionally, understanding the transport mechanisms and retention behaviors of nanomaterials is crucial for optimizing remediation strategies. Overall, the integration of nanotechnology into environmental remediation presents promising solutions for various contaminants, but ongoing research is essential to address potential risks and ensure ecological safety.
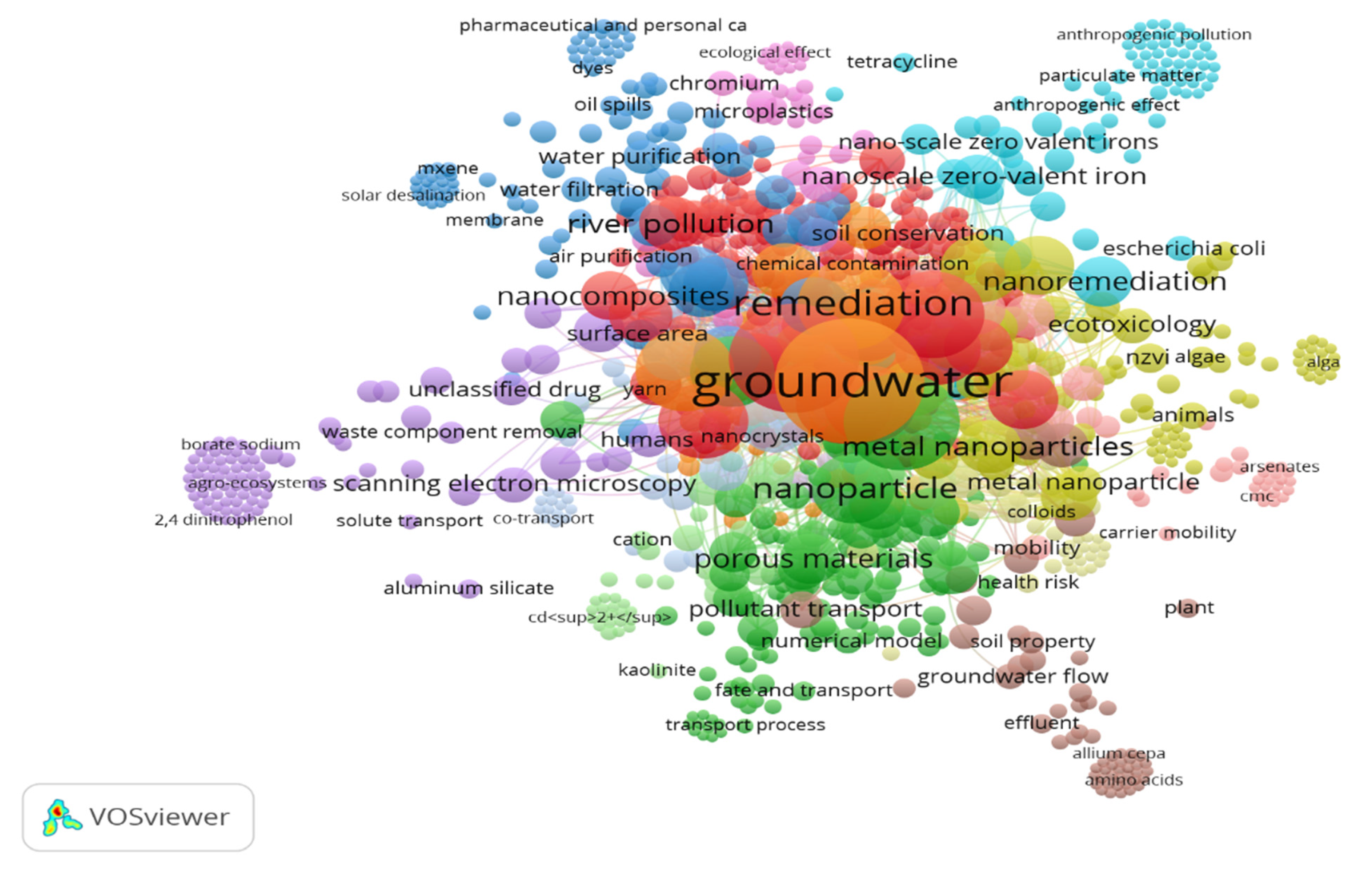
Figure 7 illustrates the network relationships among various keywords employed by researchers in the field of nanomaterials for soil and groundwater remediation. Notably, the terms “groundwater”, “groundwater pollution”, “nanoparticles”, “nanomaterial”, “remediation”, “adsorption”, and “soil pollution” are situated at the center of the network, highlighting their fundamental significance in the discourse. Surrounding these central keywords, clusters of related terms emerge, representing subfields within the broader domain of nanomaterials in soil and groundwater remediation research.
Figure 8 highlights the expanding research landscape on nanomaterials for soil and groundwater remediation, reflecting a growing awareness of water and soil pollution issues. Key terms such as “groundwater”, “groundwater pollution”, “remediation”, “nanoparticles”, and “nanomaterials” indicate a strong focus on groundwater quality and treatment technologies. The clustering of related keywords, including “adsorption”, “nanostructured materials”, and “soil pollution”, suggests significant subfields within this research area. Adsorption is recognized as a key mechanism in remediation, while soil pollution directly impacts groundwater health. This complexity in the field is driven by the need for effective solutions to environmental challenges. As research publications increase, we can expect greater funding for initiatives addressing ecological sustainability and technological advancements. Collaborative efforts are likely to lead to interdisciplinary approaches, integrating nanotechnology with traditional remediation methods. Emerging themes like “engineered nanomaterials” and “toxicity” further indicate a shift toward prioritizing safety and sustainability in nanotechnology, emphasizing the balance between innovation and ecological stewardship.
Biberci [
81] emphasized the importance of co-occurrence analysis in uncovering research topics and assessing the dynamics of research fronts within a particular field. In our investigation, we examined data from Scopus, applying a minimum occurrence threshold of 2 for keywords. This methodology enabled us to extract 46 keyword strings from a total of 825 author keywords
Table 8 presents the keywords that met or surpassed a predefined threshold for inclusion in the analysis. Specifically, the analysis focused on keywords provided by authors, rather than indexed keywords. To qualify for this analysis, keywords had to appear at least twice in the literature. The top 46 keywords are ranked in descending order based on their TLS. This ranking was determined through a comprehensive scientific analysis that considered factors such as cumulative link strength, the number of connections for each keyword, and their frequency of occurrence. By applying these criteria, we ensure that only the most relevant and impactful keywords are included in the study, allowing for a more robust and insightful analysis of the research landscape related to nanomaterials in groundwater and soil remediation.
The analysis of the keywords presented in
Table 8 reveals important insights into the current research landscape regarding nanomaterials in groundwater and soil remediation. The category of nanomaterials is foundational to the study of remediation techniques. Key terms such as “Nanomaterials”, “Nanotechnology”, and “Nanoparticles” underscore the versatility and potential applications of these materials in environmental cleanup. The prominence of these keywords, particularly “Nanomaterials” and “Nanoparticles”, indicates a growing interest in harnessing nanotechnology to enhance the efficiency of remediation efforts. Furthermore, the inclusion of specific nanomaterials, such as carbon nanotubes and zero-valent iron, highlights the ongoing exploration of tailored nanostructures designed for effective pollutant removal. The analysis reveals that treatment methods such as “Adsorption” and “Photocatalysis” are among the most frequently mentioned strategies for pollution mitigation. The keyword “Adsorption” is particularly significant as it represents a vital mechanism for the removal of contaminants from diverse matrices, including water and soil. This highlights the necessity of understanding the various mechanisms through which contaminants can be effectively managed. Additionally, terms like “Nanoremediation” and “Advanced Oxidation Processes” reflect a diverse range of methodologies employed to address contamination issues, emphasizing the need for innovative approaches in environmental remediation.
The focus on various types of pollutants is evident like “Heavy Metals”, “Arsenic”, and “Emerging Contaminants.” These keywords signify a growing awareness of specific contaminants that pose significant risks to human health and ecosystems. The substantial mention of “Heavy Metals” highlights the critical challenges posed by these pollutants, particularly in terms of their toxicity and persistence in the environment. The emergence of keywords related to “Emerging Contaminants” further underscores the need for targeted remediation strategies to address newer pollutants that may not have been adequately studied in the past. The importance of environmental resources is emphasized by the presence of keywords such as “Water”, “Wastewater”, and “Soil”. These terms highlight the significance of these resources, which are increasingly vulnerable to contamination. The keywords reflect a concentrated effort within the research community to address water-related pollution issues and to develop effective treatment strategies. The inclusion of “Environment” also points to a broader understanding of the interconnectedness of these resources and the need for holistic approaches to remediation.
The keywords associated with agricultural practices, such as “Agriculture”, “Fertilizer”, and “Nutrients”, indicate a growing interest in the role of nanomaterials in enhancing agricultural productivity while addressing soil contamination. The research in this area signifies an important shift towards integrating nanotechnology within agricultural systems, aiming to improve soil fertility and crop resilience. This dual focus on agricultural productivity and environmental health reflects a comprehensive approach to sustainability. The inclusion of keywords related to catalytic processes, such as “Catalyst”, “Iron Oxide”, and “Reductive Dechlorination”, suggests that researchers are increasingly exploring catalytic methods to enhance pollutant degradation. This focus on catalysis indicates a trend towards developing more efficient and effective remediation techniques that leverage the unique properties of nanomaterials.
In this context,
Figure 8 provides a network diagram that depicts the co-occurrence of keywords identified in research articles focusing on nanomaterials for soil and groundwater remediation.
In the diagram shown in
Figure 8, the nodes symbolize different elements, and their shapes and positions indicate the probability of co-occurrence among these elements. Analyzing the keyword co-occurrence network reveals six distinct clusters, each represented by unique colors, corresponding to various topics within the realm of nanomaterials research related to soil and groundwater remediation. The colored nodes represent these clusters, with each focusing on a specific area within this field. The size of the nodes reflects their frequency of occurrence, while the thickness of the connections between them demonstrates the strength of their relationships.
The first cluster, characterized by the red color, comprises ten keywords: toxicity, soil, water, environment, emerging contaminants, PFAS, energy, sensing, transport, and quantum dots. This cluster reflects a critical concern regarding the adverse effects of pollutants on human health and ecosystems. The emphasis on toxicity indicates a growing recognition of the risks posed by contaminants, particularly heavy metals and emerging pollutants like PFAS. The keywords soil and water highlight the importance of these natural resources, which are increasingly threatened by pollution. In this context, the terms emerging contaminants and quantum dots suggest an evolving focus on newer classes of pollutants that require innovative monitoring and remediation strategies. The presence of keywords like transport and sensing underscores the necessity for advanced methodologies to track and measure the movement and concentration of contaminants in the environment. This cluster indicates that future research should prioritize understanding toxicity pathways and developing real-time monitoring technologies that can enhance environmental health assessments.
The second cluster, represented in green, includes keywords such as nanoremediation, NZVI (nano Zero-Valent Iron), carbon nanotubes, zero-valent iron, carbon nanomaterials, iron nanoparticles, green synthesis, reductive dechlorination, and metallic nanoparticles. This cluster emphasizes the innovative applications of nanotechnology in remediation efforts. The term nanoremediation encapsulates the cutting-edge strategies being developed to tackle pollution. Specific nanomaterials, such as zero-valent iron and carbon nanotubes, are highlighted for their promising capabilities in effectively removing contaminants from water. The mention of green synthesis signifies a trend toward sustainable practices in the production of nanomaterials, reflecting a holistic approach to environmental remediation. Future research could focus on optimizing these materials for enhanced stability and reactivity in various environmental conditions, improving their practical efficacy.
The third cluster, illustrated in blue, features eight keywords: heavy metals, pollutants, nano zero-valent iron, phytoremediation, nano-phytoremediation, phytoextraction, pesticides, and phytotoxicity. This cluster reveals a significant concern regarding heavy metals as environmental pollutants. The integration of terms like phytoremediation and nano-phytoremediation suggests a blend of traditional remediation strategies with modern nanotechnology to enhance effectiveness. Research in this area must explore how nanomaterials can improve crop resilience while addressing soil contamination. It is essential to examine the potential risks associated with nanomaterials in agricultural contexts, ensuring that their application does not compromise soil health or ecosystem dynamics.
The fourth cluster, shown in yellow, includes eight keywords: nanomaterials, nanotechnology, photocatalysis, wastewater, catalyst, polymer-based nanomaterials, advanced oxidation processes, and biogenic nanoparticles. This cluster directly aligns with the principles of catalysis, showcasing the diverse applications of nanotechnology in environmental remediation. The terms photocatalysis and advanced oxidation processes are particularly noteworthy, as they indicate advanced methodologies that leverage the unique properties of nanomaterials for effective pollutant degradation. Photocatalytic processes utilize light-activated catalysts to drive chemical reactions that break down contaminants, offering a promising avenue for treating persistent pollutants. The integration of nanomaterials in these processes can significantly enhance reaction rates and broaden the spectrum of pollutants that can be effectively degraded. Moreover, the role of catalysts in these processes is critical. Catalysts can facilitate reactions without being consumed, allowing for more efficient and sustainable remediation approaches. Future research in this cluster should focus on optimizing the catalytic properties of nanomaterials, exploring their mechanisms in pollutant degradation, and assessing their long-term impacts on environmental systems.
The fifth cluster is characterized by purple and includes six keywords: adsorption, arsenic, nanocomposite, pharmaceuticals, iron oxide, and wastewater treatment. Adsorption emerges as a fundamental mechanism for removing contaminants from water and soil, highlighting its importance in achieving effective remediation. The presence of arsenic emphasizes the need for targeted strategies to address specific pollutants known to pose significant risks to health. This cluster suggests a future focus on optimizing adsorption materials and exploring their application in various treatment scenarios, especially in removing pharmaceuticals and other persistent pollutants from wastewater.
The final cluster, depicted in light blue, comprises five keywords: nanoparticles, agriculture, fertilizer, nutrients, and zinc oxide. The inclusion of agriculture and fertilizer indicates a growing interest in the role of nanomaterials in enhancing agricultural practices. Keywords like nutrients and zinc oxide suggest a focus on how nanomaterials can improve soil fertility and plant growth. Future research in this cluster should aim to explore sustainable agricultural practices that utilize nanotechnology to address both food security and environmental health.
Groundwater sources are often contaminated with heavy metals due to industrial activities, agricultural runoff, and waste disposal. The concept of “Nanoremediation” highlights the use of nanomaterials, particularly nZVI and carbon nanotubes, which have shown promise in effectively removing heavy metals from water through processes like adsorption and chemical reduction.
The transport dynamics of pollutants, particularly heavy metals like arsenic, are crucial for assessing their ecological and health risks. Research in this cluster should focus on the mechanisms of contaminant transport through soil and groundwater systems, exploring factors such as soil composition, moisture content, and microbial activity. Furthermore, the toxicity of nanomaterials themselves must be evaluated, as their interactions with existing pollutants could exacerbate environmental problems. Understanding these dynamics is vital for developing comprehensive risk assessments and effective remediation strategies that mitigate both contamination and toxicity.
Nanomaterials can enhance traditional remediation techniques by increasing reaction rates and improving the degradation of complex pollutants. Research should focus on the efficacy of various nanomaterials in degrading specific contaminants, including pharmaceuticals, pesticides, and industrial chemicals. Innovative approaches, such as photocatalysis and advanced oxidation processes, should be explored for their potential to break down persistent pollutants in wastewater.
The integration of nanomaterials in agricultural systems presents opportunities for improving crop health and productivity while simultaneously addressing soil and water contamination. Future research should investigate the dual impacts of nanomaterials—how they can enhance soil fertility and plant resilience, and their potential risks in terms of soil health and ecosystem dynamics. The interaction between nanoparticles and environmental pollutants, particularly in agricultural settings, necessitates a thorough examination to avoid negative repercussions on food safety and environmental quality. This holistic approach can lead to sustainable agricultural practices that contribute to both food security and environmental health.
Adsorption is a key mechanism in many water treatment processes, effectively capturing pollutants and improving water quality. The integration of photocatalytic technologies represents a promising avenue for enhancing the degradation of persistent contaminants. Research in this cluster should focus on optimizing adsorption materials and photocatalytic systems to increase their efficiency and effectiveness in real-world applications. Investigating the synergistic effects of combining these technologies could lead to innovative remediation solutions capable of addressing complex contamination scenarios.
The exploration of these clusters illustrates the multifaceted nature of nanomaterials and their potential applications in addressing environmental contamination. As the challenges of soil and groundwater pollution become increasingly complex, an integrated approach that considers the behavior of nanomaterials, their interactions with pollutants, and the implications for human health and ecosystems is essential.