Study of the Reaction Pathways for the Hydrogenation of Quinoline over Nickel Phosphide Catalysts
Abstract
1. Introduction
2. Results
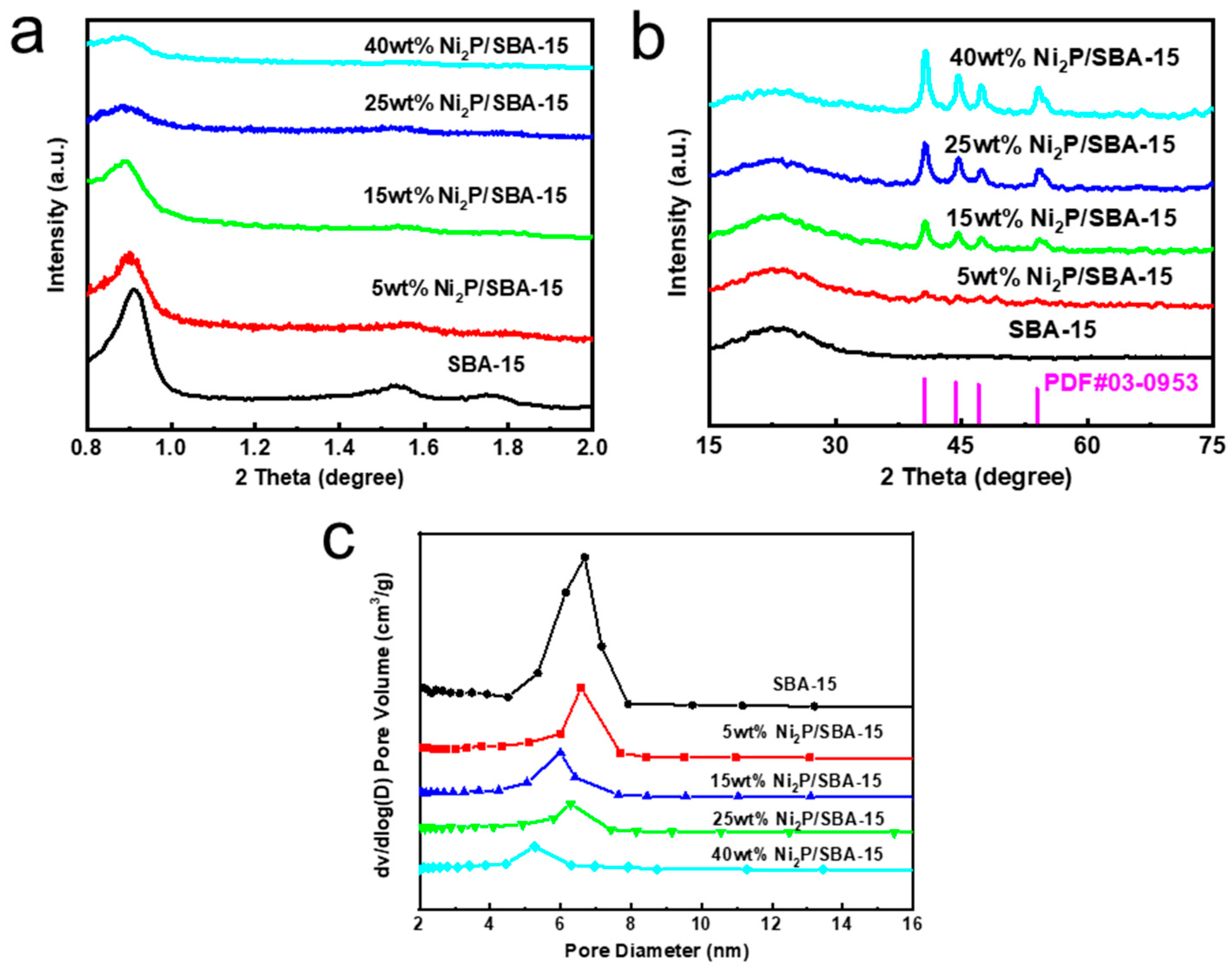
2.1. Carrier and Catalyst XRD Analysis
2.2. Study of the Denitrogenation Reaction of Quinoline
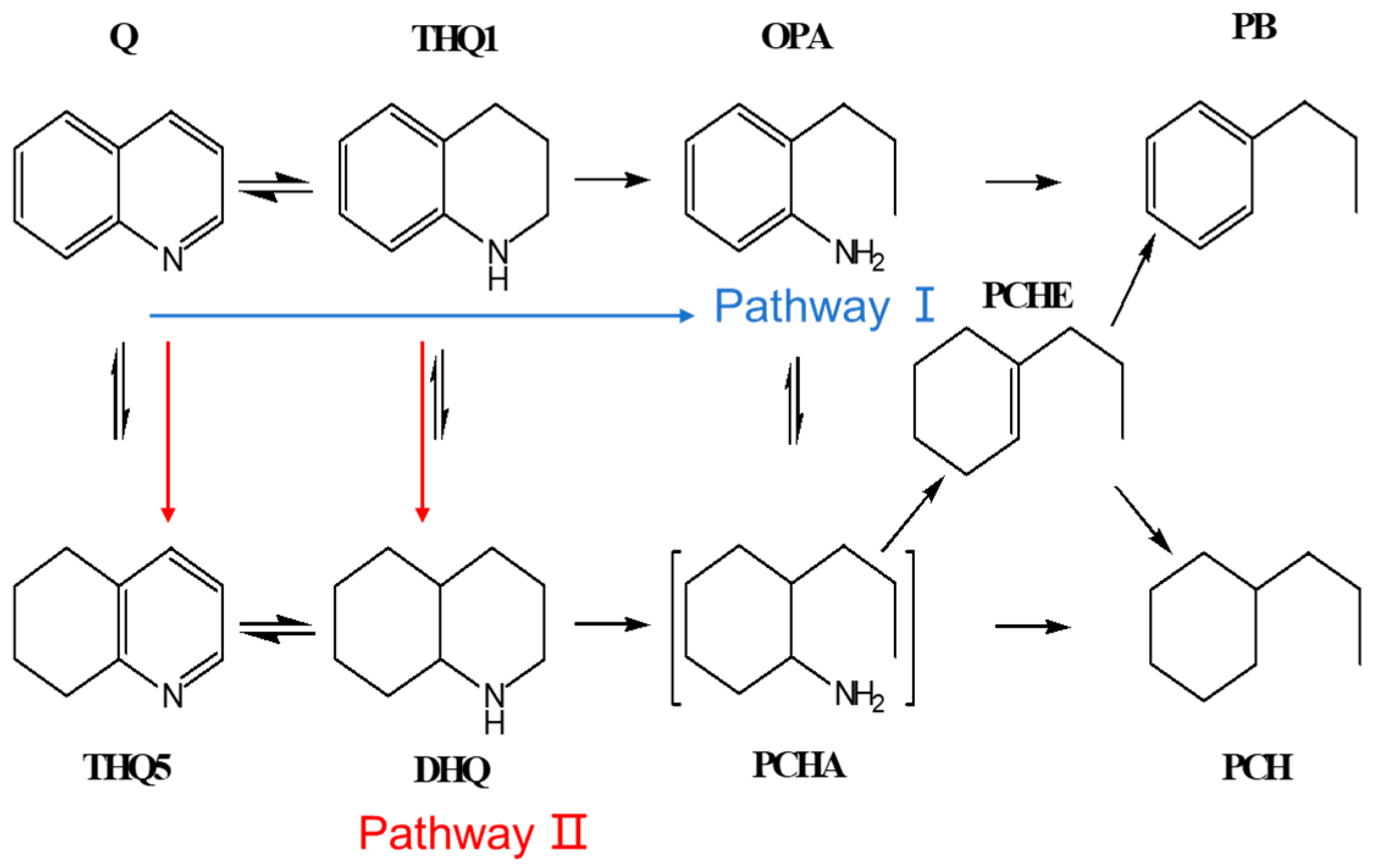
2.2.1. Hydrogenation Pathway of Quinoline
2.2.2. Effect of Different Temperatures on Conversion and Denitrogenation Rates
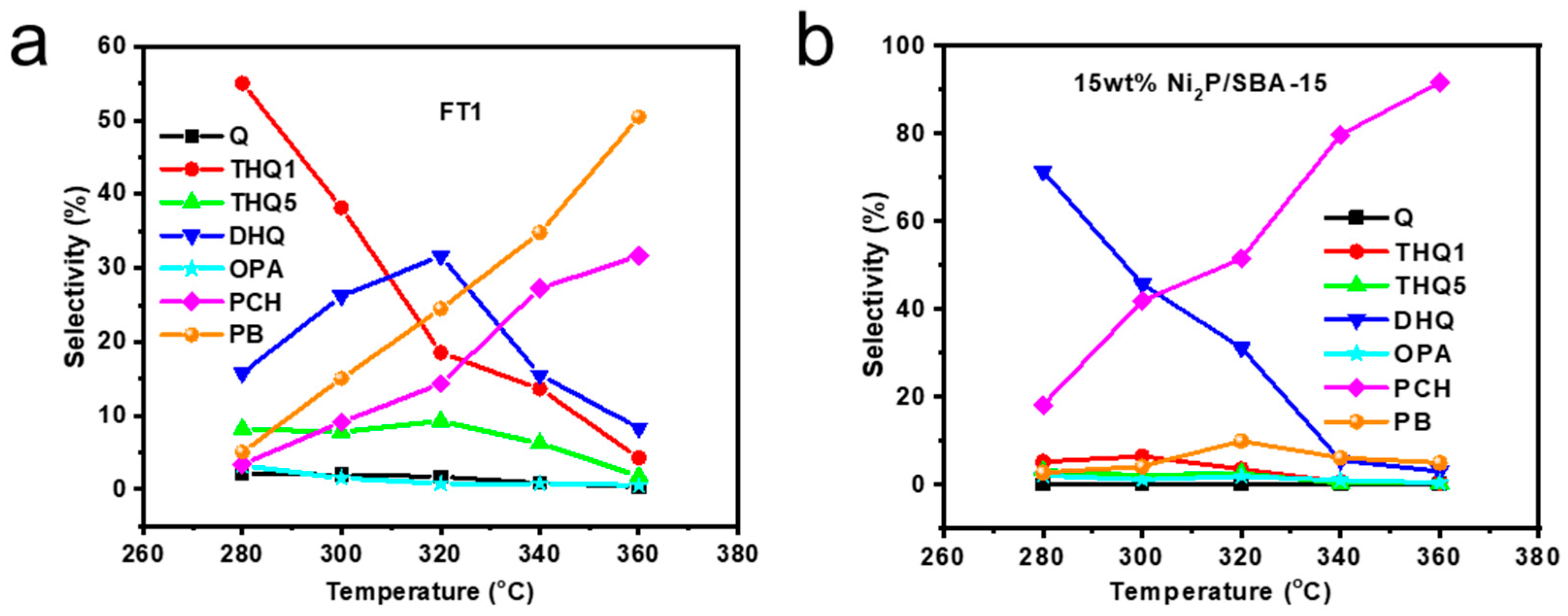
2.2.3. Selectivity Study of Quinoline Hydrogenation Products
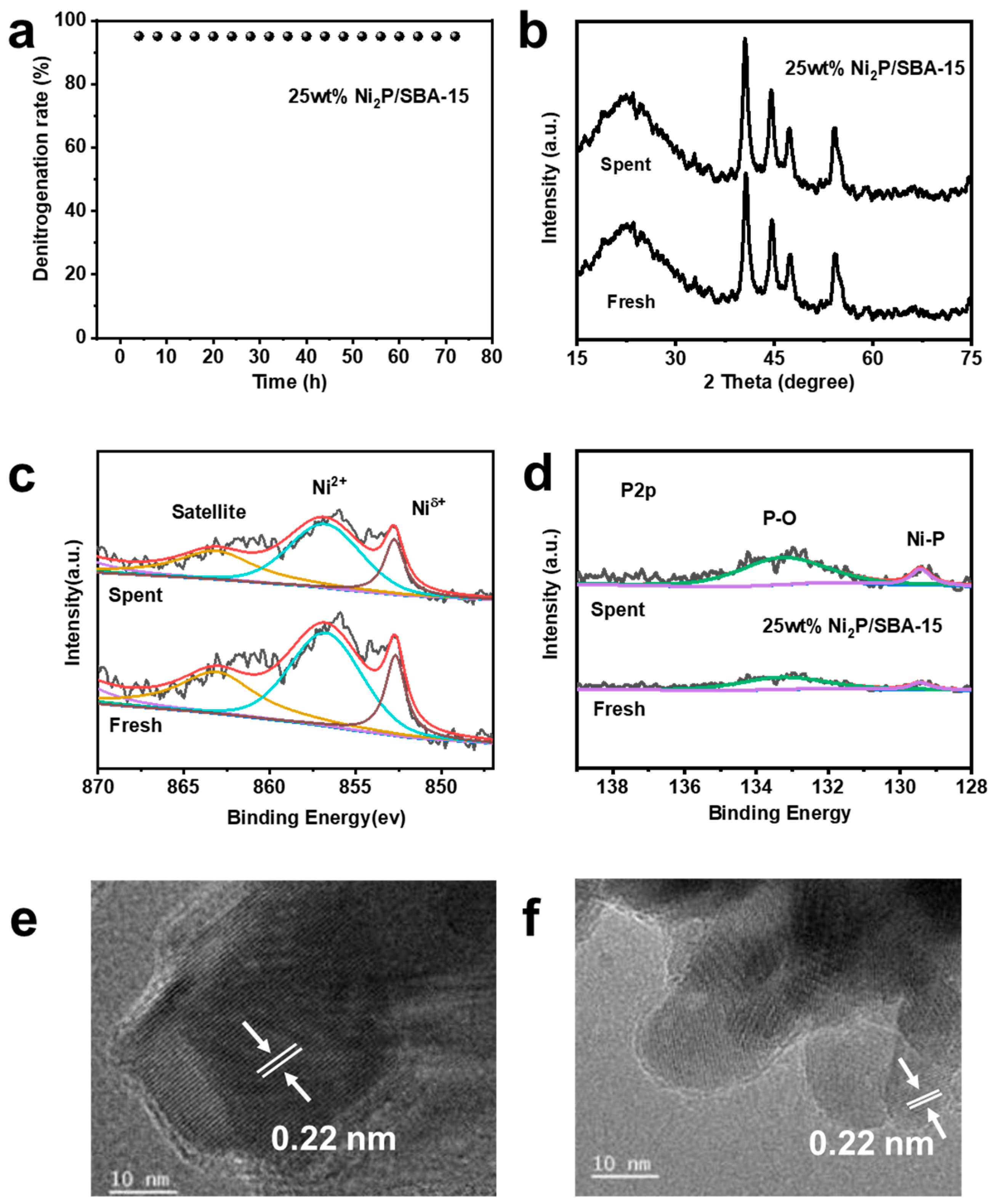
2.3. Analysis of Catalyst Durability
3. Materials and Methods
3.1. Catalyst Preparation
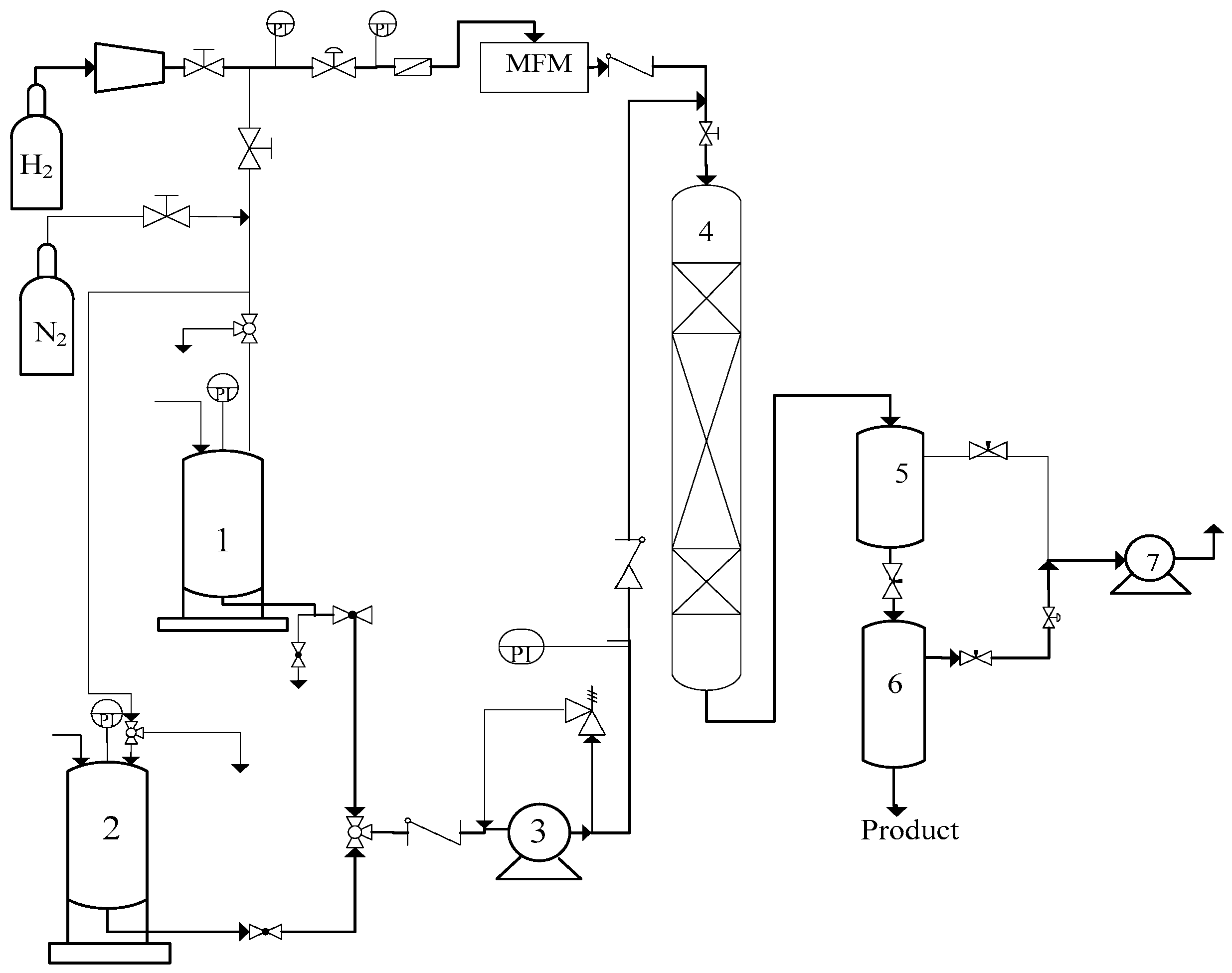
3.2. HDN Activity Evaluation Device
3.3. Reaction Materials and Conditions
3.4. Catalyst Characterization
3.4.1. X-Ray Diffractometer
3.4.2. Nitrogen Adsorption and Desorption Apparatus
3.4.3. X-Ray Photoelectron Spectroscopy
3.4.4. High-Resolution Transmission Electron Microscopy
3.4.5. Inductively Coupled Plasma
3.5. Hydrogenation Product Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Murti, S.D.S.; Sakanishi, K.; Okuma, O.; Korai, Y.; Mochida, I. Detailed characterization of heteroatom-containing molecules in light distillates derived from Tanito Harum coal and its hydrotreated oil. Fuel 2002, 81, 2241–2248. [Google Scholar] [CrossRef]
- Murti, S.D.S.; Choi, K.H.; Sakanishi, K.; Okuma, O.; Korai, Y.; Mochida, I. Analysis and removal of heteroatom containing species in coal liquid distillate over NiMo catalysts. Fuel 2005, 84, 135–142. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, D.S.; Yang, P. Removal of Basic Nitrogen of Diesel Oil by Chemical Method. J. Fushun Pet. Inst. 2002, 22, 1–4. [Google Scholar]
- Xiang, C.; Chai, Y.; Liu, Y.; Chenguang, L. Mutual Influence of Hydrodesulfurization of Dibenzothiophene and Hydrodenitrogenation of Quinoline over NiMoS/γ-Al2O3 Catalyst. Chin. J. Catal. 2008, 29, 595–601. [Google Scholar]
- Gong, H.Z.; Xiao, Z.W.; Zhuang, Y.; Liang, S.Q.; Li, X.; Zheng, W.B.; Duan, A.J.; Zhang, X.; Liu, J. Core-shell meso-beta@mesoporous aluminosilicate supported Ni2P catalyst for the hydrodenitrogenation of quinoline: Effect of core shell structure on Ni2P particle size. Fuel 2021, 302, 121131. [Google Scholar] [CrossRef]
- Gong, H.Z.; Zhuang, Y.; Zhang, X.; Liu, J.; Li, S.Y. Ni2P/Beta@SBA-16 core-shell catalyst with tunable shell thickness for the hydrodenitrogenation of quinoline. Appl. Catal. B Environ. 2023, 330, 122574. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, Z.; Li, F.; Zhao, T.; Zou, D.; Sun, G.; Li, C. Study on the preparation of γ-alumina-supported molybdenum phosphide hydrofining catalysts. J. Fuel Chem. Technol. 2006, 34, 126–128. [Google Scholar]
- Oyama, S.T. Novel catalysts for advanced hydroprocessing: Transition metal phosphides. J. Catal. 2003, 216, 343–352. [Google Scholar] [CrossRef]
- Sun, F.X.; Li, C. Advance in the Research on Hydrodesulfurization and Hydrodenitrogenation on Transition Metal Phosphides. Acta Pet. Sin. 2005, 21, 1–11. [Google Scholar]
- Liu, L.H.; Liu, S.Q.; Chai, Y.M.; Chen-guang, L. Preparation mechanism and hydrodenitrogenation performance of nickel phosphide catalyst. J. Fuel Chem. Technol. 2013, 41, 335–340. [Google Scholar]
- Robinson, W.R.A.M.; Van Gestel, J.N.M.; Koranyi, T.I.; Eijsbouts, S.; Van der Kraan, A.M.; Van Veen, J.A.R.; De Beer, V.H.J. Phosphorus promotion of Ni(Co)-containing Mo-free catalysts in quinoline hydrodenitrogenation. J. Catal. 1996, 161, 539–550. [Google Scholar] [CrossRef]
- Li, W.; Dhandapani, B.; Oyama, S.T. Molybdenum phosphide: A novel catalyst for Hydrodenitrogenation. Chem. Lett. 1998, 27, 207–208. [Google Scholar] [CrossRef]
- Stinner, C.; Prins, R.; Weber, T. Formation, structure, and HDN activity of unsupported molybdenum phosphides. J. Catal. 2000, 191, 438–444. [Google Scholar] [CrossRef]
- Zhai, Q.; Cai, J.; Yu, H.; Qin, L. SYNTHESIS OF SBA-15 MOLECULAR SIEVE. J. Chin. Ceram. Soc. 2006, 34, 385–388. [Google Scholar]
- Massoth, F.E.; Kim, S.C. Kinetics of the HDN of quinoline under vapor-phase conditions. Ind. Eng. Chem. Res. 2003, 42, 1011–1022. [Google Scholar] [CrossRef]
- Satterfield, C.N.; Cocchetto, J.F. Reaction network and kinetics of the vapor-phase catalytic hydrodenitrogenation of quinoline. Ind. Eng. Chem. Process Des. Dev. 1981, 20, 53–62. [Google Scholar] [CrossRef][Green Version]
- Gong, H.Z.; Zhuang, Y.; Liu, J.; Zhang, X. Three-dimensionally ordered macroporous ZSM-5 zeolite supported high-dispersed Ni2P: Highly active catalyst for quinoline hydrodenitrogenation. Chem. Eng. J. 2024, 480, 148236. [Google Scholar] [CrossRef]
- Meng, Q.; Wang, A.C.; Liu, C.; Wang, E.; Duan, A.; Zhao, Z.; Jiang, G. Preparation of Beta-KIT-5 composite material supported ternary metal catalyst and its hydrodenitrogenation performance of quinoline. Fuel 2022, 326, 125084. [Google Scholar] [CrossRef]
- Perot, G.; Brunet, S.; Canaff, C.; Toulhoat, H. Transformation of Quinolines and Anilines Over NiMo-Al2O3 Catalysts. Bull. Sociétés Chim. Belg. 1987, 96, 865–870. [Google Scholar] [CrossRef]
- Zheng, X.C.; Yuan, C.Y.; Zhao, W.P. Synthesis and Characterization of Mesoporous Molecular Sieves SBA-15. J. Zhengzhou Univ. 2008, 40, 101–106. [Google Scholar]
- Oyama, S.T.; Wang, X.; Lee, Y.-K.; Bando, K.; Requejo, F.G. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques. J. Catal. 2002, 210, 207–217. [Google Scholar] [CrossRef]
- Korányi, T.I.; Vít, Z.; Poduval, D.G.; Ryoo, R.; Kim, H.S.; Hensen, E.J.M. SBA-15-supported nickel phosphide hydrotreating catalysts. J. Catal. 2008, 253, 119–131. [Google Scholar] [CrossRef]

| Catalyst | Ni (wt%) | P (wt%) | Ni2P (wt%) | Ni/P(at%) |
|---|---|---|---|---|
| 5wt%Ni2P/SBA-15 | 3.5 | 1.4 | 4.9 | 1.3 |
| 15wt%Ni2P/SBA-15 | 11.5 | 3.2 | 14.7 | 1.9 |
| 25wt%Ni2P/SBA-15 | 19.9 | 5.5 | 25.4 | 1.9 |
| 40wt%Ni2P/SBA-15 | 31.5 | 8.3 | 39.8 | 2 |
| Catalyst | Ni (wt%) | Specific Surface (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|---|
| SBA-15 | 0 | 715 | 1.16 | 6.8 |
| Ni2P/SBA-15 | 5 | 431 | 0.65 | 6.3 |
| 15 | 342 | 0.49 | 5.9 | |
| 25 | 255 | 0.40 | 5.4 | |
| 40 | 167 | 0.33 | 4.9 |
| Catalysts | Feed | Reaction Conditions | HDN/% | Reference |
|---|---|---|---|---|
| Ni2P/meso-beta@MAS | 1.0 wt% Q in cyclohexane | 0.5 g cat, T = 340–400 °C, P = 4.0 MPa, WHSV = 12.8 h−1, H2/Oil = 600. | 78.3 | [5] |
| Ni2P/H-beta@SBA-16-2 | 1.0 wt% Q in cyclohexane | 0.5 g cat, P = 4 MPa, H2/feed = 400, WHSV = 12.8 h−1, 400 °C | 81.6 | [6] |
| Ni2P/3DOMZSM-5 | 1.0 wt% Q in cyclohexane | 0.5 g cat, P = 4 MPa, H2/feed = 400, WHSV P = 12.8 h−1, 400 °C | 91 | [17] |
| NiMoW/BK-3 | 0.2 wt% Q in cyclohexane | P = 4 MPa, H2/feed = 400, WHSV = 10 h−1, 280 °C | 52.4 | [18] |
| Ni2P/SBA-15 | 1.0 wt% Q in decahydronaphthalene | P = 6.0 MPa, T = 280–360 °C, WHSV = 20 h−1. | 92 | This work |
| Pore Volume (mL/g) | Specific Surface (m2/g) | Average Pore Size (nm) | Intensity (N/cm) | Effective Component/(wt/%) | |||
|---|---|---|---|---|---|---|---|
| WO3 | MoO3 | NiO | P | ||||
| ≮0.25 | ≮150 | 8.13 | ≮182 | ≮24.0 | ≮2.4 | ≮2.5 | 0.3–1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiao, Y.; Xu, C.; Lv, Z.; Zhao, Y.; Huang, P. Study of the Reaction Pathways for the Hydrogenation of Quinoline over Nickel Phosphide Catalysts. Catalysts 2025, 15, 976. https://doi.org/10.3390/catal15100976
Qiao Y, Xu C, Lv Z, Zhao Y, Huang P. Study of the Reaction Pathways for the Hydrogenation of Quinoline over Nickel Phosphide Catalysts. Catalysts. 2025; 15(10):976. https://doi.org/10.3390/catal15100976
Chicago/Turabian StyleQiao, Yuan, Chunming Xu, Zhao Lv, Yuan Zhao, and Peng Huang. 2025. "Study of the Reaction Pathways for the Hydrogenation of Quinoline over Nickel Phosphide Catalysts" Catalysts 15, no. 10: 976. https://doi.org/10.3390/catal15100976
APA StyleQiao, Y., Xu, C., Lv, Z., Zhao, Y., & Huang, P. (2025). Study of the Reaction Pathways for the Hydrogenation of Quinoline over Nickel Phosphide Catalysts. Catalysts, 15(10), 976. https://doi.org/10.3390/catal15100976







