Abstract
Terpenoids, as a class of natural products with extensive biological activities, hold broad application prospects in the fields of medicine, food, materials, and energy, with the global market scale projected to reach USD 10 billion by 2030. Traditional chemical synthesis and plant extraction methods rely on petroleum and plant resources, suffering from problems such as environmental pollution, cumbersome procedures, low yields from plant sources, enantioselectivity, geographical constraints, and competition for resources. Biocatalytic conversion of biomass feedstocks via microbial cell factories serves as an environmentally friendly alternative for the synthesis of terpenoids, but current production mostly depends on starch-based glucose, which triggers issues of food security and competition for arable land and water resources. This review focuses on the biocatalytic conversion of non-food alternative carbon sources (namely lignocellulose, acetate, glycerol, and waste oils) in the microbial synthesis of terpenoids, systematically summarizing the current research status and cutting-edge advances. These carbon sources exhibit potential for sustainable production due to their low cost, wide availability, and ability to reduce resource competition, but they also face significant technical bottlenecks. We systematically analyze the current problems in the biocatalytic conversion process and put forward some available solutions. It is hoped that this study will provide theoretical and technical suggestions for breaking through the bottlenecks in the biocatalytic conversion of non-food carbon sources and promoting the efficient and sustainable production of terpenoids.
1. Introduction
Terpenoids are a class of natural products with broad bioactivities and significant application value, which are categorized into monoterpenes (C10), sesquiterpenes (C15), diterpenes (C20), triterpenes (C30), and tetraterpenes (C40) [1]. Their structural diversity and biological functions render them indispensable in pharmaceuticals, food, materials, and energy [2,3,4]. For example, sesquiterpenes are usually considered the monomer in the synthesis of natural rubber [5]. According to the Association of Natural Rubber Producing Countries (ANRPC), global demand for natural rubber (polyisoprene) is projected to reach 15.6 million tons by 2025 [6]. In the field of pharmaceuticals, paclitaxel (a kind of diterpene) is a broad-spectrum anticancer drug with global annual sales exceeding USD 2 billion [7], while artemisinin (a kind of sesquiterpene lactone) remains the core drug for malaria treatment, with an annual demand of approximately 400 tons. The food and cosmetics industries widely utilize limonene (a kind of monoterpene) as a natural flavoring agent, as well as ganoderic acid (a kind of triterpene) as a functional additive. Due to the huge market demand, the global terpenoid value is forecasted to reach USD 10 billion by 2030 [8,9].
Currently, the production methods for terpenoids mainly include chemical synthesis, plant extraction, and biotransformation methods. Chemical synthesis methods involve complex steps, generate numerous by-products, and rely heavily on non-renewable petroleum resources, raising serious concerns about resource depletion and environmental pollution [10]. Plant extraction methods are limited by long production cycles, geographical constraints, low compound yields, and competition for arable land, which makes it difficult to conduct large-scale industrial production [10]. The biocatalytic conversion of renewable biomass using microbial cell factories is an emerging approach developed in recent years for the production of terpenoids, which exhibits various advantages, including mild reaction conditions, environmental friendliness, low-carbon sustainability, and the use of renewable feedstocks [11]. Compared with traditional plant extraction or chemical synthesis, the production of terpenoids by microbial cell factories driven by renewable carbon sources such as glucose has demonstrated advantages in terms of raw material and environmental protection costs: chemical synthesis requires multi-step protection/deprotection and the use of toxic solvents, with equipment depreciation and the treatment of waste gas, wastewater, and solid waste accounting for approximately 45–55% of the total cost. In contrast, when microbial cell factories are used to produce artemisinic acid, squalane, and other terpenoids in 50 m3 fermenters, the total production cost can be reduced to USD 11,000–14,000 per ton, which is 20–30% lower than that of the chemical method, with a simultaneous reduction of approximately 60% in CO2 emissions [12].
The biosynthesis of terpenoids in microbes involves two distinct pathways: the mevalonate (MVA) pathway in eukaryotes, and the methyl-D-erythritol-4-phosphate (MEP) pathway in prokaryotes [8,13]. The MVA pathway primarily occurs in the cytoplasm, starting with acetyl-CoA as the initial substrate and generating isopentenyl pyrophosphate (IPP) and dimethylallyl pyrophosphate (DMAPP) through multiple enzymatic steps (Figure 1). The key rate-limiting enzyme of the MVA pathway is 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), which catalyzes the conversion of HMG-CoA to MVA [14]. The MEP pathway starts with pyruvate and glyceraldehyde-3-phosphate (G3P), with methylerythritol-4-phosphate (MEP) as the key intermediate converted to IPP and DMAPP (Figure 1). The key rate-limiting enzymes of the MEP pathway are 1-deoxy-D-xylulose-5-phosphate synthase (DXS) and reductoisomerase (DXR), catalyzing the first two steps in the pathway [15]. There are many natural microorganisms that can synthesize terpenoids, including bacteria, yeast, and filamentous fungi [15,16,17]. In current terpenoid biosynthesis research and industrial practices, filamentous fungi are not commonly used as engineered hosts for terpenoid production, because they often suffer from limitations such as complex genetic manipulation and long culture cycles, which make their application far less widespread than that of yeast and bacterial systems [17,18]. Thus, the most widely studied and practically applicable microbial hosts are yeast and bacteria, such as Saccharomyces cerevisiae, Yarrowia lipolytica, Ogataea polymorpha, Escherichia coli, Bacillus spp., and so on [15,19,20]. These systems are preferred for terpenoid production due to their clear genetic backgrounds, established genetic manipulation tools, rapid growth, and controllable metabolic flux. A growing number of terpenoids have already been successfully synthesized via construction of various microbial cell factories, including artemisinic acid, valencene, β-carotene, limonene, farnesene, and squalene [21,22,23,24,25,26].
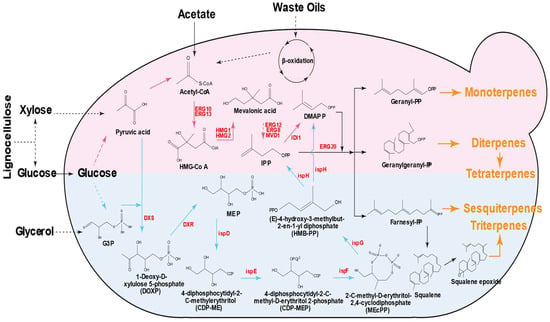
Figure 1.
Synthesis pathways of different terpenoids from different carbon sources. The upper section illustrates the MVA pathway, while the lower section depicts the MEP pathway. HMG-CoA, 3-hydroxy-3-methylglutaryl-CoA; IPP, isopentenyl pyrophosphate; DMAPP, dimethylallyl pyrophosphate; G3P, glyceraldehyde-3-phosphate; MEP, methylerythritol-4-phosphate; DXS, DOXP synthase; DXR, DOXP reductoisomerase; ispD, MEP cytidylyltransferase; ispE, 4-diphophocytidyl-2-C-methyl-D-erythritol kinase; ispF, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate synthase; ispG, HMB-PP synthase; ispH, HMB-PP reductase.
In the bioproduction of terpenoids, glucose is the commonly used carbon source, playing an important role in the cultivation of microbial cell factories. Yet using glucose as the feedstock leads to a significant issue in arable land and water occupation. Because glucose is mainly derived from the starch hydrolysis of corn or wheat, approximately 1.2 tons of corn are consumed to produce 1 ton of industrial glucose [27]. Meanwhile, corn cultivation consumes about 5000–9000 cubic meters of water per hectare, and the substantial use of fertilizers and pesticides leads to soil degradation and water pollution [27,28,29]. Large-scale utilization of glucose from starch-based feedstocks in the fermentation field would intensify competition for food and water resources among humans.
Therefore, developing more sustainable biomass to replace glucose as the feedstock is considered a potential way to produce high-value biochemicals, including terpenoids. So far, some studies have explored the agricultural waste, lignocellulose, and industrial wastes like acetate, glycerol, and waste oils as the feedstocks to produce terpenoids. However, research on these alternative carbon sources remains relatively limited, and terpenoid yields are generally lower than those achieved with glucose. This review summarizes the current research status, cutting-edge advances, and key challenges in the field of biocatalytic conversion of these alternative carbon sources for terpenoid synthesis. Some current challenges and potential solutions are discussed, with the hope of guiding future efforts to enhance the conversion efficiency of these feedstocks for terpenoids synthesis.
2. Biocatalytic Conversion of Different Carbon Sources for Terpenoids Production
2.1. Efficient Conversion of Starch-Based Glucose for Terpenoids Production
Glucose is a commonly used, efficient fermentable sugar in microbial cultivation processes. It is metabolized through the Embden Meyerhof pathway (EMP) and the pentose phosphate pathway (PPP), generating key precursors such as acetyl-CoA, pyruvate, and glyceraldehyde-3-phosphate, which provide the foundation for terpenoids synthesis. In recent years, researchers have focused on metabolically engineering various host strains to enhance the production efficiency of terpenoids from glucose (Table 1).
Ginsenosides are a class of triterpenoids found exclusively in plants of the Panax genus, which exhibit anti-tumor, neuroprotective, cardiovascular- and cerebrovascular-protective, immunomodulatory, antioxidant, and anti-fatigue activities [30]. Gan et al. [31] first isolated a dammarenediol synthase from Mugwort, and achieved the heterologous biosynthesis of dammarenediol, a critical precursor of polyene-type ginsenosides, from glucose, which was a critical precursor of polyene-type ginsenosides. Kong et al. [24] obtained the highest reported yield of limonene, 2.63 g/L, in S. cerevisiae by dynamically regulating glucose and galactose supply, employing mitochondrial compartmentalization engineering and toxicity balancing strategies. Wu et al. [32] improved the production of geraniol to 2 g/L in E. coli using glucose as the carbon source by enhancing the precursor supply, modifying the activity of key enzymes and regulating metabolic flux. Zhao et al. [21] optimized the synthesis pathway and fermentation process, which efficiently led to a yield of artemisinic acid of up to 25 g/L using glucose as the carbon source. Chen et al. [33] increased the yield of valencene to 539.3 mg/L in glucose-based fermentation by enhancing the mevalonate (MVA) pathway, overexpressing key enzymes in the FPP synthesis pathway, and optimizing the expression cassette for valencene synthase. Tao et al. [34] successfully increased patchoulol production to 1.95 g/L from glucose by enhancing the MVA pathway, optimizing the acetyl-CoA pathway and fermentation process. Zhou et al. [22] constructed an LsLTC2 (germacrene A synthase)-Erg20 (FPP synthase) fusion protein connected by a linker peptide (GGGGS) to form a “metabolic channel” to reduce the competitive consumption of FPP. Coupled with the enhancement of cofactor and acetyl-CoA supply and downregulation of competing pathways, the production of β-elemene reached 4.7 g/L for the engineered O. polymorpha using glucose as the carbon source. Ge et al. [35] introduced an isopentenol utilization pathway (IUP) in S. cerevisiae to promote the synthesis of militradiene. Combined with the increase in the IPP/DMAPP pool and the modification of the key enzyme activities, the titer reached 1.02 g/L in a 5 L fermenter using glucose as the carbon source. Chang et al. [36] also constructed an artificial IUP in E. coli to increase the supply of the precursor geranylgeranyl diphosphate (GGPP), which led to the efficient synthesis of geranyllinalool, and the titer was up to 2.7 g/L in a 5 L fermenter. Zhou et al. [26] significantly increased squalene production in Y. lipolytica using glucose as the carbon source by modifying the key enzymes in the MVA pathway, applying the peroxisomal compartmentalization strategy, regulating lipid metabolism to increase acetyl-CoA supply, and enhancing cofactor supply. The final titer reached 51.2 g/L in a 5 L fermenter, which was the highest yield reported to date. Zhu et al. [37] introduced α-amyrin synthase from Catharanthus roseus into S. cerevisiae to achieve the synthesis of ursolic acid, and the titer was up to 8.59 g/L using glucose as the carbon source after genetic optimization of the synthesis pathway, which was also the highest yield so far. These studies demonstrate that the efficient synthesis of various high-value terpenoids has been successfully achieved using glucose as the carbon source.

Table 1.
Efficient conversion of glucose for the production of high-value terpenoids by microbial cell factories.
Table 1.
Efficient conversion of glucose for the production of high-value terpenoids by microbial cell factories.
| Terpenoids | Strains | Titers (g/L) | Fermentation Scale | Application and Market Demand | Reference |
|---|---|---|---|---|---|
| Limonene | S. cerevisiae | 2.63 | 3 L Bioreactor | applied in cleaning and fragrances fields, with a market demand of 68,200 tons | [24] |
| Geraniol | E. coli | 2.0 | Shake Flask | applied in fragrances and insect repellents fields, with a market demand of 18,000 tons | [32] |
| Artemisinic acid | S. cerevisiae | 25 | 5 L Bioreactor | applied in anti-malarial drug synthesis, with a market demand of 7200 tons | [21] |
| Valencene | S. cerevisiae | 0.54 | 3 L Bioreactor | applied in citrus flavors and cleaning fields, with a market demand of 1230 tons | [33] |
| Patchoulol | S. cerevisiae | 1.95 | 5 L Bioreactor | applied in perfumes and anti-inflammation production, with a market demand of 7500 tons | [34] |
| β-Elemene | O. polymorpha | 4.7 | 3–5 L Bioreactor | applied in anti-tumor fields, with a Chinese market demand of 12,970 tons | [22] |
| Miltiradiene | S. cerevisiae | 1.02 | 5 L Bioreactor | applied in cardiovascular drug synthesis, with a Chinese market demand of 410 tons | [35] |
| Geranyllinalool | E. coli | 2.7 | 5 L Bioreactor | applied in flavor and anti-virus fields, with a projected demand of 1720 tons | [36] |
| Dammaradienol | S. cerevisiae | 1.04 | 3.6 L Bioreactor | applied in liver protection and anti-aging fields, with a market demand of 500–800 tons | [31] |
| Squalene | Y. lipolytica | 51.2 | 5 L Bioreactor | applied in moisturizers field, with a market demand of 54,600 tons | [26] |
| Ursolic Acid | S. cerevisiae | 8.59 | 5 L Bioreactor | applied in anti-inflammation and anti-tumor fields, with a market demand of 650 tons | [37] |
Although glucose exhibits high metabolic flux and yield as the carbon source for terpenoids production, it also presents significant disadvantages. For instance, it easily triggers the Crabtree effect in microorganisms, leading to excessively high glycolytic flux and diversion of substantial carbon flux towards by-products instead of the target terpenoids. It thus requires genetic modification or fermentation regulation to balance metabolic flux. Li et al. [38] demonstrated that using glucose as the carbon source resulted in significant ethanol accumulation in S. cerevisiae, which led to the valencene yield and carbon conversion efficiency being only one-third and one-fifth of those achieved with mannitol, respectively. Furthermore, the terpenoid yield in wild-type S. cerevisiae is limited by precursor flux competition (such as in sterol synthesis) and toxicity accumulation. The authors finally achieved efficient valencene synthesis by substituting glucose with mannitol to enhance precursor supply, obtaining a maximum valencene yield of 5.61 g/L in a mannitol-fed batch fermentation [34]. Moreover, terpenoid synthesis requires substantial cofactors, NADPH and ATP. Glucose metabolism primarily generates NADH via the glycolysis pathway, while NADPH regeneration relies on the pentose phosphate pathway (PPP). The co-expression of cofactor-recycling enzymes is commonly used to modulate cofactor balance, but simple enzyme co-expression often provides limited assistance to the supply of cofactors. More regulation strategies need to be implemented to enhance cofactor supply in the synthesis of terpenoids using glucose as the carbon source [39]. Additionally, due to the issue of the glucose effect, the utilization of other carbon sources is inhibited when glucose is present, which limits the development of strategies for the synergistic utilization of multiple carbon sources [40]. More importantly, glucose dependence on starch-based materials entails resource competition. Therefore, some researchers have focused on developing non-conventional fermentable carbon sources (such as acetate, glycerol, lignocellulose hydrolysates, and waste oils) to overcome these bottlenecks.
2.2. Development of Processes Based on Non-Conventional Fermentable Feedstocks for the Synthesis of Terpenoids
2.2.1. Lignocellulose
Lignocellulose is the most abundant renewable biomass resource on Earth, and has gained significant attention in recent years as an inexpensive agricultural waste. Lignocellulose consists of cellulose, hemicellulose, and lignin, forming a complex and highly ordered structure that confers high stability and recalcitrance to degradation, making it difficult to utilize directly [41]. Therefore, effective pretreatment of lignocellulose is necessary before subsequent biocatalytic conversion processes can occur. Currently, lignocellulose pretreatment approaches mainly include chemical, biological, and physical methods [41,42]. Acid pretreatment, as a commonly used chemical method, can destroy lignin, dissolve hemicellulose, and degrade cellulose into glucose with high efficiency. However, the hydrolysates contain many toxic by-products, which require additional detoxification treatment before they can be used as carbon sources [43,44]. Biological pretreatment mainly uses enzymes to decompose and transform lignocellulose, and the hydrolysates are relatively pure, with low contents of toxic by-products. However, the price of enzymes is expensive, and this is the bottleneck in the enzymatic conversion of lignocellulose [45].
So far, some studies have used lignocellulose hydrolysates as carbon sources to realize the synthesis of terpenoids [25,46]. However, the low efficiency of xylose utilization remains a bottleneck in the conversion of lignocellulose hydrolysates at present. Current studies have enhanced the xylose oxidoreductase pathway by introducing exogenous genes or strengthening endogenous pathways, thereby breaking the bottleneck of xylose metabolism [47]. Jin et al. [48] engineered S. cerevisiae to utilize xylose and acetate from lignocellulose hydrolysates of corn stover. Due to the inherently low utilization efficiency of xylose in S. cerevisiae, xylose reductase (XR) and xylitol dehydrogenase (XDH) genes from Scheffersomyces stipitis were introduced, and endogenous xylulokinase (XK) was overexpressed to successfully achieve the efficient utilization of xylose. Wei et al. [46] genetically engineered Y. lipolytica to synthesize β-ionone by introducing carotenoid cleavage dioxygenase (CCD) from Osmanthus fragrans and optimizing the mevalonate pathway. Subsequently, a heterologous xylose oxidoreductase pathway was introduced, which enabled the engineered Y. lipolytica to produce β-ionone using xylose as the sole carbon source. Meanwhile, the engineered strain was also found to have the potential for use of lignocellulose hydrolysates as the carbon source, and the productivity of β-ionone reached 7.6 mg/g of dry cell weight (DCW). Tan et al. [25] metabolically engineered Y. lipolytica to achieve efficient β-farnesene production using lignocellulose hydrolysates. Corn stover was subjected to steam explosion pretreatment and enzymatic hydrolysis to produce hydrolysates containing 40.3 g/L glucose and 14.7 g/L xylose, and these were utilized as the carbon sources to produce β-farnesene by the engineered Y. lipolytica. The titer reached 7.38 g/L in the fed-batch fermentation, which was the highest level reported to date for β-farnesene production from lignocellulose hydrolysates. This demonstrated the capability of the engineered Y. lipolytica in the conversion of lignocellulose hydrolysates into terpenoids.
Junko et al. [49] investigated the potential of the oleaginous yeast Rhodosporidium toruloides as a platform organism for converting lignocellulose into terpenoids. They found that R. toruloides could efficiently convert glucose and xylose generated from lignocellulose hydrolysates into two non-native sesquiterpenes, bisabolene and amorphadiene. James et al. [50] also explored the synthesis of bisabolene and 1,8-cineole from lignocellulose hydrolysates by engineered R. toruloides, and the titers reached 2.2 g/L and 1.4 g/L, respectively. Unlike many conventional hosts, R. toruloides exhibited preferable growth and production efficiency in lignocellulose hydrolysates. Additionally, R. toruloides was able to assimilate p-coumaric acid released from acetylated grassy lignin in hydrolysates, and it could consume several aromatic compounds in the lignin fraction [49]. These results demonstrate the significant potential of R. toruloides for the production of terpenoids from lignocellulose raw materials.
Although lignocellulose, a renewable feedstock, has been successfully explored for the synthesis of various terpenoids, it still faces multiple challenges. The “reinforced concrete” structure formed by cellulose, hemicellulose, and lignin in lignocellulose results in strong recalcitrance to degradation. Traditional chemical pretreatments easily cause cellulose hornification, reducing enzymatic hydrolysis efficiency and generating inhibitors such as acetate, furfural, and phenolics. These inhibitors suppress microbial growth, leading to a 30–50% decrease in terpenoid yield [51,52]. Furthermore, when S. cerevisiae was used as the host, the metabolic efficiency of xylose derived from hemicellulose was low due to the lack of efficient transporters, while the xylose reductase–xylitol dehydrogenase pathway (XR-XDH) easily causes cofactor (NADP+/NAD+) imbalance, resulting in xylitol accumulation and xylose residues reaching 20–40% [51,53]. Multi-dimensional improvement strategies have been developed to address the above problems. For pretreatment, Wang et al. [53] developed the Catalytic Lignin Aryl Functionalization (CLAF) technology, which introduced highly nucleophilic phenolic compounds to directionally control lignin arylation to prevent its disordered condensation, while co-producing high-purity dissolving pulp (purity >95%) and depolymerizable lignin-based bisphenols. This method can improve the hydrolysis efficiency of cellulose and reduce the formation of by-products. Moreover, to mitigate the inhibition caused by toxic by-products, some genetic modifications were explored, including the expression of glyoxylate reductase isozyme to convert furfural to less toxic alcohols, the introduction of acetylating acetaldehyde dehydrogenases to convert acetate to ethanol, screening tolerant strains through adaptive evolution, and so on [48]. To address low xylose metabolic efficiency, the xylose isomerase (XI) pathway was replaced by the XR-XDH pathway. Combined with the overexpression of xylulose-5-phosphoketolase (XfpK), xylose could be efficiently converted to acetyl-CoA, directly supplying terpenoid precursors [51,53] (Table 2).

Table 2.
Biocatalytic conversion of lignocellulose for the production of terpenoids by the engineered microbes.
Furthermore, future work can be focused on the integration of catalysis science, synthetic biology, and process engineering: (1) constructing an in vitro Multi-enzyme Coordinated Expression and Self-recycling System (iMECS) integrating Cell-Free Protein Synthesis (CFPS) modules [54]; (2) developing Lytic Polysaccharide Monooxygenase (LPMO)-cellulase fusion proteins to penetrate lignin barriers; (3) developing machine learning-optimized multi-parameter models for pretreatment–enzymatic hydrolysis–fermentation; and (4) constructing artificial microbial consortia with specialized roles for inhibitor degradation and terpenoid synthesis.
2.2.2. Acetate
Acetate is a major product of syngas (CO2 and H2) bioelectrocatalysis, and it is also a main by-product in anaerobic fermentation and lignocellulose hydrolysates. Its utilization helps reduce environmental pollution and resource waste. Acetate can be directly converted to acetyl-CoA to synthesize high-value acetyl-CoA-derived biochemicals. Compared to glucose, acetate has a shorter metabolic path to acetyl-CoA, thus exhibiting significant advantages for acetyl-CoA derivative production [55].
There have been some reports on the synthesis of terpenoids in microbes using acetate as the carbon source. Xu et al. constructed a mevalonate biosynthesis pathway in recombinant E. coli, and 1.06 g/L mevalonate was produced in a 5 L bioreactor using acetate as the carbon source [56]. You et al. [57] explored squalene synthesis in Candida glycerinogenes using acetate as the sole carbon source. They found that acetate exhibited toxicity to microbial growth. High concentrations of acetate (>5 g/L) disrupted microbial cell membranes, inhibited enzyme activity, and reduced growth rates. Therefore, they overexpressed HOG1 (high-osmolarity glycerol pathway kinase) to enhance strain tolerance to acetate toxicity, alleviating growth inhibition. Simultaneously, acetate gradient feeding and pH self-regulation strategies were applied to achieve high squalene yield. Lu et al. [58] metabolically engineered S. cerevisiae to obtain a chassis strain with high acetyl-CoA accumulation. Then, directed evolution was performed on β-caryophyllene synthase (CPS) to enhance its affinity for acetyl-CoA-derived precursors FPP (Kcat/Kₘ increased by 35.5%). Meanwhile, a two-stage culture strategy was employed: the first stage used glucose to promote biomass accumulation and the second stage switched to acetate as the carbon source to induce terpenoid synthesis. Ultimately, the β-caryophyllene yield reached 594.05 mg/L.
As a promising biomass-derived carbon source, acetate can enable efficient terpenoid transformation through the design of engineered strains with efficient acetate metabolic pathways and high acetate tolerance based on synthetic biology. However, there are currently few studies on the biocatalytic conversion of acetate into terpenoids via microbial cell factories, and some problems still remain. For example, acetate has low energy efficiency and its ATP yield is only one-third that of glucose. However, terpenoid synthesis requires a large amount of ATP and the cofactor NADPH. The deficiency in energy supply constitutes a bottleneck, restricting the efficient conversion of acetate into terpenoids. In the future, more effective strategies can be developed to address the energy supply issue, including the construction of dual-substrate co-utilization systems and the enhancement of the respiratory chain energy conversion rate so as to improve the conversion efficiency of acetate.
2.2.3. Glycerol
Crude glycerol, a waste by-product of the biodiesel industry, poses growing environmental challenges due to its excessive accumulation, with land and water contamination being particularly prevalent [59]. The market value of crude glycerol (80% purity) was as low as USD 0.04–0.09 per pound, while pure glycerol is USD 0.27–0.41 per pound [60]. Accordingly, the utilization of low-cost crude glycerol or glycerol as a viable feedstock for bioconversion has garnered significant global attention, as this not only reduces the production costs of biochemicals but also mitigates environmental pollution [61].
In microbes, glycerol is phosphorylated by glycerol kinase to form 3-phosphoglycerol, which is then oxidized to dihydroxyacetone phosphate involving in the glycolytic pathway to generate acetyl-CoA, providing precursors for the synthesis of acetyl-CoA derivatives [62]. Meanwhile, glycerol metabolism generates dihydroxyacetone phosphate (DHAP) accompanied by NADH/NADPH formation, which can support the rate-limited steps catalyzed by HMG-CoA reductase in the MVA pathway and improve the synthesis of terpenoids [63]. Therefore, glycerol was considered a suitable feedstock for the production of terpenoids, and there have been a number of studies on the conversion of glycerol to terpenoids by the microbial cell factories [64,65,66].
Wang et al. [64] achieved the synthesis of linalool by engineered E. coli using glycerol as the carbon source by introducing an efficient linalool biosynthetic enzyme coupling geranyl diphosphate synthase with linalool synthase (LIS) from Mentha aquatica. Based on the optimization of the exogenous MVA pathway and the regulation of central carbon flux, the titer of linalool reached 1.07 g/L and 4.16 g/L in shake-flask and fed-batch fermentation, respectively, which were the highest linalool yields reported in E. coli to date. Julie et al. [67] proposed an artificially designed non-natural terpenoid synthesis pathway, the Isoprenoid Alcohol Pathway (IPA), and glycerol was used as the carbon source for efficient synthesis of terpenoid precursors, ultimately achieving a monoterpenoid yield of 0.6 g/L. Sun et al. engineered Y. lipolytica to produce betulinic acid from glycerol. They found that glycerol was superior to glucose in terpenoid biosynthesis due to the enhanced acetyl-CoA and metabolic flux, and the titer of the betulinic acid reached 26.53 mg/L when using glycerol as the carbon source [66]. Liu et al. [68] achieved the synthesis of trans-nerolidol in Y. lipolytica, and the highest titer reached 11.1 g/L in the fed-batch fermentation through the implementation of three strategies: (1) rational design of nerolidol synthase to enhance catalytic efficiency; (2) overexpression of carnitine acetyltransferase (CAT2) and compartmentalization of the MVA pathway within peroxisomes to increase the supply of acetyl-CoA and FPP; (3) carbon-restricted feeding to reduce the formation of metabolic by-products. These works indicated that glycerol was also a potential low-cost feedstock for the production of terpenoids.
Additionally, when glucose is used as the carbon source, the content of cyclic adenosine monophosphate (cAMP) in cells decreases, which leads to a reduction in the transcription of some enzymes [69]. Meanwhile, the problem of the glucose effect will also hinder the co-utilization of other fermentable sugar feedstocks [40]. However, as a kind of unconventional feedstock, no inhibitory effect has been observed when glycerol was used as the carbon source, which provides conditions for the construction of a system for the synergistic utilization of multiple carbon sources. Tan et al. [65] introduced nerolidol synthase (NES) from strawberries into E. coli and employed a glucose-lactose-glycerol co-utilization system: glucose provided rapid growth energy, lactose induced pathway gene expression, and glycerol provided a high content of NADPH. The synthesis of nerolidol was significantly enhanced, and the titer reached 3.3 g/L in shake flasks. This demonstrated the advantage of glycerol as a carbon source in constructing a multi-carbon source co-utilization system, which is a strategy that could be applied to improve the synthesis of terpenoids and other high-value biochemicals in the future.
Glycerol exhibits huge potential as a low-cost carbon source. However, there are also some issues in the utilization of glycerol: (1) the uptake rate of glycerol is lower than that of glucose; and (2) high concentrations of glycerol inhibit growth, leading to a slowdown in metabolic rate. In future work, the glycerol uptake rate can be increased by enhancing the glycerol transport system. For example, overexpressing the glpFK operon (containing the glycerol uptake facilitator gene, glpF, and the glycerol kinase gene, glpK) in E. coli can increase the uptake rate by 50% [23]. Furthermore, ultraviolet mutagenesis can be used to screen for highly active transporters to enhance glycerol absorption efficiency. To address the problem of low tolerance, more hosts with high glycerol tolerance can be developed, such as Y. lipolytica, while laboratory adaptive evolution (LAE) strategies or room temperature plasma (ARTP) mutagenesis methods can be employed to screen for highly tolerant mutant strains [70]. On this basis, a multi-carbon-source synergistic utilization system can be constructed to improve the carbon source utilization rate, enhance the growth and metabolic level of cells, and thereby increase the synthesis efficiency of products.
2.2.4. Waste Oils
Waste oil refers to oil substances that have lost their original use value and are unsuitable for direct reuse in food processing or consumption. It has a wide range of sources, including catering waste oil (such as frying waste oil from restaurants and home kitchens, oil residues from food processing, and floating oil in sewers), industrial waste oil (such as industrial-grade waste oil from food processing plants and slaughterhouses, and process waste oil from the cosmetics and pharmaceutical industries), as well as agricultural and other sources (such as waste oil from animal processing and oil in expired food) [71]. The global annual output exceeds 100 million tons, of which about 60% comes from the catering industry [72]. As a major catering country, China produces about 10–15 million tons of waste oil every year, with catering waste oil alone reaching more than 8 million tons [72]. Due to the imperfect recycling system, about 30% of the waste oil is not properly treated.
Traditional treatment methods for waste oils include chemical conversion and incineration. Chemical conversion mainly depends on the acid–base neutralization and transesterification, a process in which the triacylglycerols in waste oils are converted into fatty acid methyl esters for the production of biodiesel, as well as soap and other chemical products [73]. The incineration method depends on the burning of waste oils at high temperatures, which can be used for power generation or heating [74]. However, both methods pose significant environmental pollution risks. With the development of biotechnology, the emerging biocatalytic conversion method is more environmentally friendly and valuable in resource utilization, and it uses microorganisms to convert waste oils into high-value biochemicals [75,76,77].
Triacylglycerols in waste oils can be hydrolyzed by lipase into glycerol and free fatty acids, both of which can be utilized as carbon sources by microbes [78]. Since the fatty acids can generate acetyl-CoA via the β-oxidation pathway, waste oils have been explored as low-cost carbon sources to produce a large amount of acetyl-CoA-based biochemicals, including terpenoids [78]. Qi et al. [75] targeted α-humulene synthase (HumS) to peroxisomes in Y. lipolytica, leveraging the high acetyl-CoA concentration characteristic of this organelle to enhance terpenoid precursor supply. Transcriptome-guided metabolic regulation was employed and enabled efficient α-humulene synthesis using waste cooking oil as the carbon source. A total of 5.9 g/L of α-humulene was achieved in a 5 L bioreactor, setting the current record for microbial α-humulene synthesis. Zhu et al. [76] used a similar approach by targeting farnesyl diphosphate synthase and α-bisabolene synthase to the mitochondria so as to utilize the high concentration of acetyl-CoA in mitochondria to enhance precursor supply. They further overexpressed the mitochondrial fusion-related protein Mgm1p, a factor that maintains a constant mitochondrial fusion state and sustains a continuously high ATP supply, to increase mitochondrial metabolic capacity for α-bisabolene production. α-bisabolene production reached 1058.1 mg/L in a 5 L bioreactor when using fed-batch fermentation with waste oil as the carbon source, which was the highest yield of α-bisabolene in Y. lipolytica to date. Liu et al. [77] enhanced precursor supply by strengthening acetyl-CoA supply and optimizing the MVA pathway by knocking out the acyltransferase to inhibit lipid accumulation and blocking competing pathways. Simultaneously, a heterologous malic enzyme from Mucor circinelloides was introduced and polyphosphate kinase was overexpressed to achieve ATP/NADPH regeneration. Ultimately, the β-farnesene yield reached 31.9 g/L in a 5 L bioreactor when using waste oil as the feedstock (Table 3).

Table 3.
Production of terpenoids from acetate, glycerol, and waste oils by microbial cell factories.
Although waste oils have significant advantages in the synthesis of terpenoids, the current research on this topic is still in the preliminary stage, and some issues remain to be further addressed. For example, waste oils contain free fatty acids, glycerides, and aldehyde–ketone oxides, which inhibit the growth of microorganisms. Moreover, the accumulation of long-chain fatty acids (such as oleic acid) can damage the integrity of the cell membranes of most microorganisms. In the future, pretreatment methods such as activated carbon adsorption and enzymatic hydrolysis can be developed for waste oils. More oleaginous species similar to Y. lipolytica, which possess high tolerance and efficient oil utilization efficiency, can be developed and applied to the conversion of waste oils [79,80]. Meanwhile, microorganisms naturally prefer fermentable sugar carbon sources. It is necessary to strengthen the expression of related enzymes to broaden the metabolic pathways for the reuse of waste oils, and dynamic regulatory gene circuits can be further developed to improve carbon conversion efficiency.
3. Summary and Perspectives
Terpenoids, with their rich variety and diverse biological activities, have demonstrated enormous application potential and market value in multiple fields [81]. As the global demand for natural products has continued to grow, traditional chemical synthesis methods and plant extraction methods have struggled to meet the needs of large-scale industrial production due to issues like cumbersome procedures, environmental pollution, resource dependence, and limited yields [82]. As emerging production platforms, microbial cell factories have shown significant advantages of high efficiency, sustainability, and environmental friendliness. The application of these platforms involves the metabolic engineering modification of eukaryotic or prokaryotic microorganisms to efficiently synthesize terpenoids based on their endogenous basic metabolic pathways, incorporated with the introduction of terpenoid synthase [83]. So far, a variety of microbial chassis have been developed for the synthesis of terpenoids, including S. cerevisiae, E. coli, Y. lipolytica, and O. polymorpha [84]. However, the carbon sources they rely on are mainly fermentable sugars derived from starch, which leads to issues including the utilization of arable land and water resource occupation, as well as food supply problems [85]. Non-food-based alternative carbon sources are actively developed for terpenoid production, such as lignocellulose, acetate, glycerol, and waste oils. These biomass carbon sources not only feature low cost and wide availability but also offer the advantages of reducing environmental pollution and resource competition [65,75]. Specifically, the production costs of terpenoids vary significantly depending on the carbon source and the target molecule. The use of conventional starch-based glucose typically results in higher substrate costs, which can account for 30–50% of the total production expenses, thereby limiting the economic feasibility of bulk terpenoid production. In contrast, non-food carbon sources such as lignocellulose, acetate, glycerol, and waste oils offer substantial cost advantages due to their low prices and abundant availability. For instance, the production of α-humulene and β-farnesene using waste oils has demonstrated a significant reduction in raw material costs, potentially decreasing the overall production cost by 20–40% compared to glucose-based processes [75,77]. Nevertheless, current research on the utilization of these unconventional carbon sources is still in its preliminary stage, facing numerous challenges, such as the cumbersome chemical or enzymatic pretreatment of lignocellulose leading to high production costs and a large number of inhibitors in the hydrolysates, cytotoxicity and low energy efficiency in the utilization of acetate, low absorption rate and growth inhibition at high concentrations of glycerol, the cytotoxicity of impurities, and hydrophobicity limitations of waste oils [86]. Moreover, economic benefits are often counterbalanced by challenges including the requirement for extensive pretreatment, lower microbial conversion efficiency, and higher downstream processing costs.
With the continuous development and interdisciplinary integration of synthetic biology, metabolic engineering, and systems biology, as well as the wide application of new gene-editing tools, high-throughput screening technologies, and computational simulation methods, a variety of integrated strategies can be adopted to solve the above problems [87]. For the pretreatment issues of lignocellulose, low-cost and high-efficiency enzyme preparations or microbial co-cultivation strategies can be developed, which can significantly reduce pretreatment costs and the production of toxic by-products [88]. Microorganisms with high cellulase production as the chassis can be developed to construct microbial cell factories that synchronously saccharify and convert lignocellulose into biochemicals. To address problems such as the cytotoxicity of acetate and low energy efficiency, microorganisms can be modified through metabolic engineering to enhance their tolerance and assimilation capacity for acetate. Meanwhile, developing a multi-carbon source co-utilization system, first using other carbon sources for cell growth and generating abundant cofactors, then switching to acetate for product synthesis, can effectively alleviate the problem of insufficient energy supply from acetate. Regarding issues like the low absorption rate of glycerol and growth inhibition, the expression of glycerol transporters can be modified to improve the absorption rate of glycerol [89]. At the same time, laboratory adaptive evolution or genetic engineering strategies can be developed to improve the cell growth and metabolic capacity of microorganisms in high-concentration glycerol cultivation. For the high content of toxic impurities and hydrophobicity limitations of waste oils, efficient pretreatment technologies can be developed to remove impurities and reduce their inhibitory effects on microbial growth. Additionally, new oil emulsification technologies and microbial surface display technologies can also be developed to improve the dispersibility of waste oils in fermentation broth and the conversion efficiency of waste oils. Furthermore, a novel fermentation system capable of synergistically converting multiple carbon sources can be established, enabling microbial hosts to simultaneously utilize glycerol and free fatty acids in waste oils, thus enhancing the overall utilization efficiency of carbon sources.
Moreover, the titer of terpenoids is generally low, while the fermentation broth contains a large number of impurities (such as proteins and structurally similar terpenoid precursors). Traditional separation technologies struggle to efficiently distinguish these components, resulting in the recovery rates of target products often being below 60% [90]. The separation and purification process consumes significant amounts of solvents and energy, which leads to a high downstream cost, accounting for 50–80% of the total cost [91]. Therefore, in addition to using metabolic engineering to modify the cell factories and reduce impurity formation, technologies such as “in situ extraction–adsorption coupling” and “aqueous two-phase extraction–chromatography combination” can be integrated into the separation and purification process so as to simplify the procedure and improve the efficiency of separation and purification, thereby achieving the goal of cost reduction [92].
It is believed that the efficient, sustainable, and low-cost microbial production of terpenoids can be achieved in the future through the aforementioned interdisciplinary technological innovations and targeted solutions.
Author Contributions
H.L. and L.D.: conception of the topic; H.L., J.Z. and R.C.: writing, original draft; J.Z. and R.C.: searching references and accessing information; L.D. and F.W.: writing, review and editing, supervision, funding acquisition, conceptualization, project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Key Research and Development Program of China (2022YFC2106100), and the National Natural Science Foundation of China (22208011).
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Paramasivan, K.; Mutturi, S. Progress in terpene synthesis strategies through engineering of Saccharomyces cerevisiae. Crit. Rev. Biotechnol. 2017, 37, 974–989. [Google Scholar] [CrossRef]
- Carsanba, E.; Pintado, M.; Oliveira, C. Fermentation Strategies for Production of Pharmaceutical Terpenoids in Engineered Yeast. Pharmaceuticals 2021, 14, 295. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, C.; Qin, L.; Lv, B.; Zhang, G.; Li, C. Mining and engineering of terpene synthases and their applications in biomanufacturing. Chin. J. Chem. Eng. 2025; in press. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, J.; Hu, J.; Zhou, M. Terpenoid-based supramolecular materials: Fabrications, performances, applications. Supramol. Chem. 2023, 34, 105–131. [Google Scholar] [CrossRef]
- Meadows, A.L.; Hawkins, K.M.; Tsegaye, Y.; Antipov, E.; Kim, Y.; Raetz, L.; Dahl, R.H.; Tai, A.; Mahatdejkul-Meadows, T.; Xu, L.; et al. Rewriting yeast central carbon metabolism for industrial isoprenoid production. Nature 2016, 537, 694–697. [Google Scholar] [CrossRef]
- Association of Natural Rubber Producing Countries. Monthly Rubber Statistical Bulletin; ANRPC: Kuala Lumpur, Malaysia, 2025. [Google Scholar]
- Jiang, B.; Gao, L.; Wang, H.; Sun, Y.; Zhang, X.; Ke, H.; Liu, S.; Ma, P.; Liao, Q.; Wang, Y.; et al. Characterization and heterologous reconstitution of Taxus biosynthetic enzymes leading to baccatin III. Science 2024, 383, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Chi, H.; Er, L.; Liu, T. Efficient discovery and industrialized manufacture of terpenoids. Bull. Chin. Acad. Sci. 2025, 40, 47–66. [Google Scholar]
- Prerna; Chadha, J.; Khullar, L.; Mudgil, U.; Harjai, K. A comprehensive review on the pharmacological prospects of Terpinen-4-ol: From nature to medicine and beyond. Fitoterapia 2024, 176, 106051. [Google Scholar] [CrossRef] [PubMed]
- Nevarez, D.M.; Mengistu, Y.A.; Nawarathne, I.N.; Walker, K.D. An N-aroyltransferase of the BAHD superfamily has broad aroyl CoA specificity in vitro with analogues of N-dearoylpaclitaxel. J. Am. Chem. Soc. 2009, 131, 5994–6002. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-C.; Xu, L.; Sun, Y.; Yuan, L.; Xu, H.; Song, X.; Sun, L. Progress in the Metabolic Engineering of Yarrowia lipolytica for the Synthesis of Terpenes. BioDesign Res. 2024, 6, 0051. [Google Scholar] [CrossRef]
- Jin, K.; Xia, H.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Compartmentalization and transporter engineering strategies for terpenoid synthesis. Microb. Cell Factories 2022, 21, 92. [Google Scholar] [CrossRef]
- Sharma, S.; Negi, S.; Kumar, P.; Irfan, M. Cellular strategies for surviving the alpine extremes: Methylerythritol phosphate pathway-driven isoprenoid biosynthesis and stress resilience. Protoplasma 2025, 262, 1053–1072. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, M.; Hoshyar, R.; Ande, S.R.; Chen, Q.M.; Solomon, C.; Zuse, A.; Naderi, M. Mevalonate Cascade and its Regulation in Cholesterol Metabolism in Different Tissues in Health and Disease. Curr. Mol. Pharmacol. 2017, 10, 13–26. [Google Scholar] [CrossRef]
- Yang, D.; Park, S.Y.; Park, Y.S.; Eun, H.; Lee, S.Y. Metabolic Engineering of Escherichia coli for Natural Product Biosynthesis. Trends Biotechnol. 2020, 38, 745–765. [Google Scholar] [CrossRef]
- Yuan, Y.; Cheng, S.; Bian, G.; Yan, P.; Ma, Z.; Dai, W.; Chen, R.; Fu, S.; Huang, H.; Chi, H.; et al. Efficient exploration of terpenoid biosynthetic gene clusters in filamentous fungi. Nat. Catal. 2022, 5, 277–287. [Google Scholar] [CrossRef]
- Yuan, W.; Jiang, C.; Wang, Q.; Fang, Y.; Wang, J.; Wang, M.; Xiao, H. Biosynthesis of mushroom-derived type II ganoderic acids by engineered yeast. Nat. Commun. 2022, 13, 7740. [Google Scholar] [CrossRef]
- Liu, D.; Garrigues, S.; de Vries, R.P. Heterologous protein production in filamentous fungi. Appl. Microbiol. Biotechnol. 2023, 107, 5019–5033. [Google Scholar] [CrossRef]
- Rahmat, E.; Kang, Y. Yeast metabolic engineering for the production of pharmaceutically important secondary metabolites. Appl. Microbiol. Biotechnol. 2020, 104, 4659–4674. [Google Scholar] [CrossRef] [PubMed]
- Pramastya, H.; Song, Y.; Elfahmi, E.; Sukrasno, S.; Quax, W.J. Positioning Bacillus subtilis as terpenoid cell factory. J. Appl. Microbiol. 2020, 130, 1839–1856. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, Y.; Jia, H.; Han, Y.; Zheng, X.; Wang, M.; Feng, W. From Plant to Yeast—Advances in Biosynthesis of Artemisinin. Molecules 2022, 27, 6888. [Google Scholar] [CrossRef]
- Ye, M.; Gao, J.; Zhou, Y.J. Global metabolic rewiring of the nonconventional yeast Ogataea polymorpha for biosynthesis of the sesquiterpenoid β-elemene. Metab. Eng. 2023, 76, 225–231. [Google Scholar] [CrossRef]
- Dong, Y.; Hu, K.; Li, X.; Li, Q.; Zhang, X. Improving β-carotene production in Escherichia coli by metabolic engineering of glycerol utilization pathway. Chin. J. Biotechnol. 2017, 33, 247–260. [Google Scholar]
- Kong, X.; Wu, Y.; Yu, W.; Liu, Y.; Li, J.; Du, G.; Lv, X.; Liu, L. Efficient Synthesis of Limonene in Saccharomyces cerevisiae Using Combinatorial Metabolic Engineering Strategies. J. Agric. Food Chem. 2023, 71, 7752–7764. [Google Scholar] [CrossRef]
- Bi, H.; Xv, C.; Su, C.; Feng, P.; Zhang, C.; Wang, M.; Fang, Y.; Tan, T. β-Farnesene Production from Low-Cost Glucose in Lignocellulosic Hydrolysate by Engineered Yarrowia lipolytica. Fermentation 2022, 8, 532. [Google Scholar] [CrossRef]
- Ning, Y.; Liu, M.; Ru, Z.; Zeng, W.; Liu, S.; Zhou, J. Efficient synthesis of squalene by cytoplasmic-peroxisomal engineering and regulating lipid metabolism in Yarrowia lipolytica. Bioresour. Technol. 2024, 395, 130379. [Google Scholar] [CrossRef]
- Chiu, Y.-W.; Walseth, B.; Suh, S. Water Embodied in Bioethanol in the United States. Environ. Sci. Technol. 2009, 43, 2688–2692. [Google Scholar] [CrossRef]
- Wang, X.B.; Yan, X.; Li, X.Y.; Tu, C.; Sun, Z.K. Environmental Safety Risks in Agricultural Application of Effluents from Sugar Molasses-Based Fermentation Industries. Sci. Agric. Sin. 2023, 56, 490–507. [Google Scholar]
- March, H. Sustainability in Austerity. How Local Government Can Deliver During Times of Crisis. J. Clean. Prod. 2011, 19, 1770–1771. [Google Scholar] [CrossRef]
- Fan, W.; Fan, L.; Wang, Z.; Mei, Y.; Liu, L.; Li, L.; Yang, L.; Wang, Z. Rare ginsenosides: A unique perspective of ginseng research. J. Adv. Res. 2024, 66, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Li, Z.; Fan, B.; Ji, Z.; Yang, L.; Wu, Y.; Ye, Q.; Ji, A.; Liu, Z.; Duan, L. De Novo Biosynthesis of a Polyene-Type Ginsenoside Precursor Dammaradienol in Saccharomyces cerevisiae. ACS Synth. Biol. 2024, 13, 4015–4026. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Xu, X.; Zhang, R.; Cheng, T.; Cao, Y.; Li, X.; Guo, J.; Liu, H.; Xian, M. Engineering Escherichia coli for high-yield geraniol production with biotransformation of geranyl acetate to geraniol under fed-batch culture. Biotechnol. Biofuels 2016, 9, 58. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, C.; Zhu, M.; Xiong, J.; Ma, H.; Zhuo, M.; Li, S. High production of valencene in Saccharomyces cerevisiae through metabolic engineering. Microb. Cell Factories 2019, 18, 195. [Google Scholar] [CrossRef]
- Tao, Q.; Du, G.; Chen, J.; Zhang, J.; Peng, Z. Metabolic Engineering for Efficient Synthesis of Patchoulol in Saccharomyces cerevisiae. Fermentation 2024, 10, 211. [Google Scholar] [CrossRef]
- Ge, W.; Pai, H.; Zhang, J.; Zhang, C.; Lu, W. Construction of isopentenol utilization pathway and artificial multifunctional enzyme for miltiradiene synthesis in Saccharomyces cerevisiae. Bioresour. Technol. 2025, 419, 132065. [Google Scholar] [CrossRef]
- Chang, J.; Wei, X.; Liu, D.; Li, Q.; Li, C.; Zhao, J.; Cheng, L.; Wang, G. Engineering Escherichia coli via introduction of the isopentenol utilization pathway to effectively produce geranyllinalool. Microb. Cell Factories 2024, 23, 292. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, X.; Li, W.; Qiao, J.; Zhao, G.-R. Modular Metabolic Engineering of Saccharomyces cerevisiae for Enhanced Production of Ursolic Acid. J. Agric. Food Chem. 2025, 73, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; You, X.; Wu, T.; Li, W.; Chen, H.; Cha, Y.; Zhuo, M.; Chen, B.; Li, S. Efficient utilization of carbon to produce aromatic valencene in Saccharomyces cerevisiae using mannitol as the substrate. Green Chem. 2022, 24, 4614–4627. [Google Scholar] [CrossRef]
- Li, M.; Hou, F.; Wu, T.; Jiang, X.; Li, F.; Liu, H.; Xian, M.; Zhang, H. Recent advances of metabolic engineering strategies in natural isoprenoid production using cell factories. Nat. Prod. Rep. 2019, 37, 80–99. [Google Scholar] [CrossRef]
- Kim, S.R.; Ha, S.-J.; Wei, N.; Oh, E.J.; Jin, Y.-S. Simultaneous co-fermentation of mixed sugars: A promising strategy for producing cellulosic ethanol. Trends Biotechnol. 2012, 30, 274–282. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X. Efficient sugar production from plant biomass: Current status, challenges, and future directions. Renew. Sustain. Energy Rev. 2022, 164, 112583. [Google Scholar] [CrossRef]
- Guo, H.; Zhao, Y.; Chang, J.-S.; Lee, D.-J. Enzymes and enzymatic mechanisms in enzymatic degradation of lignocellulosic biomass: A mini-review. Bioresour. Technol. 2022, 367, 128252. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Su, K.; Mohan, M.; Chen, J.; Xu, Y.; Zhou, X. Research progress on organic acid pretreatment of lignocellulose. Int. J. Biol. Macromol. 2025, 307, 142325. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.; Liang, M.; Guo, Z.; Cai, Y.; Zhu, C.; Wang, Z.; Wang, S.; Xu, J.; Ying, H. Physical–Chemical–Biological Pretreatment for Biomass Degradation and Industrial Applications: A Review. Waste 2024, 2, 451–473. [Google Scholar] [CrossRef]
- Shi, J.; Wu, Y.-Y.; Sun, R.-Z.; Hua, Q.; Wei, L. Synthesis of β-ionone from xylose and lignocellulosic hydrolysate in genetically engineered oleaginous yeast Yarrowia lipolytica. Biotechnol. Lett. 2024, 46, 1219–1236. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Liu, Y.; Jing, X.; Ning, Y.; Xu, P.; Deng, L.; Wang, F. Efficient Production of Triacetic Acid Lactone from Lignocellulose Hydrolysate by Metabolically Engineered Yarrowia lipolytica. J. Agric. Food Chem. 2023, 71, 18909–18918. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Quarterman, J.; Kim, S.R.; Cate, J.H.; Jin, Y.S. Enhanced biofuel production through coupled acetic acid and xylose consumption by engineered yeast. Nat. Commun. 2013, 4, 2580. [Google Scholar] [CrossRef]
- Yaegashi, J.; Kirby, J.; Ito, M.; Sun, J.; Dutta, T.; Mirsiaghi, M.; Sundstrom, E.R.; Rodriguez, A.; Baidoo, E.; Tanjore, D.; et al. Rhodosporidium toruloides: A new platform organism for conversion of lignocellulose into terpene biofuels and bioproducts. Biotechnol. Biofuels 2017, 10, 241. [Google Scholar] [CrossRef] [PubMed]
- Kirby, J.; Geiselman, G.M.; Yaegashi, J.; Kim, J.; Zhuang, X.; Tran-Gyamfi, M.B.; Prahl, J.-P.; Sundstrom, E.R.; Gao, Y.; Munoz, N.; et al. Further engineering of R. toruloides for the production of terpenes from lignocellulosic biomass. Biotechnol. Biofuels 2021, 14, 101. [Google Scholar] [CrossRef]
- Ojo, A.O. An Overview of Lignocellulose and Its Biotechnological Importance in High-Value Product Production. Fermentation 2023, 9, 990. [Google Scholar] [CrossRef]
- Ao, T.-J.; Wu, J.; Chandra, R.; Zhang, H.-Y.; Yuan, Y.-F.; Luo, Y.-P.; Li, D.; Liu, C.-G.; Renneckar, S.; Saddler, J. Influence of hemicellulose and lignin on the effect of drying of cellulose and the subsequent enzymatic hydrolysis. Green Chem. 2025, 27, 8901–8913. [Google Scholar] [CrossRef]
- Li, N.; Yan, K.; Rukkijakan, T.; Liang, J.; Liu, Y.; Wang, Z.; Nie, H.; Muangmeesri, S.; Castiella-Ona, G.; Pan, X.; et al. Selective lignin arylation for biomass fractionation and benign bisphenols. Nature 2024, 630, 381–386. [Google Scholar] [CrossRef]
- Liu, L.; Wang, X.; Wu, Y.; Li, H.; Wang, J.; Li, C.; Zheng, H.; Ni, J. Chemoenzymatic platform with coordinated cofactor self-circulation for lignin valorization. Nat. Synth. 2025, 4, 1151–1160. [Google Scholar] [CrossRef]
- Qian, Q.; Zhang, J.; Cui, M.; Han, B. Synthesis of acetic acid via methanol hydrocarboxylation with CO2 and H2. Nat. Commun. 2016, 7, 11481. [Google Scholar] [CrossRef]
- Xu, X.; Xie, M.; Zhao, Q.; Xian, M.; Liu, H. Microbial production of mevalonate by recombinant Escherichia coli using acetic acid as a carbon source. Bioengineered 2018, 9, 116–123. [Google Scholar] [CrossRef]
- You, Z.; Du, X.; Zong, H.; Lu, X.; Zhuge, B. Acetic acid stress and utilization synergistically enhance squalene biosynthesis in Candida glycerinogenes. Biochem. Eng. J. 2024, 210, 109413. [Google Scholar] [CrossRef]
- Lu, S.; Deng, H.; Zhou, C.; Du, Z.; Guo, X.; Cheng, Y.; He, X. Enhancement of β-Caryophyllene Biosynthesis in Saccharomyces cerevisiae via Synergistic Evolution of β-Caryophyllene Synthase and Engineering the Chassis. ACS Synth. Biol. 2023, 12, 1696–1707. [Google Scholar] [CrossRef] [PubMed]
- Alphy, M.P.; Anjali, K.B.; Vivek, N.; Thirumalesh, B.V.; Sindhu, R.; Pugazhendi, A.; Pandey, A.; Binod, P. Sweet sorghum juice as an alternative carbon source and adaptive evolution of Lactobacillus brevis NIE9.3.3 in sweet sorghum juice and biodiesel derived crude glycerol to improve 1, 3 propanediol production. J. Environ. Chem. Eng. 2021, 9, 106086. [Google Scholar] [CrossRef]
- Abdul Raman, A.A.; Tan, H.W.; Buthiyappan, A. Two-Step Purification of Glycerol as a Value Added by Product from the Biodiesel Production Process. Front. Chem. 2019, 7, 774. [Google Scholar] [CrossRef]
- Liu, H.; Liu, S.; Ning, Y.; Zhang, R.; Deng, L.; Wang, F. Metabolic engineering of Escherichia coli for efficient production of 1,4-butanediol from crude glycerol. J. Environ. Chem. Eng. 2023, 12, 111660. [Google Scholar] [CrossRef]
- Schmerling, C.; Schroeder, C.; Zhou, X.; Bost, J.; Waßmer, B.; Ninck, S.; Busche, T.; Montero, L.; Kaschani, F.; Schmitz, O.J.; et al. An unusual glycerol-3-phosphate dehydrogenase in Sulfolobus acidocaldarius elucidates the diversity of glycerol metabolism across Archaea. Commun. Biol. 2025, 8, 539. [Google Scholar] [CrossRef]
- Li, D.-X.; Guo, Q.; Yang, Y.-X.; Jiang, S.-J.; Ji, X.-J.; Ye, C.; Wang, Y.-T.; Shi, T.-Q. Recent Advances and Multiple Strategies of Monoterpenoid Overproduction in Saccharomyces cerevisiae and Yarrowia lipolytica. ACS Synth. Biol. 2024, 13, 1647–1662. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Zhang, J.; Xiao, L.; Zhou, Y.; Wang, F.; Li, X. Metabolic engineering of Escherichia coli for efficient production of linalool from biodiesel-derived glycerol by targeting cofactors regeneration and reducing acetate accumulation. Ind. Crops Prod. 2023, 203, 117082. [Google Scholar] [CrossRef]
- Tan, N.; Ong, L.; Shukal, S.; Chen, X.; Zhang, C. High-Yield Biosynthesis of trans-Nerolidol from Sugar and Glycerol. J. Agric. Food Chem. 2023, 71, 8479–8487. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, C.; Nan, W.; Li, D.; Ke, D.; Lu, W. Glycerol improves heterologous biosynthesis of betulinic acid in engineered Yarrowia lipolytica. Chem. Eng. Sci. 2019, 196, 82–90. [Google Scholar] [CrossRef]
- Couillaud, J.; Leydet, L.; Duquesne, K.; Iacazio, G. The Terpene Mini-Path, a New Promising Alternative for Terpenoids Bio-Production. Genes 2021, 12, 1974. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, S.-C.; Qi, Y.-K.; Liu, Z.; Chen, J.; Wei, L.-J.; Hua, Q. Enhancing Trans-Nerolidol Productivity in Yarrowia lipolytica by Improving Precursor Supply and Optimizing Nerolidol Synthase Activity. J. Agric. Food Chem. 2022, 70, 15157–15165. [Google Scholar] [CrossRef]
- Ojima, Y.; Saito, H.; Miyuki, S.; Fukunaga, K.; Tsuboi, T.; Azuma, M. Induction conditions that promote the effect of glycerol on recombinant protein production in Escherichia coli. Biotechnol. Rep. 2025, 46, e00898. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xie, X.; Zhang, Y.; Li, X.; Wang, F. Atmospheric and room temperature plasma treatment to improve methanol tolerance and catalytic activity of Mucor circinelloides for one-step biodiesel production. Fuel 2025, 387, 134343. [Google Scholar] [CrossRef]
- Täuber, S.; Riedel, S.L.; Junne, S. Polyhydroxyalkanoate production from food residues. Appl. Microbiol. Biotechnol. 2025, 109, 171. [Google Scholar] [CrossRef] [PubMed]
- QY Research. 2025–2031 Global and China Waste Grease Recovery Industry Research and 15th Five-Year Plan Analysis Report; QY Research: Shanghai, China, 2025; p. 105. [Google Scholar]
- Khosa, S.; Rani, M.; Saeed, M.; Ali, S.D.; Alhodaib, A.; Waseem, A. A Green Nanocatalyst for Fatty Acid Methyl Ester Conversion from Waste Cooking Oil. Catalysts 2024, 14, 244. [Google Scholar] [CrossRef]
- Vasileiadou, A. Advancements in waste-to-energy (WtE) combustion technologies: A review of current trends and future developments. Discov. Appl. Sci. 2025, 7, 457. [Google Scholar] [CrossRef]
- Guo, Q.; Peng, Q.Q.; Chen, Y.Y.; Song, P.; Ji, X.J.; Huang, H.; Shi, T.Q. High-yield α-humulene production in Yarrowia lipolytica from waste cooking oil based on transcriptome analysis and metabolic engineering. Microb. Cell Factories 2022, 21, 271. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhao, B.; Zhang, Y.; Kong, J.; Rong, L.; Liu, S.; Wang, Y.; Zhang, C.-Y.; Xiao, D.; Foo, J.L.; et al. Mitochondrial Engineering of Yarrowia lipolytica for Sustainable Production of α-Bisabolene from Waste Cooking Oil. ACS Sustain. Chem. Eng. 2022, 10, 9644–9653. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, Q.; Wang, Z.; Cui, Z.; Su, T.; Lu, X.; Qi, Q.; Hou, J. Engineering Yarrowia lipolytica for the sustainable production of β-farnesene from waste oil feedstock. Biotechnol. Biofuels Bioprod. 2022, 15, 101. [Google Scholar] [CrossRef]
- Worland, A.M.; Han, Z.; Maruwan, J.; Wang, Y.; Du, Z.-Y.; Tang, Y.J.; Su, W.W.; Roell, G.W. Elucidation of triacylglycerol catabolism in Yarrowia lipolytica: How cells balance acetyl-CoA and excess reducing equivalents. Metab. Eng. 2024, 85, 1–13. [Google Scholar] [CrossRef]
- Palande, A.S.; Kulkarni, S.V.; León-Ramirez, C.; Campos-Góngora, E.; Ruiz-Herrera, J.; Deshpande, M.V. Dimorphism and hydrocarbon metabolism in Yarrowia lipolytica var. indica. Arch. Microbiol. 2014, 196, 545–556. [Google Scholar] [CrossRef]
- Laribi, A.; Zieniuk, B.; Bouchedja, D.N.; Hafid, K.; Elmechta, L.; Becila, S. Valorization of Olive Mill Wastewater via Yarrowia lipolytica: Sustainable Production of High-Value Metabolites and Biocompounds—A Review. Fermentation 2025, 11, 326. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, K. Production of Terpenoids by Synthetic Biology Approaches. Front. Bioeng. Biotechnol. 2020, 8, 347. [Google Scholar] [CrossRef]
- Wang, J.; Ji, X.; Yi, R.; Li, D.; Shen, X.; Liu, Z.; Xia, Y.; Shi, S. Heterologous Biosynthesis of Terpenoids in Saccharomyces cerevisiae. Biotechnol. J. 2025, 20, e202400712. [Google Scholar] [CrossRef]
- Khanam, S.; Mishra, P.; Faruqui, T.; Alam, P.; Albalawi, T.; Siddiqui, F.; Rafi, Z.; Khan, S. Plant-based secondary metabolites as natural remedies: A comprehensive review on terpenes and their therapeutic applications. Front. Pharmacol. 2025, 16, 1587215. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Z.; Ma, W.; Ma, W.; Jiang, Y.; Jiang, W.; Wan, Y.; Xin, F.; Zhang, W.; Jiang, M. Metabolic Engineering of Yarrowia lipolytica for Efficient Production of Terpenoids. Ind. Eng. Chem. Res. 2024, 63, 14469–14479. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, W.; You, C.; Fan, C.; Ji, W.; Park, J.-T.; Kwak, J.; Chen, H.; Zhang, Y.-H.P.J.; Ma, Y. Biosynthesis of artificial starch and microbial protein from agricultural residue. Sci. Bull. 2023, 68, 214–223. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Sankaran, R.; Show, P.L.; Ibrahim, T.N.B.T.; Chew, K.W.; Culaba, A.; Chang, J.-S. Pretreatment methods for lignocellulosic biofuels production: Current advances, challenges and future prospects. Biofuel Res. J. 2020, 7, 1115–1127. [Google Scholar] [CrossRef]
- Wang, J.; Li, S.; Xiong, Z.; Wang, Y. Pathway mining-based integration of critical enzyme parts for de novo biosynthesis of steviolglycosides sweetener in Escherichia coli. Cell Res. 2015, 26, 258–261. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, H.; Wang, Y.; He, B.; Lu, J.; Zhu, W.; Peng, L.; Wang, Y. Challenges and perspectives of green-like lignocellulose pretreatments selectable for low-cost biofuels and high-value bioproduction. Bioresour. Technol. 2022, 369, 128315. [Google Scholar] [CrossRef]
- Lv, P.J.; Qiang, S.; Liu, L.; Hu, C.Y.; Meng, Y.H. Dissolved-oxygen feedback control fermentation for enhancing β-carotene in engineered Yarrowia lipolytica. Sci. Rep. 2020, 10, 17114. [Google Scholar] [CrossRef]
- Zhu, K.; Kong, J.; Rong, L.; Liu, S.; Xiao, D.; Yu, A. Research Progress in Microbial Metabolic Engineering for Producing Monoterpene Aroma Products. Sci. Technol. Food Ind. 2021, 42, 390–398. [Google Scholar]
- Matsumoto, M.; Okuno, R.; Kondo, K. Extraction of 2,3-Butanediol with Aqueous Two-Phase Systems Formed by Water-Miscible Organic Solvents and Inorganic Salts. Solvent Extr. Res. Dev. Jpn. 2014, 21, 181–190. [Google Scholar] [CrossRef][Green Version]
- Zhou, X.; Zhang, Y.; Gao, H.; Zhang, Q.; Qin, L.; Dong, Y.; Li, C.; Sun, Y.; Xiu, Z. In-situ microextractive adsorption of α-pinene from fermentation broths by a recombinant yeast. Sep. Purif. Technol. 2025, 376, 134043. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).