Study on the Effect of Precious Metal Loading and Pt/Pd Ratio on Gaseous Pollutant Emissions from Diesel Engines
Abstract
1. Introduction
2. Results and Discussion
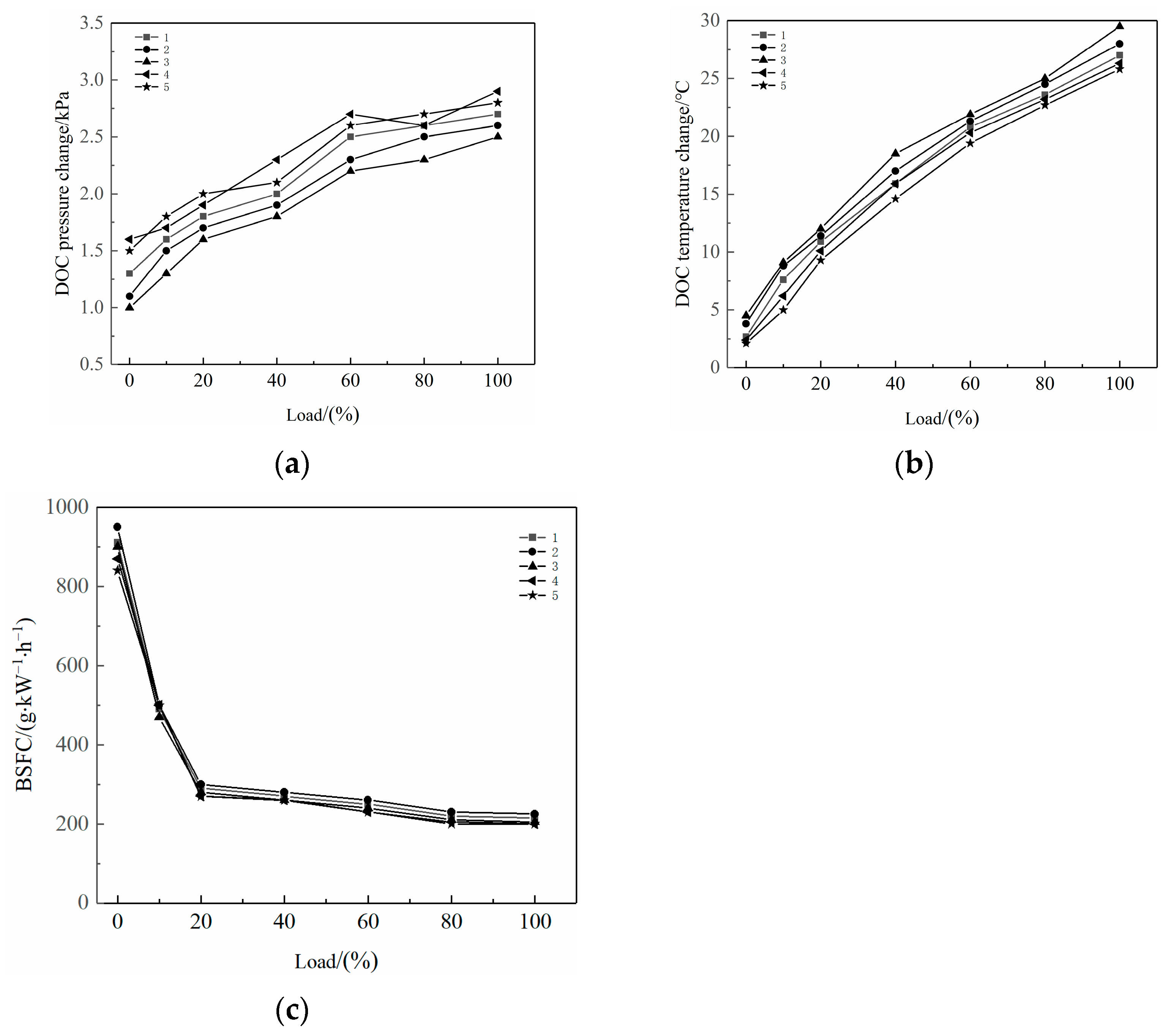
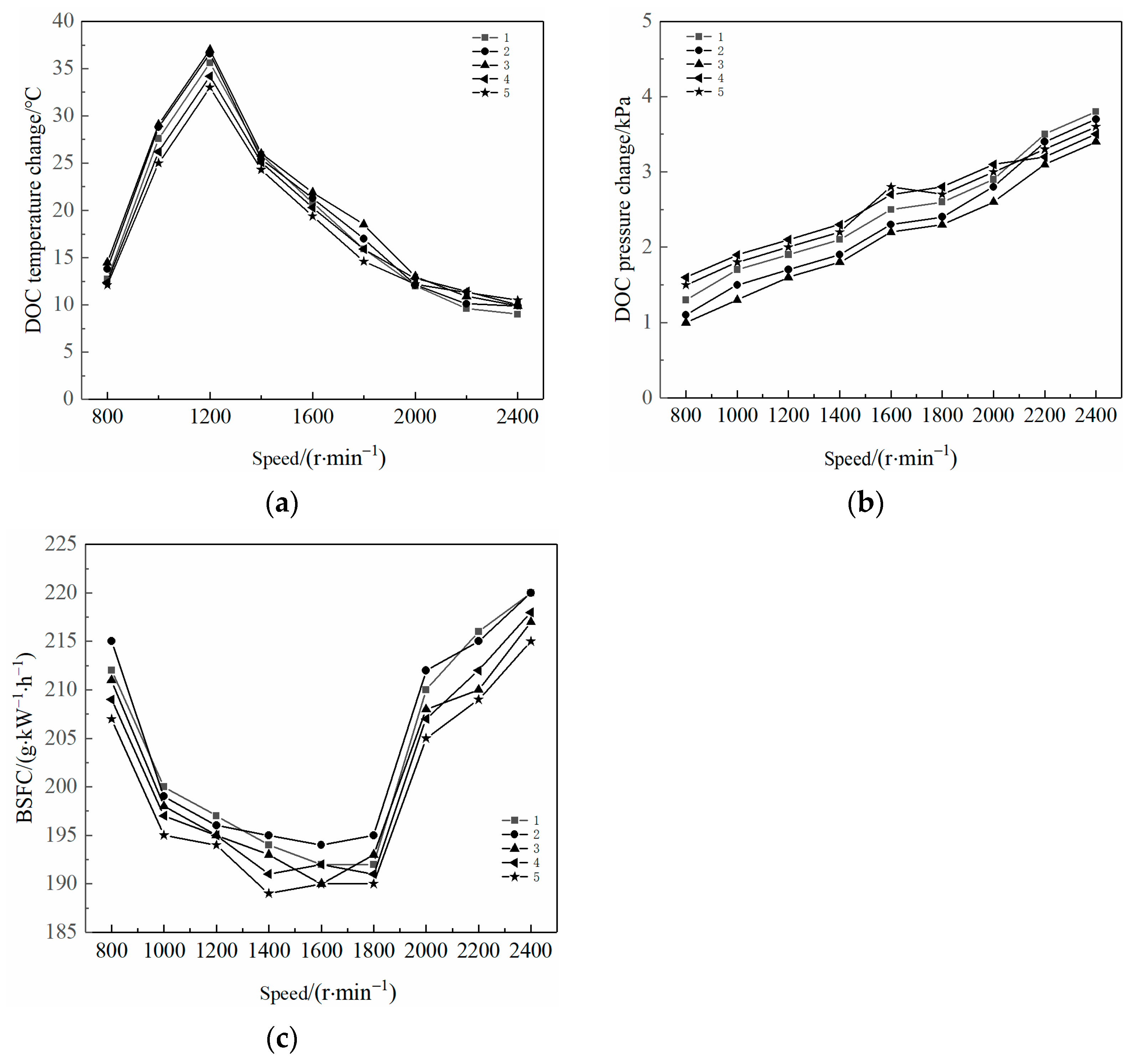
2.1. Effects on DOC Temperature Change, DOC Pressure Change, and BSFC
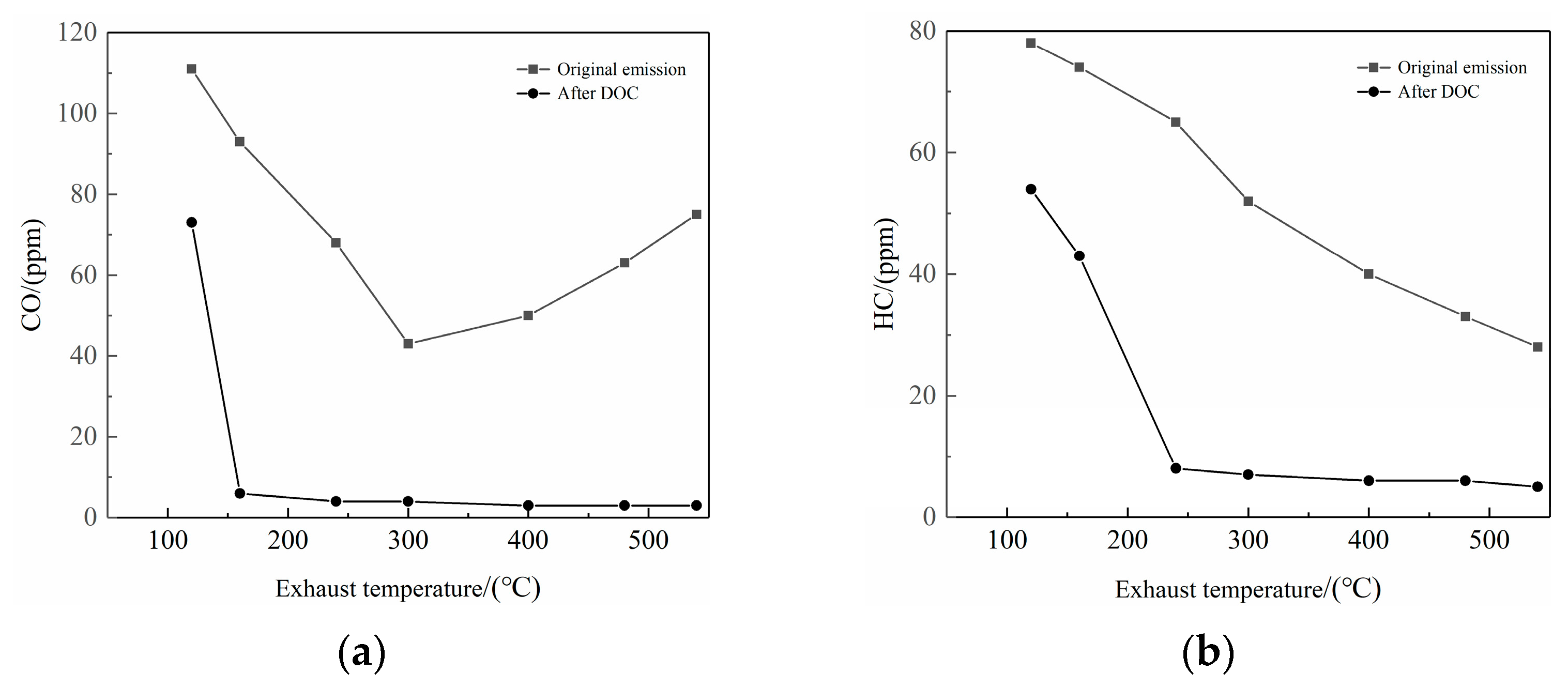
2.2. Effects on CO and HC Emissions
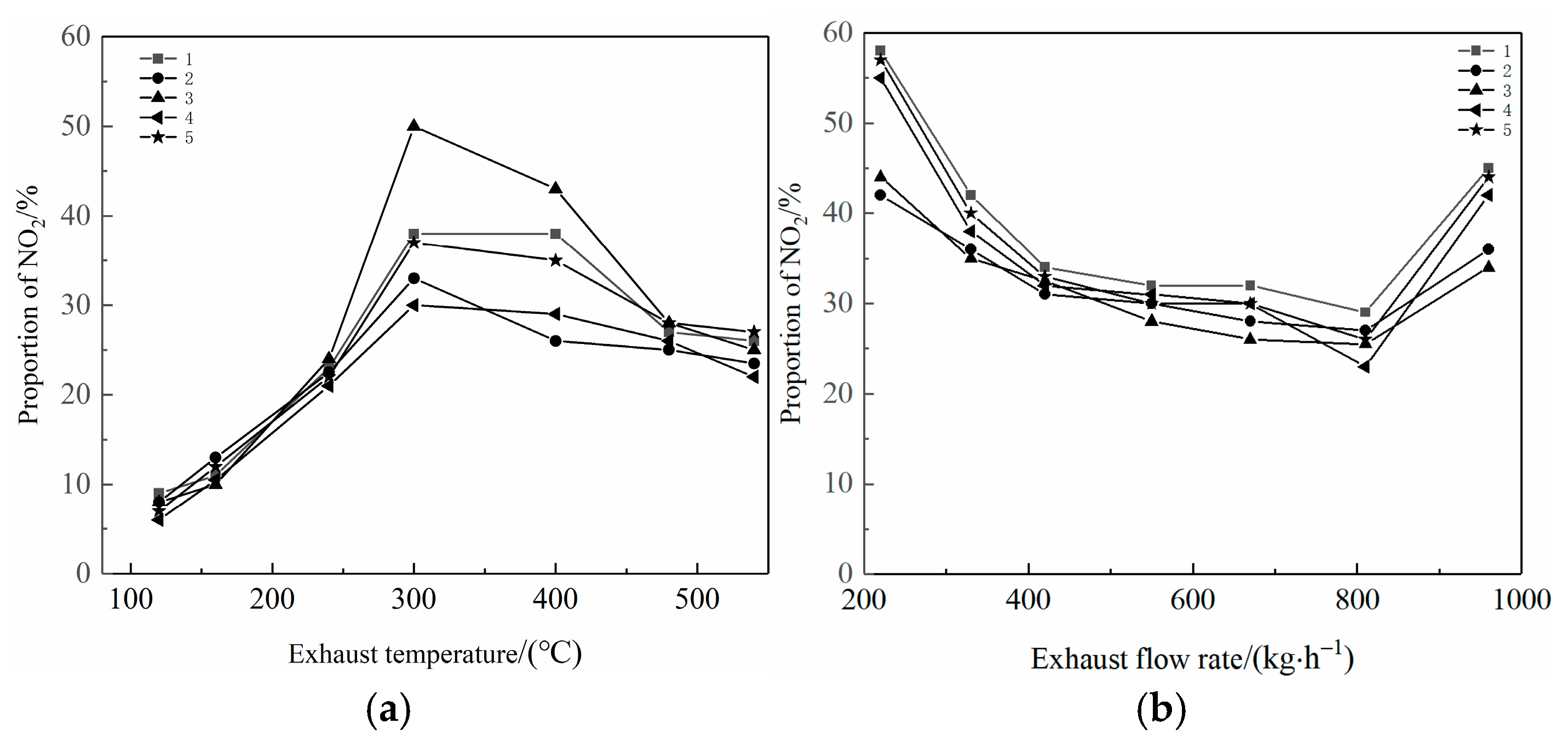
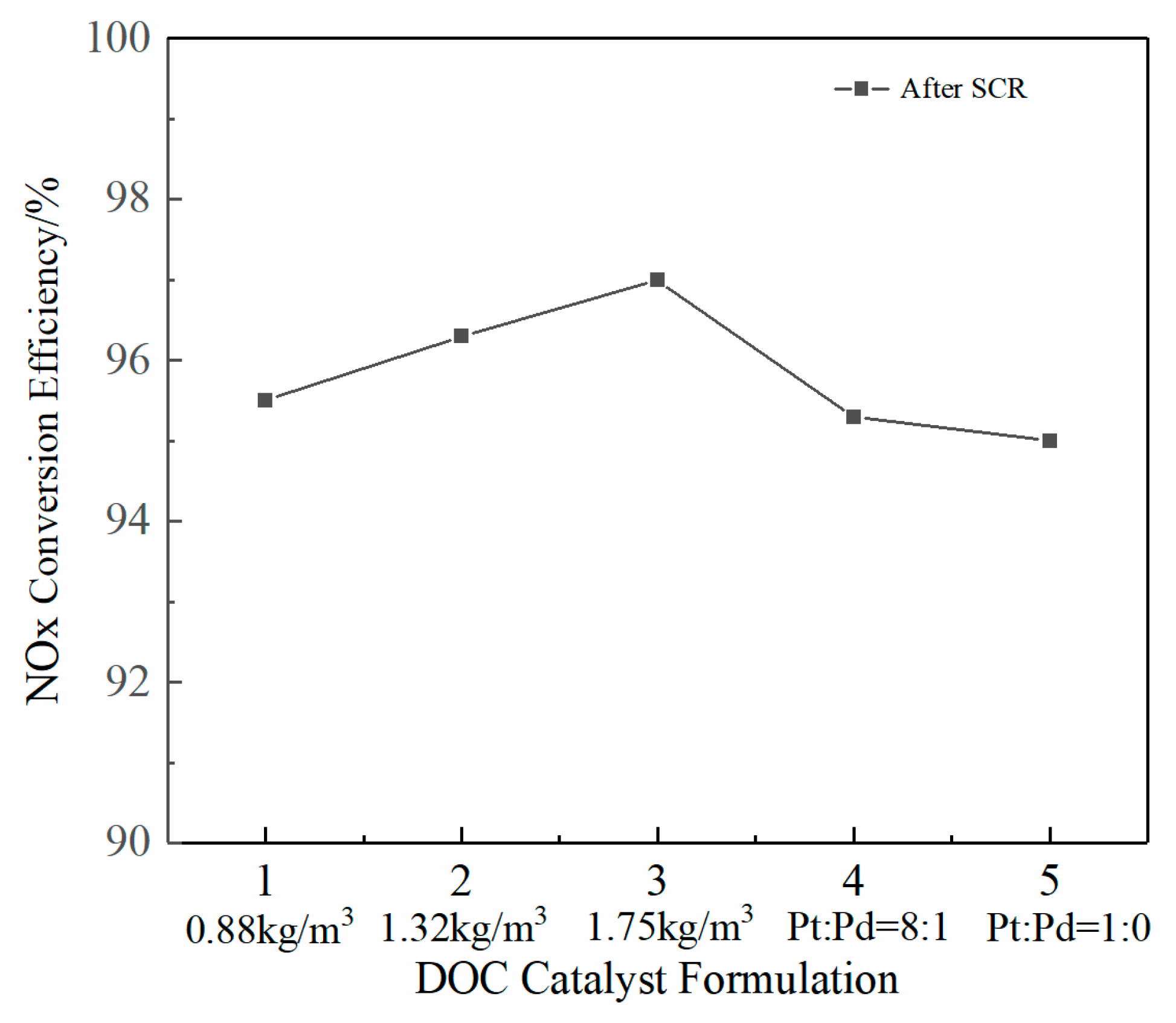
2.3. Effect on NOx Emissions
3. Experimental Design
3.1. Equipment Parameters
3.2. Experimental Design
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Caliskan, H.; Ergun, Y.; Karali, H.I.; Caglayan, H.; Hong, H.; Kale, U.; Matijošius, J. Investigating the diesel engine emission performances with various novel emission filters. Energy 2025, 318, 134775. [Google Scholar] [CrossRef]
- Wu, H.; Xie, F.; Han, Y. Effect of cetane coupled with various engine conditions on diesel engine combustion and emission. Fuel 2022, 322, 124164. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Lin, Y.; Fang, L.; Lou, D. Particle filter performance of soot-loaded diesel particulate filter and the effect of its regeneration on the particle number and size distribution. J. Clean. Prod. 2024, 461, 142651. [Google Scholar] [CrossRef]
- Zou, Y.; Xiao, L. DOC Study on the Effects of Catalyst Active Component Loading and Carrier Properties on the Catalytic Conversion Efficiency of Key Gaseous Pollutants. Sustainability 2025, 17, 6354. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, D.; Tan, P.; Hu, Z.; Fang, L. Effect of SCR downsizing and ammonia slip catalyst coating on the emissions from a heavy-duty diesel engine. Energy Rep. 2022, 8, 749–757. [Google Scholar] [CrossRef]
- Zhang, Y.; Lou, D.; Tan, P.; Hu, Z.; Fang, L. Effect of catalyzed diesel particulate filter and its catalyst loading on emission characteristics of a non-road diesel engine. J. Environ. Sci. 2023, 126, 794–805. [Google Scholar] [CrossRef]
- Hu, J.; Liao, J.; Hu, Y.; Lei, J.; Zhang, M.; Zhong, J.; Yan, F.; Cai, Z. Experimental investigation on emission characteristics of non-road diesel engine equipped with integrated DOC+ CDPF+ SCR aftertreatment. Fuel 2021, 305, 121586. [Google Scholar] [CrossRef]
- He, D.; Chen, Y.; Li, S.; Liu, Y.; Zhang, H.; Jiao, Y.; Zhao, M.; Wang, J.; Chen, Y. Unidirectional electron transfer on bismuth-doped Pt/YMn2O5 for efficient CO oxidation as diesel oxidation catalysts. ACS Catal. 2024, 14, 7353–7368. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, J.; Li, J.; Cao, C.; Wang, S.; Lv, J.; Zheng, W.; Tan, D. The development of diesel oxidation catalysts and the effect of sulfur dioxide on catalysts of metal-based diesel oxidation catalysts: A review. Fuel Process. Technol. 2022, 233, 107317. [Google Scholar] [CrossRef]
- Li, R.; Yang, D.; Liu, F.; Zhu, J.; Hu, Q. Study on the nano-structure characteristics of particle before and after diesel oxidation catalyst for diesel engines. Energy Sources Part A 2024, 46, 275–293. [Google Scholar] [CrossRef]
- Liang, Y.; He, D.; Ding, X.; Wang, J.; Zhao, M.; Chen, Y. Effect of MnOx phase on Pt-based catalyst for enhancing CO/C3H6/NO oxidation performance. Int. J. Hydrogen Energy 2022, 47, 30722–30731. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, K.; Liang, X.; Cui, L.; Wang, Y. Analysis of the correlation between mechanical and physicochemical properties of particles based on a diesel oxidation catalytic system. Sci. Total Environ. 2024, 926, 171898. [Google Scholar] [CrossRef]
- Lefort, I.; Herreros, J.M.; Tsolakis, A. Reduction of low temperature engine pollutants by understanding the exhaust species interactions in a diesel oxidation catalyst. Environ. Sci. Technol. 2014, 48, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.B.; Hazlett, M.; Balakotaiah, V.; Kalamaras, C.; Epling, W. Effect of Pt: Pd ratio on CO and hydrocarbon oxidation. Appl. Catal. B 2018, 223, 67–75. [Google Scholar] [CrossRef]
- Karre, A.V.; Garlapalli, R.K.; Jena, A.; Tripathi, N. State of the art developments in oxidation performance and deactivation of diesel oxidation catalyst (DOC). Catal. Commun. 2023, 179, 106682. [Google Scholar] [CrossRef]
- Resitoglu, I.A.; Altinisik, K.; Keskin, A.; Ocakoglu, K. The effects of Fe2O3 based DOC and SCR catalyst on the exhaust emissions of diesel engines. Fuel 2020, 262, 116501. [Google Scholar] [CrossRef]
- Caliskan, H.; Mori, K. Environmental, enviroeconomic and enhanced thermodynamic analyses of a diesel engine with diesel oxidation catalyst (DOC) and diesel particulate filter (DPF) after treatment systems. Energy 2017, 128, 128–144. [Google Scholar] [CrossRef]
- Anguita, P.; García-Vargas, J.M.; Gaillard, F.; Iojoiu, E.; Gil, S.; Giroir-Fendler, A. Effect of Na, K, Ca and P-impurities on diesel oxidation catalysts (DOCs). Chem. Eng. J. 2018, 352, 333–342. [Google Scholar] [CrossRef]
- Epling, W.S.; Campbell, L.E.; Yezerets, A.; Currier, N.W.; Parks, J.E. Overview of the fundamental reactions and degradation mechanisms of NOx storage/reduction catalysts. Catal. Rev. 2004, 46, 163–245. [Google Scholar] [CrossRef]
- Papeta, O.P.; Zubkov, I.N.; Kataria, Y.V.; Saliev, A.N.; Svetogorov, R.D.; Agliullin, M.R.; Chemes, A.A.; Savost’yAnov, A.P.; Yakovenko, R.E. Deactivation of Hybrid Cobalt Catalyst Based on Hierarchical Porous Zeolite in Fischer–Tropsch Synthesis. Energy Fuels 2025, 39, 6968–6980. [Google Scholar] [CrossRef]
- Wang, J.; Ren, D.; Zhang, N.; Lang, J.; Du, Y.; He, W.; Norinaga, K.; Huo, Z. Boosting in-situ hydrodeoxygenation of fatty acids over a fine and oxygen-vacancy-rich NiAl catalyst. Renew. Energy 2023, 202, 952–960. [Google Scholar] [CrossRef]
- Zuo, Q.; Tang, Y.; Zhu, G.; Wei, K.; Guan, Q.; Zhang, B.; Shen, Z. Investigations on the soot combustion performance enhancement of a catalytic gasoline particulate filter in equilibrium state for reducing the BSFC of gasoline direct injection engine. Fuel 2021, 284, 119032. [Google Scholar] [CrossRef]
- Ferreira, J.; Andrade, D.I.; Fuziki, M.E.K.; de Almeida, L.N.B.; Colpini, L.M.S.; Lenzi, G.G.; Tusset, A.M. Catalytic Systems in the Reduction of Nitrogen Oxide Emissions in Diesel-Powered Trucks. Sustainability 2022, 14, 6662. [Google Scholar] [CrossRef]
- Atzl, B.A.; Pupp, M.; Rupprich, M. The Use of Photocatalysis and Titanium Dioxide on Diesel Exhaust Fumes for NOx Reduction. Sustainability 2018, 10, 4031. [Google Scholar] [CrossRef]
- Giechaskiel, B.; Selleri, T.; Gioria, R.; Melas, A.D.; Franzetti, J.; Ferrarese, C.; Suarez-Bertoa, R. Assessment of a euro VI step E heavy-duty vehicle’s aftertreatment system. Catalysts 2022, 12, 1230. [Google Scholar] [CrossRef]




| Property | Numerical Value |
|---|---|
| Displacement | 8/L |
| Rating speed | 2400 r·min−1 |
| Maximum torque speed | 2000 r·min−1 |
| Rated Power | 187 kW |
| Maximum torque | 1345 N·m |
| Compression ratio | 18.5 |
| Property | Numerical Value |
|---|---|
| Cell density | 400 cpsi |
| Carrier diameter | 304 mm |
| Carrier length | 101 mm |
| Wall thickness | 4 mm |
| Pore diameter | 1~10 µm |
| Catalytic washcoat | γ-Al2O3 |
| Carrier material | Cordierite |
| Property | Numerical Value |
|---|---|
| Cell density | 300 cpsi |
| Carrier material | Cordierite |
| Wall thickness | 3 mm |
| Precious Metal Loading | 0.65 kg/m3 |
| Pt:Pd | 4:1 |
| Property | Numerical Value |
|---|---|
| Cell density | 500 cpsi |
| Active Component | Cu-SSZ-13 |
| Wall thickness | 3 mm |
| Washcoat | γ-Al2O3 |
| Carrier material | Cordierite |
| Property | Resolution | Uncertainty |
|---|---|---|
| Dynamometer (speed measurement) | 1 rpm | ±0.3% |
| Dynamometer (torque measurement) | 0.01 N m | ±0.2% |
| Flow meter sensor | 0.01 g | ±0.3% |
| Pressure transducer | 0.01 MPa | ±0.3% |
| Gas analyzer | ||
| CO measurement | 0.01% | <0.2% |
| HC measurement | 2 ppm | <0.2% |
| NOx measurement | 1 ppm | <0.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, K.; Wu, H.; Zou, Y. Study on the Effect of Precious Metal Loading and Pt/Pd Ratio on Gaseous Pollutant Emissions from Diesel Engines. Catalysts 2025, 15, 974. https://doi.org/10.3390/catal15100974
Shao K, Wu H, Zou Y. Study on the Effect of Precious Metal Loading and Pt/Pd Ratio on Gaseous Pollutant Emissions from Diesel Engines. Catalysts. 2025; 15(10):974. https://doi.org/10.3390/catal15100974
Chicago/Turabian StyleShao, Kun, Heng Wu, and Yantao Zou. 2025. "Study on the Effect of Precious Metal Loading and Pt/Pd Ratio on Gaseous Pollutant Emissions from Diesel Engines" Catalysts 15, no. 10: 974. https://doi.org/10.3390/catal15100974
APA StyleShao, K., Wu, H., & Zou, Y. (2025). Study on the Effect of Precious Metal Loading and Pt/Pd Ratio on Gaseous Pollutant Emissions from Diesel Engines. Catalysts, 15(10), 974. https://doi.org/10.3390/catal15100974





