Synergistic Integration of TiO2 Nanorods with Carbon Cloth for Enhanced Photocatalytic Hydrogen Evolution and Wastewater Remediation
Abstract
1. Introduction
2. Results and Discussion
2.1. Surface Structure of TiO2 NRs/CC
2.2. Surface Morphology of TiO2 NRs/CC
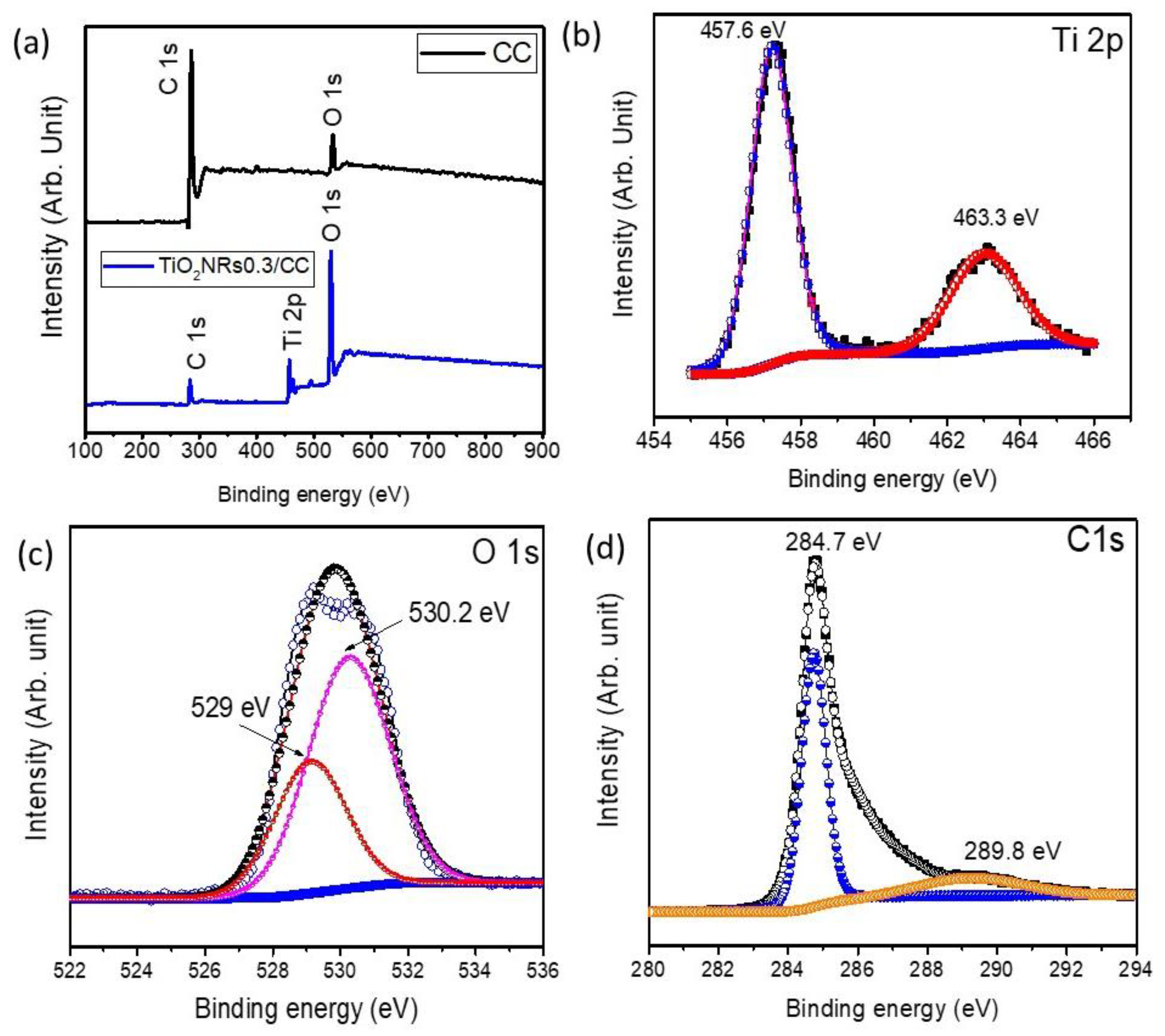
2.3. Surface Chemical Composition and States of TiO2 NRs/CC
2.4. Optical and Photophysical Properties of TiO2 NRs/CC
2.5. Charge Transport Resistance and Semiconductor Characteristics of TiO2 NRs/CC
2.6. Photocatalytic Performance of TiO2NRs/CC
2.6.1. H2 Evolution
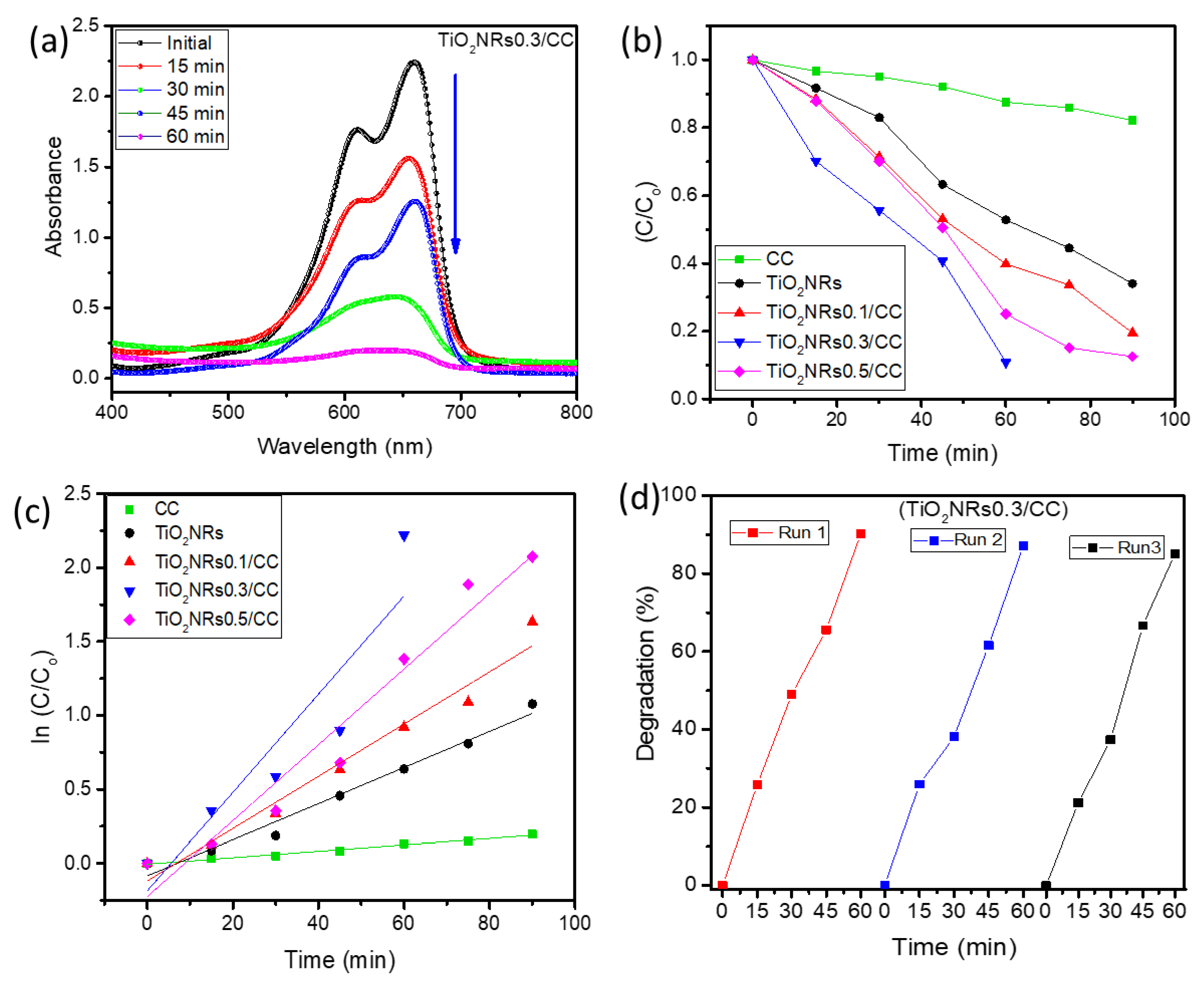
2.6.2. Dye Degradation
2.7. Photocatalytic Mechanism
2.7.1. H2 Evolution Pathway
2.7.2. Dye Degradation Pathway
3. Materials and Methodology
3.1. Materials
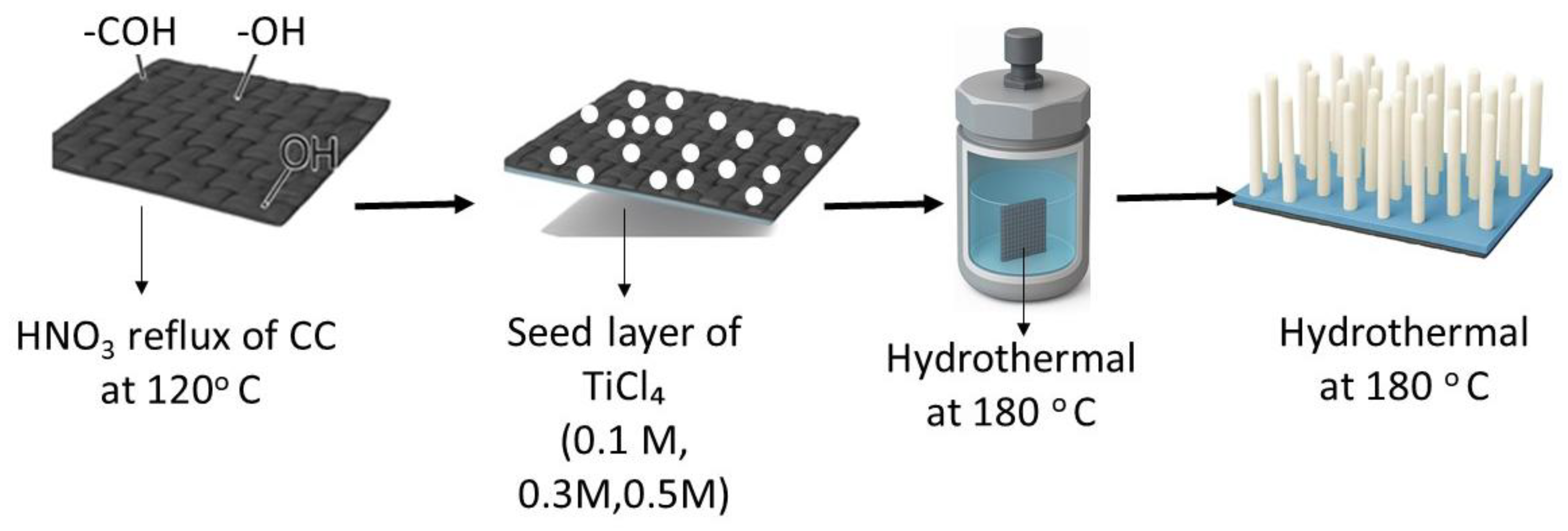
3.2. Synthesis of TiO2NRs/CC
- Hydrolysis of TiCl4 in ethanol (during seeding),
- Hydrolysis of TBT (in precursor solution),
- Condensation of hydroxide to TiO2,
3.3. Characterization of TiO2NRs/CC
3.4. Photocatalytic Hydrogen (H2) Evolution Using TiO2NRs/CC
3.5. Electrochemical Measurement
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Shakeelur Raheman, A.R.; Mane, R.S.; Wilson, H.M.; Jha, N. CdSe quantum dot/white graphene hexagonal porous boron nitride sheet (h-PBNs) heterostructure photocatalyst for solar driven H2 production. J. Mater. Chem. C Mater. 2021, 9, 8524–8536. [Google Scholar] [CrossRef]
- Joshy, D.; Narendranath, S.B.; Ismail, Y.A.; Periyat, P. Recent progress in one dimensional TiO2 nanomaterials as photoanodes in dye-sensitized solar cells. Nanoscale Adv. 2022, 4, 5202–5232. [Google Scholar] [CrossRef] [PubMed]
- Ghibaudo, N.; Ferretti, M.; Al-Hetlani, E.; Madkour, M.; Amin, M.O.; Alberti, S. Synthesis and characterization of TiO2-based supported materials for industrial application and recovery in a pilot photocatalytic plant using chemometric approach. Environ. Sci. Pollut. Res. 2024, 31, 20556–20567. [Google Scholar] [CrossRef]
- Fernández-Ibáñez, P.; Blanco, J.; Malato, S.; De Las Nieves, F.J. Application of the colloidal stability of TiO2 particles for recovery and reuse in solar photocatalysis. Water Res. 2003, 37, 3180–3188. [Google Scholar] [CrossRef]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Murthy, R.; Neelakantan, S.C. Graphitic Carbon Cloth-Based Hybrid Molecular Catalyst: A Non-conventional, Synthetic Strategy of the Drop Casting Method for a Stable and Bifunctional Electrocatalyst for Enhanced Hydrogen and Oxygen Evolution Reactions. ACS Omega 2022, 7, 32604–32614. [Google Scholar] [CrossRef]
- Xi, X.; Lu, Z.; Chen, H.J.; Tian, W.; Yang, Y.L. Flexible carbon-fiber/g-C3N4 nanosheet array as recyclable photocatalysts and photoelectrocatalysts. Sci. Rep. 2025, 15, 21090. [Google Scholar] [CrossRef]
- Juang, S.E.; Chin, N.C.; Chang, Y.C.; Chou, C.M. Fabrication of ZnCo2O4-Zn(OH)2 Microspheres on Carbon Cloth for Photocatalytic Decomposition of Tetracycline. Molecules 2024, 29, 4054. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Kao, Y.C.; Lin, K.S.; Chen, C.Y.; Kang, C.W.; Yang, T.H. Carbon fiber cloth@BiOBr/CuO as immobilized membrane-shaped photocatalysts with enhanced photocatalytic H2 production activity. J. Taiwan. Inst. Chem. Eng. 2023, 149, 104998. [Google Scholar] [CrossRef]
- Wan, W.; Li, Y.; Bai, S.; Yang, X.; Chi, M.; Shi, Y.; Liu, C.; Zhang, P. Three-Dimensional Porous ZnO-Supported Carbon Fiber Aerogel with Synergistic Effects of Adsorption and Photocatalysis for Organics Removal. Sustainability 2023, 15, 13088. [Google Scholar] [CrossRef]
- Jiang, L.; Gao, X.; Chen, S.; Ashok, J.; Kawi, S. Oxygen-Deficient WO3/TiO2/CC Nanorod Arrays for Visible-Light Photocatalytic Degradation of Methylene Blue. Catalysts 2021, 11, 1349. [Google Scholar] [CrossRef]
- Yi, Y.; Guan, Q.; Wang, W.; Jian, S.; Li, H.; Wu, L.; Zhang, H.; Jiang, C. Recyclable Carbon Cloth-Supported ZnO@Ag3PO4 Core–Shell Structure for Photocatalytic Degradation of Organic Dye. Toxics 2023, 11, 70. [Google Scholar] [CrossRef]
- Jian, S.; Wang, W.; Wu, L.; Li, H.; Hong, C.; Long, S.; Zhou, W.; Guo, Y. Construction of highly efficient carbon cloth-supported S-scheme Co3O4/AgIO4 heterojunction for photocatalytic degradation of Rhodamine B organic dye. J. Photochem. Photobiol. A Chem. 2024, 452, 115598. Available online: https://ssrn.com/abstract=4729376 (accessed on 4 September 2025). [CrossRef]
- He, D.; Li, X.; Jia, B.; Li, M.; Tang, H.; Li, H.; Ma, Y.; Wang, X. Photocatalytic removal of volatile organic compounds by monolithic heterojunction materials: A review. Catal. Sci. Technol. 2025, 15, 5202–5225. [Google Scholar] [CrossRef]
- Shi, H.; Wen, G.; Nie, Y.; Zhang, G.; Duan, H. Flexible 3D carbon cloth as a high-performing electrode for energy storage and conversion. Nanoscale 2020, 12, 5261–5285. [Google Scholar] [CrossRef]
- Ferrari, A.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2010, 46, 855–874. [Google Scholar] [CrossRef]
- Yang, H.G.; Zeng, H.C. Preparation of hollow anatase TiO2 nanospheres via Ostwald ripening. J. Phys. Chem. B 2004, 108, 3492–3495. [Google Scholar] [CrossRef]
- Chen, C.; Mao, S.S. Titanium dioxide nanomaterials: Synthesis, properties, modifications and applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef] [PubMed]
- Kavan, L.; Grätzel, M.; Gilbert, S.E.; Klemenz, C.; Scheel, H.J. Electrochemical and photoelectrochemical investigation of single-crystal anatase. J. Am. Chem. Soc. 1996, 118, 6716–6723. [Google Scholar] [CrossRef]
- Liu, B.; Aydil, E.S. Growth of oriented single-crystalline rutile TiO2 nanorods on transparent conducting substrates for dye-sensitized solar cells. J. Am. Chem. Soc. 2009, 131, 3985–3990. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Parmon, V.N.; Lapkin, A.A.; Walsh, F.C. The effect of hydrothermal conditions on the mesoporous structure of TiO2 nanotubes. J. Mater. Chem. 2004, 14, 3370–3377. [Google Scholar] [CrossRef]
- Ji, Y. Facile route for synthesis of TiO2 nanorod arrays by high-temperature calcinations. Mater. Lett. 2013, 108, 208–211. [Google Scholar] [CrossRef]
- Yu, J.; Yu, H.; Cheng, B.; Zhou, M.; Zhao, X. Enhanced photocatalytic activity of TiO2 powder (P25) by hydrothermal treatment. J. Mol. Catal. A Chem. 2006, 253, 112–118. [Google Scholar] [CrossRef]
- Pakpahan, S.; Gultom, R. Optimizing Photovoltaic Performance in p-Cu2O/n-TiO2 Heterojunction Solar Cells: The Impact of Annealing Temperature, Layer Thickness, and Carbon Doping. Indones. J. Chem. Res. 2025, 13, 23–29. [Google Scholar] [CrossRef]
- Negoescu, D.; Bratan, V.; Gherendi, M.; Atkinson, I.; Culita, D.C.; Neacsu, A.; Baran, A.; Petrescu, S.; Parvulescu, V. Iron Promoted TiO2-Activated Carbon Nanocomposites for Photocatalytic Degradation of Congo Red in Water. Catalysts 2024, 14, 844. [Google Scholar] [CrossRef]
- Lee, S.F.; Jimenez-Relinque, E.; Martinez, I.; Castellote, M. Effects of Mott–Schottky Frequency Selection and Other Controlling Factors on Flat-Band Potential and Band-Edge Position Determination of TiO2. Catalysts 2023, 13, 1000. [Google Scholar] [CrossRef]
- Trinh, N.B.; Nguyen, T.A.; Van Vu, S.; Vo, H.G.T.; Lo, T.N.H.; Park, I.; Vo, K.Q. Modified hydrothermal method for synthesizing titanium dioxide-decorated multiwalled carbon nanotube nanocomposites for the solar-driven photocatalytic degradation of dyes. RSC Adv. 2024, 14, 34037–34050. [Google Scholar] [CrossRef]
- Sriramoju, J.B.; Muniyappa, M.; Marilingaiah, N.R.; Sabbanahalli, C.; Shetty, M.; Mudike, R.; Chitrabanu, C.P.; Shivaramu, P.D.; Nagaraju, G.; Rangappa, K.S.; et al. Carbon-based TiO2-x heterostructure nanocomposites for enhanced photocatalytic degradation of dye molecules. Ceram. Int. 2021, 47, 10314–10321. [Google Scholar] [CrossRef]
- Hossen, M.A.; Ikreedeegh, R.R.; Aziz, A.A.; Zerga, A.Y.; Tahir, M. Carbon-based nanomaterials (CNMs) modified TiO2 nanotubes (TNTs) photo-driven catalysts for sustainable energy and environmental applications: A comprehensive review. J. Env. Chem. Eng. 2024, 12, 114088. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, C.-C.; Zakaria, R.; Ying, J.Y. Role of Particle Size in Nanocrystalline TiO2-Based Photocatalysts. J. Phys. Chem. B 1998, 102, 10871–10878. [Google Scholar] [CrossRef]
- Xia, W.; Qian, H.; Zeng, X.; Sun, J.; Wang, P.; Luo, M.; Dong, J. TiO2@Sn3O4 nanorods vertically aligned on carbon fiber papers for enhanced photoelectrochemical performance. RSC Adv. 2019, 9, 23334–23342. [Google Scholar] [CrossRef]
- Tahir, M.; Alesayi, M.T.H.; Alshehhi, S.M.S. Recycling Carbon Fiber-Reinforced Polymers (CFRPs) to Construct CFs/TiO2 Nanotexture with Efficient Interface Charge Transfer for Stimulating Photocatalytic Hydrogen Production. Energy Fuels 2023, 37, 12319–12334. [Google Scholar] [CrossRef]
- Behpour, M.; Shirazi, P.; Rahbar, M. Immobilization of the Fe2O3/TiO2 photocatalyst on carbon fiber cloth for the degradation of a textile dye under visible light irradiation, Reaction Kinetics. Mech. Catal. 2019, 127, 1073–1085. [Google Scholar] [CrossRef]
- Wang, Y.; Omsinsombon, J.; Prateepmaneerak, N.; Li, S.; Zhu, G.; Khan, M.Z.; Taghavian, H.; Venkataraman, M.; Militký, J.; Chaiyasat, A. UV-activated TiO2-coated carbon felt for photocatalytic dye degradation and antibacterial applications. Surf. Interfaces 2025, 60, 106018. [Google Scholar] [CrossRef]
- Chennam, P.K.; Sepúlveda, M.; Rihova, M.; Alijani, M.; Kachlík, M.; Zazpe, R.; Pavlinak, D.; Maca, K.; Macak, J.M. Carbon fibers decorated with TiO2 nanoparticles for photocatalytic degradation of methylene blue dye. Front. Nanotechnol. 2024, 6, 1483917. [Google Scholar] [CrossRef]
- Tian, Q.; Yi, S.; Li, C.; Liu, Y.; Niu, Z.; Yue, X.; Liu, Z. Design of charge transfer channels: Defective TiO2/MoP supported on carbon cloth for solar-light-driven hydrogen generation. Inorg. Chem. Front. 2021, 8, 2017–2026. [Google Scholar] [CrossRef]
- Wang, L.; Liu, N.; Guo, Z.; Wu, D.; Chen, W.; Chang, Z.; Yuan, Q.; Hui, M.; Wang, J. Nitric Acid-Treated Carbon Fibers with Enhanced Hydrophilicity for Candida tropicalis Immobilization in Xylitol Fermentation. Materials 2016, 9, 206. [Google Scholar] [CrossRef]
- Tamilselvan, V.; Yuvaraj, D.; Kumar, R.R.; Rao, K.N. Growth of rutile TiO2 nanorods on TiO2 seed layer deposited by electron beam evaporation. Appl. Surf. Sci. 2012, 258, 4283–4287. [Google Scholar] [CrossRef]
- Guo, W.; Xu, C.; Wang, X.; Wang, S.; Pan, C.; Lin, C.; Wang, Z.L. Rectangular Bunched Rutile TiO2 Nanorod Arrays Grown on Carbon Fiber for Dye-Sensitized Solar Cells. J. Am. Chem. Soc. 2012, 134, 4437–4441. [Google Scholar] [CrossRef] [PubMed]
- Diao, W.; He, J.; Wang, Q.; Rao, X.; Zhang, Y. Na and Cl co-doped TiO2 nanorod arrays on carbon cloth for efficient photocatalytic degradation of formaldehyde under UV/visible LED irradiation. Catal. Sci. Technol. 2021, 11, 230–238. [Google Scholar] [CrossRef]
- Han, X.; Kuang, Q.; Jin, M.; Xie, Z.; Zheng, L. Synthesis of titania nanosheets with a high percentage of exposed (001) facets and related photocatalytic properties. J. Am. Chem. Soc. 2009, 131, 3152–3153. [Google Scholar] [CrossRef] [PubMed]





| Photocatalyst System | Light Source | Photocatalytic H2 Evolution | Photocatalytic Dye Degradation | Reference |
|---|---|---|---|---|
| TiO2@Sn3O4 nanorods | Visible-light PEC | 5.23 µmol h−1 (vs. 1.13 µmol h−1 for TiO2 NRs) | [33] | |
| Carbon fiber-reinforced polymers/TiO2 composite (powdered CFs mixed with TiO2) | UV–Vis, MeOH sacrificial | 85.4 µmol g−1 h−1 (3 wt% CFs/TiO2), 2.87× vs. bare TiO2 | [34] | |
| Fe2O3/TiO2 immobilized on CFC | Visible light | 97.54% color removal; ~84% COD removal (240 min) | [35] | |
| UV-activated TiO2 coated felt (covalently anchored) | UV | 95% Orange II in 180 min | [36] | |
| TiO2 nanoparticles decorated on CFs | UV (λ ≈ 365 nm) | First-order kinetics | [37] | |
| Defective TiO2/MoP heterojunction on CC | Solar light (panel; immobilized sheet) | 14.7 µmol h−1 cm−2; ~19× higher than TiO2/CC | [38] | |
| TiO2 nanorods (powder) | Vis (λ ≥ 400 nm), TEOA | 0.66 mmol h−1 g−1 | Present research | |
| TiO2NR0.1/CC | Vis (λ ≥ 400 nm), TEOA | 0.90 mmol h−1 g−1 | Present research | |
| TiO2NR0.3/CC | Vis (λ ≥ 400 nm), TEOA | 2.66 mmol h−1 g−1 | TiO2NR0.3/CC achieved 90.2% in 90 min | Present research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AR, S.R.; Ansari, K.B.; Lee, S.J.; Salunke, N. Synergistic Integration of TiO2 Nanorods with Carbon Cloth for Enhanced Photocatalytic Hydrogen Evolution and Wastewater Remediation. Catalysts 2025, 15, 961. https://doi.org/10.3390/catal15100961
AR SR, Ansari KB, Lee SJ, Salunke N. Synergistic Integration of TiO2 Nanorods with Carbon Cloth for Enhanced Photocatalytic Hydrogen Evolution and Wastewater Remediation. Catalysts. 2025; 15(10):961. https://doi.org/10.3390/catal15100961
Chicago/Turabian StyleAR, Shakeelur Raheman, Khursheed B. Ansari, Sang Joon Lee, and Nilesh Salunke. 2025. "Synergistic Integration of TiO2 Nanorods with Carbon Cloth for Enhanced Photocatalytic Hydrogen Evolution and Wastewater Remediation" Catalysts 15, no. 10: 961. https://doi.org/10.3390/catal15100961
APA StyleAR, S. R., Ansari, K. B., Lee, S. J., & Salunke, N. (2025). Synergistic Integration of TiO2 Nanorods with Carbon Cloth for Enhanced Photocatalytic Hydrogen Evolution and Wastewater Remediation. Catalysts, 15(10), 961. https://doi.org/10.3390/catal15100961









