Catalytic Biomass Conversion into Fuels and Materials: Sustainable Technologies and Applications
Abstract
1. Introduction
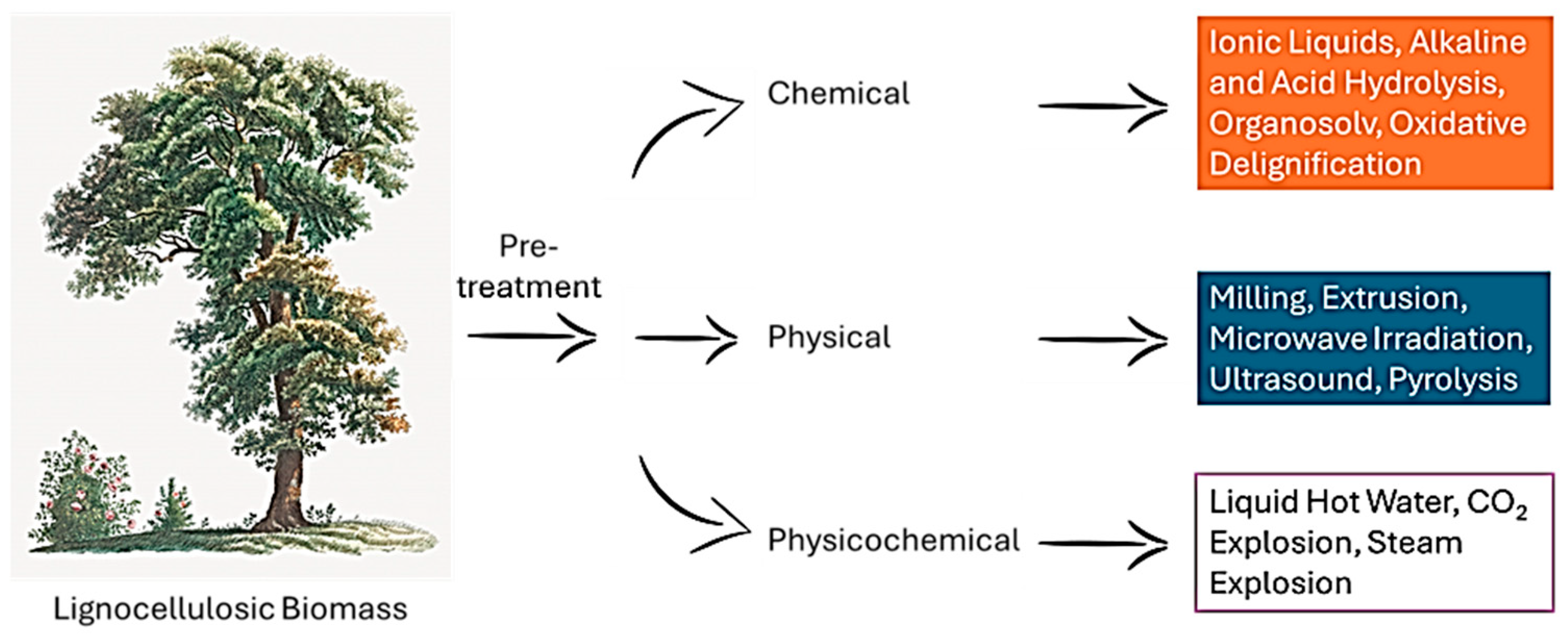
2. Biomass Feedstocks and Their Catalytic Pretreatments
2.1. Lignocellulosic Biomass (Agricultural Residues, Forestry Waste)
2.2. Aquatic Biomass
2.3. Waste Biomass (Municipal Solid Wastes, Industrial Byproducts)
3. Heterogeneous Catalysts and Their Use in Biomass Treatments
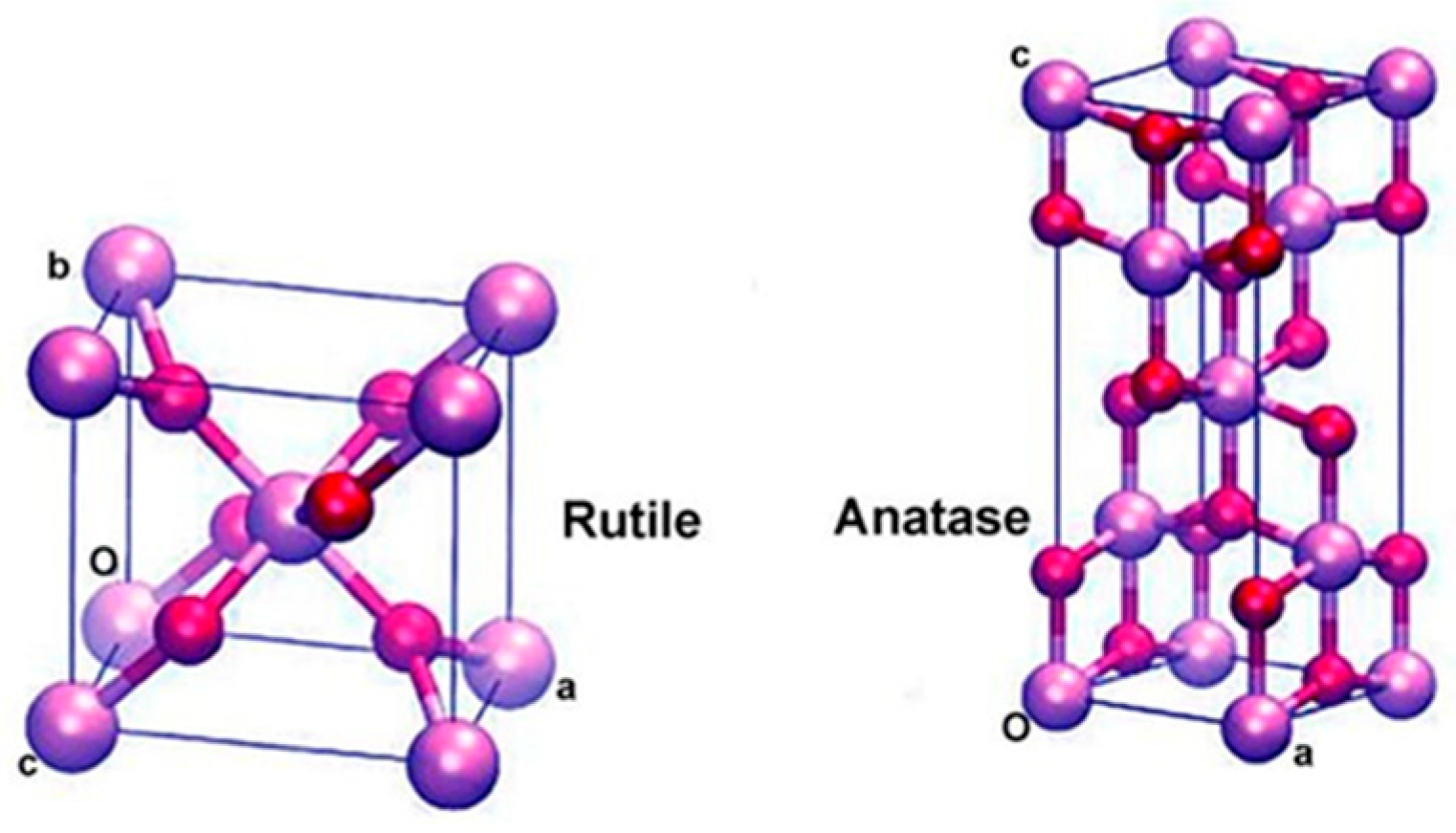
3.1. Solid Acid Catalysts
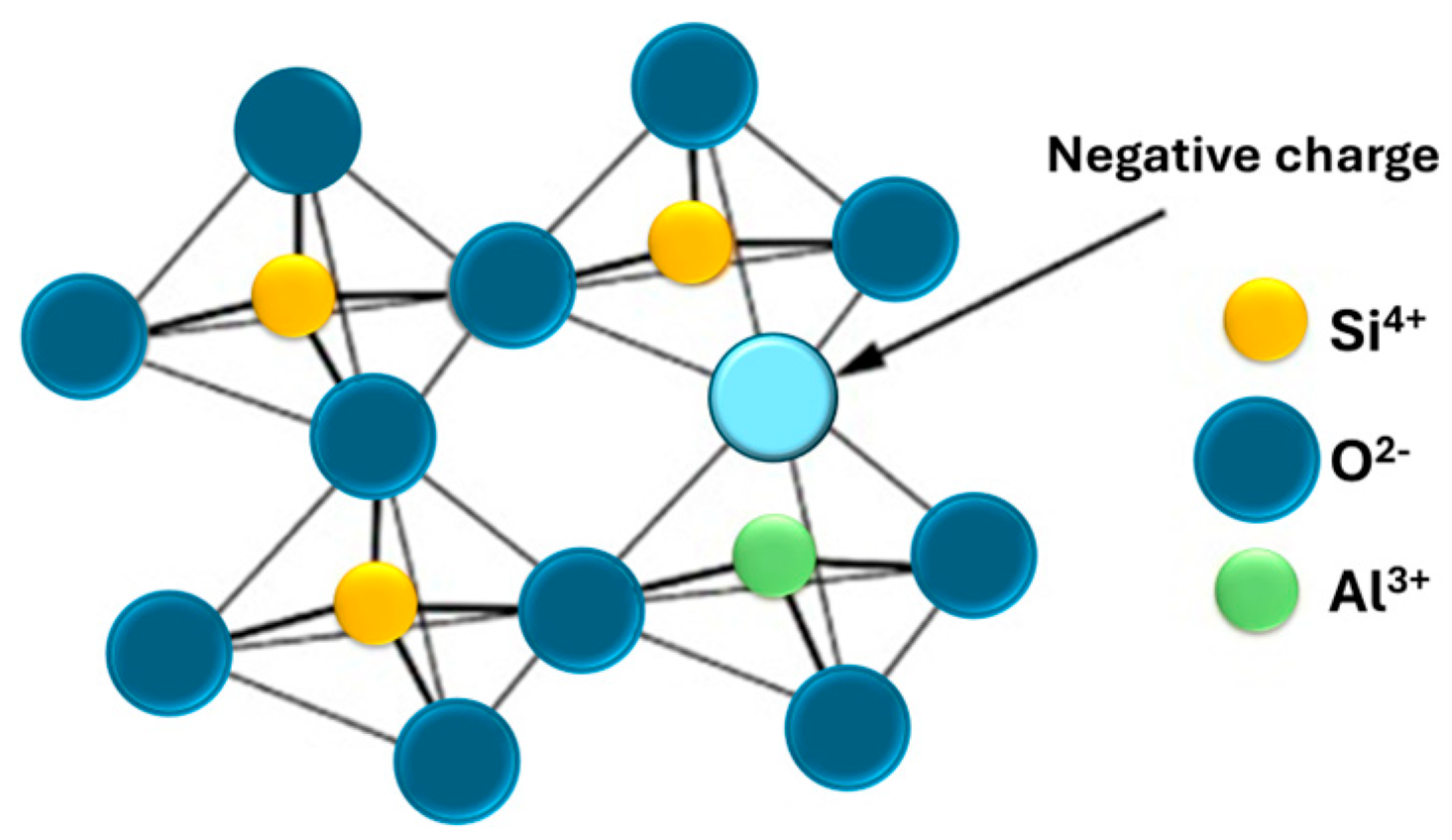
3.2. Zeolites
3.3. Solid Alkaline Catalysts
3.4. Supported Metal Catalysts
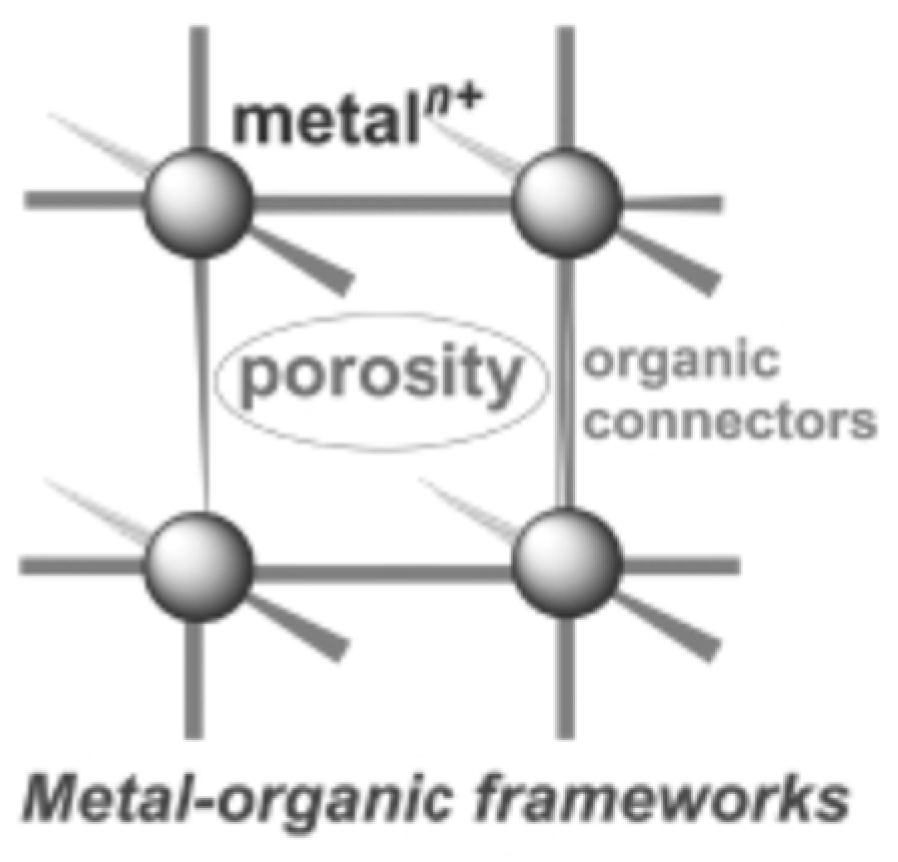
3.5. Functionalized Porous Catalysts
3.6. Single-Atom Catalysts (SACs)
4. Catalytic Technologies for Biomass Conversion
4.1. Thermochemical Processes
4.1.1. Catalytic Pyrolysis and Cracking
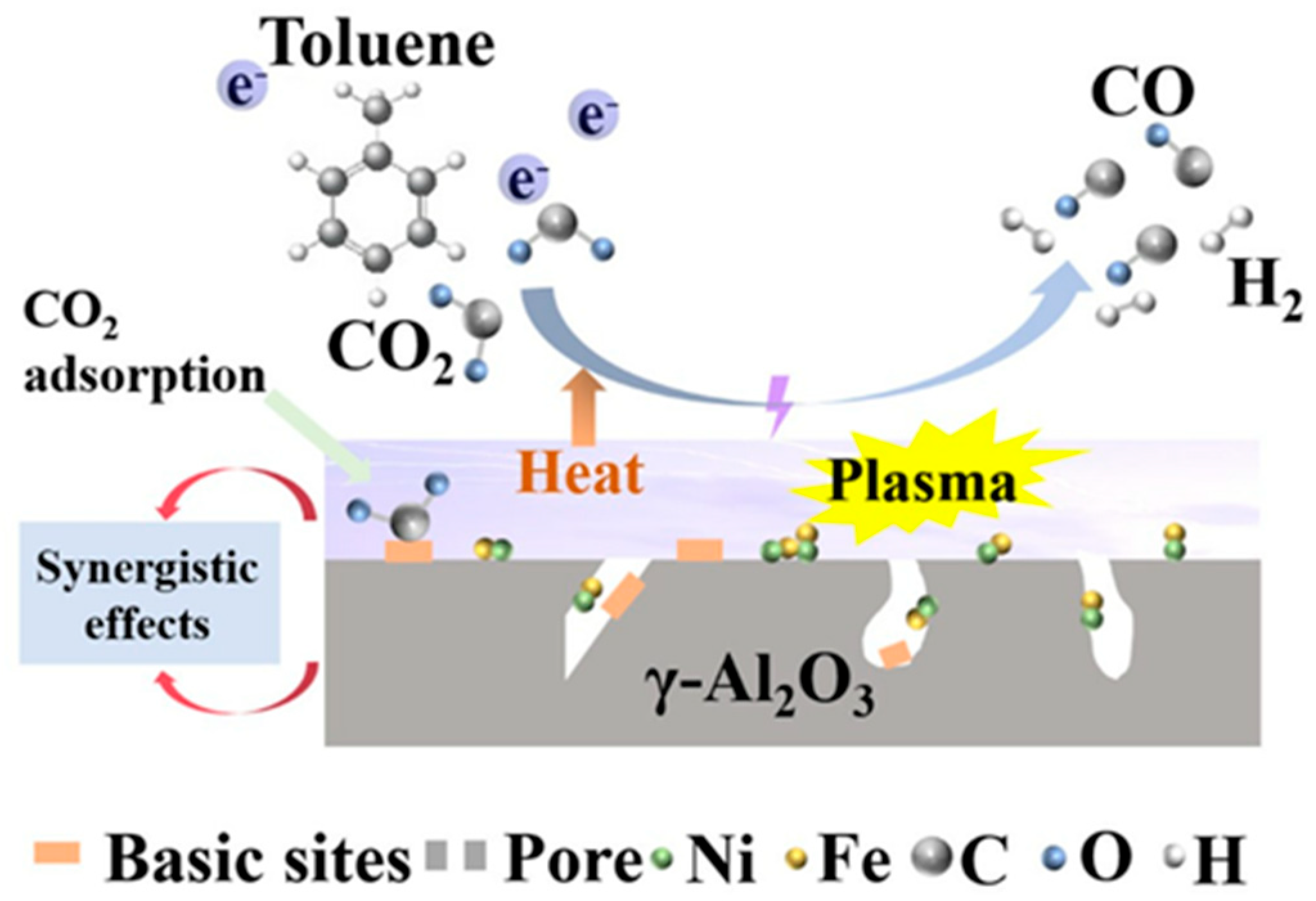
4.1.2. Gasification and Fischer–Tropsch Process
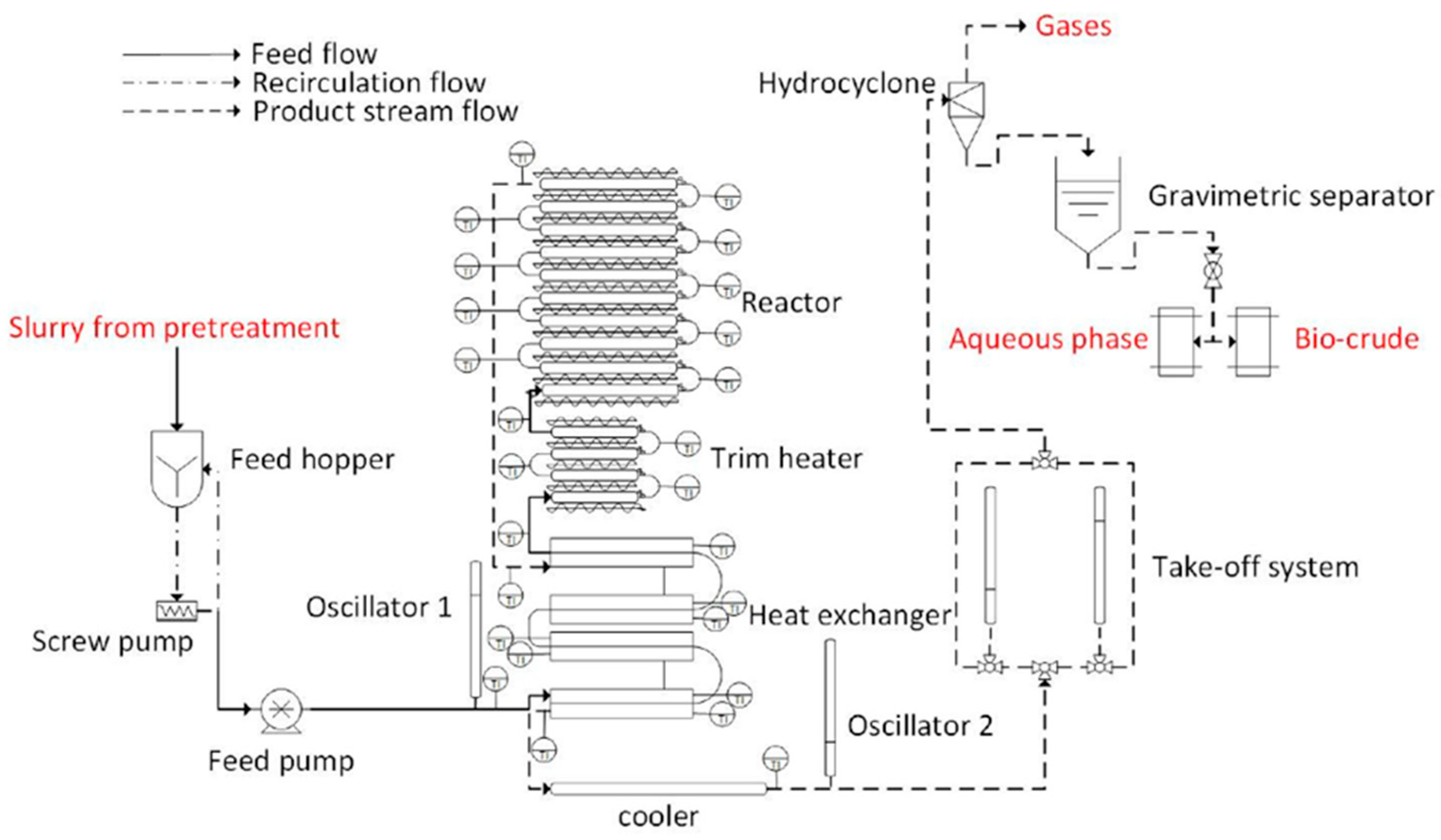
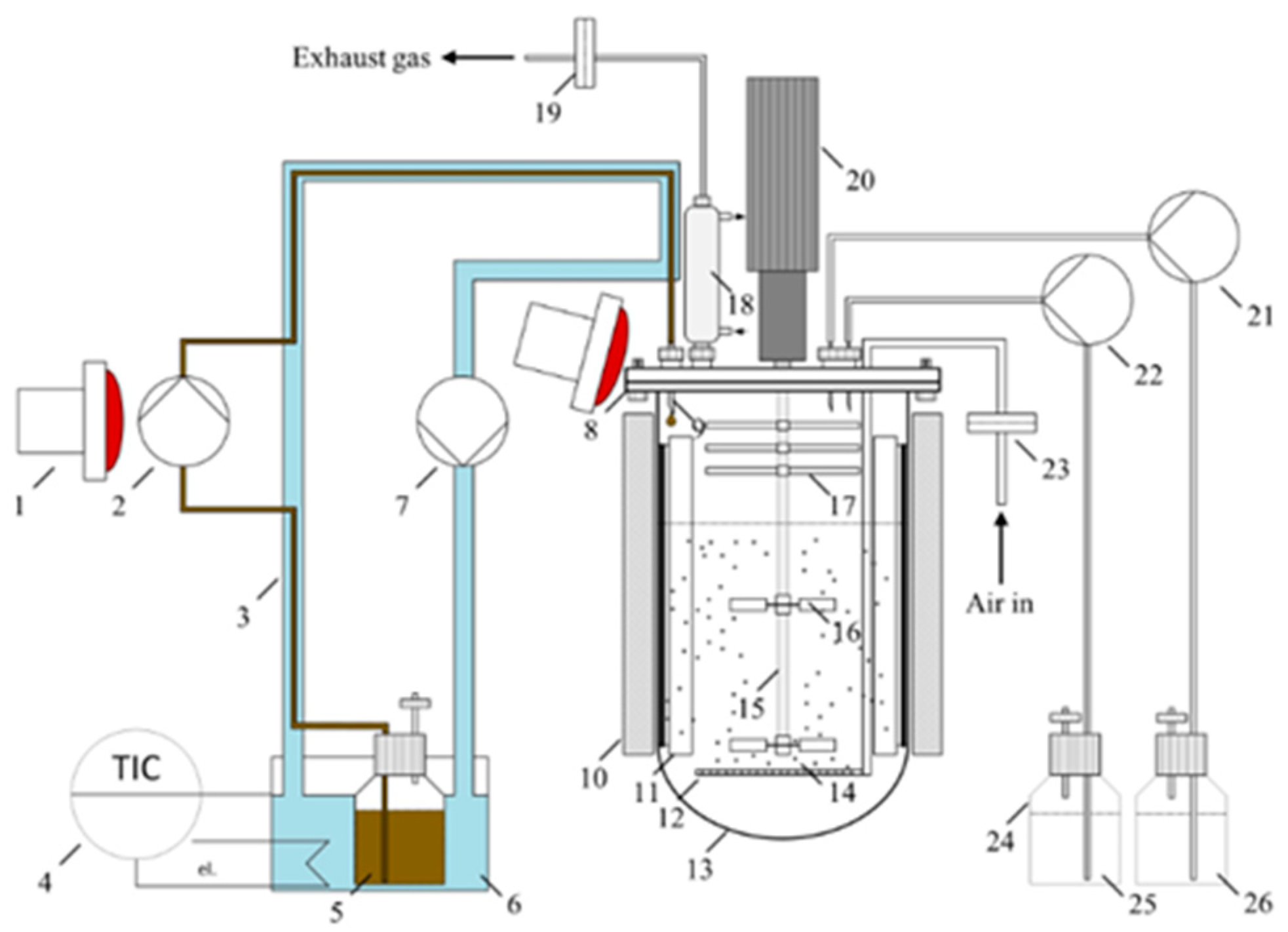
4.2. Aqueous-Phase Processes: Hydrothermal Liquefaction (HTL)
4.3. Chemical Upgrading
4.3.1. Hydrodeoxygenation (HDO)
4.3.2. Transesterification
4.3.3. Hydrogenolysis
5. Catalytic Technologies for Biomass-Platform Chemical Production
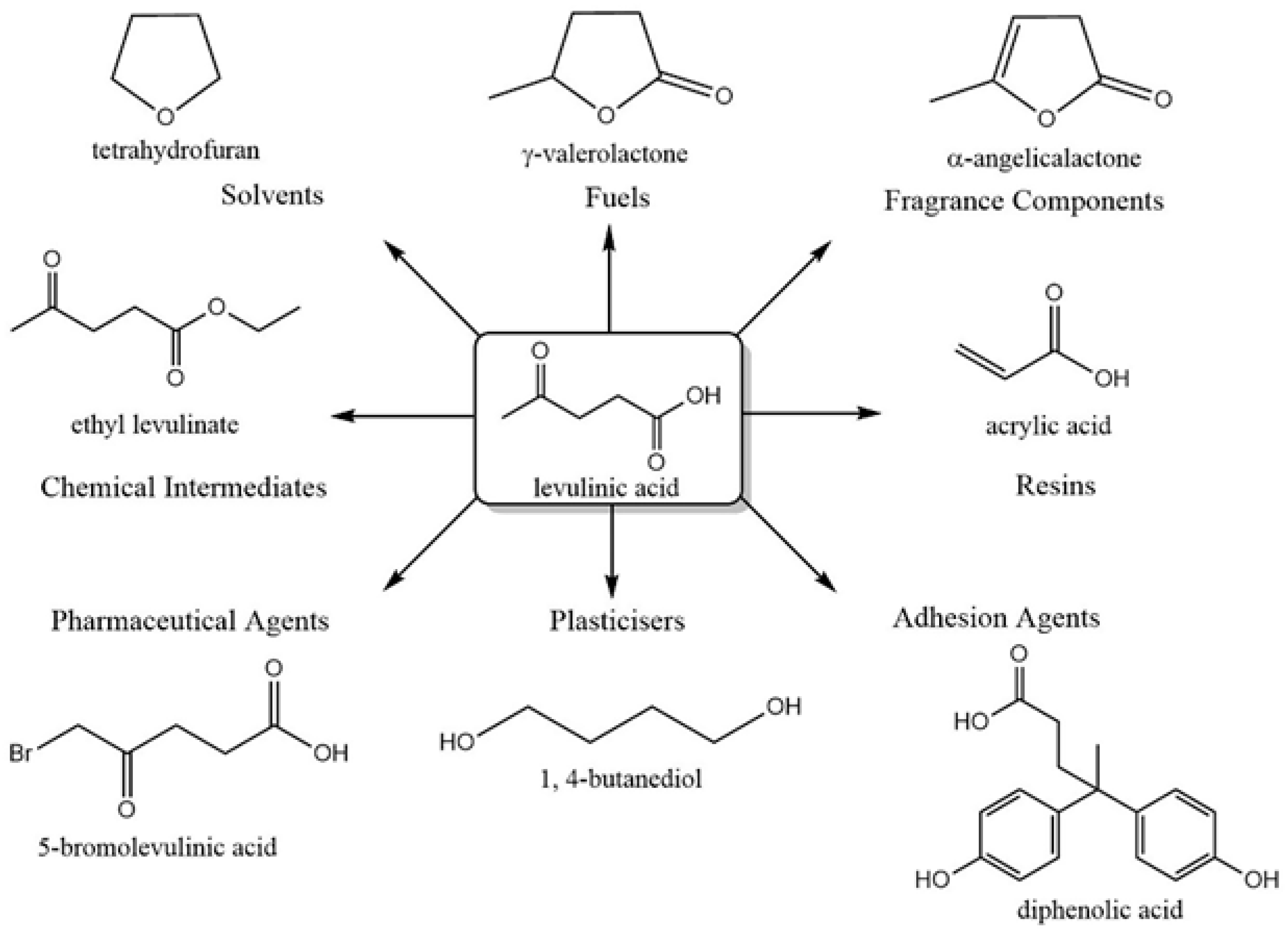
5.1. 5-HMF, Furfural, and LA
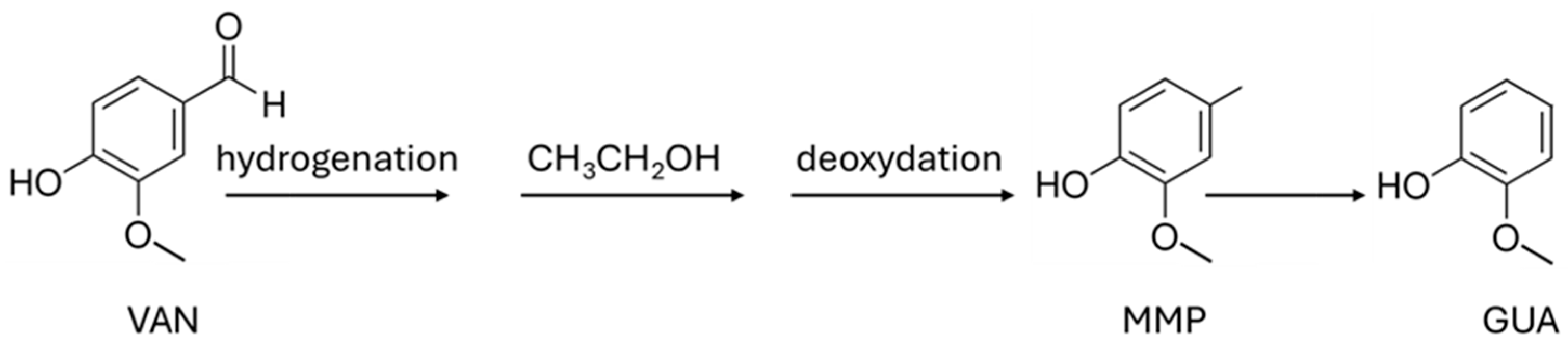
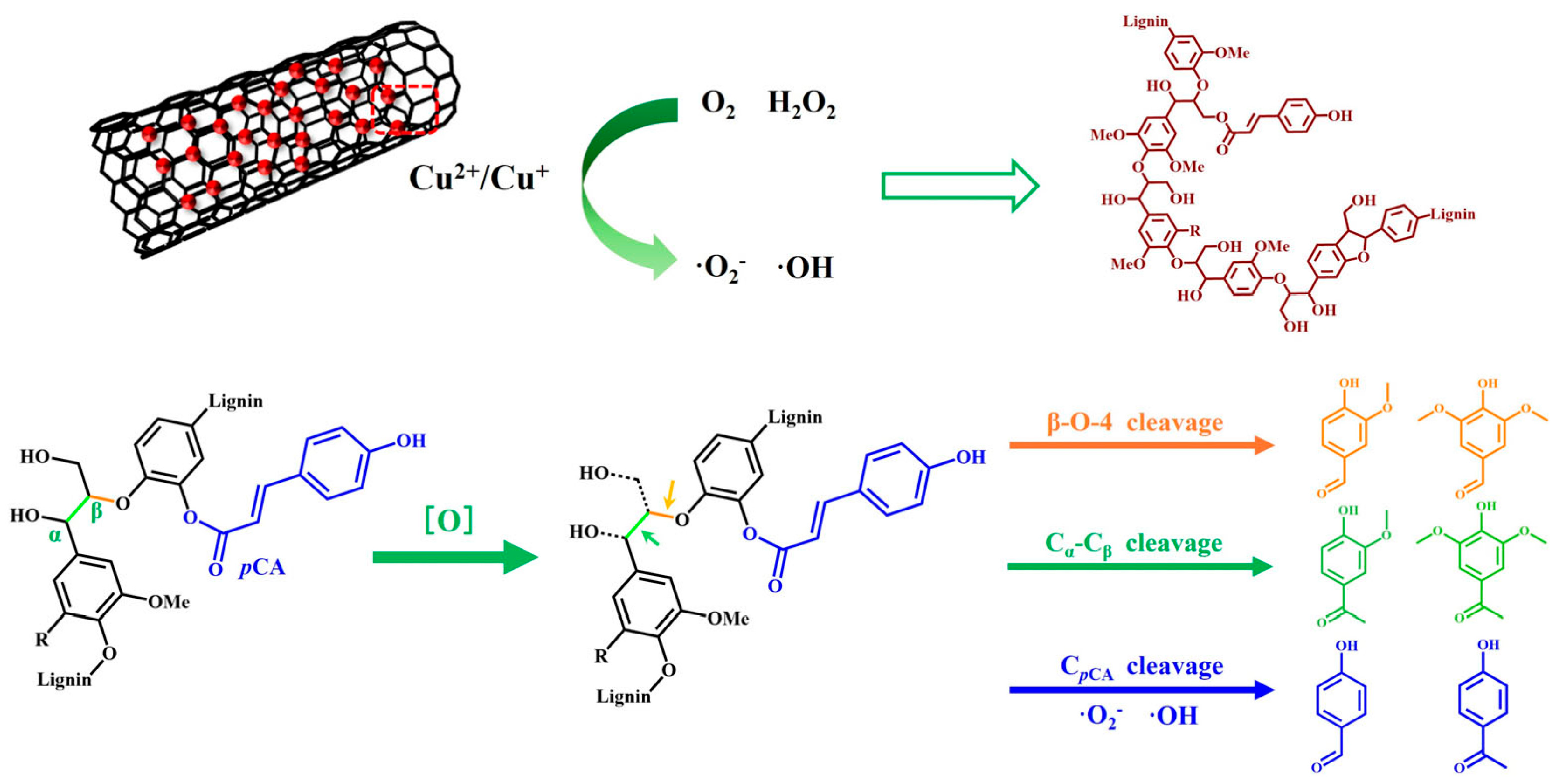
5.2. Lignin-Derived Aromatics
5.3. Biopolymers and Composites—Polylactic Acid (PLA) and Polyhydroxyalkanoates (PHA)
6. Non-Catalytic Technologies for Biomass Conversion into Carbon Materials for Catalytic Use
7. Catalyst Stability and Deactivation
8. Economic Feasibility and Process Intensification
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOFs | Metal–Organic Frameworks |
| SACs | Single-atom catalysts |
| DES | Deep Eutectic Solvents |
| LHW | Liquid Hot Water |
| HTL | Hydrothermal Liquefaction |
| LA | Levulinic Acid |
| HDO | Hydrodeoxygenation |
| FAMES | Fatty Acid Methyl esters |
| SSAC | Sulfonated hierarchical Sucrose-based Activated Carbon |
| SSUAC | N-doped SSAC |
| GVL | γ-valerolactone |
| VAN | Vanillin |
| MMP | 2-methoxy-4-methylphenol |
| GUA | Guaiacol |
| SILnPs | Supported Ionic Liquid Nanoparticle |
| STPA | 2-sulfoterephthalate monosodium salt |
| mSBA | 3-sulfobenzoate sodium salt |
| pSBA | 4-sulfobenzoic acid monopotassium salt |
| pClSBA | 4-(chlorosulfonyl)benzoic acid |
| CFP | Catalytic Fast Pyrolysis |
| CHP | Catalytic Hydropyrolysis |
| BJF | Bio-Jet Fuel |
| FCC | Fluid Catalytic Cracking |
| F-T | Fischer–Tropsch |
| HAP | Hydroxyapatite |
| MSS | Modified Steel Slag |
| HDS | Hydrosulfurization |
| FFA | Free Fatty Acids |
| 5-HMF | 5-Hydroxymethylfurfural |
| BH | Barley Hulls |
| RCF | Reductive Catalytic Fractionation |
| PLA | Polylactic Acid |
| PHA | Polyhydroxyalkanoates |
| ROP | Ring-Opening Polymerization |
| PET | Polyethylene Terephthalate |
| PS | Polystyrene |
| CVD | Chemical Vapor Deposition |
| AC | Activated Carbon |
| HTC | Hydrothermal Carbonization |
| PAN | Polyacrylonitrile |
| TEAs | Techno-Economic Analyses |
References
- Mignogna, D.; Szabó, M.; Ceci, P.; Avino, P. Biomass energy and biofuels: Perspective, potentials, and challenges in the energy transition. Sustainability 2024, 16, 7036. [Google Scholar] [CrossRef]
- Tun, M.M.; Juchelkova, D.; Win, M.M.; Thu, A.M.; Puchor, T. Biomass energy: An overview of biomass sources, energy potential, and management in Southeast Asian countries. Resources 2019, 8, 81. [Google Scholar] [CrossRef]
- Solarte-Toro, J.C.; Ortiz-Sanchez, M.; Inocencio-García, P.J.; Cardona Alzate, C.A. Sustainable biorefineries based on catalytic biomass conversion: A review. Catalysts 2023, 13, 902. [Google Scholar] [CrossRef]
- Aristizábal-Marulanda, V.; Solarte-Toro, J.C.; Cardona Alzate, C.A. Study of Biorefineries Based on Experimental Data: Production of Bioethanol, Biogas, Syngas, and Electricity Using Coffee-Cut Stems as Raw Material. Environ. Sci. Pollut. Res. 2020, 28, 24590. [Google Scholar] [CrossRef] [PubMed]
- Morakile, T.; Mandegari, M.; Farzad, S.; Görgens, J.F. Comparative Techno-Economic Assessment of Sugarcane Biorefineries Producing Glutamic Acid, Levulinic Acid and Xylitol from Sugarcane. Ind. Crop. Prod. 2022, 184, 115053. [Google Scholar] [CrossRef]
- Xiao, A.; Xu, H.; Cui, H.; Cheng, Z.; Zhou, Z. Bimetallic and trimetallic Pt-based catalysts for selective hydrogenation of p-chloronitrobenzene to p-chloroaniline. Appl. Catal. A Gen. 2023, 666, 119424. [Google Scholar] [CrossRef]
- Jun, H.; Oh, S.; Lee, G.; Oh, M. Enhanced catalytic activity of MOF-74 via providing additional open metal sites for cyanosilylation of aldehydes. Sci. Rep. 2022, 12, 14735. [Google Scholar] [CrossRef]
- Wang, B.; Yan, X.; Zhou, M.; Li, H. Tailoring crystal planes and oxygen vacancies of ceria for enhanced catalytic performance of single-atom Ru in hydrogenative dearomatization of lignin-derived phenols. Energy Mater. 2025, 5, 500084. [Google Scholar] [CrossRef]
- Liu, Y.; Deak, N.; Wang, Z.; Yu, H.; Hameleers, L.; Jurak, E.; Deuss, P.J.; Barta, K. Tunable and functional deep eutectic solvents for lignocellulose valorization. Nat. Commun. 2021, 12, 5424. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Farrauto, R.J. Fundamentals of Industrial Catalytic Processes; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Pérez-Almada, D.; Galán-Martín, Á.; del Mar Contreras, M.; Castro, E. Integrated techno-economic and environmental assessment of biorefineries: Review and future research directions. Sustain. Energy Fuels 2023, 7, 4031–4050. [Google Scholar] [CrossRef]
- Ragonnaud, G.; Macsai, G.; Chahri, S. Implementing the EU’s Critical Raw Materials Act. Available online: https://www.europarl.europa.eu/RegData/etudes/BRIE/2024/766253/EPRS_BRI(2024)766253_EN.pdf (accessed on 1 August 2025).
- Nopparat, S.; Khatiya, W.; Supawan, U.P.K.; Wanwitoo, W.; Navadol, L.; Verawat, C.; Kowit, S.; Saksit, I. Efficiency of catalytic liquid hot water pretreatment for conversion of corn stover to bioethanol. ACS Omega 2020, 5, 29872–29881. [Google Scholar] [CrossRef]
- Oliveira, L.; Pereira, M.; Pacheli Heitman, A.; Filho, J.; Oliveira, C.; Ziolek, M. Niobium: The focus on catalytic application in the conversion of biomass and biomass derivatives. Molecules 2023, 28, 1527. [Google Scholar] [CrossRef]
- Dong, L.; Lin, L.; Han, X.; Si, X.; Liu, X.; Guo, Y.; Lu, F.; Rudic, F.; Parker, F.S.; Yang, S.; et al. Breaking the limit of lignin monomer production via cleavage of interunit carbon–carbon linkages. Chem 2019, 5, 1521–1536. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.; Zhao, W.; Li, L.; Liu, S.; Long, J.; Li, X. Selective depolymerization of lignin catalyzed by nickel supported on zirconium phosphate. Green Chem. 2019, 21, 658–668. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Lian, Y.; Li, Y.; Huang, B.; Lu, Q. Zirconium Phosphate Assisted Phosphoric Acid Co-Catalyzed Hydrolysis of Lignocellulose for Enhanced Extraction of Nanocellulose. Polymers 2023, 15, 447. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Wang, T.; Zhang, X.; Long, J.; Zhang, Q.; Ma, L. High yield of renewable hexanes by direct hydrolysis–hydrodeoxygenation of cellulose in an aqueous phase catalytic system. RSC Adv. 2014, 5, 11649–11657. [Google Scholar] [CrossRef]
- Park, Y.; Jeong, G.T. Production of levulinic acid from macroalgae by hydrothermal conversion with ionic resin catalyst. Bioresour. Technol. 2024, 402, 130778. [Google Scholar] [CrossRef] [PubMed]
- Dibenedetto, A.; Colucci, A.; Aresta, M. The need to implement an efficient biomass fractionation and full utilization based on the concept of “biorefinery” for a viable economic utilization of microalgae. Environ. Sci. Pollut. Res. 2016, 23, 22274–22283. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Colucci, A. Aquatic Biomass for the Production of Fuel and Chemicals in Materials for a Sustainable Future, Chapter 8; Letcher, T.M., Scott, J.L., Davidson, M.G., Eds.; Royal Society of Chemistry: London, UK, 2012; p. 215. [Google Scholar]
- Aresta, M.; Dibenedetto, A.; Dumeignil, F. From Biomass to Chemicals and Fuels, Berlin, Boston: De Gruyter, 2012. [CrossRef]
- Shang, S.; Guo, C.; Lan, K.; Qin, Z. Hydrogen-rich syngas production via catalytic gasification of sewage sludge and wheat straw using corn stalk char-supported catalysts. BioResources 2020, 15, 4294–4313. [Google Scholar] [CrossRef]
- Alam, T.; Park, S.W.; Lee, S.Y.; Jeong, Y.O.; De Girolamo, A.; Seo, Y.C.; Choi, H.S. Co-gasification of treated solid recovered fuel residue by using minerals bed and biomass waste blends. Energies 2020, 13, 2081. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Nocito, F.; Ferragina, C. Valorization of bio-glycerol: New catalytic materials for the synthesis of glycerol carbonate via glycerolysis of urea. J. Catal. 2009, 268, 106. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Angelini, A.; Aresta, M.; Ethiraj, J.; Fragale, C.; Nocito, F. Converting wastes into added value products: From glycerol to glycerol carbonate, glycidol and epichlorohydrin using environmentally friendly synthetic routes. Tetrahedron 2011, 67, 1308. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Nocito, F.; Papai, I.; Angelini, A.; Mancuso, R.; Aresta, M. Catalytic synthesis of hydroxymetyl-2-oxazolidinones from glycerol or glycerol carbonate and urea. ChemSusChem 2012, 6, 345. [Google Scholar] [CrossRef]
- Mondal, S.; Biswas, P. Conversion of bio-glycerol to propylene glycol over basic oxides (MgO, La2O3, MgO-La2O3, CaO, and BaO2) supported Cu–Zn bimetallic catalyst: A reaction kinetic study. Environ. Technol. Innov. 2022, 27, 102367. [Google Scholar] [CrossRef]
- Aresta, M.; Dibenedetto, A.; Dumeignil, F. Biorefinery: From Biomass to Chemicals and Fuels: Towards Circular Economy, Berlin, Boston: De Gruyter, 2022. [CrossRef]
- Zheng, B.; Fang, Z.; Cheng, J.; Jiang, Y. Microwave-assisted conversion of carbohydrates into 5-hydroxymethylfurfural catalyzed by ZnCl2. Z. Naturforschung Sect. B 2010, 65, 168. [Google Scholar] [CrossRef]
- Huang, C.W.; Nguyen, B.S.; Wu, J.C.S.; Nguyen, V.H. A current perspective for photocatalysis towards the hydrogen production from biomass-derived organic substances and water. Int. J. Hydrogen Energy 2020, 45, 18144. [Google Scholar] [CrossRef]
- Kou, J.; Lu, C.; Wang, J.; Chen, Y.; Xu, Z.; Varma, R.S. Selectivity enhancement in heterogeneous photocatalytic transformations. Chem. Rev. 2017, 117, 1445. [Google Scholar] [CrossRef] [PubMed]
- Absalan, Y.; Shad, N.N.; Gholizadeh, M. The Complex Compound of Amino Acids with Titanium (III) as a Method to Control and Synthesis of Different Structures of TiO2 Nanoparticles; Usage as Photocatalysts to Oxidize Alcohols to Aldehyde. Available via license: CC BY 4.0. 2021. Available online: https://d1wqtxts1xzle7.cloudfront.net/88537288/v1-libre.pdf?1657698767=&response-content-disposition=inline%3B+filename%3DThe_Complex_Compound_of_Amino_Acids_With.pdf&Expires=1759388110&Signature=KzuIykSKea35ohfoPkmp09NdipnnRXWRCwXx19Aqs8hgrsQzeS3b8UMuVkV0co5FiHmaNkZFZa6ABc9FDxhDlz1cwRghViwA6pV08OhaG9mU~4ZB3MOngW43ybtOy8QnL~Kn7OFtTDxgS40HZU2PJYd9~27iWNQIHlob0P14YSzEUCZruN1adxtRngxbmF6NlJlC6vZQoTaOsr13yubjI2KuDuYFR63LHygmUCausoUrLHffSNq0PD10OmDbKtNM37KowQLIso-1Hr6SXD9RnmU6uAd~ngxDWCu2ICmprMHgTmq15TgVYII6N9Yd6joywZh38PXSBR8kPecrMetvqA__&Key-Pair-Id=APKAJLOHF5GGSLRBV4ZA (accessed on 4 September 2025).
- Liu, X.; Duan, X.; Wei, W.; Wang, S.; Ni, B.J. Photocatalytic conversion of lignocellulosic biomass to valuable products. Green Chem. 2019, 21, 4266. [Google Scholar] [CrossRef]
- Li, X.; Guo, Z.; He, T. The doping mechanism of Cr into TiO2 and its influence on the photocatalytic performance. Phys. Chem. Chem. Phys. 2013, 15, 20037. [Google Scholar] [CrossRef]
- Mittal, N.; Kaur, M.; Singh, V.; Babbar, A.; Dua, T.; Singh, V. Accelerated depolymerization of rice straw-derived lignin to vanillin and guaiacol using γ-Fe2O3/N, Fe-TiO2 as novel photocatalyst under visible light in aqueous media. J. Photochem. Photobiol. A Chem. 2025, 467, 116440. [Google Scholar] [CrossRef]
- Ren, F.; Li, H.; Wang, Y.; Yang, J. Enhanced photocatalytic oxidation of propylene over V-doped TiO2 photocatalyst: Reaction mechanism between V5+ and single-electron-trapped oxygen vacancy. Appl. Catal. B Environ. 2015, 176, 160. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, H.; Chen, Q.; Li, J.; Wang, P. Construction of N, S codoped TiO2 NCs decorated TiO2 nano-tube array photoelectrode and its enhanced visible light photocatalytic mechanism. Electrochim. Acta 2013, 103, 134. [Google Scholar] [CrossRef]
- Bellardita, M.; Virtù, D.; Di Franco, F.; Loddo, V.; Palmisano, L.; Santamaria, M. Heterogeneous photocatalytic aqueous succinic cid formation from maleic acid reduction. Chem. Eng. J. 2022, 431, 134131. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Z.; Huang, J.; Jiang, Y. Zirconia-based solid acid catalysts for biomass conversion. Energy Fuels 2021, 35, 9209. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; Pastore, C.; Di Bitonto, L.; Angelini, A.; Quaranta, E. Conversion of fructose into 5-HMF: A study on the behaviour of heterogeneous cerium-based catalysts and their stability in aqueous media under mild conditions. RSC Adv. 2015, 5, 26941. [Google Scholar] [CrossRef]
- Ventura, M.; Nocito, F.; de Giglio, E.; Cometa, S.; Altomare, A.; Dibenedetto, A. Tunable mixed oxides based on CeO2 for the selective aerobic oxidation of 5-(hydroxymethyl) furfural to FDCA in water. Green Chem. 2018, 20, 3921. [Google Scholar] [CrossRef]
- Guo, X.; Shen, S.; Wang, B.; Jing, S.; Wu, B.; Sun, R.; Chen, C.; Liu, Y.; Li, J. Al-induced orthorhombic SnO2 and oxygen vacancies synergistically promote lactic acid production from dihydroxyacetone. J. Alloy. Compd. 2024, 1014, 178358. [Google Scholar] [CrossRef]
- Park, Y.M.; Lee, J.Y.; Chung, S.H.; Park, I.S.; Lee, S.Y.; Kim, D.K.; Lee, J.S.; Lee, K.Y. Esterification of used vegetable oils using the heterogeneous WO3/ZrO2 catalyst for production of biodiesel. Bioresour. Technol. 2010, 101, S59. [Google Scholar] [CrossRef] [PubMed]
- Dibenedetto, A.; Angelini, A.; Colucci, A.; di Bitonto, L.; Pastore, C.; Aresta, B.M.; Giannini, C.; Comparelli, R. Tunable mixed oxides: Efficient agents for the simultaneous trans-esterification of lipids and esterification of free fatty acids from bio-oils for the effective production of FAMEs. International Int. J. Renew. Energy Biofuels 2016, 2016, 204112. [Google Scholar] [CrossRef]
- Mo, X.; López, D.E.; Suwannakarn, K.; Liu, Y.; Lotero, E.; Goodwin, J.G., Jr.; Lu, C. Activation and deactivation characteristics of sulfonated carbon catalysts. J. Catal. 2008, 254, 332. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, H.; Gao, J.; Chen, S.; Jin, Y.; Deng, C.; Wu, S.; Xiao, H.; Li, W. Synthesis of sulfonated hierarchical carbons and theirs application on the production of furfural from wheat straw. Mol. Catal. 2022, 517, 112034. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of cellulose by amorphous carbon bearing SO3H, COOH, and OH groups. J. Am. Chem. Soc. 2008, 130, 12787. [Google Scholar] [CrossRef]
- Ausavasukhi, A.; Sooknoi, T. Hydrothermal sulfonation of palm kernel shells to produce a carbon-based solid acid catalyst for the glycerol etherification. Diam. Relat. Mater. 2025, 154, 112217. [Google Scholar] [CrossRef]
- Wei, C.; Wang, W.; Wang, H.; Zhu, J.; He, Z.H.; Ma, Y.; Guo, N.; Liu, Z.T. ZrO2 modified sulfonated charcoal-based catalysts for hydrolysis of biomass sugars and agricultural residues. Mol. Catal. 2024, 569, 114622. [Google Scholar] [CrossRef]
- Zhong, R.; Sels, B.F. Sulfonated mesoporous carbon and silica-carbon nanocomposites for biomass conversion. Appl. Catal. B Environ. 2018, 236, 518. [Google Scholar] [CrossRef]
- Choudhary, V.R.; Mantri, K. AlClx-grafted Si-MCM-41 prepared by reacting anhydrous AlCl3 with terminal Si–OH groups: An active solid catalyst for benzylation and acylation reactions. Microporous Mesoporous Mater. 2002, 56, 317–320. [Google Scholar] [CrossRef]
- Brunel, D.; Blanc, A.C.; Galarneau, A.; Fajula, F. New trends in the design of supported catalysts on mesoporous silicas and their applications in fine chemicals. Catal. Today 2002, 73, 139–152. [Google Scholar] [CrossRef]
- Hegde, V.; Pandit, P.; Rananaware, P.; Brahmkhatri, V.P. Sulfonic acid-functionalized mesoporous silica catalyst with different morphology for biodiesel production. Front. Chem. Sci. Eng. 2022, 16, 1198. [Google Scholar] [CrossRef]
- El Bojaddayni, I.; Küçük, M.E.; El Ouardi, Y.; Jilal, I.; El Barkany, S.; Moradi, K.; Repo, E.; Laatikainen, K.; Ouammou, A. A review on synthesis of zeolites from natural clay resources and waste ash: Recent approaches and progress. Miner. Eng. 2023, 198, 108086. [Google Scholar] [CrossRef]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3. [Google Scholar] [CrossRef]
- Susanti, Y.; Maghfirah, A.; Fajar, A.T.; Mukti, R.R.; Kadja, G.T. Hydrophobic HY zeolite with enhanced stability in hot liquid water. J. Iran. Chem. Soc. 2025, 22, 397. [Google Scholar] [CrossRef]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164. [Google Scholar] [CrossRef]
- Romµn-Leshkov, Y.; Moliner, M.; Labinger, J.A.; Davis, M.E. Mechanism of glucose isomerization using a solid Lewis acid catalyst in water. Angew. Chem. Int. Ed. 2010, 49, 8954. [Google Scholar] [CrossRef]
- Islam, M.K.; Kongparakul, S.; Guan, G.; Chanlek, N.; Samart, C. Oxidative fractionation of palm kernel shell waste biomass over bimetallic Co-Cu/Zeolite HY catalyst. Biomass Bioenergy 2025, 193, 107609. [Google Scholar] [CrossRef]
- Xiang, H.; Zhang, Y.; Zhong, X.; Wang, B.; Drivas, C.; Isaacs, M.A.; Syahida, A.; Zhipeng, Q.J.; Zhang, Q.; Fan, X. Sulfonic acid-unctionalised zeolites for fructose dehydration into 5-hydroxymethylfurfural. Sustain. Sci. Technol. 2025, 2, 034002. [Google Scholar] [CrossRef]
- Tang, J.; Zou, J.; Li, Q.; Wu, Q.; Zheng, X.; Fang, J.; Xiao, Z. Alkaline catalytic liquefaction of pig manure fermentation residue in ethanol solvent for the production of high-quality biocrude oil. Waste Manag. 2025, 197, 86. [Google Scholar] [CrossRef]
- Zuo, X.; Tang, X. Glucose Isomerization to Fructose Catalyzed by MgZr Mixed Oxides in Aqueous Solution. Catalysts 2024, 14, 332. [Google Scholar] [CrossRef]
- Guo, F.; Peng, Z.G.; Dai, J.Y.; Xiu, Z.L. Calcined sodium silicate as solid base catalyst for biodiesel production. Fuel Process. Technol. 2010, 91, 322. [Google Scholar] [CrossRef]
- Zhao, C.; Kou, Y.; Lemonidou, A.A.; Li, X.; Lercher, J.A. Hydrodeoxygenation of bio-derived phenols to hydrocarbons using RANEY® Ni and Nafion/SiO2 catalysts. Chem. Commun. 2009, 46, 412. [Google Scholar] [CrossRef]
- Astuti, M.D.; Mustikasari, K.; Husain, S. Recent progress in the direct synthesis of γ-valerolactone from biomass-derived sugars catalyzed by RANEY® Ni–Sn alloy supported on aluminium hydroxide. Catal. Sci. Technol. 2020, 10, 7768. [Google Scholar] [CrossRef]
- Siyao, C.; Jiajie, H.; Ying, L.; Shubin, W.; Junping, Z. Catalytic conversion of cellulose to 5-Hydroxymethylfurfural using montmorillonite-supported transition metal catalysts. Ind. Crop. Prod. 2025, 225, 120428. [Google Scholar] [CrossRef]
- Al Alwan, B.; Alamri, S.; El Jery, A.; Shah, M.; Sahlabji, T. Development of Novel Mussel-Shell-Derived CaO-Based Transition Metal Catalysts for Efficient Microwave-Assisted Biodiesel Production. Processes 2025, 13, 522. [Google Scholar] [CrossRef]
- Chen, C.; Ji, X.; Xiong, Y.; Jiang, J. Ni/Ce co-doping metal–organic framework catalysts with oxygen vacancy for catalytic transfer hydrodeoxygenation of lignin derivatives vanillin. Chem. Eng. J. 2024, 481, 148555. [Google Scholar] [CrossRef]
- Ge, F.; Li, H.; Wu, B.; Yang, X.; Jiang, J.; Zhang, Y.; Zhou, M. MOFs-derived carbon-covered nickel phosphide for catalytic transfer hydrodeoxygenation of lignin-derived vanillin. Chem. Eng. J. 2024, 489, 151367. [Google Scholar] [CrossRef]
- Xia, D.; Yu, H.; Li, H.; Huang, P.; Li, Q.; Wang, Y. Carbon-based and carbon-supported nanomaterials for the catalytic conversion of biomass: A review. Environ. Chem. Lett. 2022, 20, 1719–1744. [Google Scholar] [CrossRef]
- Frontera, P.; Antonucci, P.L.; Macario, A. Focus on Materials for Sulfur-Resistant Catalysts in the Reforming of Biofuels. Catalysts 2021, 11, 1029. [Google Scholar] [CrossRef]
- Kuang, Y.; Li, H. Metal@ hollow carbon sphere nanoreactors for sustainable biomass and CO2 valorization. J. Mater. Chem. A 2022, 10, 7557. [Google Scholar] [CrossRef]
- Nocito, F.; Ditaranto, N.; Linsalata, D.; Naschetti, M.; Comparelli, R.; Aresta, M.; Dibenedetto, A. Selective aerobic oxidation of furfural into furoic Acid over a highly recyclable MnO2@CeO2 core–shell oxide: The role of the morphology of the catalyst. ACS Sustain. Chem. Eng. 2022, 10, 8615. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Qin, B.; Zheng, J.; Dai, W.; Zhang, T.; Du, Y.; Li, W.; Wang, Y.; Li, R. Core-shell bifunctional catalysts: Controllable intimacy between metals and acids within nanometer-scale for n-alkane conversion. J. Catal. 2025, 443, 115958. [Google Scholar] [CrossRef]
- Kang, P.; Gabrijelčič, M.; Krajnc, A.; Črnivec, I.G.O.; Likozar, B.; Sharma, R.K. Organically functionalized mesoporous silica network for one-pot synthesis of 5-hydroxymethylfurfural from glucose in water. ACS Sustain. Chem. Eng. 2025, 13, 4997. [Google Scholar] [CrossRef]
- Sidhpuria, K.B.; Daniel-da-Silva, A.L.; Trindade, T.; Coutinho, J.A. Supported ionic liquid silica nanoparticles (SILnPs) as an efficient and recyclable heterogeneous catalyst for the dehydration of fructose to 5-hydroxymethylfurfural. Green Chem. 2011, 13, 340. [Google Scholar] [CrossRef]
- Zhong, J.; Pérez-Ramírez, J.; Yan, N. Biomass valorisation over polyoxometalate-based catalysts. Green Chem. 2020, 23, 18. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Liu, Y.; Zhao, L.; Peng, F.; Ren, J. Unlocking Precision in Biomass Conversion through Functional Ligand Engineering of Lewis Acidic MOFs. Angew. Chem. Int. Ed. Engl. 2025, 64, e202508256. [Google Scholar] [CrossRef]
- Nishchakova, A.D.; Bulusheva, L.G.; Bulushev, D.A. Supported Ni single-atom catalysts: Synthesis, structure, and applications in thermocatalytic reactions. Catalysts 2023, 13, 845. [Google Scholar] [CrossRef]
- Fan, Y.; Zhuang, C.; Li, S.; Wang, Y.; Zou, X.; Liu, X.; Huang, W.; Zhu, G. Efficient single-atom Ni for catalytic transfer hydrogenation of furfural to furfuryl alcohol. J. Mater. Chem. A 2020, 9, 1110. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X.; Luo, M.; Jiang, Q.; Li, X.; Yang, C.; Zhang, Q.; Ma, L.; Yan, L. Selective Hydrogenation of Furfural Under Mild Conditions Over Single-Atom Pd1/α-MoC Catalyst. ChemSusChem 2024, 18, e202401802. [Google Scholar] [CrossRef]
- Qi, H.; Yang, J.; Liu, F.; Zhang, L.; Yang, J.; Liu, X.; Li, L.; Su, Y.; Liu, Y.; Hao, R.; et al. Highly selective and robust ingle-atom catalyst Ru1/NC for reductive amination of aldehydes/ketones. Nat. Commun. 2021, 12, 3295. [Google Scholar] [CrossRef]
- Ma, D.; Lu, S.; Liu, X.; Guo, Y.; Wang, Y. Depolymerization and hydrodeoxygenation of lignin to aromatic hydrocarbons with a Ru catalyst on a variety of Nb-based supports. Chin. J. Catal. 2019, 40, 609. [Google Scholar] [CrossRef]
- Lin, L.; Sheveleva, A.M.; da Silva, I.; Parlett, C.M.; Tang, Z.; Liu, Y.; Fan, M.; Han, X.; Carter, J.H.; Tuna, F.; et al. Quantitative production of butenes from biomass-derived γ-valerolactone catalysed by hetero-atomic MFI zeolite. Nat. Mater. 2019, 19, 86. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Pang, J.; Gao, P.; Zheng, M.; Hou, G. Recent Advances in Metal–Zeolite Catalysts for Ethanol to 1, 3-Butadiene Conversion: Active Metal Sites, Mechanisms, and Future Challenges. ACS Catal. 2025, 15, 5053. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Yang, J.; Liu, Y.; Hei, J.; Huang, S.; Gao, D. Production of aromatic hydrocarbons from catalytic fast pyrolysis of microalgae over Fe-modified HZSM-5 catalysts. RSC Adv. 2024, 14, 36970. [Google Scholar] [CrossRef]
- Yuan, H.; Li, C.; Shan, R.; Zhang, J.; Chen, Y. Aromatics production from catalytic pyrolysis of waste cassava residue using La and P modified ZSM-5: Experimental and kinetic study. Ind. Crop. Prod. 2023, 198, 116753. [Google Scholar] [CrossRef]
- Zhong, S.; Zhang, J.; Li, C.; Shan, R.; Yuan, H.; Chen, Y. Selective production of aromatics from catalytic fast pyrolysis of cassava residues over vanadium modified ZSM-5: Experimental and kinetic study. J. Anal. Appl. Pyrolysis 2024, 177, 106334. [Google Scholar] [CrossRef]
- Shafaghat, H.; Butt, W. Direct conversion of sawdust into biobased aviation fuel precursor via atmospheric catalytic hydropyrolysis process. Biomass Bioenergy 2025, 198, 107892. [Google Scholar] [CrossRef]
- Stummann, M.Z.; Høj, M.; Gabrielsen, J.; Clausen, L.R.; Jensen, P.A.; Jensen, A.D. A perspective on catalytic hydropyrolysis of biomass. Renew. Sustain. Energy Rev. 2021, 143, 110960. [Google Scholar] [CrossRef]
- Corma, A.; Wojciechowski, B.W. The chemistry of catalytic cracking. Catal. Rev. 1985, 27, 29. [Google Scholar] [CrossRef]
- Dou, X.; Yue, J.; Guan, Y.; Liu, W.; Zhang, Y.; Chen, Z.; Luo, G.; Xu, G. Characteristics and kinetics of in-situ catalytic cracking of biomass tar in a micro dual bed reactor. Energy 2024, 306, 132470. [Google Scholar] [CrossRef]
- Parrillo, F.; Ardolino, F.; Boccia, C.; Arconati, V.; Ruoppolo, G.; Arena, U. Waste-derived catalysts for tar cracking in hot syngas cleaning. Waste Manag. 2024, 179, 163. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, Y.; Zou, L.; Xiu, H.; Liu, T.; Zhang, X. Experimental study on hydrogen production from heavy tar in biomass gasification furnace catalyzed by carbon-based catalysts. Fuel 2023, 361, 130718. [Google Scholar] [CrossRef]
- Neto, A.S.; Wainaina, S.; Chandolias, K.; Piatek, P.; Taherzadeh, M.J. Exploring the potential of syngas fermentation for recovery of high-value resources: A comprehensive review. Curr. Pollut. Rep. 2024, 11, 1. [Google Scholar] [CrossRef]
- Świerczyński, D.; Libs, S.; Courson, C.; Kiennemann, A. Steam reforming of tar from a biomass gasification process over Ni/olivine catalyst using toluene as a model compound. Appl. Catal. B Environ. 2007, 74, 211. [Google Scholar] [CrossRef]
- Luo, Q.X.; Guo, L.P.; Yao, S.Y.; Bao, J.; Liu, Z.T.; Liu, Z.W. Cobalt nanoparticles confined in carbon matrix for probing the size dependence in Fischer-Tropsch synthesis. J. Catal. 2019, 369, 143. [Google Scholar] [CrossRef]
- Low, C.W.; Veksha, A.; Aryal, R.; Chan, W.P.; Lisak, G. Catalytic reforming of biomass pyrolysis gas over Ni catalysts: Alumina, spent fluid catalytic cracking catalyst and char as supports. Appl. Catal. A Gen. 2024, 691, 120074. [Google Scholar] [CrossRef]
- Li, Y.; Di, H.; Zhang, T.; Hou, J.; Yang, J.; Li, K.; Han, J.; Zhao, R. Catalytic cracking of tar model compounds over char-based catalysts. J. Energy Inst. 2025, 119, 102017. [Google Scholar] [CrossRef]
- Mohamed, D.K.B.; Veksha, A.; Ha, Q.L.M.; Chan, W.P.; Lim, T.T.; Lisak, G. Advanced Ni tar reforming catalysts resistant to syngas impurities: Current knowledge, research gaps and future prospects. Fuel 2022, 318, 123602. [Google Scholar] [CrossRef]
- Jiang, L.; Hu, S.; Wang, Y.; Su, S.; Sun, L.; Xu, B.; He, L.; Xiang, J. Catalytic effects of inherent alkali and alkaline earth metallic species on steam gasification of biomass. Int. J. Hydrogen Energy 2015, 40, 15460. [Google Scholar] [CrossRef]
- Wang, W.; Lemaire, R.; Bensakhria, A.; Luart, D. Review on the catalytic effects of alkali and alkaline earth metals (AAEMs) including sodium, potassium, calcium and magnesium on the pyrolysis of lignocellulosic biomass and on the co-pyrolysis of coal with biomass. J. Anal. Appl. Pyrolysis 2022, 163, 105479. [Google Scholar] [CrossRef]
- de Oliveira, D.C.; Lora, E.E.; Venturini, O.J.; Maya, D.M.; Garcia-Pérez, M. Gas cleaning systems for integrating biomass gasification with Fischer-Tropsch synthesis-A review of impurity removal processes and their sequences. Renew. Sustain. Energy Rev. 2022, 172, 113047. [Google Scholar] [CrossRef]
- Jeske, K.; Kizilkaya, A.C.; López-Luque, I.; Pfänder, N.; Bartsch, M.; Concepción, P.; Priet, G. Design of Cobalt Fischer–Tropsch Catalysts for the Combined Production of Liquid Fuels and Olefin Chemicals from Hydrogen-Rich Syngas. ACS Catal. 2021, 11, 4784–4798. [Google Scholar] [CrossRef]
- Nirmal Kumar, S.; Appari, S.; Kuncharam, B.V.R. Techniques for overcoming sulfur poisoning of catalyst employed in hydrocarbon reforming. Catal. Surv. Asia 2021, 25, 362. [Google Scholar] [CrossRef]
- Sadeqzadeh, M.; Chambrey, S.; Piché, S.; Fongarland, P.; Luck, F.; Curulla-Ferré, D.; Schweich, D.; Bousquet, J.; Khodakov, A.Y. Deactivation of a Co/Al2O3 Fischer–Tropsch catalyst by water-induced sintering in slurry reactor: Modeling and experimental investigations. Catal. Today 2013, 215, 52. [Google Scholar] [CrossRef]
- Jiang, F.; Wang, S.; Zheng, J.; Liu, B.; Xu, Y.; Liu, X. Fischer-Tropsch synthesis to lower α-olefins over cobalt-based catalysts: Dependence of the promotional effect of promoter on supports. Catal. Today 2021, 369, 158. [Google Scholar] [CrossRef]
- Chen, J.Y.; Xiao, Y.; Guo, F.S.; Li, K.M.; Huang, Y.B.; Lu, Q. Single-atom metal catalysts for catalytic chemical conversion of biomass to chemicals and fuels. ACS Catal. 2024, 14, 5198. [Google Scholar] [CrossRef]
- Mahinpey, N.; Abdalla, A.; Farooqui, A. Biomass gasification for hydrogen production: A pathway to cleaner energy transition. In Biomass to Bioenergy; Woodhead Publishing: Cambridge, UK, 2024; p. 205. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal liquefaction of various biomass and waste feedstocks for biocrude production: A state of the art review. Renew. Sustain. Energy Rev. 2017, 68, 113. [Google Scholar] [CrossRef]
- Cheikhwafa, J.; Torrens, E.; Bengoa, C. Influence of catalysts on the hydrothermal liquefaction of municipal primary sludge: Upgrading to a higher quality biocrude. Fuel 2024, 369, 131715. [Google Scholar] [CrossRef]
- Shah, A.A.; Sharma, K.; Haider, M.S.; Toor, S.S.; Rosendahl, L.A.; Pedersen, T.H.; Castello, D. The role of catalysts in biomass hydrothermal liquefaction and biocrude upgrading. Processes 2022, 10, 207. [Google Scholar] [CrossRef]
- Zhao, B.; Hu, Y.; Qi, L.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Promotion effects of metallic iron on hydrothermal liquefaction of cornstalk in ethanol-water mixed solvents for the production of biocrude oil. Fuel 2021, 285, 119150. [Google Scholar] [CrossRef]
- de Caprariis, B.; Bavasso, I.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; Scarsella, M. Enhanced bio-crude yield and quality by reductive hydrothermal liquefaction of oak wood biomass: Effect of iron addition. J. Anal. Appl. Pyrolysis 2019, 139, 123. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Wang, H.; Hu, Y.; Gao, J.; Zhao, G.; Ray, M.B.; Xu, C.C. Synergistic effects of metallic Fe and other homogeneous/heterogeneous catalysts in hydrothermal liquefaction of woody biomass. Renew. Energy 2021, 176, 543. [Google Scholar] [CrossRef]
- Satiada, G.B.D.; Carpio, R.B.; Guerrero, G.A.M.; Detras, M.C.M.; Bambase, M.E. Influence of alkali catalysts on product yield and Si-containing products from hydrothermal liquefaction of corn stover. Heliyon 2024, 10, e37520. [Google Scholar] [CrossRef]
- LeClerc, H.O.; Tompsett, G.A.; Paulsen, A.D.; McKenna, A.M.; Niles, S.F.; Reddy, C.M.; Nelson, R.K.; Cheng, F.; Teixeira, A.R.; Timko, M.T. Hydroxyapatite catalyzed hydrothermal liquefaction transforms food waste from an environmental liability to renewable fuel. iScience 2022, 25, 104916. [Google Scholar] [CrossRef]
- Manan, W.N.; Wan Isahak, W.N.R.; Yaakob, Z. CeO2-based heterogeneous catalysts in dry reforming methane and steam reforming methane: A short review. Catalysts 2022, 12, 452. [Google Scholar] [CrossRef]
- Rouf, H.; Ramli, A.; Anuar, N.A.S.I.K.; Yunus, N.M. Ce–Zr-based mixed oxide catalyst for oxidative depolymerization of kenaf stalk (biomass) into vanillin. Bioresour. Bioprocess. 2023, 10, 76. [Google Scholar] [CrossRef]
- Mazhkoo, S.; Norouzi, O.; Pourali, O.; Sarvestani, M.E.; Hayder, A.; Maria, F.D.; Dutta, A. Catalytic Hydrothermal Liquefaction of Grape Pomace Using Ni–ZrO2–MSS and Ni–HZSM5 in a Water–Crude Glycerol Cosolvent. ACS Omega 2025, 10, 11836. [Google Scholar] [CrossRef]
- Ghavami, N.; Özdenkçi, K.; Salierno, G.; Björklund-Sänkiaho, M.; De Blasio, C. Analysis of operational issues in hydrothermal liquefaction and supercritical water gasification processes: A review. Biomass Convers. Biorefin. 2023, 13, 12367. [Google Scholar] [CrossRef]
- Anastasakis, K.; Biller, P.; Madsen, R.B.; Glasius, M.; Johannsen, I. Continuous hydrothermal liquefaction of biomass in a novel pilot plant with heat recovery and hydraulic oscillation. Energies 2018, 11, 2695. [Google Scholar] [CrossRef]
- Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Continuous hydrothermal liquefaction of biomass: A critical review. Energies 2018, 11, 3165. [Google Scholar] [CrossRef]
- Arora, P.; Abdolahi, H.; Cheah, Y.W.; Salam, M.A.; Grennfelt, E.L.; Rådberg, H.; Creaser, D.; Olsson, L. The role of catalyst poisons during hydrodeoxygenation of renewable oils. Catal. Today 2021, 367, 28. [Google Scholar] [CrossRef]
- Han, Q.; Rehman, M.U.; Wang, J.; Rykov, A.; Gutiérrez, O.Y.; Zhao, Y.; Wang, S.; Ma, X.; Lercher, J.A. The synergistic effect between Ni sites and Ni-Fe alloy sites on hydrodeoxygenation of lignin-derived phenols. Appl. Catal. B Environ. 2019, 253, 348. [Google Scholar] [CrossRef]
- Dou, X.; Li, W.; Zhu, C.; Jiang, X. Catalytic waste Kraft lignin hydrodeoxygenation to liquid fuels over a hollow Ni-Fe catalyst. Appl. Catal. B Environ. 2021, 287, 119975. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, J.W.; Kim, H.U.; Park, Y.K.; Lam, S.S.; Ha, J.M.; Jae, J. Bimetallic Ni-Re catalysts for the efficient hydrodeoxygenation of biomass-derived phenols. Int. J. Energy Res. 2021, 45, 16349. [Google Scholar] [CrossRef]
- Arora, P.; Ojagh, H.; Woo, J.; Grennfelt, E.L.; Olsson, L.; Creaser, D. Investigating the effect of Fe as a poison for catalytic HDO over sulfided NiMo alumina catalysts. Appl. Catal. B Environ. 2018, 227, 240. [Google Scholar] [CrossRef]
- Ali, A.; Zhao, C. Ru nanoparticles supported on hydrophilic mesoporous carbon catalyzed low-temperature hydrodeoxygenation of microalgae oil to alkanes at aqueous-phase. Chin. J. Catal. 2020, 41, 1174. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, Q.; Wu, H.; Yan, J.; Mei, X.; An, P.; Zheng, L.; Zhang, J.; He, M.; Han, B. Selective hydrodeoxygenation of aromatics to cyclohexanols over Ru single atoms supported on CeO2. J. Am. Chem. Soc. 2022, 144, 20834. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Y.; Wang, H.; Liu, K.; Jiang, N.; Li, J.; Song, H.; Chen, Y. Enhanced hydrodeoxygenation performance through novel Al-doped radial channel silica-supported carbon-encapsulated nickel catalysts. Chem. Eng. J. 2024, 495, 153118. [Google Scholar] [CrossRef]
- Jin, W.; Pastor-Pérez, L.; Villora-Picó, J.J.; Sepúlveda-Escribano, A.; Gu, S.; Reina, T.R. Investigating new routes for biomass upgrading: “H2-free” hydrodeoxygenation using Ni-based catalysts. ACS Sustain. Chem. Eng. 2019, 7, 16041. [Google Scholar] [CrossRef]
- Haider, M.S.; Isik, M.A.; Castello, D.; Pedersen, T.H.; Rosendahl, L.A. Demineralization of miscanthus biocrude obtained from catalytic hydrothermal liquefaction: Conditioning through acid washing. Processes 2021, 9, 1035. [Google Scholar] [CrossRef]
- Soares, A.V.H.; Perez, G.; Passos, F.B. Alumina supported bimetallic Pt–Fe catalysts applied to glycerol hydrogenolysis and aqueous phase reforming. Appl. Catal. B Environ. 2016, 185, 77. [Google Scholar] [CrossRef]
- Liu, X.; Xing, S.; Yang, L.; Fu, J.; Lv, P.; Zhang, X.; Li, M.; Wang, Z. Highly active and durable Ca-based solid base catalyst for biodiesel production. Fuel 2021, 302, 121094. [Google Scholar] [CrossRef]
- Mustafa, M.G.; Singh, B.; Singh, G.P.; Dey, R.K. Sustainable biodiesel production from waste cooking oil using highly effective CaO/hectorite catalyst: Process optimization, kinetic and thermodynamic studies. Sustain. Chem. One World 2025, 5, 100034. [Google Scholar] [CrossRef]
- Supeno, M.; Sihotang, J.P.; Panjaitan, Y.V.; Damanik, D.S.; Tarigan, J.B.; Sitepu, E.K. Room temperature esterification of high-free fatty acid feedstock into biodiesel. RSC Adv. 2023, 13, 33107. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, S.; Wei, W.; Yu, S.; Wang, Z.; Zheng, J. Improved catalytic stability of immobilized Candida antarctica lipase B on macroporous resin with organic polymer coating for biodiesel production. Bioprocess Biosyst. Eng. 2025, 48, 147. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, L.; Li, Y.; Hu, Y.; Li, H.; Xu, C.C.; Yang, S. Functionalized organic–inorganic hybrid porous coordination polymer-based catalysts for biodiesel production via trans/esterification. Green Chem. 2022, 24, 7763–7786. [Google Scholar] [CrossRef]
- Pandey, D.K.; Biswas, P. Continuous production of propylene glycol (1, 2-propanediol) by the hydrogenolysis of glycerol over a bi-functional Cu–Ru/MgO catalyst. React. Chem. Eng. 2020, 5, 2221. [Google Scholar] [CrossRef]
- Trivedi, J.B.; Rana, P.H. Selective double dehydration of sorbitol to isosorbide using sulfated SnO2 and sulfated SnO2/Hβ as heterogeneous catalyst. Res. Chem. Intermed. 2025, 51, 1935–1949. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Ma, R.; Song, G. Catalytic hydrogenolysis of lignin into serviceable products. Acc. Chem. Res. 2025, 58, 529. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, K.; Li, H.; Xiao, L.P.; Song, G. Selective hydrogenolysis of catechyl lignin into propenylcatechol over an atomically dispersed ruthenium catalyst. Nat. Commun. 2021, 12, 416. [Google Scholar] [CrossRef]
- Wang, Z.; Deuss, P.J. Catalytic hydrogenolysis of lignin: The influence of minor units and saccharides. ChemSusChem 2021, 14, 5186. [Google Scholar] [CrossRef]
- Ventura, M.; Lobefaro, F.; de Giglio, E.; Distaso, M.; Nocito, F.; Dibenedetto, A. Selective Aerobic Oxidation of 5-Hydroxymethylfurfural to 2,5-Diformylfuran or 2-Formyl-5-furancarboxylic Acid in Water by using MgO⋅CeO2 Mixed Oxides as Catalysts. ChemSusChem 2018, 11, 1305. [Google Scholar] [CrossRef]
- Ventura, M.; Dibenedetto, A.; Aresta, M. Heterogeneous catalysts for the selective aerobic oxidation of 5-hydroxymethylfurfural to added value products in water. Inorganica Chim. Acta 2018, 470, 11. [Google Scholar] [CrossRef]
- Ventura, M.; Williamson, D.; Lobefaro, F.; Jones, M.D.; Mattia, D.; Nocito, F.; Aresta, M.; Dibenedetto, A. Sustainable Synthesis of Oxalic and Succinic Acid through Aerobic Oxidation of C6 Polyols Under Mild Conditions. ChemSusChem 2018, 11, 1073. [Google Scholar] [CrossRef]
- Nocito, F.; Ventura, M.; Aresta, M.; Dibenedetto, A. Selective Oxidation of 5-(Hydroxymethyl)furfural to DFF Using Water as Solvent and Oxygen as Oxidant with Earth-Crust-Abundant Mixed Oxides. ACS Omega 2018, 3, 18724. [Google Scholar] [CrossRef] [PubMed]
- Ventura, M.; Aresta, M.; Dibenedetto, A. Selective aerobic oxidation of 5-(hydroxymethyl)furfural to 5-formyl-2-furancarboxylic acid in water. ChemSusChem 2016, 9, 1096. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Wang, X.; Wang, H.; Yao, J.; Kong, F.; Ren, J.; Wang, S. Efficient conversion of xylose and corncob to furfural using a novel carbon-based solid acid derived from black liquor lignin-tin complexes. J. Environ. Chem. Eng. 2024, 12, 114516. [Google Scholar] [CrossRef]
- Yang, X.; Guo, H.; Cao, X.; Ma, Y.; Wang, W.; Guo, N. Solid acid catalyst derived from cotton for conversion of xylose and corn cob to furfural. ChemistrySelect 2022, 7, e202203762. [Google Scholar] [CrossRef]
- Fan, B.; Kong, L.; He, Y. Highly efficient production of furfural from corncob by Barley hull biochar-based solid acid in cyclopentyl methyl ether–water system. Catalysts 2024, 14, 583. [Google Scholar] [CrossRef]
- Dibenedetto, A.; Aresta, M.; di Bitonto, L.; Pastore, C. Organic carbonates as efficient extracting solvents in the synthesis of 5-HMF from fructose in aqueous media using cerium phosphates as catalysts. ChemSusChem 2016, 9, 118. [Google Scholar] [CrossRef]
- Chen, C.; Lv, M.; Hu, H.; Huai, L.; Zhu, B.; Fan, S.; Wang, Q.; Zhang, J. 5-Hydroxymethylfurfural and its downstream chemicals: A review of catalytic routes. Adv. Mater. 2024, 36, 2311464. [Google Scholar] [CrossRef]
- Wang, K.; Rezayan, A.; Si, L.; Zhang, Y.; Nie, R.; Lu, T.; Wang, J.; Xu, C. Highly efficient 5-hydroxymethylfurfural production from glucose over bifunctional SnO x/C catalyst. ACS Sustain. Chem. Eng. 2021, 9, 11351. [Google Scholar] [CrossRef]
- Du, Q.; Guo, X.; Zhu, H.; Cheng, Y.; Wang, L.; Li, X. One-pot conversion of cellulose to HMF under mild conditions through decrystallization and dehydration in dimethyl sulfoxide/tetraethylammonium chloride. Chem. Eng. J. 2023, 475, 146217. [Google Scholar] [CrossRef]
- Souzanchi, S.; Nazari, L.; Rao, K.T.V.; Yuan, Z.; Tan, Z.; Xu, C.C. Development of a continuous-flow tubular reactor for synthesis of 5-hydroxymethylfurfural from fructose using heterogeneous solid acid catalysts in biphasic reaction medium. New J. Chem. 2021, 45, 8479. [Google Scholar] [CrossRef]
- Ma, C.; Cai, B.; Zhang, L.; Feng, J.; Pan, H. Acid-Catalyzed conversion of cellulose into levulinic acid with biphasic solvent system. Front. Plant Sci. 2021, 12, 630807. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Guan, Q.; Jin, F. Catalytic conversion of cellulosic biomass to harvest high-valued organic acids. iScience 2023, 26, 107933. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.gfbiochemicals.com/technology (accessed on 1 August 2025).
- Su, X.; Zhou, L.; Zhang, L.; Li, J.; Xiao, T.; Gong, Q.; Cheng, H.; Zhao, F. The transfer hydrogenation of levulinic acid to γ-valerolactone over CuNiAl catalyst. Chem. Synth. 2025, 5, 16. [Google Scholar] [CrossRef]
- Sajid, M.; Farooq, U.; Bary, G.; Azim, M.M.; Zhao, X. Sustainable production of levulinic acid and its derivatives for fuel additives and chemicals: Progress, challenges, and prospects. Green Chem. 2021, 23, 9198–9238. [Google Scholar] [CrossRef]
- Liu, C.; Lu, X.; Yu, Z.; Xiong, J.; Bai, H.; Zhang, R. Production of Levulinic Acid from Cellulose and Cellulosic Biomass in Different Catalytic Systems. Catalysts 2020, 10, 1006. [Google Scholar] [CrossRef]
- Velasco, J.O.; Van Der Linden, I.; Deuss, P.J.; Heeres, H.J. Efficient depolymerization of lignins to alkylphenols using phosphided NiMo catalysts. Catal. Sci. Technol. 2021, 11, 5158. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, L.; Hou, Q.; Mo, Z.; Liu, X.; Li, W. A review on catalytic depolymerization of lignin towards high-value chemicals: Solvent and catalyst. Fermentation 2023, 9, 386. [Google Scholar] [CrossRef]
- Tang, C.; Cao, Y.; Gao, J.; Luo, G.; Fan, J.; Clark, J.H.; Zhang, S. Oxidative Catalytic Depolymerization of Lignin into Value-Added Monophenols by Carbon Nanotube-Supported Cu-Based Catalysts. Molecules 2024, 29, 4762. [Google Scholar] [CrossRef]
- Van den Bosch, S.; Schutyser, W.; Vanholme, R.; Driessen, T.; Koelewijn, S.F.; Renders, T.; De Meester, B.; Huijgen, W.J.J.; Dehaen, W.; Courtin, C.M.; et al. Reductive lignocellulose fractionation into soluble lignin-derived phenolic monomers and dimers and processable carbohydrate pulps. Energy Environ. Sci. 2015, 8, 1748. [Google Scholar] [CrossRef]
- Fan, M.H.; Deng, S.M.; Wang, T.J.; Li, Q.X. Production of BTX through catalytic depolymerization of lignin. Chin. J. Chem. Phys. 2014, 27, 221. [Google Scholar] [CrossRef]
- Huang, S.; Xue, Y.; Yu, B.; Wang, L.; Zhou, C.; Ma, Y. A review of the recent developments in the bioproduction of polylactic acid and its precursors optically pure lactic acids. Molecules 2021, 26, 6446. [Google Scholar] [CrossRef]
- Guo, M.; Zhou, C.; Cui, Y.; Jiang, W.; Han, G.; Jiang, Z.; Ben, H.; Yang, X. Sustainable Production of Lactic Acid from Cellulose Using Au/W-ZnO Catalysts. Polymers 2023, 15, 4235. [Google Scholar] [CrossRef]
- Widjaja, T.; Hendrianie, N.; Nurkhamidah, S.; Altway, A.; Yusuf, B.; Rohma, A.A.Z.; Pahlevi, A. Poly lactic acid production using the ring opening polymerization (ROP) method using Lewis acid surfactant combined iron (Fe) catalyst (Fe(DS)3). Heliyon 2023, 9, e17985. [Google Scholar] [CrossRef]
- Santulli, F.; Gravina, G.; Lamberti, M.; Tedesco, C.; Mazzeo, M. Zinc and magnesium catalysts for the synthesis for PLA and its degradation: Clues for catalyst design. Mol. Catal. 2022, 528, 112480. [Google Scholar] [CrossRef]
- Van Thuoc, D.; My, D.N.; Loan, T.T.; Sudesh, K. Utilization of waste fish oil and glycerol as carbon sources for polyhydroxyalkanoate production by Salinivibrio sp. M318. Int. J. Biol. Macromol. 2019, 141, 885. [Google Scholar] [CrossRef]
- Dubey, S.; Mishra, S. Natural sea salt based polyhydroxyalkanoate production by wild Halomonas hydrothermalis strain. Fuel 2022, 311, 122593. [Google Scholar] [CrossRef]
- Gutschmann, B.; Maldonado Simões, M.; Schiewe, T.; Schröter, E.S.; Münzberg, M.; Neubauer, P.; Bockisch, A.; Riedel, S.L. Continuous feeding strategy for polyhydroxyalkanoate production from solid waste animal fat at laboratory- and pilot-scale. Microb. Biotechnol. 2023, 16, 295. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Sabita, A.; Putri, N.A.; Azliza, N.; Illiyanasafa, N.; Darmokoesoemo, H.; Amenaghawon, A.N.; Kurniawan, T.A. Waste to wealth: Polyhydroxyalkanoates (PHA) production from food waste for a sustainable packaging paradigm. Food Chem. Mol. Sci. 2024, 9, 100225. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647. [Google Scholar] [CrossRef]
- Zhu, X.; Lin, L.; Pang, M.; Jia, C.; Xia, L.; Shi, G.; Zhang, S.; Lu, Y.; Sun, L.; Yu, F.; et al. Continuous and low-carbon production of biomass flash graphene. Nat. Commun. 2024, 15, 1. [Google Scholar] [CrossRef]
- Gan, Y.X. Activated Carbon from Biomass Sustainable Sources. J. Carbon Res. 2021, 7, 39. [Google Scholar] [CrossRef]
- Malini, K.; Selvakumar, D.; Kumar, N.S. Activated carbon from biomass: Preparation, factors improving basicity and surface properties for enhanced CO2 capture capacity—A review. J. CO2 Util. 2023, 67, 102318. [Google Scholar] [CrossRef]
- Doczekalska, B.; Bartkowiak, M.; Łopatka, H.; Zborowska, M. Activated Carbon Prepared from Corn Biomass by Chemical Activation with Potassium Hydroxide. BioResources 2022, 17, 1794. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Bahrudin, N.N.; Hum, N.N.M.F.; Surip, S.N.; Syed-Hassan, S.S.A.; Yousif, E.; Sabar, S. Microporous activated carbon developed from KOH activated biomass waste: Surface mechanistic study of methylene blue dye adsorption. Water Sci. Technol. 2021, 84, 1858. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Chen, Y.; Chen, H.; Zhao, Y.; Tian, C.; Chen, W.; Chang, C.; Pang, S.; Li, P. Characterization and mechanistic insights into coke formation on biochar-based catalysts under microwave-assisted biomass pyrolysis. Ind. Crops Prod. 2025, 226, 120645. [Google Scholar] [CrossRef]
- Gao, B.; Cao, G.; Feng, Y.; Jiao, Y.; Li, C.; Zhao, J.; Fang, Y. CO2 Reforming of Biomass Gasification Tar over Ni-Fe-Based Catalysts in a DBD Plasma Reactor. Molecules 2025, 30, 1032. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Lu, Y.; Unocic, K.A.; Habas, S.E.; Griffin, M.B.; Schaidle, J.A.; Meyer, H.M.; Wang, Y.; Wang, H. Deactivation by Potassium Accumulation on a Pt/TiO2 Bifunctional Catalyst for Biomass Catalytic Fast Pyrolysis. ACS Catal. 2022, 12, 465. [Google Scholar] [CrossRef]
- Kass, M.D.; Kaul, B.; Armstrong, B.; Lamm, B.W.; Lobodin, V.; Ramasamy, K.K.; Olarte, M.V.; Wang, H.; Subramaniam, S.; Santosa, D.; et al. Rheology and Engine Performance of Very Low Sulfur Fuel Oil Blended with 10% Fast Pyrolysis and Hydrothermal Liquefaction Oils in a 2-Stroke Crosshead Engine. SSEN 2025, 5399034. [Google Scholar] [CrossRef]
- Lin, F.; Xu, M.; Ramasamy, K.K.; Li, Z.; Klinger, J.L.; Schaidle, J.A.; Wang, H. Catalyst Deactivation and Its Mitigation during Catalytic Conversions of Biomass. ACS Catal. 2022, 12, 13555. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Musademba, D. Techno-economic analysis and environmental impact assessment of biodiesel production from bio-oil derived from microwave-assisted pyrolysis of pine sawdust. Heliyon 2023, 9, e22261. [Google Scholar] [CrossRef]
- Jez, S.; Spinelli, D.; Fierro, A.; Dibenedetto, A.; Aresta, M.; Busi, E.; Basosi, R. Comparative life cycle assessment study on environmental impact of oil production from micro-algae and terrestrial oilseed crops. Bioresour. Technol. 2017, 239, 266. [Google Scholar] [CrossRef] [PubMed]
- Foust, T.; Beckham, G.; Tao, L.; Hannon, J.R.; Wyman, C.E.; Lynd, L.R.; Laser, M.S.; Andrade, O.; Benavides, P.T.; Wang, M.; et al. Technoeconomic and Life-Cycle Analysis of Single-Step Catalytic Conversion of Wet Ethanol into Fungible Fuel Blendstocks. Proc. Natl. Acad. Sci. USA 2019, 117, 12576–12583. [Google Scholar] [CrossRef]
- Vallejo-Blancas, D.; Sánchez-Ramirez, E.; Ponce-Ortega, J.M.; Segovia-Hernández, J.G.; Quiroz Ramírez, J.J.; Contreras-Zarazúa, G. A process intensification 4.0 approach to determine the feasibility and sustainability of producing biojet-fuel by alcohol to jet route. A case of study of Mexico. Chem. Eng. Process. Process Intensif. 2025, 208, 110078. [Google Scholar] [CrossRef]





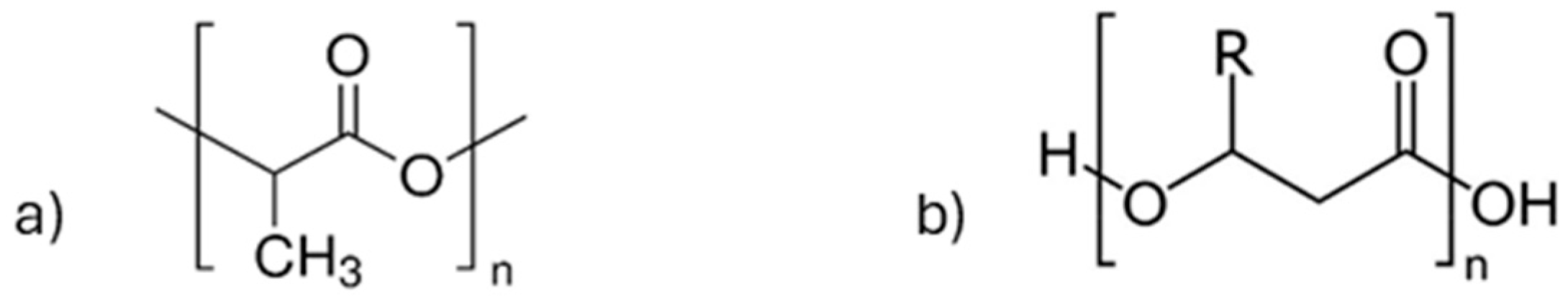
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nocito, F.; Daraselia, D.; Dibenedetto, A. Catalytic Biomass Conversion into Fuels and Materials: Sustainable Technologies and Applications. Catalysts 2025, 15, 948. https://doi.org/10.3390/catal15100948
Nocito F, Daraselia D, Dibenedetto A. Catalytic Biomass Conversion into Fuels and Materials: Sustainable Technologies and Applications. Catalysts. 2025; 15(10):948. https://doi.org/10.3390/catal15100948
Chicago/Turabian StyleNocito, Francesco, Diana Daraselia, and Angela Dibenedetto. 2025. "Catalytic Biomass Conversion into Fuels and Materials: Sustainable Technologies and Applications" Catalysts 15, no. 10: 948. https://doi.org/10.3390/catal15100948
APA StyleNocito, F., Daraselia, D., & Dibenedetto, A. (2025). Catalytic Biomass Conversion into Fuels and Materials: Sustainable Technologies and Applications. Catalysts, 15(10), 948. https://doi.org/10.3390/catal15100948








