Nitrile-Converting Enzymes: Industrial Perspective, Challenges and Emerging Strategies
Abstract
1. Introduction
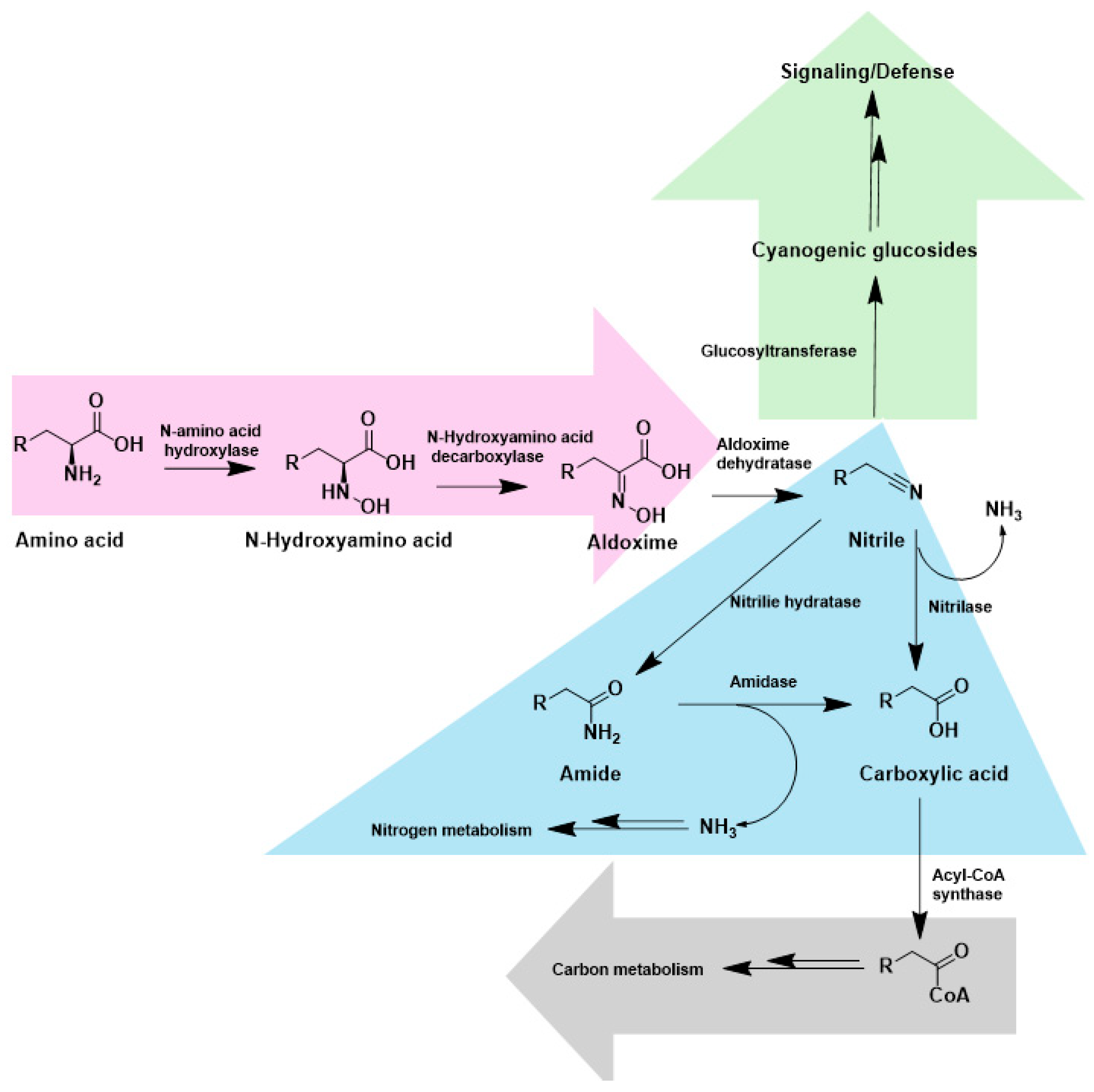
2. Nitrilases and NHases
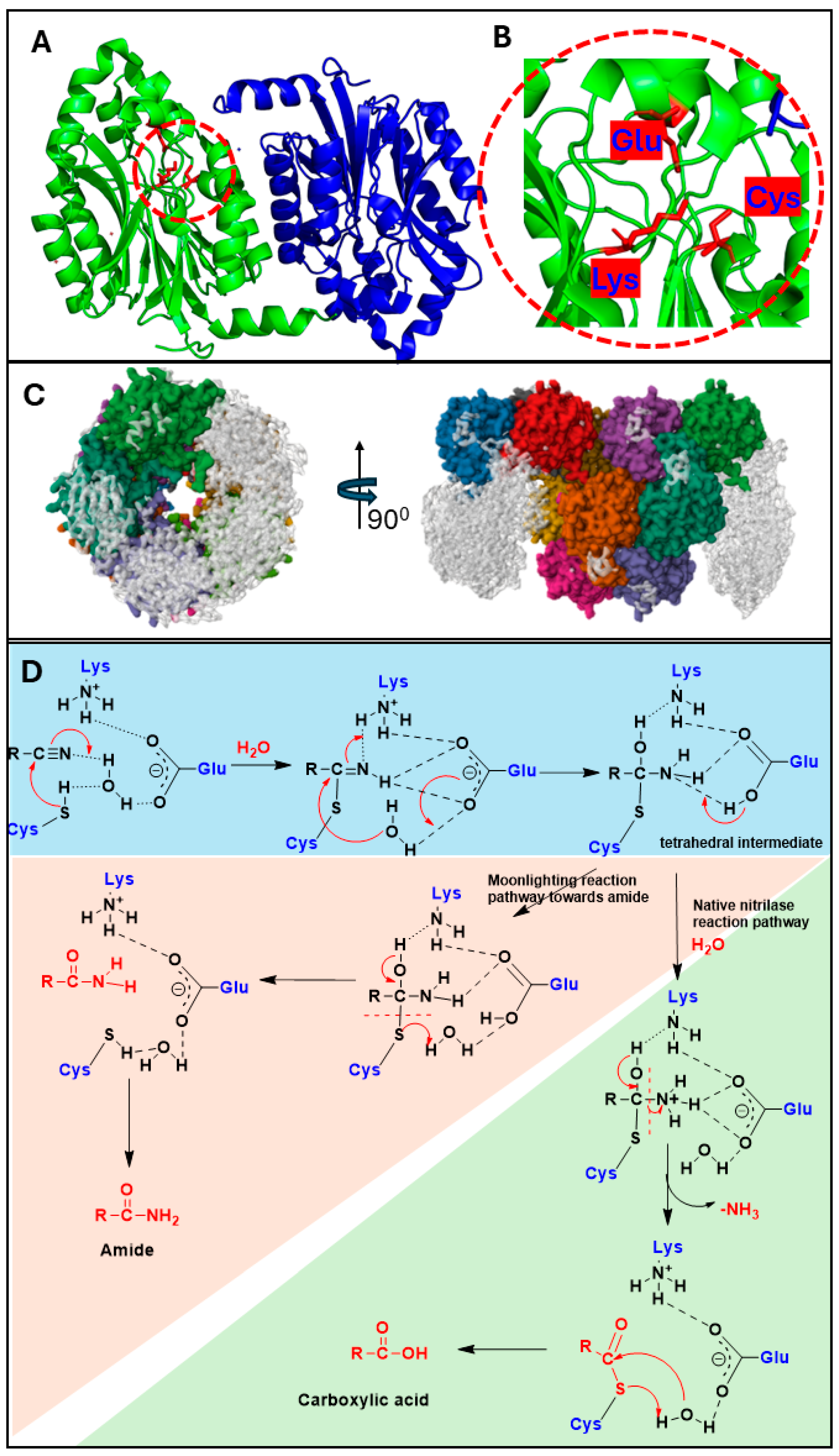
3. Nitrilases and Controlled Nitrile Hydrolysis via the Catalytic Triad
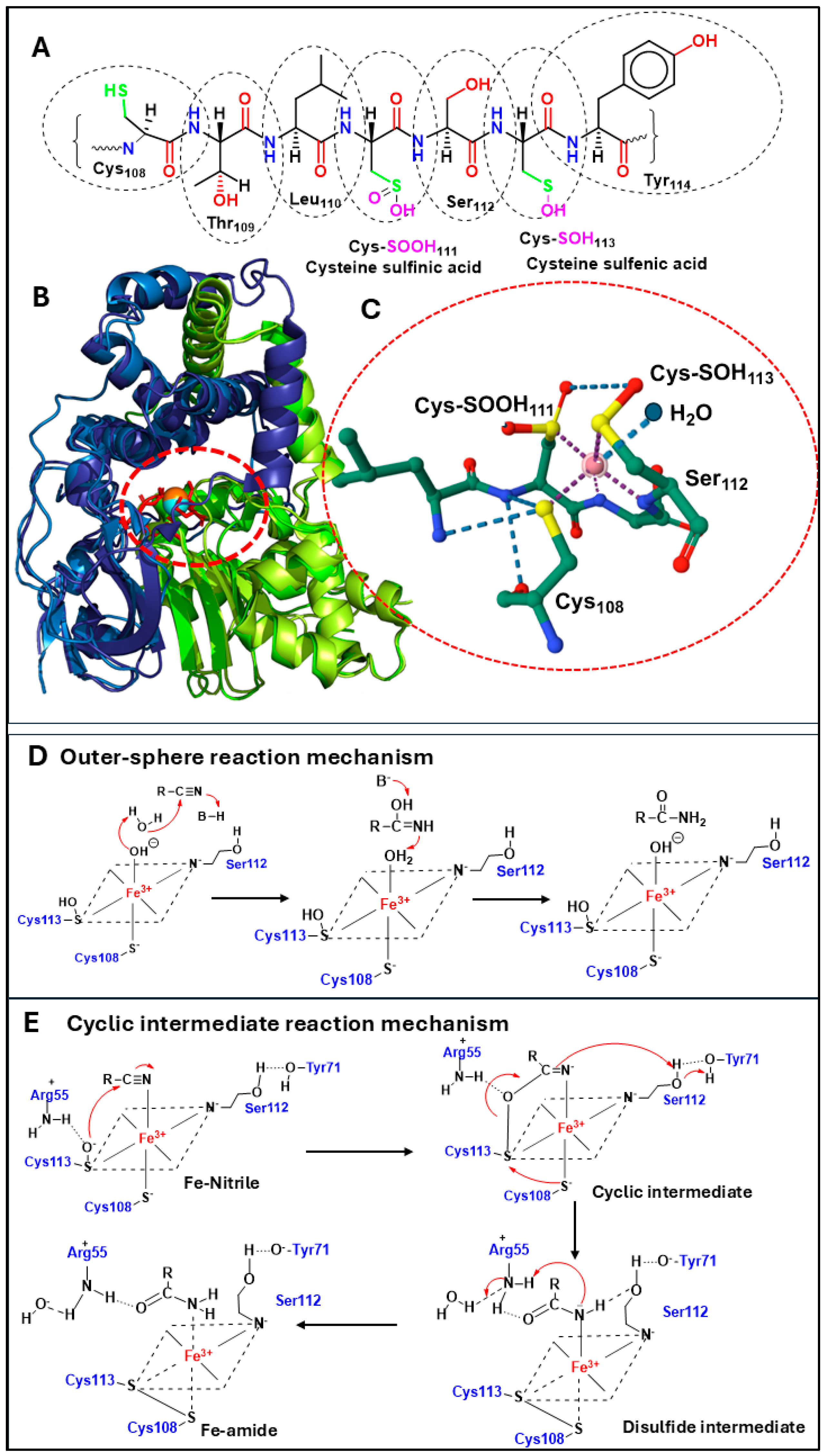
4. NHases and Metal-Catalysed Nitrile Hydrolysis
5. Metagenomics and Identification of Nitrilases and NHases
6. Screening and Assay Development for Nitrilases and NHases
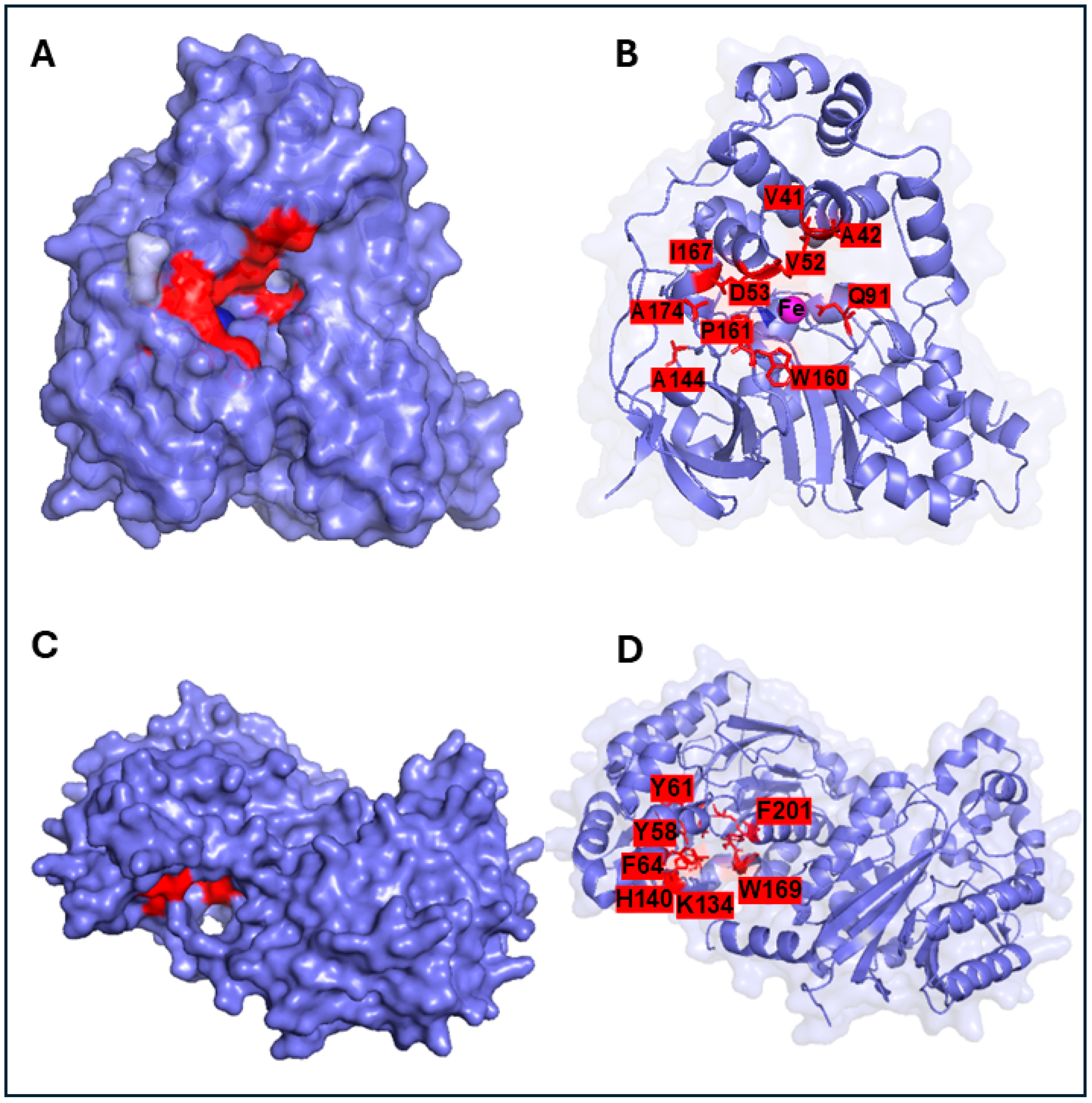
7. Industrial Substrate Scope and Current Limitations
8. Improving the Efficiency of Nitrile-Converting Enzyme for Industrial Applications and Future Perspectives
9. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scotti, C.; Barlow, J.W. Natural Products Containing the Nitrile Functional Group and Their Biological Activities. Nat. Prod. Commun. 2022, 17, 1934578X221099973. [Google Scholar] [CrossRef]
- Rimmer, P.B.; Shorttle, O. A Surface Hydrothermal Source of Nitriles and Isonitriles. Life 2024, 14, 498. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Li, X.; Yu, Z.; Song, C.; Du, Y. Nitrile-Containing Pharmaceuticals: Target, Mechanism of Action, and Their SAR Studies. RSC Med. Chem. 2021, 12, 1650–1671. [Google Scholar] [CrossRef] [PubMed]
- Sruthi, P.R.; Anas, S. An Overview of Synthetic Modification of Nitrile Group in Polymers and Applications. J. Polym. Sci. 2020, 58, 1039–1061. [Google Scholar] [CrossRef]
- Fleming, F.F.; Yao, L.; Ravikumar, P.C.; Funk, L.; Shook, B.C. Nitrile-Containing Pharmaceuticals: Efficacious Roles of the Nitrile Pharmacophore. J. Med. Chem. 2010, 53, 7902–7917. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y. Metal–mediated C–CN Bond Activation in Organic Synthesis. Chem. Rev. 2020, 121, 327–344. [Google Scholar] [CrossRef]
- Peter, J.K.; Singh, R.; Yadav, A.K.; Kothari, R.; Mehta, P.K. Toxicity of Nitriles/Amides-Based Products in the Environment and Their Enzymatic Bioremediation. J. Hazard. Mater. Adv. 2023, 13, 100389. [Google Scholar] [CrossRef]
- Egelkamp, R.; Zimmermann, T.; Schneider, D.; Hertel, R.; Daniel, R. Impact of Nitriles on Bacterial Communities. Front. Environ. Sci. 2019, 7, 103. [Google Scholar] [CrossRef]
- Jeschke, P. Current Status of Chirality in Agrochemicals. Pest. Manag. Sci. 2018, 74, 2389–2404. [Google Scholar] [CrossRef]
- Martínková, L. Nitrile-Converting Enzymes and their Synthetic Applications. In Green Biocatalysis; Wiley: Hoboken, NJ, USA, 2016; pp. 331–349. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Asano, Y. Nitrile-Synthesizing Enzymes and Biocatalytic Synthesis of Volatile Nitrile Compounds: A Review. J. Biotechnol. 2024, 384, 20–28. [Google Scholar] [CrossRef]
- Sun, S.; Zhou, J.; Jiang, J.; Dai, Y.; Sheng, M. Nitrile Hydratases: From Industrial Application to Acetamiprid and Thiacloprid Degradation. J. Agric. Food Chem. 2021, 69, 10440–10449. [Google Scholar] [CrossRef]
- Pace, H.C.; Brenner, C. The Nitrilase Superfamily: Classification, Structure and Function. Genome Biol. 2001, 2, 1–9. [Google Scholar] [CrossRef]
- Feng, C.; Chen, J.; Ye, W.; Wang, Z. Nitrile Hydratase as a Promising Biocatalyst: Recent Advances and Future Prospects. Biotechnol. Lett. 2024, 46, 1171–1185. [Google Scholar] [CrossRef] [PubMed]
- O'Reilly, C.; Turner, P.D. The Nitrilase Family of CN Hydrolysing Enzymes-a Comparative Study. J. Appl. Microbiol. 2003, 95, 1161–1174. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Xia, Y.; Zhou, Z. Recent Advances and Promises in Nitrile Hydratase: From Mechanism to Industrial Applications. Front. Bioeng. Biotechnol. 2020, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-S.; Shi, J.-S.; Lu, Z.-M.; Li, H.; Zhou, Z.-M.; Xu, Z.-H. Nitrile-Converting Enzymes as a Tool to Improve Biocatalysis in Organic Synthesis: Recent Insights and Promises. Crit. Rev. Biotechnol. 2015, 37, 69–81. [Google Scholar] [CrossRef]
- Bhatt, A.; Prajapati, D.; Gupte, A. Current Status and Future of Nitrile Catalysis Using Key Nitrilases Enzymes and Their Biotechnological Impact. Open Biotechnol. J. 2021, 15, 71–81. [Google Scholar] [CrossRef]
- Martínková, L.; Kulik, N.; Sedova, A.; Křístková, B.; Bojarová, P. Recent Progress in the Production of Cya-nide-Converting Nitrilases—Comparison with Nitrile-Hydrolyzing Enzymes. Catalysts 2023, 13, 500. [Google Scholar] [CrossRef]
- Martínková, L.; Rucká, L.; Nešvera, J.; Pátek, M. Recent Advances and Challenges in the Heterologous Production of Microbial Nitrilases for Biocatalytic Applications. World J. Microbiol. Biotechnol. 2016, 33, 1–11. [Google Scholar] [CrossRef]
- Martínková, L.; Vejvoda, V.; Kaplan, O.; Kubáč, D.; Malandra, A.; Cantarella, M.; Bezouška, K.; Křen, V. Fungal Nitrilases as Biocatalysts: Recent Developments. Biotechnol. Adv. 2009, 27, 661–670. [Google Scholar] [CrossRef]
- Woodward, J.D.; Trompetter, I.; Sewell, B.T.; Piotrowski, M. Substrate Specificity of Plant Nitrilase Complexes Is Affected by Their Helical Twist. Commun. Biol. 2018, 1, 186. [Google Scholar] [CrossRef]
- Aguirre-Sampieri, S.; Casañal, A.; Emsley, P.; Garza-Ramos, G. Cryo-EM Structure of Bacterial Nitrilase Reveals Insight into Oligomerization, Substrate Recognition, and Catalysis. J. Struct. Biol. 2024, 216, 108093. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jiang, S.; Sun, Y.; Yang, Z.; Chen, Z.; Wang, H.; Wei, D. Switching the Secondary and Natural Activity of Nitrilase from Acidovorax Facilis 72 W for the Efficient Production of 2-Picolinamide. Biotechnol. Lett. 2021, 43, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.-S.; Li, H.; Lu, Z.-M.; Zhang, X.-J.; Zhang, Q.; Yu, J.-H.; Zhou, Z.-M.; Shi, J.-S.; Xu, Z.-H. Engineering of a Fungal Nitrilase for Improving Catalytic Activity and Reducing By-Product Formation in the Absence of Structural Infor-mation. Catal. Sci. Technol. 2016, 6, 4134–4141. [Google Scholar] [CrossRef]
- Jiang, S.; Zhang, L.; Yao, Z.; Gao, B.; Wang, H.; Mao, X.; Wei, D. Switching a Nitrilase from Syechocystis Sp. PCC6803 to a Nitrile Hydratase by Rationally Regulating Reaction Pathways. Catal. Sci. Technol. 2017, 7, 1122–1128. [Google Scholar] [CrossRef]
- Black, G.W.; Brown, N.L.; Perry, J.J.B.; Randall, P.D.; Turnbull, G.; Zhang, M. A High-Throughput Screening Method for Determining the Substrate Scope of Nitrilases. Chem. Commun. 2014, 51, 2660–2662. [Google Scholar] [CrossRef]
- Kushwaha, M.; Kumar, V.; Mahajan, R.; Bhalla, T.C.; Chatterjee, S.; Akhter, Y. Molecular Insights into the Activity and Mechanism of Cyanide Hydratase Enzyme Associated with Cyanide Biodegradation by Serratia Marcescens. Arch. Microbiol. 2018, 200, 971–977. [Google Scholar] [CrossRef]
- Lipowicz, B.; Hanekop, N.; Schmitt, L.; Proksch, P. An Aeroplysinin-1 Specific Nitrile Hydratase Isolated from the Marine Sponge Aplysina Cavernicola. Mar. Drugs 2013, 11, 3046–3067. [Google Scholar] [CrossRef]
- Okamoto, S.; Eltis, L.D. Purification and Characterization of a Novel Nitrile Hydratase from Rhodococcus Sp. RHA1. Mol. Microbiol. 2007, 65, 828–838. [Google Scholar] [CrossRef]
- Noguchi, T.; Honda, J.; Nagamune, T.; Sasabe, H.; Inoue, Y.; Endo, I. Photosensitive nitrile hydratase intrinsically possesses nitric oxide bound to the non-heme iron center: Evidence by Fourier transform infrared spectroscopy. FEBS Lett. 1995, 358, 9–12. [Google Scholar] [CrossRef]
- Rose, M.J.; Betterley, N.M.; Oliver, A.G.; Mascharak, P.K. Binding of Nitric Oxide to a Synthetic Model of Iron-Containing Nitrile Hydratase (Fe-NHase) and Its Photorelease: Relevance to Photoregulation of Fe-NHase by NO. Inorg. Chem. 2010, 49, 1854–1864. [Google Scholar] [CrossRef] [PubMed]
- Nagamune, T.; Kurata, H.; Hirata, M.; Honda, J.; Hirata, A.; Endo, I. Photosensitive Phenomena of Nitrile Hydratase of Rhodococcus sp. N-771. Photochem. Photobiol. 1990, 51, 87–90. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Metalloenzyme nitrile hydratase: Structure, regulation, and application to biotechnology. Nat. Biotechnol. 1998, 16, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, Z.; Zhou, Z. An Archaeal Nitrile Hydratase from the Halophilic Archaeon A07HB70 Exhibits High Tolerance to 3-Cyanopyridine and Nicotinamide. Protein Expr. Purif. 2023, 214, 106390. [Google Scholar] [CrossRef]
- Yang, X.; Bennett, B.; Holz, R.C. Analyzing the function of the insert region found between the and-subunits in the eukaryotic nitrile hydratase from Monosiga brevicollis. Arch. Biochem. Biophys. 2018, 657, 1–7. [Google Scholar] [CrossRef]
- Hussain, A.; Ogawa, T.; Saito, M.; Sekine, T.; Nameki, M.; Matsushita, Y.; Hayashi, T.; Katayama, Y. Cloning and expression of a gene encoding a novel thermostable thiocyanate-degrading enzyme from a mesophilic alphaproteobacteria strain THI201. Microbiology 2013, 159, 2294–2302. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, Z.; Berdychowska, J.; Zhu, X.; Wang, L.; Peplowski, L.; Zhou, Z. Effect and Mechanism Analysis of Different Linkers on Efficient Catalysis of Subunit-Fused Nitrile Hydratase. Int. J. Biol. Macromol. 2021, 181, 444–451. [Google Scholar] [CrossRef]
- Wu, Y.; Chang, C.; Wang, C.; Hsieh, C.; Horng, Y. C=N Bond Activation and Hydration by an Iron (III) Complex with Asymmetric Sulfur Oxygenation. Eur. J. Inorg. Chem. 2017, 2017, 840–843. [Google Scholar] [CrossRef]
- Light, K.M.; Yamanaka, Y.; Odaka, M.; Solomon, E.I. Spectroscopic and Computational Studies of Nitrile Hydratase: Insights into Geometric and Electronic Structure and the Mechanism of Amide Synthesis. Chem. Sci. 2015, 6, 6280–6294. [Google Scholar] [CrossRef]
- Hopmann, K.H.; Himo, F. Theoretical Investigation of the Second-Shell Mechanism of Nitrile Hydratase. Eur. J. Inorg. Chem. 2008, 2008, 1406–1412. [Google Scholar] [CrossRef]
- MacDonald, C.A.; Boyd, R.J. Competing Nitrile Hydratase Catalytic Mechanisms: Is Cysteine-Sulfenic Acid Acting as a Nucleophile? Comput. Theor. Chem. 2015, 1070, 48–54. [Google Scholar] [CrossRef]
- Nelp, M.T.; Song, Y.; Wysocki, V.H.; Bandarian, V. A Protein-Derived Oxygen Is the Source of the Amide Oxygen of Nitrile Hydratases. J. Biol. Chem. 2016, 291, 7822–7829. [Google Scholar] [CrossRef] [PubMed]
- Hopmann, K.H. Full Reaction Mechanism of Nitrile Hydratase: A Cyclic Intermediate and an Unexpected Disulfide Switch. Inorg. Chem. 2014, 53, 2760–2762. [Google Scholar] [CrossRef] [PubMed]
- Kayanuma, M.; Shoji, M.; Yohda, M.; Odaka, M.; Shigeta, Y. Catalytic Mechanism of Nitrile Hydratase Subsequent to Cyclic Intermediate Formation: A QM/MM Study. J. Phys. Chem. B 2016, 120, 3259–3266. [Google Scholar] [CrossRef]
- Yamanaka, Y.; Kato, Y.; Hashimoto, K.; Iida, K.; Nagasawa, K.; Nakayama, H.; Dohmae, N.; Noguchi, K.; Noguchi, T.; Yohda, M.; et al. Time-Resolved Crystallography of the Reaction Intermediate of Nitrile Hydratase: Revealing a Role for the Cysteinesulfenic Acid Ligand as a Catalytic Nucleophile. Angew. Chem. Int. Ed. Engl. 2015, 54, 10763–10767. [Google Scholar] [CrossRef]
- Hashimoto, K.; Suzuki, H.; Taniguchi, K.; Noguchi, T.; Yohda, M.; Odaka, M. Catalytic Mechanism of Nitrile Hydratase Proposed by Time-Resolved X-Ray Crystallography Using a Novel Substrate, Tert-Butylisonitrile. J. Biol. Chem. 2008, 283, 36617–36623. [Google Scholar] [CrossRef]
- Yu, H.; Liu, J.; Shen, Z. Modeling Catalytic Mechanism of Nitrile Hydratase by Semi-Empirical Quantum Mechanical Calculation. J. Mol. Graph. Model. 2008, 27, 522–528. [Google Scholar] [CrossRef]
- Thimann, K.V.; Mahadevan, S. Nitrilase. Arch. Biochem. Biophys. 1964, 105, 133–141. [Google Scholar] [CrossRef]
- Hook, R.H.; Robinson, W.G. Ricinine Nitrilase. J. Biol. Chem. 1964, 239, 4263–4267. [Google Scholar] [CrossRef]
- Kobayashi, M.; Shimizu, S. Versatile Nitrilases: Nitrile-Hydrolysing Enzymes. FEMS Microbiol. Lett. 1994, 120, 217–223. [Google Scholar] [CrossRef]
- Asano, Y.; Tani, Y.; Yamada, H. A new enzyme "Nitrile hydratase" which degrades acetonitrile in combination with amidase. Agric. Biol. Chem. 1980, 44, 2251–2252. [Google Scholar] [CrossRef]
- Yamada, H.; Kobayashi, M. Nitrile Hydratase and Its Application to Industrial Production of Acrylamide. Biosci. Biotechnol. Biochem. 1996, 60, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Robertson, D.E.; Chaplin, J.A.; DeSantis, G.; Podar, M.; Madden, M.; Chi, E.; Richardson, T.; Milan, A.; Miller, M.; Weiner, D.P.; et al. Exploring Nitrilase Sequence Space for Enantioselective Catalysis. Appl. Environ. Microbiol. 2004, 70, 2429–2436. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.; Birkemeyer, C.; Ballschmiter, M. A Nitrilase from a Metagenomic Library Acts Regioselectively on Aliphatic Dinitriles. Appl. Microbiol. Biotechnol. 2010, 89, 91–98. [Google Scholar] [CrossRef]
- Sonbol, S.A.; Ferreira, A.J.S.; Siam, R. Red Sea Atlantis II Brine Pool Nitrilase with Unique Thermostability Profile and Heavy Metal Tolerance. BMC Biotechnol 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Dennett, G.V.; Blamey, J.M. A New Thermophilic Nitrilase from an Antarctic Hyperthermophilic Microorganism. Front. Bioeng. Biotechnol. 2016, 4, 5. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Baker, P.J.; Cheng, F.; Xue, Y.-P.; Zheng, Y.-G.; Shen, Y.-C. Screening and Improving the Recombinant Nitrilases and Application in Biotransformation of Iminodiacetonitrile to Iminodiacetic Acid. PLoS ONE 2013, 8, e67197. [Google Scholar] [CrossRef]
- Riffiani, R.; Sulistinah, N.; Sunarko, B. Gene Encoding Nitrilase from Soil Sample of Lombok Gold Mine Industry Using Metagenomics Approach. KnE Life Sci. 2017, 201, 201–207. [Google Scholar] [CrossRef]
- Dooley-Cullinane, T.; O'REilly, C.; Aslam, B.; Weiner, D.P.; O'NEill, D.; Owens, E.; O'MEara, D.; Coffey, L. The use of clade-specific PCR assays to identify novel nitrilase genes from environmental isolates. Microbiologyopen 2018, 8, e00700. [Google Scholar] [CrossRef]
- Veselá, A.B.; Rucká, L.; Kaplan, O.; Pelantová, H.; Nešvera, J.; Pátek, M.; Martínková, L. Bringing Nitrilase Sequences from Databases to Life: The Search for Novel Substrate Specificities with a Focus on Dinitriles. Appl. Microbiol. Biotechnol. 2015, 100, 2193–2202. [Google Scholar] [CrossRef]
- Zhu, D.; Mukherjee, C.; Biehl, E.R.; Hua, L. Discovery of a Mandelonitrile Hydrolase from Bradyrhizobium Japonicum USDA110 by Rational Genome Mining. J. Biotechnol. 2007, 129, 645–650. [Google Scholar] [CrossRef]
- Seffernick, J.L.; Samanta, S.K.; Louie, T.M.; Wackett, L.P.; Subramanian, M. Investigative Mining of Sequence Data for Novel Enzymes: A Case Study with Nitrilases. J. Biotechnol. 2009, 143, 17–26. [Google Scholar] [CrossRef]
- Precigou, S.; Goulas, P.; Duran, R. Rapid and Specific Identification of Nitrile Hydratase (NHase)-Encoding Genes in Soil Samples by Polymerase Chain Reaction. FEMS Microbiol. Lett. 2001, 204, 155–161. [Google Scholar] [CrossRef][Green Version]
- Marron, A.O.; Akam, M.; Walker, G. Nitrile Hydratase Genes Are Present in Multiple Eukaryotic Supergroups. PLoS ONE 2012, 7, e32867. [Google Scholar] [CrossRef]
- Zhao, Y.-X.; Yang, W.-L.; Guo, L.; Jiang, H.-Y.; Cheng, X.; Dai, Y.-J. Bioinformatics of a Novel Nitrile Hydratase Gene Cluster of the N 2-Fixing Bacterium Microvirga Flocculans CGMCC 1. 16731 and Characterization of the Enzyme. J. Agric. Food Chem. 2020, 68, 9299–9307. [Google Scholar] [CrossRef] [PubMed]
- Bragança, S.C.; Dooley-Culliname, T.; O’Reilly, C.; Coffey, L. Applying Functional Metagenomics to Search for Novel Nitrile-Hydrolyzing Enzymes Using Environmental Samples. Biomaterials Tissue Technol. 2017, 1, 10–15761. [Google Scholar]
- An, X.; Cheng, Y.; Miao, L.; Chen, X.; Zang, H.; Li, C. Characterization and Genome Functional Analysis of an Efficient Nitrile-Degrading Bacterium, Rhodococcus Rhodochrous BX2, to Lay the Foundation for Potential Bioaugmentation for Remediation of Nitrile-Contaminated Environments. J. Hazard. Mater. 2020, 389, 121906. [Google Scholar] [CrossRef] [PubMed]
- Roldán, M.D.; Olaya-Abril, A.; Sáez, L.P.; Cabello, P.; Luque-Almagro, V.M.; Moreno-Vivián, C. Bioremediation of cyanide-containing wastes: The potential of systems and synthetic biology for cleaning up the toxic leftovers from mining. EMBO Rep. 2021, 22, e53720. [Google Scholar] [CrossRef]
- Amrutha, M.; Nampoothiri, K.M. In Silico Analysis of Nitrilase-3 Protein from Corynebacterium Glutamicum for Bioremediation of Nitrile Herbicides. J. Genet. Eng. Biotechnol. 2022, 20, 51. [Google Scholar] [CrossRef]
- Achudhan, A.B.; Kannan, P.; Saleena, L.M. Functional Metagenomics Uncovers Nitrile-Hydrolysing Enzymes in a Coal Metagenome. Front. Mol. Biosci. 2023, 10, 1123902. [Google Scholar] [CrossRef]
- Kumari, P.; Poddar, R. A comparative multivariate analysis of nitrilase enzymes: An ensemble based computational approach. Comput. Biol. Chem. 2019, 83, 107095. [Google Scholar] [CrossRef] [PubMed]
- Jallageas, J.C.; Arnaud, A.; Galzy, P. Nitrilases and Amidases: Determination of Activity by Proton Magnetic Resonance Spectrometry. Anal. Biochem. 1979, 95, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Fan, A.; Wang, Y.; Zhu, X.; Wang, Z.; Wu, M.; Zheng, Y. Novel Sensitive High-Throughput Screening Strategy for Nitrilase-Producing Strains. Appl. Environ. Microbiol. 2007, 73, 6053–6057. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Angelini, L.M.L.; Da Silva, A.R.M.; Rocco, L.d.F.C.; Milagre, C.D.d.F. A High-Throughput Screening Assay for Distinguishing Nitrile Hydratases from Nitrilases. Braz. J. Microbiol. 2015, 46, 113–116. [Google Scholar] [CrossRef]
- Lin, Z.-J.; Zheng, R.-C.; Lei, L.-H.; Zheng, Y.-G.; Shen, Y.-C. Ferrous and Ferric Ions-Based High-Throughput Screening Strategy for Nitrile Hydratase and Amidase. J. Microbiol. Methods 2011, 85, 214–220. [Google Scholar] [CrossRef]
- Sahu, R.; Meghavarnam, A.K.; Janakiraman, S. A Simple, Efficient and Rapid Screening Technique for Differentiating Nitrile Hydratase and Nitrilase Producing Bacteria. Biotechnol. Rep. 2019, 24, e00396. [Google Scholar] [CrossRef]
- Nichols, M.L.; Willits, C.O. Reactions of Nessler’s Solution1. J. Am. Chem. Soc. 1934, 56, 769–774. [Google Scholar] [CrossRef]
- Banerjee, A.; Kaul, P.; Sharma, R.; Banerjee, U.C. A High-Throughput Amenable Colorimetric Assay for Enantioselective Screening of Nitrilase-Producing Microorganisms Using pH Sensitive Indicators. Slas Discov. Adv. Sci. Drug Discov. 2003, 8, 559–565. [Google Scholar] [CrossRef][Green Version]
- Singh, R.; Pandey, D.; Dhariwal, S.; Sood, P.; Chand, D. Bioconversion of Acrylonitrile Using Nitrile Hydratase Activity of Bacillus Sp. APB-6. 3 Biotech 2018, 8, 225. [Google Scholar] [CrossRef]
- Hjort, C.M.; Godtfredsen, S.E.; Emborg, C. Isolation and Characterization of a Nitrile Hydratase from a Rhodococcus Sp. J. Chem. Technol. Biotechnol. 1990, 48, 217–226. [Google Scholar] [CrossRef]
- Egelkamp, R.; Friedrich, I.; Hertel, R.; Daniel, R. From Sequence to Function: A New Workflow for Nitrilase Identification. Appl. Microbiol. Biotechnol. 2020, 104, 4957–4970. [Google Scholar] [CrossRef]
- Yazbeck, D.R.; Durao, P.J.; Xie, Z.; Tao, J. A Metal Ion-Based Method for the Screening of Nitrilases. J. Mol. Catal. B Enzym. 2006, 39, 156–159. [Google Scholar] [CrossRef]
- He, Y.-C.; Ma, C.-L.; Xu, J.-H.; Zhou, L. A High-Throughput Screening Strategy for Nitrile-Hydrolyzing Enzymes Based on Ferric Hydroxamate Spectrophotometry. Appl. Microbiol. Biotechnol. 2010, 89, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Roth, M. Fluorimetric methods in a laboratory of clinical chemistry. Ann. Dell'istituto Super. Sanità 1971, 7, 199–207. [Google Scholar]
- Lloret, S.; Andres, J.; Legua, C.; Falco, P. Determination of ammonia and primary amine compounds and Kjeldahl nitrogen in water samples with a modified Roth’s fluorimetric method. Talanta 2005, 65, 869–875. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharma, R.; Banerjee, U.C. A Rapid and Sensitive Fluorometric Assay Method for the Determination of Nitrilase Activity. Biotechnol. Appl. Biochem. 2003, 37, 289–293. [Google Scholar] [CrossRef]
- Lasch, P.; Beyer, W.; Bosch, A.; Borriss, R.; Drevinek, M.; Dupke, S.; Ehling-Schulz, M.; Gao, X.; Grunow, R.; Jacob, D.; et al. A MALDI-ToF Mass Spectrometry Database for Identification and Classification of Highly Pathogenic Bacteria. Sci. Data 2025, 12, 187. [Google Scholar] [CrossRef]
- Iqfath, M.; Wali, S.N.; Amer, S.; Hernly, E.; Laskin, J. Nanospray Desorption Electrospray Ionization Mass Spectrometry Imaging (Nano-DESI MSI): A Tutorial Review. ACS Meas. Sci. Au 2024, 4, 475–487. [Google Scholar] [CrossRef]
- Martinez, S.; Kuhn, M.L.; Russell, J.T.; Holz, R.C.; Elgren, T.E. Acrylamide production using encapsulated nitrile hydratase from Pseudonocardia thermophila in a sol–gel matrix. J. Mol. Catal. B Enzym. 2014, 100, 19–24. [Google Scholar] [CrossRef]
- Raj, J.; Prasad, S.; Bhalla, T.C. Rhodococcus Rhodochrous PA-34: A Potential Biocatalyst for Acrylamide Synthesis. Process. Biochem. 2006, 41, 1359–1363. [Google Scholar] [CrossRef]
- Jiao, S.; Li, F.; Yu, H.; Shen, Z. Advances in Acrylamide Bioproduction Catalyzed with Rhodococcus Cells Harboring Nitrile Hydratase. Appl. Microbiol. Biotechnol. 2019, 104, 1001–1012. [Google Scholar] [CrossRef]
- Luo, T.; Yu, S. Control and Communication Mechanisms of a SoftRouter. In Proceedings of the COIN-NGNCON 2006-The Joint International Conference on Optical Internet and Next Generation Network, Jeju, Republic of Korea, 13 September 2006. [Google Scholar] [CrossRef]
- Nagasawa, T.; Nakamura, T.; Yamada, H. Production of Acrylic Acid and Methacrylic Acid Using Rhodococcus Rhodochrous J1 Nitrilase. Appl. Microbiol. Biotechnol. 1990, 34, 322–324. [Google Scholar] [CrossRef]
- Bhatt, A.; Prajapati, D.; Gupte, A. Production, Purification and Characterization of Nitrilase from Bacillus Subtilis-AGAB-2 and Chemoenzymatic Synthesis of Acrylic Acid from Acrylonitrile. Catal. Lett. 2024, 154, 4302–4319. [Google Scholar] [CrossRef]
- Kamal, A. Bioconversion of Acrylonitrile to Acrylic Acid by Rhodococcus Ruber Strain AKSH-84. J. Microbiol. Biotechnol. 2011, 21, 37–42. [Google Scholar] [CrossRef]
- Wu, S.; Snajdrova, R.; Moore, J.C.; Baldenius, K.; Bornscheuer, U.T. Biocatalysis: Enzymatic Synthesis for Industrial Applications. Angew. Chem. Int. Ed. Engl. 2021, 60, 88–119. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Jiao, S.; Wang, M.; Yu, H.; Shen, Z. A CRISPR/Cas9-Based Genome Editing System for Rhodococcus Ruber TH. Metab. Eng. 2020, 57, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, K.V.; Shemyakina, A.O.; Grechishnikova, E.G.; Gerasimova, T.V.; Kalinina, T.I.; Novikov, A.D.; Leonova, T.E.; Ryabchenko, L.E.; Bayburdov, T.A.; Yanenko, A.S. A New Concept of Biocatalytic Synthesis of Acrylic Monomers for Obtaining Water-Soluble Acrylic Heteropolymers. Metab. Eng. Commun. 2023, 18, e00231. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zheng, Y.; Li, J.; Du, Y.; Wei, Y.; Yu, H. Engineering a Non-Pigmented Rhodococcus Ruber Strain for Enhanced Bio-Production of Acrylamide. Chem. Eng. J. 2025, 508, 160950. [Google Scholar] [CrossRef]
- Prasad, S.; Raj, J.; Bhalla, T.C. Bench Scale Conversion of 3-Cyanopyidine to Nicotinamide Using Resting Cells of Rhodococcus Rhodochrous PA-34. Indian J. Microbiol. 2007, 47, 34–41. [Google Scholar] [CrossRef]
- Zhu, X.-Y.; Gong, J.-S.; Li, H.; Lu, Z.-M.; Shi, J.-S.; Xu, Z.-H. Bench-Scale Biosynthesis of Isonicotinic Acid from 4-Cyanopyridine by Pseudomonas Putida. Chem. Pap. 2014, 68, 739–744. [Google Scholar] [CrossRef]
- Sharma, N.N.; Sharma, M.; Bhalla, T.C. Nocardia Globerula NHB-2 Nitrilase Catalysed Biotransformation of 4-Cyanopyridine to Isonicotinic Acid. AMB Express 2012, 2, 25. [Google Scholar] [CrossRef]
- Cantarella, L.; Gallifuoco, A.; Malandra, A.; Martínková, L.; Spera, A.; Cantarella, M. High–yield continuous production of nicotinic acid via nitrile hydratase–amidase cascade reactions using cascade CSMRs. Enzym. Microb. Technol. 2011, 48, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, M.; Li, X.; Zhang, J.; Sun, H.; Luo, J. Highly efficient synthesis of 5-cyanovaleramide by Rhodococcus ruber CGMCC3090 resting cells. J. Chem. Technol. Biotechnol. 2012, 87, 1396–1400. [Google Scholar] [CrossRef]
- Wang, L.; Liu, S.; Du, W.; Dou, T.; Liang, C. High Regioselectivity Production of 5-Cyanovaleramide from Adiponitrile by a Novel Nitrile Hydratase Derived from Rhodococcus Erythropolis CCM2595. ACS Omega 2020, 5, 18397–18402. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Mehta, P.K.; Bhatia, R.K.; Bhalla, T.C. Optimization of Arylacetonitrilase Production from Alcaligenes Sp. MTCC 10675 and Its Application in Mandelic Acid Synthesis. Appl. Microbiol. Biotechnol. 2013, 98, 83–94. [Google Scholar] [CrossRef]
- Pawar, S.V.; Yadav, G.D. Enantioselective Enzymatic Hydrolysis of rac–Mandelonitrile to R–Mandelamide by Nitrile Hydratase Immobilized on Poly(vinyl alcohol)/Chitosan–Glutaraldehyde Support. Ind. Eng. Chem. Res. 2014, 53, 7986–7991. [Google Scholar] [CrossRef]
- Yu, H.; Jiao, S.; Wang, M.; Liang, Y.; Tang, L. Biodegradation of Nitriles by Rhodococcus. In Biology of Rhodococcus; Springer: Cham, Switzerland, 2019; Volume 16, pp. 173–202. [Google Scholar] [CrossRef]
- Li, M.; Ma, D.; Qiao, J.; Cheng, Z.; Wang, Q.; Zhou, Z.; Han, L. Development of High-Performance Nitrile Hydratase Whole-Cell Catalyst by Automated Structure-and Sequence-Based Design and Mechanism Insights. Syst. Microbiol. Biomanufacturing 2024, 4, 882–894. [Google Scholar] [CrossRef]
- Cantarella, M.; Cantarella, L.; Gallifuoco, A.; Spera, A. Nitrile Bioconversion by Microbacterium Imperiale CBS 498-74 Resting Cells in Batch and Ultrafiltration Membrane Bioreactors. J. Ind. Microbiol. Biotechnol. 2005, 33, 208–214. [Google Scholar] [CrossRef]
- Srinivasan, K.; Hariharapura, R.C.; Mallikarjuna, S.V. Pharmaceutical Waste Management through Microbial Bioremediation. Environ. Monit. Assess. 2025, 197, 1–14. [Google Scholar] [CrossRef]
- Langdahl, B.R.; Bisp, P.; Ingvorsen, K. Nitrile Hydrolysis by Rhodococcus Erythropolis BL1, an Acetonitrile-Tolerant Strain Isolated from a Marine Sediment. Microbiology 1996, 142, 145–154. [Google Scholar] [CrossRef]
- Gong, J.-S.; Lu, Z.-M.; Li, H.; Shi, J.-S.; Zhou, Z.-M.; Xu, Z.-H. Nitrilases in Nitrile Biocatalysis: Recent Progress and Forthcoming Research. Microb. Cell Factories 2012, 11, 142. [Google Scholar] [CrossRef]
- Torri, D.; Bering, L.; Yates, L.R.L.; Angiolini, S.M.; Xu, G.; Cuesta-Hoyos, S.; Shepherd, S.A.; Micklefield, J. Enzymatic Cascades for Stereoselective and Regioselective Amide Bond Assembly. Angew. Chem. Int. Ed. 2025, 64, e202422185. [Google Scholar] [CrossRef]
- Wang, L.; Song, Y.; Guo, Y.; Chen, G.; Zhang, H.; Liang, C. Modification of Nitrile Hydratase by Substrate Channel Engineering Regulates Monocyanamide Synthesis. Catal. Lett. 2025, 155, 1–12. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, Z.; Peplowski, L.; Zhou, Z. Highly Efficient Biosynthesis of Isonicotinamide through a Substrate Access Tunnel Engineered Nitrile Hydratase from Carbonactinospora Thermoautotrophicus. New J. Chem. 2023, 47, 13279–13285. [Google Scholar] [CrossRef]
- Ma, D.; Cheng, Z.; Peplowski, L.; Han, L.; Xia, Y.; Hou, X.; Guo, J.; Yin, D.; Rao, Y.; Zhou, Z. Insight into the Broadened Substrate Scope of Nitrile Hydratase by Static and Dynamic Structure Analysis. Chem. Sci. 2022, 13, 8417–8428. [Google Scholar] [CrossRef]
- Cheng, Z.; Peplowski, L.; Cui, W.; Xia, Y.; Liu, Z.; Zhang, J.; Kobayashi, M.; Zhou, Z. Identification of key residues modulating the stereoselectivity of nitrile hydratase toward rac-mandelonitrile by semi-rational engineering. Biotechnol. Bioeng. 2017, 115, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Cui, W.; Xia, Y.; Peplowski, L.; Kobayashi, M.; Zhou, Z. Modulation of Nitrile Hydratase Regioselectivity towards Dinitriles by Tailoring the Substrate Binding Pocket Residues. ChemCatChem 2018, 10, 449–458. [Google Scholar] [CrossRef]
- Cheng, Z.; Cui, W.; Liu, Z.; Zhou, L.; Wang, M.; Kobayashi, M.; Zhou, Z. A switch in a substrate tunnel for directing regioselectivity of nitrile hydratases towards,-dinitriles. Catal. Sci. Technol. 2016, 6, 1292–1296. [Google Scholar] [CrossRef]
- Zheng, R.-C.; Yin, X.-J.; Zheng, Y.-G. Highly Regioselective and Efficient Production of 1-Cyanocyclohexaneacetamide by Rhodococcus Aetherivorans ZJB1208 Nitrile Hydratase. J. Chem. Technol. Biotechnol. 2015, 91, 1314–1319. [Google Scholar] [CrossRef]
- Van Pelt, S.; Quignard, S.; Kubáč, D.; Sorokin, D.Y.; Van Rantwijk, F.; Sheldon, R.A. Nitrile Hydratase CLEAs: The Immobilization and Stabilization of an Industrially Important Enzyme. Green Chem. 2007, 10, 395–400. [Google Scholar] [CrossRef]
- Van Pelt, S.; Zhang, M.; Otten, L.G.; Holt, J.; Sorokin, D.Y.; Van Rantwijk, F.; Black, G.W.; Perry, J.J.; Sheldon, R.A. Probing the Enantioselectivity of a Diverse Group of Purified Cobalt-Centred Nitrile Hydratases. Org. Biomol. Chem. 2011, 9, 3011–3019. [Google Scholar] [CrossRef]
- Angiolini, S.; Bruton, I.; Bering, L.; Thomas, S.S.; Thompson, J.; Shepherd, S.A.; Micklefield, J. Integrating Enzymes with Photoredox Catalysis for Conversion of Nitriles into Fluorinated Products. ChemCatChem 2025, e202500240. [Google Scholar] [CrossRef]
- Bian, S.-Q.; Wang, Z.; Gong, J.-S.; Su, C.; Li, H.; Xu, Z.-H.; Shi, J.-S. Enhancing the Substrate Specificity of Nitrilase toward Aliphatic Nitriles Based on Substrate Channel Design. Acs Sustain. Chem. Eng. 2025, 13, 5332–5344. [Google Scholar] [CrossRef]
- Jones, L.B.; Wang, X.; Gullapalli, J.S.; Kunz, D.A. Characterization of the Nit6803 Nitrilase Homolog from the Cyanotroph Pseudomonas Fluorescens NCIMB 11764. Biochem. Biophys. Rep. 2021, 25, 100893. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.; Kumar, V.; Thakur, S.; Sharma, N.; Sheetal; Bhalla, T.C. Biotransformation of 4-Hydroxyphenylacetonitrile to 4-Hydroxyphenylacetic Acid Using Whole Cell Arylacetonitrilase of Alcaligenes Faecalis MTCC 12629. Process. Biochem. 2018, 73, 117–123. [Google Scholar] [CrossRef]
- Pai, O.; Banoth, L.; Ghosh, S.; Chisti, Y.; Banerjee, U.C. Biotransformation of 3-Cyanopyridine to Nicotinic Acid by Free and Immobilized Cells of Recombinant Escherichia Coli. Process. Biochem. 2014, 49, 655–659. [Google Scholar] [CrossRef]
- Duca, D.; Rose, D.R.; Glick, B.R. Characterization of a Nitrilase and a Nitrile Hydratase from Pseudomonas Sp. Strain UW4 That Converts Indole-3-Acetonitrile to Indole-3-Acetic Acid. Appl. Environ. Microbiol. 2014, 80, 4640–4649. [Google Scholar] [CrossRef]
- Layh, N.; Parratt, J.; Willetts, A. Characterization and Partial Purification of an Enantioselective Arylacetonitrilase from Pseudomonas Fluorescens DSM 7155. J. Mol. Catal. B Enzym. 1998, 5, 467–474. [Google Scholar] [CrossRef]
- Gilligan, T.; Yamada, H.; Nagasawa, T. Production of S-(+)-2-Phenylpropionic Acid from (R, S)-2-Phenylpropionitrile by the Combination of Nitrile Hydratase and Stereoselective Amidase in Rhodococcus Equi TG328. Appl. Microbiol. Biotechnol. 1993, 39, 720–725. [Google Scholar] [CrossRef]
- Xue, Y.-P.; Yang, Y.-K.; Lv, S.-Z.; Liu, Z.-Q.; Zheng, Y.-G. High-Throughput Screening Methods for Nitrilases. Appl. Microbiol. Biotechnol. 2016, 100, 3421–3432. [Google Scholar] [CrossRef]
- Zheng, W.; Yang, L.; Meng, Y.; Yang, L.; Wu, J. Enhancing the Catalytic Efficiency of Nitrilase for Sterically Hindered Substrates by Distal Sites Engineering. Green Synth. Catal. 2024. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Gong, J.-S.; Feng, D.-T.; Su, C.; Li, H.; Rao, Z.-M.; Lu, Z.-M.; Shi, J.-S.; Xu, Z.-H. Geometric Remodeling of Nitrilase Active Pocket Based on ALF-Scanning Strategy To Enhance Aromatic Nitrile Substrate Preference and Catalytic Efficiency. Appl. Environ. Microbiol. 2023, 89, e00220-23. [Google Scholar] [CrossRef]
- Tang, X.-L.; Wen, P.-F.; Zheng, W.; Zhu, X.-Y.; Zhang, Y.; Diao, H.-J.; Zheng, R.-C.; Zheng, Y.-G. Bidirectional Regulation of Nitrilase Reaction Specificity by Tuning the Characteristic Distances between Key Residues and Substrate. ACS Catal. 2023, 13, 10282–10294. [Google Scholar] [CrossRef]
- Wang, Z.-K.; Feng, D.-T.; Su, C.; Li, H.; Rao, Z.-M.; Rao, Y.-J.; Lu, Z.-M.; Shi, J.-S.; Xu, Z.-H.; Gong, J.-S. Designing ASSMD Strategy for Exploring and Engineering Extreme Thermophilic Ancestral Nitrilase for Nitriles Biocatalysis. ACS Catal. 2024, 14, 13825–13838. [Google Scholar] [CrossRef]
- Jin, Y.; Shen, J.; Wang, Y.; Shao, H.; Xia, H.; Lin, J.; Liu, Z.; Zheng, Y. Modification of nitrilase based on computer screening and efficient biosynthesis of 4-cyanobenzoic acid. Biotechnol. J. 2024, 19, 2300706. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lan, Y.; Guo, J.; Ma, D.; Jiang, S.; Lai, Q.; Zhou, Z.; Peplowski, L. Computational Design of Nitrile Hydratase from Pseudonocardia Thermophila JCM3095 for Improved Thermostability. Molecules 2020, 25, 4806. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Qiao, J.; Cheng, Z.; Guo, J.; Wang, Q.; Zhou, Z.; Han, L. Computational Design of Coevolutionary Residues for Improved Stability and Activity of Nitrile Hydratase. Biochem. Biophys. Res. Commun. 2025, 750, 151400. [Google Scholar] [CrossRef]
- Chen, J.; Yu, H.; Liu, C.; Liu, J.; Shen, Z. Improving Stability of Nitrile Hydratase by Bridging the Salt-Bridges in Specific Thermal-Sensitive Regions. J. Biotechnol. 2013, 164, 354–362. [Google Scholar] [CrossRef]
- Liu, Y.; Cui, W.; Liu, Z.; Cui, Y.; Xia, Y.; Kobayashi, M.; Zhou, Z. Enhancement of Thermo-Stability and Product Tolerance of Pseudomonas Putida Nitrile Hydratase by Fusing with Self-Assembling Peptide. J. Biosci. Bioeng. 2014, 118, 249–252. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, W.; Liu, Z.; Zhou, L.; Cui, Y.; Kobayashi, M.; Zhou, Z. Construction of a Subunit-Fusion Nitrile Hydratase and Discovery of an Innovative Metal Ion Transfer Pattern. Sci. Rep. 2016, 6, 19183. [Google Scholar] [CrossRef]
- Xia, Y.; Peplowski, L.; Cheng, Z.; Wang, T.; Liu, Z.; Cui, W.; Kobayashi, M.; Zhou, Z. Metallochaperone function of the self-subunit swapping chaperone involved in the maturation of subunit-fused cobalt-type nitrile hydratase. Biotechnol. Bioeng. 2018, 116, 481–489. [Google Scholar] [CrossRef]
- Xia, Y.; Cui, W.; Cheng, Z.; Peplowski, L.; Liu, Z.; Kobayashi, M.; Zhou, Z. Improving the Thermostability and Catalytic Efficiency of the Subunit-Fused Nitrile Hydratase by Semi-Rational Engineering. ChemCatChem 2018, 10, 1370–1375. [Google Scholar] [CrossRef]
- Wang, L.; Guan, S.; Bai, J.; Jiang, Y.; Song, Y.; Zheng, X.; Gao, J. Enzyme Immobilized in BioMOFs: Facile Synthesis and Improved Catalytic Performance. Int. J. Biol. Macromol. 2020, 144, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Chiyanzu, I.; Cowan, D.A.; Burton, S.G. Immobilization of Geobacillus Pallidus RAPc8 Nitrile Hydratase (NHase) Reduces Substrate Inhibition and Enhances Thermostability. J. Mol. Catal. B Enzym. 2010, 63, 109–115. [Google Scholar] [CrossRef]
- Sheldon, R.A. Characteristic Features and Biotechnological Applications of Cross-Linked Enzyme Aggregates (CLEAs). Appl. Microbiol. Biotechnol. 2011, 92, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Mohan, U.; Kamble, A.L.; Pawar, S.; Banerjee, U.C. Cross-Linked Enzyme Aggregates of Recombinant Pseudomonas Putida Nitrilase for Enantioselective Nitrile Hydrolysis. Bioresour. Technol. 2010, 101, 6856–6858. [Google Scholar] [CrossRef]
- Pawar, S.V.; Yadav, G.D. PVA/chitosan–glutaraldehyde cross–linked nitrile hydratase as reusable biocatalyst for conversion of nitriles to amides. J. Mol. Catal. B Enzym. 2014, 101, 115–121. [Google Scholar] [CrossRef]
- D'ANtona, N.; Morrone, R.; Gambera, G.; Pedotti, S. Enantiorecognition of planar metallocenic chirality by a nitrile hydratase/amidase bienzymatic system. Org. Biomol. Chem. 2016, 14, 4393–4399. [Google Scholar] [CrossRef]
- Peplowski, L.; Kubiak, K.; Nowak, W. Mechanical Aspects of Nitrile Hydratase Enzymatic Activity. Steered Molecular Dynamics Simulations of Pseudonocardia Thermophila JCM 3095. Chem. Phys. Lett. 2008, 467, 144–149. [Google Scholar] [CrossRef]
- Mashweu, A.R.; Chhiba-Govindjee, V.P.; Bode, M.L.; Brady, D. Substrate Profiling of the Cobalt Nitrile Hydratase from Rhodococcus Rhodochrous ATCC BAA 870. Molecules 2020, 25, 238. [Google Scholar] [CrossRef]
- Wang, M.-X. Enantioselective Biotransformations of Nitriles in Organic Synthesis. Top. Catal. 2005, 35, 117–130. [Google Scholar] [CrossRef]
- Mulelu, A.E.; Kirykowicz, A.M.; Woodward, J.D. Cryo-EM and Directed Evolution Reveal How Arabidopsis Nitrilase Specificity Is Influenced by Its Quaternary Structure. Commun. Biol. 2019, 2, 1–11. [Google Scholar] [CrossRef]
- Luo, H.; Ma, J.; Chang, Y.; Yu, H.; Shen, Z. Directed Evolution and Mutant Characterization of Nitrilase from Rhodococcus Rhodochrous Tg1-A6. Appl. Biochem. Biotechnol. 2015, 178, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Nadarajan, S.P.; Sundaramoorthy, U.; Jeon, H.; Chung, T.; Yun, H. Biotransformation of-keto nitriles to chiral (S)--amino acids using nitrilase and-transaminase. Biotechnol. Lett. 2016, 39, 535–543. [Google Scholar] [CrossRef]
- Bering, L.; Craven, E.J.; Thomas, S.A.S.; Shepherd, S.A.; Micklefield, J. Merging enzymes with chemocatalysis for amide bond synthesis. Nat. Commun. 2022, 13, 1–10. [Google Scholar] [CrossRef]
- Craven, E.J.; Latham, J.; Shepherd, S.A.; Khan, I.; Diaz-Rodriguez, A.; Greaney, M.F.; Micklefield, J. Programmable late-stage CH bond functionalization enabled by integration of enzymes with chemocatalysis. Nat. Catal. 2021, 4, 385–394. [Google Scholar] [CrossRef]
| (A) NHase-Catalysed Reactions | |||||
| Origin/Engineering strategy | Substrates | Purpose | Conversion rate | Main product | References |
| NHase from Rhodococcus sp. (integrated cascade with amidase, NHase, nitrilase, amide bond synthetase, decarboxylase, metal/photocatalysts) | Aromatic nitriles | Regioselectivity and stereoselectivity | >90% | Enantioenriched amides | Torri et al., 2025 [115] |
| NHase βM40A mutant (substrate channel engineered for selective monocyanamide synthesis) | Malononitrile | Regioselectivity (monocyanamide enrichment) | ~92% | Cyanoacetamide | Wang et al., 2025 [116] |
| Tunnel engineered NHase from Carbonactinospora thermoautotrophicus | 4-cyanopyridine | Product yield | 100% | Isonicotinamide | Guo et al., 2023 [117] |
| NHase variant with tunnel mutations | Phenylacetonitrile/benzonitriles | Product yield | 85–95% | Corresponding amides | Ma et al., 2022 [118] |
| Rhodococcus erythropolis CCM2595 | Adiponitrile | Product yield | >95% | 5-cyanovaleramide | Wang et al., 2020 [106] |
| Rhodococcus rhodochrous J1 | Adiponitrile, malononitrile, terephthalonitrile, phthalonitrile | Product yield | >98% | Adipamide Malonamide Terephthalamide Phthalamide | Cheng et al., 2018 [119,120] |
| Pseudomonas putida NRRL-18668 | Adiponitrile/Malononitrile | Product yield | >99% | 5-cyanovaleramide/Cyanoacetamide | Cheng et al., 2018 [121] |
| βL37 mutants of Pseudomonas putida NRRL-18668 and C. testosteroni | Adiponitrile/Malononitrile α,ω-dinitriles | Product yield/ Diamide synthesis | >98% | Adipoamide/Malonamide α,ω-diamides | Cheng et al., 2016 [121] |
| Rhodococcus aetherivorans ZJB1208 | 1-cyanocyclohexaneacetonitrile | Regioselectivity/ Product yield | 100% | 1-cyanocyclohexaneacetamide | Zheng et al., 2016 [122] |
| Rhodococcus ruber CGMCC3090 | Adiponitrile | Product yield | 100% | 5-cyanovaleramide | Shen et al., 2012 [105] |
| Rhodopseudomonas palustris HaA2 | 2-phenylpropionitrile/2-phenylbutyronitrile | Stereoselectivity | — | (S)-2-phenylpropionamide/(S)-2-phenylbutyramide | van Pelt et al., 2011 [123,124] |
| Rhodococcus rhodochrous DSM43269 | 2-phenylpropionitrile | Stereoselectivity | E-value >100 | (S)-2-phenylpropionamide | van Pelt et al., 2011 [123,124] |
| (B) Nitrilase-Catalysed Reactions | |||||
| Origin/Engineering strategy | Substrate | Regio-/stereoselectivity | Conversion rate | Main product | References |
| Engineered nitrilase with photoredox catalysts (integrated reaction) | Aromatic nitriles | Regioselectivity | ~80% | Fluorinated acids | Angiolini et al., 2025 [125] |
| Substrate channel engineered mutants of PpNit nitrilase gene from Pseudomonas putida | 3-chloropropionitrile and various aliphatic nitriles | Product yield | ~100% | Corresponding aliphatic carboxylic acids | Bian et al., 2025 [126] |
| Nit6803 nitrilase homologue from Pseudomonas fluorescens NCIMB 11764 | Succinonitrile/fumaronitrile/Sebaconitrile | Dicarboxylic acid synthesis | ~90–20% | Dicarboxylic acids | Jones et al., 2021 [127] |
| Variovorax boronicumulans J1 nitrilase (arylacetonitrilase Nit09) | Phenylacetonitrile | Product yield | ~90% | Phenylacetic acid | Egelkamp et al. 2020 [82] |
| Alcaligenes faecalis MTCC 12629 | 4-hydroxyphenylacetonitrile | Product yield | 90% | 4-hydroxyphenylacetic acid | Thakur et al., 2018 [128] |
| Recombinant E. coli JM109 cells harbouring nitrilase gene from Alcaligenes faecalis MTCC 126 | 3-cyanopyridine | Product yield | 98% | Nicotinic acid | Pai et al. 2014 [129] |
| Pseudomonas sp Strain UW4 | Indole-3-acetonitrile | Product yield | — | Indole-3-acetic acid | Duca et al., 2014 [130] |
| Nocardia globerula NHB-2 | 4-cyanopyridine Aliphatic nitriles | Product yield | 100% | Isonicotinic acid Corresponding acids | Sharma et al., 2012 [103] |
| Pseudomonas fluorescens DSM 50106 | Mandelonitrile | Stereoselectivity | 95% | (R)-Mandelic acid | Layh et al., 1998 [131] |
| Rhodococcus rhodochrous ATCC BAA-870 | 2-phenylpropionitrile | Stereoselectivity | E-value >100 | (S)-2-phenylpropionic acid | Gilligan et al., 1993 [132] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menon, B.R.K.; Philpin, J.D.; Scaife, J.J.; Chua, T. Nitrile-Converting Enzymes: Industrial Perspective, Challenges and Emerging Strategies. Catalysts 2025, 15, 939. https://doi.org/10.3390/catal15100939
Menon BRK, Philpin JD, Scaife JJ, Chua T. Nitrile-Converting Enzymes: Industrial Perspective, Challenges and Emerging Strategies. Catalysts. 2025; 15(10):939. https://doi.org/10.3390/catal15100939
Chicago/Turabian StyleMenon, Binuraj R. K., James David Philpin, Joe James Scaife, and Thomas Chua. 2025. "Nitrile-Converting Enzymes: Industrial Perspective, Challenges and Emerging Strategies" Catalysts 15, no. 10: 939. https://doi.org/10.3390/catal15100939
APA StyleMenon, B. R. K., Philpin, J. D., Scaife, J. J., & Chua, T. (2025). Nitrile-Converting Enzymes: Industrial Perspective, Challenges and Emerging Strategies. Catalysts, 15(10), 939. https://doi.org/10.3390/catal15100939






