Abstract
Lithium-oxygen batteries (LOBs) are limited by sluggish oxygen redox kinetics and cathode instability. Herein, we report a cobalt particle catalyst encapsulated in nitrogen-doped carbon (Co@NC) with a three-dimensional hierarchical architecture, synthesized via a chitosan-derived hierarchical porous carbon framework. This innovative design integrates uniformly dispersed ultra-thin carbon shells (11.7 nm), pyridinic nitrogen doping, and Co particles (1.41 μm) stabilized through carbon-support electronic coupling. The hierarchical porosity facilitates rapid O2/Li+ mass transport, while pyridinic N sites act as dual-function electrocatalytic centers for Li2O2 nucleation and charge transfer kinetics. Co@NC achieves 11,213 mAh g−1 at 200 mA g−1 (126.5% higher than nitrogen-doped carbon) and maintains 1.54 V overpotential (500 mAh g−1). These metrics outperform benchmark catalysts, addressing kinetic and stability challenges in LOBs. The study advances electrocatalyst design by integrating structural optimization, heteroatom doping, and electronic coupling strategies for high-performance metal–air batteries.
1. Introduction
LOBs represent a paradigm-shifting energy storage technology due to their ultrahigh theoretical energy density, yet practical applications remain hindered by sluggish oxygen reduction/evolution reaction (ORR/OER) and cathode degradation [1,2,3]. Conventional catalysts, including platinum group metals and transition metal oxides, suffer from limited activity, poor stability, and inefficient mass transport under electrochemical stress [4,5].
Recent efforts have focused on developing effective catalysts—including noble metals [6,7], non-noble metal compounds [8], and hybrid metal materials [9]—to accelerate the kinetics of catalytic reactions while inhibiting parasitic reactions. Among these, cobalt-based catalysts emerge as prime candidates for reducing ORR/OER polarization and enhancing dischage–charge dynamics, as validated by both experimental and theoretical frameworks. Xia pioneered a urea-glass route for Co4N nanoparticles, enhancing ORR/OER kinetics via nitrogen-doped carbon coupling to achieve 6813 mAh g−1 discharge capacity [10]. Wang introduced atomically dispersed Co-N4 sites on carbon nanosheets, reducing polarization to 0.40 V and enabling 260 cycles at 400 mA g−1 [11]. Meng advanced Co-S@NC nanocrystals with 3d-orbital regulation, delivering 34,587 mAh g−1 at 1000 mA g−1 through hierarchical pore design [12]. Wu engineered 3D interconnected carbon using ZnCo-ZIF templates, embedding Co-Nx/pyridine-N sites to sustain 180 cycles at 28,000 mAh g−1 [13]. Collectively, these synergistic strategies—spanning atomic-scale design to 3D architecture—address kinetic barriers, stability, and scalability, advancing LOBs toward commercialization via optimized electron transfer, active site engineering, and structural innovation.
However, a persistent challenge persists: Both exposed Co particles and atomically dispersed Co-Nx sites face inevitable deactivation risks from continuous redox-induced structural changes or Li2O2 accumulation blocking active sites. Catalytic sites thus require protective encapsulation via external media like carbon matrices. Yet critical ambiguities remain—the interfacial electron coupling between carbon supports and metallic centers, as well as the precise tuning of reaction active sites, demand meticulous optimization to balance charge transfer kinetics with catalytic activity [14]. The unclear interplay between carbon encapsulation thickness, pore architecture, and metal-support bonding strength necessitates future research to refine encapsulation parameters—including porosity, layer thickness, and interfacial chemistry—to synchronize rapid electron transport with sustained catalytic efficiency, ensuring durable performance without compromising reaction accessibility. This nuanced optimization of electron coupling and active site protection is pivotal to advancing Li-O2 catalysts from lab-scale innovation to commercial viability.
This study integrates hierarchical porosity engineering, conformal carbon encapsulation, and heteroatom doping to create a synergistic electrocatalytic architecture. Chitosan-derived carbon matrices with interconnected micropores and mesopores form a three-dimensional transport network enabling rapid O2/Li+ diffusion kinetics (Scheme 1). Ultra-thin (11.7 nm) carbon layers stabilize monodisperse cobalt particles (1.41 μm) through steric hindrance, preventing sintering-induced aggregation while preserving metallic conductivity. Pyridinic nitrogen species are strategically incorporated to generate catalytic hotspots for Li2O2 nucleation, enhancing intrinsic activity through electronic structure modulation. Structural validation via high-resolution transmission electron microscopy (HR-TEM) reveals face-centered cubic (fcc) cobalt domains embedded within nitrogen-doped carbon frameworks. This integrated architecture synergistically addresses kinetic limitations and stability challenges in LOB cathodes.
Scheme 1.
Schematic illustration of the synthetic process of the Co@NC sample.
Electrochemical evaluations reveal that Co@NC outperforms benchmark catalysts across key metrics: achieving 11,213 mAh g−1 specific capacity at 200 mA g−1 (126.5% higher than nitrogen-doped carbon) and exhibiting only 1.54 V overpotential at a capacity limit of 500 mAh g−1. Mechanistic studies attribute this superiority to the synergistic effects of dual-active sites (Co-Nx and pyridinic N) and the three-dimensional conductive network that minimizes charge transfer resistance. These findings establish Co@NC as a next-generation catalyst platform for long-cycle-life metal-air batteries.
2. Results and Discussion
In this comprehensive investigation, a meticulously engineered synthetic paradigm for fabricating nitrogen-doped chitosan-derived carbon-encapsulated cobalt composites was developed. Systematic optimization of annealing time parameters (2 h (Co@NC-2h), 4 h (Co@NC), 6 h (Co@NC-6h)) was conducted to enhance LOB performance, with evaluations based on single-cycle charge–discharge tests and transmission electron microscopy (TEM) analysis. Elemental composition analysis of three samples synthesized under varying annealing times (2 h, 4 h, 6 h) was meticulously conducted via inductively coupled plasma atomic emission spectroscopy (ICP-AES), utilizing a detection limit of ±0.05 ppm. The results unequivocally confirmed a uniform cobalt content of 16 wt% across all composites, ensuring identical metal element composition. This homogeneity in cobalt loading effectively eliminates variations in catalytic activity attributable to differences in metal content, thereby establishing carbon layer thickness—developed under distinct annealing durations—as the decisive factor for catalytic performance.
Building upon this elemental uniformity, the study further elucidated the critical role of annealing duration in systematically balancing carbonization completeness, structural integrity, and electrocatalytic kinetics (Figure 1a–g).
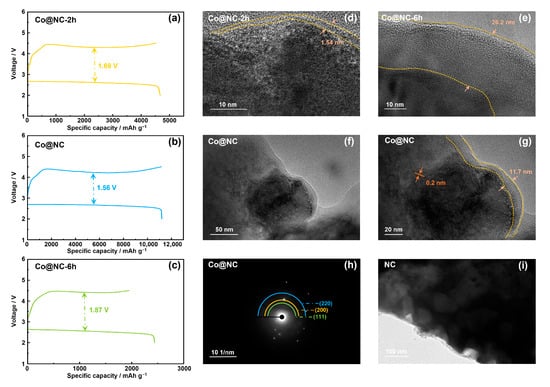
Figure 1.
Discharge/charge curves of LOB with (a) Co@NC-2h, (b) Co@NC and (c) Co@NC-6h at different current density of 200 mA g−1 between 2.0 and 4.5 V, TEM images of (d) Co@NC-2h, (e) Co@NC-6h and (f,g) Co@NC, (h) Selected area electron diffraction pattern of Co@NC, (i) TEM images of NC.
Annealing time significantly impacted performance metrics. At 2 h, incomplete carbonization resulted in a thin carbon layer (1.54 nm), insufficient Co particle encapsulation, compromised structural integrity, and reduced ORR/OER kinetics. This manifested in lower discharge capacity (~4678 mAh/g) and elevated overpotential (~1.69 V) due to impaired charge transfer and limited catalytic site availability. Conversely, 6 h annealing caused excessive carbon layer growth (26.2 nm), increasing electron transfer resistance, obscuring Co active sites, and inducing partial Co particle coalescence. Performance deteriorated further, with discharge capacity dropping to ~2443 mAh/g and overpotential rising to ~1.87 V, reflecting hindered ionic diffusion and mass transport limitations.
The 4 h annealing condition achieved an optimal balance, enabling complete carbonization while maintaining particle dispersion. This yielded peak performance: discharge capacity of 11,213 mAh/g and overpotential of 1.56 V. Structural characterization confirmed sufficient carbon layer formation (~11.7 nm) to stabilize Co particles without impeding mass transport—a critical energy-efficient advantage over both shorter and longer durations [15].
The lattice-resolved HR-TEM images exhibited sharply defined crystalline fringes with an interplanar spacing of 0.20 nm, precisely matching the (111) plane of face-centered cubic (fcc) cobalt [16]. The well-preserved crystal lattice attests to the gentle yet effective carbonization protocol, minimizing thermal stress-induced lattice distortions. Selected-area electron diffraction (SAED) analysis revealed distinct diffraction spots corresponding to the (111), (200), and (220) crystallographic planes of cobalt particles in the Co@NC sample (Figure 1h), confirming its crystalline nature [17].
The stratified pores of the original nitrogen-doped carbon matrix (Figure 1i), engineered through crosslinking of chitosan precursors, facilitate mass transport while providing abundant anchoring sites for cobalt particles. The microporous framework enhances gas adsorption capacity, critical for electrocatalytic applications, whereas the mesoporous channels ensure rapid electrolyte penetration.
Building upon the optimized annealing parameters previously established—particularly the 4 h pyrolysis condition that achieved peak LOBs performance (discharge capacity 11,213 mAh/g, overpotential 1.56 V)—this investigation now details the meticulously engineered synthetic paradigm for fabricating nitrogen-doped chitosan-derived carbon-encapsulated cobalt composites (Co@NC). The three-dimensional hierarchical porous architecture of the carbon matrix, strategically designed to optimize mass transport dynamics and electron conductivity, was achieved through a dual-stage pyrolysis protocol under controlled nitrogen atmosphere. This protocol, featuring precise temperature ramping at 5 °C/min and a 4 h annealing duration, ensures the formation of monodisperse Co particles with an average diameter of ~1.41 μm (Figure 2a–d) while developing a nitrogen-doped carbon matrix with stratified porosity—comprising mesoporous channels for rapid electrolyte penetration and micropores for enhanced gas adsorption critical to electrocatalytic performance. The pristine nitrogen-doped carbon matrix (NC), synthesized under identical pyrolysis conditions without a metal precursor, served as a critical reference for evaluating catalytic performance differentials (Figure 2e,f).
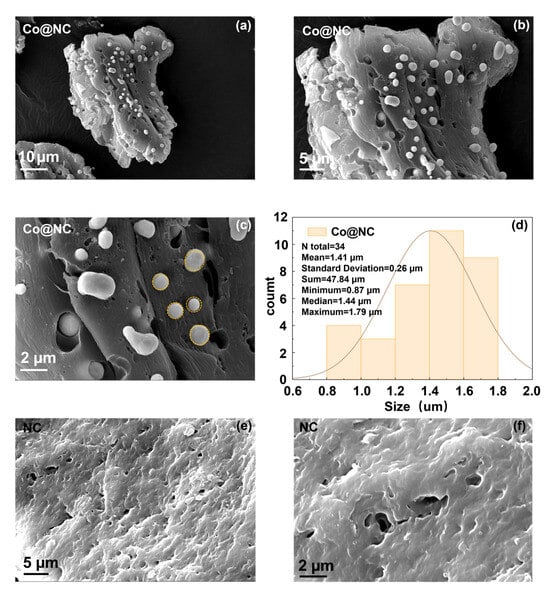
Figure 2.
(a–c) SEM images of Co@NC, (d) The granularity statistics histogram of Co@NC, (e,f) SEM and images of NC.
The N2 adsorption–desorption isotherm measurements (Figure 3) reveal that Co@NC and NC exhibit similar porosity characteristics. Co@NC demonstrates a BET surface area of 24.6 m2/g. The high surface area of Co@NC, combined with its hierarchical pore structure featuring the coexistence of micropores and mesopores, provides significant advantages for facilitating electrocatalytic charge transfer and mass transport during catalytic processes.
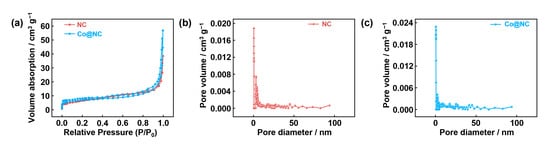
Figure 3.
(a) Nitrogen adsorption–desorption isotherm curve; pore size distribution curve of (b) NC and (c) Co@NC.
Energy-dispersive X-ray spectroscopy (EDX) elemental mapping (Figure 4), acquired at 100,000× magnification, demonstrated homogeneous distribution of C, N, and Co across the composite matrix.
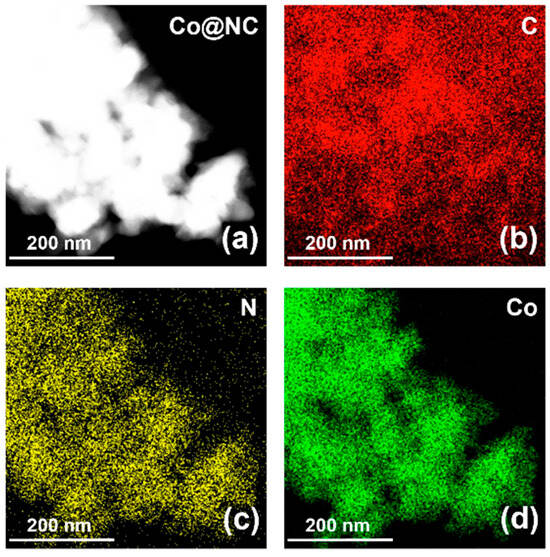
Figure 4.
EDS mappings of (a) Co@NC with a scale bar of 200 nm. Panels labeled as (b) C, (c) N and (d) Co are EDS mappings of corresponding elements.
The crystalline architecture of the Co@NC and pristine NC composites was meticulously analyzed via X-ray diffraction (XRD). The XRD patterns (Figure 5a) revealed broad diffraction peaks at 26.2° and 44.6°, corresponding to the (002) and (100) planes of graphitized carbon [18], indicative of a partially ordered carbon matrix with turbostratic stacking. Notably, the Co@NC composite exhibited sharp diffraction peaks at 44.2°, 51.6°, and 75.9°, precisely matching the (111), (200), and (220) reflections of face-centered cubic (fcc) cobalt (JCPDS No. 15-0806) [19]. Complementary Raman spectroscopy (Figure 5b) provided insights into carbon structural defects. Both Co@NC and NC exhibited characteristic D-band (1337 cm−1) and G-band (1590 cm−1) vibrations, attributed to disordered sp3 hybridized carbons and ordered sp2 graphitic domains, respectively. The intensity ratio (ID/IG) increased from 1.06 in NC to 1.08 in Co@NC, indicating enhanced defect density upon cobalt particle decoration [20]. This phenomenon is ascribed to interface-induced strain and local lattice distortions at the metal-carbon heterojunction.
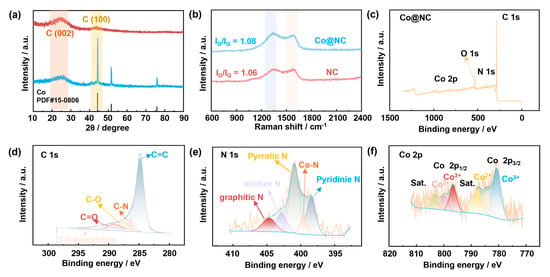
Figure 5.
(a) XRD patterns of Co@NC and NC samples, (b) Raman patterns of Co@NC and NC samples, (c) XPS spectra of Co@NC, (d) C 1s XPS spectra of Co@NC, (e) N 1s XPS spectra of Co@NC, (f) Co 2p XPS spectra of Co@NC.
The surface composition and chemical valence states of the Co@NC composite were systematically investigated via X-ray photoelectron spectroscopy (XPS). Survey scans (0–1350 eV) confirmed the coexistence of Co, N, O, and C elements on the material surface (Figure 5c).
High-resolution C 1s spectra (Figure 5d) were deconvoluted using Gaussian-Lorentzian (GL) line shapes with a Shirley background subtraction. The fitting revealed five distinct components: sp2-hybridized C=C (284.8 eV), C-N (286.5 eV), C-O (287.5 eV), C=O (288.9 eV), and π–π* shake-up satellite (291.2 eV) [21]. The dominant C=C peak indicates partial graphitization of the carbon matrix, enhancing electronic conductivity through delocalized π-bond networks. The N 1s spectrum (Figure 5e) exhibited five deconvoluted peaks: pyridinic N (398.4 eV), Co-N coordination (399.5 eV), pyrrolic N (400.9 eV), graphitic N (402.8 eV), and oxidized N (404.7 eV) [22]. The significant pyridinic N content provides coordination sites for Co atoms, facilitating the formation of Co-Nx moieties that anchor cobalt particles within the carbon framework. And pyridinic N sites act as dual-function electrocatalytic centers for Li2O2 nucleation and charge transfer kinetics [23]. The Co-N peak at 399.5 eV directly confirms covalent bonding between cobalt and nitrogen, critical for stabilizing metallic particles against aggregation. In the Co 2p XPS spectrum (Figure 5f), the core-level emission exhibits characteristic spin–orbit splitting into Co 2p3/2 and Co 2p1/2 doublets, each demonstrating further into the sub-peaks of Co3+ (780.7 eV and 796.6 eV) and Co2+ (784.2 eV and 799.8 eV) [24,25]. Notably, the detection of divalent and trivalent cobalt species (Co2+/Co3+) strongly indicates the formation of surface cobalt(II,III) oxide (Co3O4) structures—a surprising discovery arising from the partial air oxidation of encapsulated Co particles via inherent surface defects in the carbon coating. This unexpected phenomenon introduces a synergistic interplay between the oxygen vacancies and low-coordination sites of Co3O4 and the high-activity sites of metallic Co particles, effectively expanding the reaction pathways for ORR/OER [26,27,28,29]. Specifically, the oxygen vacancies within Co3O4 act as active centers for oxygen adsorption and activation, while the low-coordination sites enhance electron transfer efficiency. Simultaneously, the high-activity sites of Co particles facilitate rapid electron coupling with adsorbed oxygen intermediates, creating a dual-site catalytic mechanism that significantly improves reaction kinetics.
To unravel the structure-performance nexus of the Co@NC electrocatalyst, a rigorous multidimensional electrochemical assessment was systematically performed within a LOB framework under precisely controlled conditions. The galvanostatic charge–discharge profiles (Figure 6a,b), acquired in a Swagelok-type cell with 1 M LiTFSI/TEGDME electrolyte, revealed exceptional energy storage capabilities. At a current density of 200 mA g−1, the Co@NC cathode delivered an unprecedented discharge specific capacity of 11,213 mAh g−1, significantly surpassing the performance of pristine NC (4951 mAh g−1). This 126.5% capacity enhancement was attributed to the synergistic effects of nitrogen-doped carbon matrices and well-dispersed cobalt particles, which facilitated oxygen reduction/evolution kinetics through dual-active sites. Notably, the Co@NC electrocatalyst demonstrated remarkable rate capability across a broad current density range. Even at elevated rates of 400 and 800 mA g−1, the material maintained discharge capacities of 6536 and 1642 mAh g−1, respectively. This exceptional rate performance, outperforming benchmark catalysts like NC (Figure 6c and Table 1), stems from the hierarchical architecture’s ability to balance mass transport and electron conduction. The porous nitrogen-doped carbon framework enabled rapid O2 diffusion, while the embedded Co particles served as efficient redox mediators [30]. A comparative analysis of electrochemical performance metrics, as depicted in Figure 6d and detailed in Table 2, positions the Co@NC cathode among the most advanced LOB catalysts reported to date [31,32,33,34,35,36].
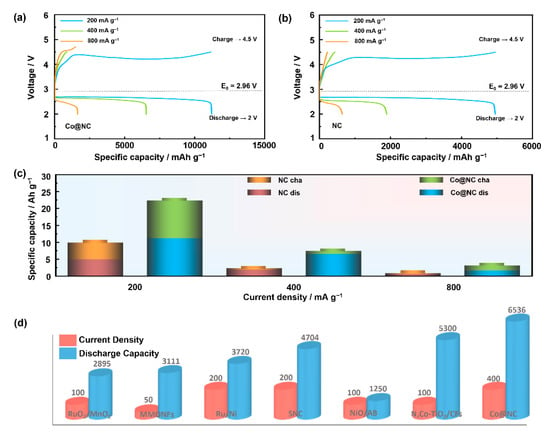
Figure 6.
Discharge/charge curves of LOB with (a) Co@NC and (b) NC at different current density of 200, 400 and 800 mA g−1 between 2.0 and 4.5 V, (c) the specific discharge/charge capacities of LOB with different catalysts evaluated across various current densities, where ‘cha’ denotes the charging stage and ‘dis’ the discharging stage, (d) performance comparisons of Co@NC and other representative published efforts.

Table 1.
Values of specific capacity of LOBs at different current densities of 200, 400 and 800 mA g−1.

Table 2.
Comparison of electrochemical properties of LOB of some similar materials and Co@NC catalyst.
At a current density of 500 mA g−1 with a capacity limit of 500 mAh g−1, the Co@NC cathode exhibited a remarkably low discharge–charge overpotential of 1.54 V, significantly outperforming benchmark NC catalysts (1.69 V). This superior performance was attributed to the synergistic interplay between nitrogen-doped carbon matrices and well-dispersed cobalt particles, which collectively enhanced ORR/OER reaction kinetics (Figure 7a). Long-term cycling stability tests (Figure 7b–d) underscored the durability of the Co@NC architecture. At 500 mA g−1 with a 500 mAh g−1 capacity limit, the material achieved an exceptional cycle life of 210 h (105 cycles). This far exceeded the performance of NC (60 h/30 cycles), establishing Co@NC as a leading LOB cathode material. The superior stability was attributed to the hierarchical carbon matrix, which prevented cobalt article aggregation and electrolyte decomposition, as confirmed by post-mortem SEM analysis showing minimal structural degradation after cycling (See Figure 8). In addition, Co@NC maintained a stable overpotential below 2.2 V even after 30 cycles, contrasting sharply with NC, which exhibited a 31.5% overpotential increase by 30 cycles. This remarkable reversibility stemmed from the dual functionality of the nitrogen-doped carbon framework: (1) graphitic domains ensured rapid electron transport, and (2) pyridinic/Co-N species provided stable catalytic sites for Li2O2 formation/decomposition [37]. The absence of sudden voltage drops or capacity fluctuations further confirmed the absence of critical parasitic reactions, such as electrolyte oxidation or carbon corrosion.
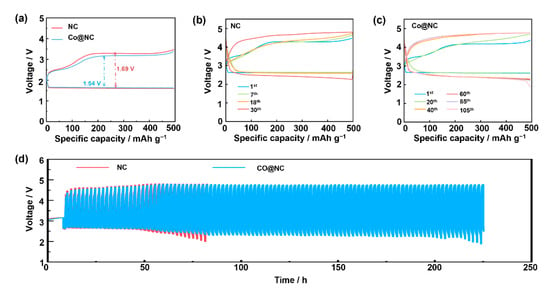
Figure 7.
(a) Initial discharge/charge profiles of different cathodes at current density of 500 mA g−1 under the limited capacities of 500 mAh/g, Discharge/charge profile of (b) NC and (c) Co@NC catalyst at current density of 500 mA g−1 under the limited capacities of 500 mAh/g, (d) Cyclic performance of Li-O2 batteries with different catalysts at current density of 500 mA g−1 under the limited capacities of 500 mAh/g.
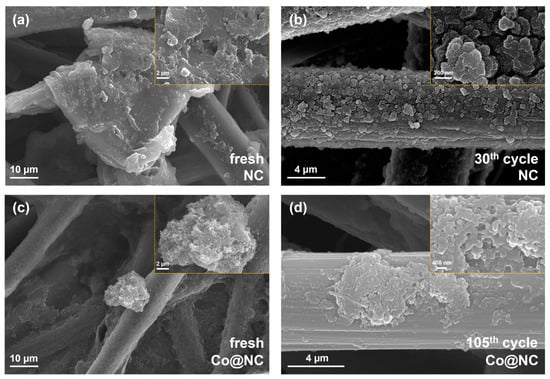
Figure 8.
SEM images of the NC at different discharge stages: (a) fresh; (b) after first discharge, SEM images of the Co@NC at different discharge stages: (c) fresh; (d) after first discharge.
Post-mortem examination of cycled LOBs provided pivotal insights into the dynamic evolution of cathode surfaces and their direct correlation with electrochemical performance metrics. Subsequent disassembly and SEM characterization of post-cycling cells revealed striking morphological disparities across the tested cathode architectures, offering visual validation of structural-performance relationships. For NC cathodes (Figure 8a,b), irregular state deposits with high packing density completely enveloped the electrode surfaces after cycling [38]. These indissoluble Li2O2/Li2CO3 hybrid products formed a passivating layer that progressively occluded catalytic active sites. The resultant three-phase interface (TPI) degradation severely impeded mass transport of electrons, Li+ ions, and superoxide radicals (O2−), creating a kinetic bottleneck [39]. This passivation phenomenon, corroborated by reduced discharge potentials (the discharge cut-off potential below 2 V after 30 cycles), accelerated parasitic reactions including electrolyte decomposition and carbon corrosion, ultimately precipitating premature cell failure. In stark contrast, the Co@NC cathode (Figure 8c,d) exhibited exceptional passivation resistance. SEM imaging revealed minimal surface deposition even after extended cycling, confirming exposed active sites. This exceptional stability arises from synergistic effects: carbon coating enhances cobalt particle stability and accelerates Li2O2 decomposition kinetics, while nitrogen-doped porous carbon maintains structural integrity under electrochemical stress. The preservation of three-phase interface (TPI) functionality, unobstructed by thick insulating layers, is evidenced by stable charge–discharge overpotentials and negligible capacity degradation throughout cycling.
To systematically investigate the root causes of cathode cycling stability disparities in LOBs, we conducted SEM analysis of discharged cathodes—a diagnostic approach explicitly designed to correlate morphological evolution with electrochemical performance. This methodical examination revealed striking differences in discharge product morphology across cathode architectures, directly linking structural features to cycling longevity.
For NC cathodes, SEM revealed dense, film-like Li2O2 deposits that progressively passivate the electrode surface (Figure 9c,d). This morphological transition follows a surface-mediated pathway: intermediate LiO2 adsorbs onto the cathode surface (LiO2), followed by direct reduction to Li2O2 (LiO2* + Li+ + e− → Li2O2*) [40,41]. The resulting insulating layer buries active sites, severely limiting ion/electron transport and increasing OER overpotentials during charging—key factors accelerating capacity decay and premature cell failure.
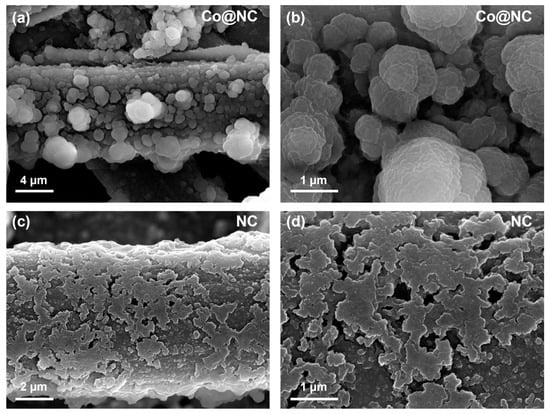
Figure 9.
Morphology in LOBs of (a,b) Co@NC and (c,d) NC cathodes after the first discharge.
In contrast, Co@NC-based cathodes exhibited µm-scale spherical secondary particles formed via solution-mediated mechanisms (Figure 9a,b). Initial LiO2 formation occurs through single-electron reduction (O2 + e− + Li+ → LiO2), followed by dissolution into the electrolyte (LiO2(sol)) and subsequent disproportionation (2LiO2(sol) → Li2O2 + O2) or direct reduction (LiO2(sol) + Li+ + e− → Li2O2) [42,43]. This solution-phase growth preserves electrode porosity and active site accessibility, maintaining efficient mass transport and enabling superior rate capability. The nitrogen-doped porous carbon matrix in Co@NC further enhances stability by preventing cobalt particle agglomeration and accelerating Li2O2 decomposition kinetics, as evidenced by minimal surface deposition even after extended cycling.
The SEM-driven morphological insights thus clarify how carbon layer engineering and pathway-specific product formation synergistically determine cycling stability. Unlike NC cathodes, where surface passivation dominates degradation, Co@NC’s hierarchical porous architecture and solution-mediated growth mechanism preserve three-phase interface functionality, ensuring stable charge–discharge overpotentials and negligible capacity degradation over hundreds of cycles. This mechanistic understanding, rooted in post-cycling microscopy, provides a rational framework for designing next-generation LOB cathodes with optimized stability and performance.
3. Materials and Methods
3.1. Materials
Chitosan ((C6H11NO4)n, deacetylation degree ≥ 95%, viscosity 100–200 mPa·s), cobalt(II) chloride hexahydrate (CoCl2·6H2O, AR, ≥99%), and zinc chloride (ZnCl2, metals basis ≥ 99.95%) were obtained from Shanghai Aladdin Bio-Chem Technology Co., Ltd., Shanghai, China. All reagents were used as received without further purification.
3.2. Electrocatalysts’ Synthesis
3.2.1. The Synthesis of Co@NC
The experimental procedure was conducted as follows: 1.2 g of chitosan was dispersed in 80 mL deionized water, and 3.2 g zinc chloride was dissolved in 20 mL deionized water with ultrasonication-assisted dissolution. The dissolved zinc chloride solution was gradually added dropwise to the chitosan dispersion under continuous stirring. The mixture was stirred overnight, followed by centrifugation, washing, collection, and dissolution. After drying, the material was ready for subsequent use. For cobalt incorporation, 1.2 g of the pretreated chitosan was dispersed in 100 mL of 0.25 M CoCl2·6H2O solution. The mixture was magnetically stirred overnight, then centrifuged for 10 min to collect the precipitate. The obtained product was dried at 70 °C for 8 h and ground into powder. Pyrolytic carbonization was performed under high-purity nitrogen atmosphere at 900 °C for 4 h with a heating rate of 5 °C min−1.
3.2.2. The Synthesis of NC
The NC sample was prepared using the same method, with the only exception being that CoCl2·6H2O was not added to the solution.
3.2.3. The Synthesis of Co@NC-2h and Co@NC-6h
The Co@NC-2h and Co@NC-6h samples were synthesized via identical procedures, differing solely in that pyrolytic carbonization for Co@NC-2h occurred over a 2-h duration and for Co@NC-6h occurred over a 6 h duration.
3.3. Characterization Techniques
3.3.1. Structural and Chemical Analysis
The crystal structure of the metal single-atom doped material was characterized using X-ray diffraction (XRD) performed on a Rigaku D/max-rb diffractometer with Cu Kα radiation (λ = 1.5406 Å, 40 kV, 20 mA). Morphological and elemental analyses were conducted through transmission electron microscopy (TEM) imaging at 300 kV using a JEOL JEM-2100 microscope, coupled with energy dispersive spectroscopy (EDS) via a NORAN System 7 detector integrated within the TEM setup. Surface composition and chemical states were investigated by X-ray photoelectron spectroscopy (XPS) on a Physical Electronics PHI 5700 instrument utilizing Al Kα radiation. Post-cycling cathode degradation was examined through scanning electron microscopy (SEM) on a Helios Nanolab 600i system operated at 20 kV, targeting specimens after full cycle protocols. Raman spectroscopy measurements employed a Renishaw Invia-Reflex system with a 532 nm excitation wavelength. Quantitative analysis of cobalt content in the Co@NC composite was determined via inductively coupled plasma emission spectrometry (ICP).
3.3.2. Li-O2 Cell Assembly and Testing
The cathode catalyst (50 wt%), Ketjen Black EC-600JD (KB, 30 wt%), and polyvinylpyrrolidone binder (PVP, 20 wt%) were homogeneously blended in a solvent mixture (isopropanol/water = 1:3 vol%) and ultrasonically dispersed to form a uniform ink. This slurry was evenly spray-coated onto a disc-shaped carbon paper current collector (diameter = 14 mm). After vacuum-drying at 80 °C for 10 h, the electrodes were transferred to an argon-filled glovebox (O2 < 0.1 ppm, H2O < 0.1 ppm) for battery assembly. 2032-type coin cells were employed for Lithium-Oxygen battery fabrication, using a 16 mm diameter lithium metal anode, Whatman GF/D glass fiber separator, and 1 M LiTFSI in TEGDME electrolyte. Electrochemical performance evaluation, including cyclic stability and rate capability, was conducted on a LANHE CT2001A system under galvanostatic conditions within a voltage window of 2.0–4.5 V or under capacity-controlled protocols. All procedures were performed at ambient temperature (25 ± 1 °C).
4. Conclusions
This study introduces a pioneering innovation in LOB cathode design through the strategic integration of a multiscale porous architecture with pyridinic nitrogen-doped carbon encapsulation, specifically engineered to overcome critical limitations in high-capacity energy storage systems. The chitosan-derived carbon matrix, characterized by hierarchical porosity and ultra-thin conformal coatings (11.7 nm), achieves dual optimization: rapid Li+/O2 transport and stabilization of cobalt particles (1.41 μm) against electrochemical degradation. The integration of pyridinic nitrogen doping creates active sites that enhance charge transfer kinetics, enabling ultra-low overpotential—a pivotal threshold for practical application. Electrochemical performance reaches over 100 cycles at a current density of 500 mA g−1, outperforming conventional catalysts. Beyond incremental gains, this work establishes a universal framework for sustainable catalyst design by leveraging biomass precursors and synergizing doping with macroscopic structural engineering, thereby advancing the commercial viability of high-energy-density metal-air batteries. The findings offer transformative insights into reconciling stability, efficiency, and scalability in next-generation energy storage systems.
Author Contributions
Conceptualization, methodology, investigation and writing—review and editing, Y.G. and M.L.; Visualization, Y.W. and S.G.; Software, Y.M. and X.X.; resources, supervision, project administration, funding acquisition, X.L. and Y.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Fundamental Research Funds for the Youth Foundation of the Shandong Natural Science Foundation (No. ZR2023QB230), the National Natural Science Foundation (No. 22309035) and the Double First-class Discipline Construction Fund Project of the Harbin Institute of Technology at Weihai (No. 2023SYLHY11).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Zhang, Z.; Xiao, X.; Yan, A.; Sun, K.; Yu, J.; Tan, P. Breaking the Capacity Bottleneck of Lithium-Oxygen Batteries through Reconceptualizing Transport and Nucleation Kinetics. Nat. Commun. 2024, 15, 9952. [Google Scholar] [CrossRef]
- Yang, R.; Zhang, G.; Yu, H.; Zhang, D.; Lian, G.; Guo, Z.; Hou, C.; Yang, X.; Wang, N.; Dang, F. Electrocatalysis Synergism Motivated by Low Energy D-Orbitals at High Spin State for Long-Lifespan Li-O2 Batteries. Appl. Catal. B Environ. Energy 2025, 381, 125831. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, D.; Zhang, P.; Hui, X.; Zhang, Z.; Wang, R.; Wang, C.; Ge, X.; Liu, X.; Li, Y.C.; et al. P-Block Element Modulated 1 T Phase MoS2 with Ru Lattice Grafting for High-Performance Li||O2 Batteries. Nat. Commun. 2025, 16, 1453. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Li, X.; Zhao, Y.; Han, G.; Lou, S.; Zhu, Y. Single-Atom Tailored Transition Metal Oxide Enhances d-p Hybridization in Catalytic Conversion for Lithium-Oxygen Batteries. Chem. Eng. J. 2024, 488, 151064. [Google Scholar] [CrossRef]
- Cao, D.; Hao, Y.; Wang, Y.; Bai, Y.; Li, Y.; Wang, X.; Chen, J.; Wu, C. Platinum Nanocrystals Embedded in Three-Dimensional Graphene for High-Performance Li–O2 Batteries. ACS Appl. Mater. Interfaces 2022, 14, 40921–40929. [Google Scholar] [CrossRef]
- Soltani, M.; Amin, H.M.A.; Cebe, A.; Ayata, S.; Baltruschat, H. Metal-Supported Perovskite as an Efficient Bifunctional Electrocatalyst for Oxygen Reduction and Evolution: Substrate Effect. J. Electrochem. Soc. 2021, 168, 034504. [Google Scholar] [CrossRef]
- Wittmaier, D.; Wagner, N.; Friedrich, K.A.; Amin, H.M.A.; Baltruschat, H. Modified Carbon-Free Silver Electrodes for the Use as Cathodes in Lithium–Air Batteries with an Aqueous Alkaline Electrolyte. J. Power Sources 2014, 265, 299–308. [Google Scholar] [CrossRef]
- Guddehalli Chandrappa, S.; Karkera, G.; Gangadharappa, V.A.; Chen, D.; Caruso, R.A.; Annigere, S.P. KNi0.8Co0.2F3 as an Efficient Electrocatalyst for Nonaqueous Li–O2 Batteries. ACS Appl. Energy Mater. 2022, 5, 14680–14686. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Baltruschat, H.; Wittmaier, D.; Friedrich, K.A. A Highly Efficient Bifunctional Catalyst for Alkaline Air-Electrodes Based on a Ag and Co3O4 Hybrid: RRDE and Online DEMS Insights. Electrochim. Acta 2015, 151, 332–339. [Google Scholar] [CrossRef]
- Xia, J.; Yin, S.; Yang, T.; Niu, Y.; Xiong, G.; Guan, X.; Li, N.; Yan, Y.; Han, F.; Hu, R.; et al. Synthesis of Co4N Nanoparticles via a Urea-Glass Route toward Bifunctional Cathode for High-Performance Li−O2 Batteries. J. Energy Storage 2023, 74, 109364. [Google Scholar] [CrossRef]
- Wang, P.; Ren, Y.; Wang, R.; Zhang, P.; Ding, M.; Li, C.; Zhao, D.; Qian, Z.; Zhang, Z.; Zhang, L.; et al. Atomically Dispersed Cobalt Catalyst Anchored on Nitrogen-Doped Carbon Nanosheets for Lithium-Oxygen Batteries. Nat. Commun. 2020, 11, 1576. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Feng, Y.; Zhao, Z.; Lian, F. Boosting the ORR/OER Activity of Cobalt-Based Nano-Catalysts by Co 3d Orbital Regulation. Small 2024, 20, 2400855. [Google Scholar] [CrossRef]
- Wu, M.; Chen, Y.; Qian, Y.; Ye, X.; Wang, N.; Alodhayb, A.; Shi, Z. High-Performance 3d Ordered Hierarchically Interconnected Co@N-Doped Porous Carbon as an Efficient Electrocatalyst in Lithium-Oxygen Batteries. J. Power Sources 2025, 652, 237653. [Google Scholar] [CrossRef]
- Guo, K.; Bao, L.; Yu, Z.; Lu, X. Carbon Encapsulated Nanoparticles: Materials Science and Energy Applications. Chem. Soc. Rev. 2024, 53, 11100–11164. [Google Scholar] [CrossRef]
- Clarysse, J.; Silva, J.D.J.; Xing, Y.; Zhang, S.B.X.Y.; Docherty, S.R.; Yazdani, N.; Yarema, M.; Copéret, C.; Wood, V. Earth-Abundant Ni-Zn Nanocrystals for Efficient Alkyne Semihydrogenation Catalysis. Nat. Commun. 2025, 16, 4378. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Zhang, S.; Liu, J.; Jiang, Y.; Zhang, Y.; Wang, G.; Zhang, H. High-Performance Electrocatalytic Nitrate Reduction into Ammonia Using a Chitosan Regulated Co Nanocatalyst. Inorg. Chem. Front. 2024, 11, 8371–8376. [Google Scholar] [CrossRef]
- Huo, M.; Liang, Y.; Liu, W.; Zhang, X.; Qin, K.; Ma, Y.; Xing, Z.; Chang, J.; Zhu, G. Synergistically Promoting Oxygen Electrocatalysis through the Precise Integration of Atomically-Dispersed Fe Sites and Co Nanoparticles. Adv. Energy Mater. 2024, 2405155. [Google Scholar] [CrossRef]
- Luo, X.; Wei, X.; Wang, H.; Gu, W.; Kaneko, T.; Yoshida, Y.; Zhao, X.; Zhu, C. Secondary-Atom-Doping Enables Robust Fe–N–C Single-Atom Catalysts with Enhanced Oxygen Reduction Reaction. Nano-Micro Lett. 2020, 12, 163. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, X.; Yu, Y.; Zhou, K.; Ye, X.; Zhang, A.; Hou, X.; Chen, B.; Fan, F.; Li, Y.; et al. Dynamic Evolution of Co Species and Morphological Reconstruction on Co-N-C during the Nitrate Reduction Reaction in Neutral Solution. Nano Res. 2025, 18, 94907038. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, C.; Wu, M.; Yu, Y.; Yu, K.; Yan, K.; Shen, C.; Gu, J.; Yan, M.; Sun, C.; et al. Boosting Bi-Directional Redox of Sulfur with Dual Metal Single Atom Pairs in Carbon Spheres Toward High-Rate and Long-Cycling Lithium–Sulfur Battery. Adv. Energy Mater. 2023, 13, 2301505. [Google Scholar] [CrossRef]
- Xu, H.; Liu, H.; Yang, W.; Li, M.; Zhao, F.; Li, C.; Qi, J.; Wang, H.; Peng, W.; Fan, X.; et al. Enhanced Electrocatalytic Conversion of Tellurium with MnO Hollow Nanospheres Modified Hierarchical N-Doped Carbon Nanosheets in High-Performance Aqueous Zn-Te Battery. Chem. Eng. J. 2024, 485, 149825. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.; Fan, K.; Lu, F.; Sun, Q.; Zhang, Q.; Li, B.; Shu, Y.; Zong, L.; Wang, L. Microporous Hard Carbon Support Provokes Exceptional Performance of Single Atom Electrocatalysts for Advanced Air Cathodes. Angew. Chem. 2025, 137, e202501307. [Google Scholar] [CrossRef]
- Huang, M.; Wang, N.; Xie, M.; Fu, Y.; Li, Z.; Lu, Y.; Liu, Q. Phase-Transfer Catalyst for Lithium-Oxygen Batteries Based on Bidirectional Coordination Catalysis: 2-Aminopyridine. Adv. Funct. Mater. 2025, 35, 2420678. [Google Scholar] [CrossRef]
- Xue, Y.; Zuo, Z.; Li, Y.; Liu, H.; Li, Y. Graphdiyne-Supported NiCo2S4 Nanowires: A Highly Active and Stable 3D Bifunctional Electrode Material. Small 2017, 13, 1700936. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Zhao, C.; Yin, X.; Zhao, Y. Co, N Co-Doped Porous Carbons as High-Performance Oxygen Reduction Electrocatalysts. New Carbon Mater. 2021, 36, 209–218. [Google Scholar] [CrossRef]
- Fan, X.; Teng, Z.; Han, L.; Shen, Y.; Wang, X.; Qu, W.; Song, J.; Wang, Z.; Duan, H.; Wu, Y.A.; et al. Boosted Charge and Proton Transfer over Ternary Co/Co3O4/CoB for Electrochemical Nitric Oxide Reduction to Ammonia. Nat. Commun. 2025, 16, 4874. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhang, C.; Zhou, P.; Han, B.; Zhang, Z.; Liu, B. Carbon-Supported Co/Co3O4 Hybrid Catalyst: An Efficient Non-Noble Metal Catalyst for the Hydrodeoxygenation of Vanillin. Catal. Sci. Technol. 2023, 13, 6233–6237. [Google Scholar] [CrossRef]
- Xu, Y.; Li, G.; Li, K.; Hai, J.; Weng, H.; Yuan, H.; Su, Y.; Hu, N.; Zhang, Y. Hierarchical Coaxial Heterostructure Enabled by Thermal Annealing Cobalt Nanowires for Stable Lithium Anodes. Chem. Eng. J. 2025, 508, 160761. [Google Scholar] [CrossRef]
- Namita; Kunal; Khan, A.; Arti; Alam, N.; Ansari, J.R. Synergistic Effect of Cobalt-Reduced Graphene Oxide Hybrid for Enhanced Hydrogen Evolution Reaction. Chemosphere 2025, 379, 144447. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.; Chen, Q.; Yang, M.; Liu, M.; Ma, J.; Zhang, J. Tuning Co-Catalytic Sites in Hierarchical Porous N-Doped Carbon for High-Performance Rechargeable and Flexible Zn-Air Battery. Adv. Energy Mater. 2023, 13, 2202871. [Google Scholar] [CrossRef]
- Lee, K.B.; Jo, S.; Zhang, L.; Kim, M.; Sohn, J.I. Hierarchically Interconnected 3D Catalyst Structure of Porous Multi-Metal Oxide Nanofibers for High-Performance Li–O2 Batteries. Small Methods 2024, 8, e2301728. [Google Scholar] [CrossRef]
- Liao, K.; Zhang, T.; Wang, Y.; Li, F.; Jian, Z.; Yu, H.; Zhou, H. Nanoporous Ru as a Carbon- and Binder-Free Cathode for Li–O2 Batteries. ChemSusChem 2015, 8, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zheng, W.; Kang, C.; Xie, B.; Qian, Z.; Wang, Y.; Ye, S.; Lou, S.; Kong, F.; Mei, B.; et al. Tailoring the p -Band Center of N–S Pair for Accelerating High-Performance Lithium–Oxygen Battery. Small 2023, 19, 2207461. [Google Scholar] [CrossRef] [PubMed]
- Bai, W.-L.; Xu, S.-M.; Xu, C.-Y.; Zhang, Q.; Wang, H.-H.; Zhang, Z.; Chen, X.; Dong, S.-Y.; Liu, Y.-S.; Xu, Z.-X.; et al. Free-Standing N,Co-Codoped TiO 2 Nanoparticles for LiO2 -Based Li–O2 Batteries. J. Mater. Chem. A 2019, 7, 23046–23054. [Google Scholar] [CrossRef]
- Cai, S.; Zheng, M.; Lin, X.; Lei, M.; Yuan, R.; Dong, Q. A Synergistic Catalytic Mechanism for Oxygen Evolution Reaction in Aprotic Li–O2 Battery. ACS Catal. 2018, 8, 7983–7990. [Google Scholar] [CrossRef]
- Tong, S.; Zheng, M.; Lu, Y.; Lin, Z.; Li, J.; Zhang, X.; Shi, Y.; He, P.; Zhou, H. Mesoporous NiO with a Single-Crystalline Structure Utilized as a Noble Metal-Free Catalyst for Non-Aqueous Li–O2 Batteries. J. Mater. Chem. A 2015, 3, 16177–16182. [Google Scholar] [CrossRef]
- Li, W.; Meng, R.; Wang, K.; Cheng, Y.; Cai, D.; Zhan, G. Engineering Pyridinic-N-Co Sites for Enhanced CO2 Hydrogenation to Methanol. Appl. Catal. B Environ. Energy 2025, 365, 124906. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, G.; Liu, R.; Yang, R.; Li, X.; Zhang, X.; Yu, H.; Zhang, P.; Li, B.; Hou, H.; et al. Mutually Activated 2D Ti0.87O2/MXene Monolayers Through Electronic Compensation Effect as Highly Efficient Cathode Catalysts of Li–O2 Batteries. Adv. Funct. Mater. 2025, 35, 2414679. [Google Scholar] [CrossRef]
- Shen, Z.; Lang, S.; Zhou, C.; Wen, R.; Wan, L. In Situ Realization of Water-Mediated Interfacial Processes at Nanoscale in Aprotic Li–O2 Batteries. Adv. Energy Mater. 2020, 10, 2002339. [Google Scholar] [CrossRef]
- Zhao, C.; Yan, Z.; Zhou, B.; Pan, Y.; Hu, A.; He, M.; Liu, J.; Long, J. Identifying the Role of Lewis-base Sites for the Chemistry in Lithium-Oxygen Batteries. Angew. Chem. Int. Ed. 2023, 62, e202302746. [Google Scholar] [CrossRef]
- Xia, Y.; Fan, S.; Jin, X.; Wang, L.; Lin, S.; Yan, J.; Han, J.; Yu, Z.; Peng, D.-L.; Yue, G. Amorphous Interface-Controlled Discharge Product Formation: A Pathway to High-Performance Lithium-Oxygen Batteries. Nano Energy 2025, 141, 111086. [Google Scholar] [CrossRef]
- Liu, Y.; Han, M.; Liu, J.; Huang, Y.; Liu, Y.; Li, W.; Liu, Y.; Li, J. Self-Reconstruction of Ultralow-Pt-Loading Co3O4 Nanoneedle Array Cathode for High-Performance Li-O2 Battery. Chem. Eng. J. 2025, 512, 162420. [Google Scholar] [CrossRef]
- Amin, H.M.A.; Molls, C.; Bawol, P.P.; Baltruschat, H. The Impact of Solvent Properties on the Performance of Oxygen Reduction and Evolution in Mixed Tetraglyme-Dimethyl Sulfoxide Electrolytes for Li-O2 Batteries: Mechanism and Stability. Electrochim. Acta 2017, 245, 967–980. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).