Abstract
This study reports on green-synthesized zinc oxide nanoparticles (ZnONPs), focusing on their physicochemical characterization, photocatalytic properties, and agricultural applications. Dynamic light scattering (DLS) analysis revealed a mean hydrodynamic diameter of 337.3 nm and a polydispersity index (PDI) of 0.400, indicating moderate polydispersity and nanoparticle aggregation, typical of biologically synthesized systems. High-resolution transmission electron microscopy (HR-TEM) showed predominantly spherical particles with an average diameter of ~28 nm, exhibiting slight agglomeration. Energy-dispersive X-ray spectroscopy (EDX) confirmed the elemental composition of zinc and oxygen, while X-ray diffraction (XRD) analysis identified a hexagonal wurtzite crystal structure with a dominant (002) plane and an average crystallite size of ~29 nm. Photoluminescence (PL) spectroscopy displayed a distinct near-band-edge emission at ~462 nm and a broad blue–green emission band (430–600 nm) with relatively low intensity. The ultraviolet–visible spectroscopy (UV–Vis) absorption spectrum of the synthesized ZnONPs exhibited a strong absorption peak at 372 nm, and the optical band gap was calculated as 2.67 eV using the Tauc method. Fourier-transform infrared spectroscopy (FTIR) analysis revealed both similarities and distinct differences to the pigeon extract, confirming the successful formation of nanoparticles. A prominent absorption band observed at 455 cm−1 was assigned to Zn–O stretching vibrations. X-ray photoelectron spectroscopy (XPS) analysis showed that raw pigeon droppings contained no Zn signals, while their extract provided organic biomolecules for reduction and stabilization, and it confirmed Zn2+ species and Zn–O bonding in the synthesized ZnONPs. Photocatalytic degradation assays demonstrated the efficient removal of pollutants from sewage water, leading to significant reductions in total dissolved solids (TDS), chemical oxygen demand (COD), and total suspended solids (TSS). These results are consistent with reported values for ZnO-based photocatalytic systems, which achieve biochemical oxygen demand (BOD) levels below 2 mg/L and COD values around 11.8 mg/L. Subsequent reuse of treated water for irrigation yielded promising agronomic outcomes. Wheat and barley seeds exhibited 100% germination rates with ZnO NP-treated water, which were markedly higher than those obtained using chlorine-treated effluent (65–68%) and even the control (89–91%). After 21 days, root and shoot lengths under ZnO NP irrigation exceeded those of the control group by 30–50%, indicating enhanced seedling vigor. These findings demonstrate that biosynthesized ZnONPs represent a sustainable and multifunctional solution for wastewater remediation and agricultural enhancement, positioning them as a promising candidate for integration into green technologies that support sustainable urban development.
1. Introduction
In the 21st century, despite technological advances, water contamination remains one of the most significant threats to public health and environmental sustainability [1]. Rapid industrialization, urban expansion, and improper waste disposal practices have led to the widespread release of hazardous organic and inorganic pollutants into aquatic ecosystems [2,3].
Conventional wastewater treatment techniques, such as adsorption, coagulation, and biological oxidation, often fail to remove persistent organic pollutants (POPs) and pharmaceutical compounds effectively [4,5]. Consequently, there is a growing need for more advanced, efficient, and environmentally friendly purification technologies [6,7,8]. Among the promising approaches is photocatalysis using metal oxide nanoparticles, notably titanium dioxide (TiO2) and zinc oxide (ZnO). These semiconducting nanomaterials possess high surface reactivity and can degrade organic pollutants into non-toxic byproducts under light irradiation [9,10,11].
Zinc oxide nanoparticles (ZnONPs) are among the most widely utilized metal oxide nanomaterials in various industrial and environmental applications. Over the past two decades, research has focused on evaluating their effects on wastewater treatment and plant systems [12,13,14,15]. Numerous studies have demonstrated the potential benefits of ZnONPs, particularly in enhancing plant growth, seed germination, and stress tolerance, thereby supporting their application in sustainable agriculture and environmental remediation. Among various semiconductors, ZnO has been extensively studied; however, its wide band gap (~3.2 eV) limits its photocatalytic activity under visible light. Compared to TiO2, which has a similar band gap energy, ZnO demonstrates superior photocatalytic activity under solar irradiation due to its higher absorption efficiency and non-stoichiometric nature [16,17].
Beyond environmental remediation, ZnONPs have shown promise in agricultural applications, particularly as nanofertilizers. Conventional nitrogen- and phosphorus-based fertilizers suffer from low nutrient use efficiency, with up to 70% of the nutrients lost through leaching or volatilization. This not only contributes to environmental pollution but also necessitates the development of more sustainable alternatives. ZnONPs can enhance nutrient uptake, seed germination, and plant growth due to their bioavailability and small size [18].
Recent studies, including that by Włodarczyk et al. [19], have demonstrated that ZnONPs positively influence germination dynamics in tomato cultivars, with smaller particles (<50 nm) being more effective. Similar findings across other crops suggest that ZnONPs at appropriate concentrations can serve dual roles in pollution mitigation and agricultural enhancement, although caution is warranted, as excessive concentrations may induce phytotoxic effects [20,21,22]. In another study, Al-Sudani and colleagues assessed the effect of ZnO and Fe2O3 NPs on sunflower seeds. While the germination percentage remained stable, smaller ZnO (7 nm) and Fe2O3 (4.5 nm) NPs enhanced total phenolic content and showed strong anti-glycation activity [23]. These findings show that nanoparticle type and size can influence not only plant growth but also biochemical properties, with potential applications in functional food development. Collectively, these insights highlight the multifaceted utility of ZnO nanomaterials in addressing pressing environmental and agricultural challenges [18,19].
This study aims to synthesize ZnONPs using an eco-friendly approach based on waste biomaterial (pigeon droppings) and investigate their optical, morphological, and structural properties. Additionally, the study evaluates the effectiveness of the synthesized ZnONPs in treating sewage water and assesses the impact of this treated water on the seed germination of two selected crop species: wheat (Triticum aestivum) and barley (Hordeum vulgare). This is the first study to explore the use of ZnONPs synthesized from pigeon droppings for wastewater treatment and to investigate the effects of the resulting treated water on seed germination in different plant species.
2. Results and Discussion
A picture of the dynamic light scattering (DLS) size distribution of green-produced ZnONPs is shown in Figure 1. The particle size distribution confirmed the existence of zinc oxide NPs, and showed an average particle size of 337.3 nm and a polydispersity index of 0.400. The particle size is larger than previous measurements due to aggregated particles. Because larger particles have a greater tendency to agglomerate, leading to accumulation, very few nanoparticle applications require a perfect distribution of small nanoparticles. Agglomeration, sometimes called secondary particles, depends on factors such as surface ligands, capping agents, and synthesis conditions. When compared to other methods, DLS often reports particles with bigger dimensions [24]. These results also confirmed that the polydispersity index values of the monodispersed ZnONPs with a very wide size range are greater than 0.7.
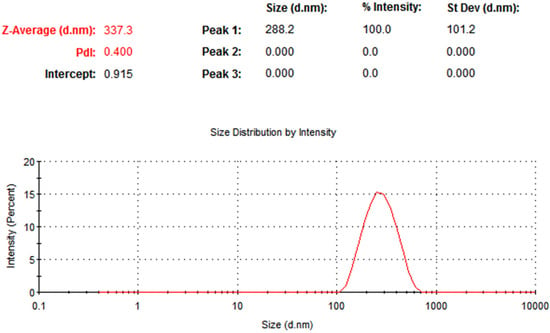
Figure 1.
Dynamic light scattering (DLS) analysis result of synthesized zinc oxide nanoparticles (ZnONPs).
Using a carbon-coated copper grid and ultra-pure water as a solvent, high-resolution transmission electron microscopy (HR-TEM) images were obtained at an accelerating voltage of 200 kV. The morphology and size of these ZnONPs were directly affected by the concentration of the extract used, as shown in Figure 2, which was confirmed by TEM analysis. This analysis also confirmed the growth pattern and crystallite distribution of the particles. The particles exhibited little aggregation and were spherical [24,25,26,27]. Their sizes ranged from 28 nm [28].
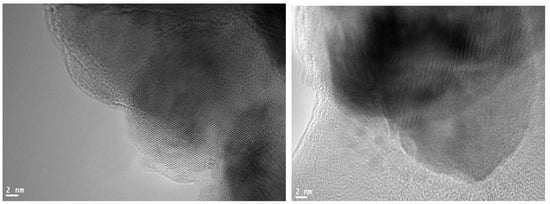
Figure 2.
Transmission electron microscopy (TEM) images of synthesized ZnONPs.
Energy-dispersive X-ray spectroscopy (EDX) was also used to analyze the elemental makeup of the ZnONPs. The EDX spectrum (Figure 3 (red arrows)) shows three characteristic zinc peaks and one oxygen peak, confirming the formation of ZnO nanoparticles [16]. Three zinc peaks and one oxygen peak are present in the EDX graph. The results agree with previous research; between 0 and 1.5, there was one zinc peak and one oxygen peak, and between 7.5 and 10.4, two additional zinc peaks emerged [29,30].
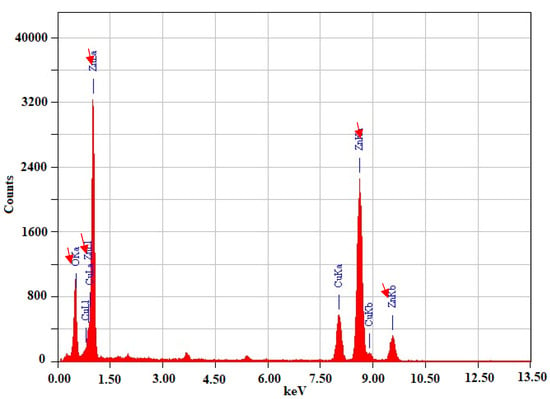
Figure 3.
Energy-dispersive X-ray spectroscopy (EDX) mapping of synthesized ZnONPs.
Figure 4 shows the X-ray diffraction (XRD) results that describe the crystal structure of the produced ZnONPs. Figure 1 shows the XRD of ZnO (ICDD card number -003-0888), which confirms the hexagonal wurtzite structure (space group P63mc, No. 186) with well-defined peaks at 2θ of 31.7°, 34.4°, 36.2°, 47.5°, 56.6°, 63.0°, and 66.56°, which correspond to the lattice planes (100), (002), (101), (102), (110), (103), and (200) of ZnO [31,32,33]. Strong diffraction peaks of ZnO occur at 2θ of 31.7°, 34.4°, and 36.2°, with the associated planes being (100), (002), and (101), respectively. According to references [33,34,35], the most intense point on the (002) plane indicates that the c-axis is the preferred direction for development. The diffraction peaks from ZnO were strong in the samples, whereas peaks from Zn were modest [36]. The presence of interstitial Zn in ZnO lattices was shown by the peak at 2θ = 46°.
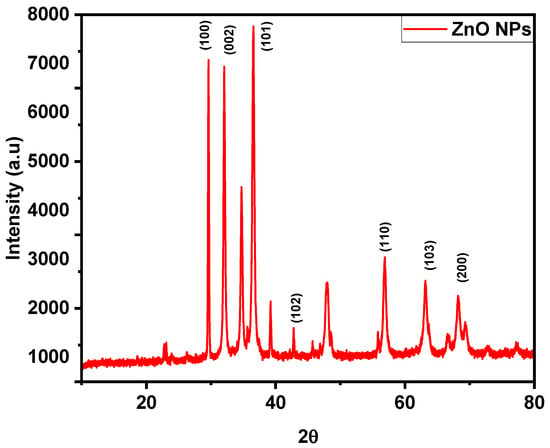
Figure 4.
X-ray diffraction (XRD) pattern of synthesized ZnONPs.
The additional peaks seen in the XRD data are potentially attributable to the biological production process of the NPs. Different diffraction peaks might be seen if residual living molecules or biomolecules interact with the ZnONPs. Because of the nature of biological production processes, very small quantities of other substances may also be present. For example, extra diffraction peaks might be introduced by organic residues or metal ions originating from biological sources [37]. Equation (1), the Debye–Scherrer formula, was used to determine the average size of the crystallites:
where D is the crystallite size, λ is the X-ray wavelength, β is the full breadth at half maximum, K is a constant = 0.9 and θ is the angle of the largest peak [38]. In line with other findings on nanoscale oxides, the ZnONPs had an average diameter of around 29 nm.
D = Kλ/βcosθ
In Figure 5, the Photoluminescence (PL) spectra of the ZnO particles are shown at room temperature. One emission band predominated in the spectrum. A single prominent emission band is observed at around 462 nm, corresponding to an energy of 2.70 eV. This emission is likely due to defect-related states or fluorescence from the biological medium. The characteristics of ZnONPs, including possible quantum confinement effects, may also influence this emission [39]. The visible emission band often associated with deep-level emission (DLE) in ZnO is the other band seen in PL spectra, according to Madler et al. [40]. For the most part, interstitial oxygen (Oi), zinc vacancies (ZnO), or oxygen vacancies (VO) are the sources of DLE. The energy transfer from the donor level of Zn interstitial (Zni) to the acceptor level of the Zn vacancy (ZnO) is the most probable source of the blue emission around 430–600 nm [41].
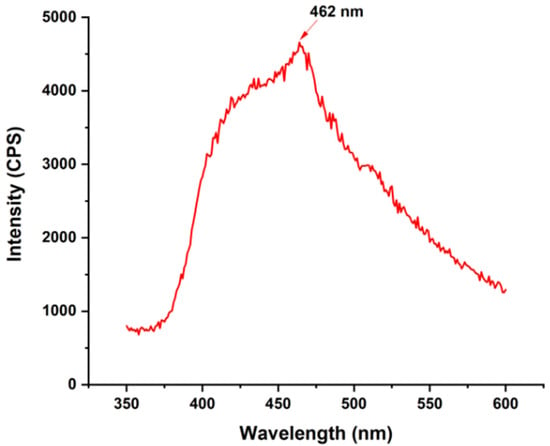
Figure 5.
Photoluminescence (PL) emission spectrum of synthesized ZnONPs.
The radiative transfer of electrons from oxygen vacancy-generated shallow donor levels to the valence band likely produced the blue–green band at around 470 nm. A photo-generated hole recombines with the singly ionized charge state of a particular defect, resulting in its emission [42]. The recombination probability of the photo-excited load carriers is directly proportional to the PL emission rate. An increase in PL strength indicates a decrease in photocatalytic activity and more e−/h+ pair recombination. In contrast, a decrease in PL intensity suggests less e−/h+ pair recombination, which in turn reveals a high competence for photoinduced charge carrier partitioning and an increase in photocatalytic activity. There appear to be fewer external defects in the crystalline structure, as indicated by the increased photocatalytic activity and decreased recombination rate resulting from the low intensity of ZnONPs [43].
The Fourier-transform infrared spectroscopy (FTIR) spectrum of the pigeon droppings extract (Figure 6A) exhibits several characteristic absorption bands corresponding to functional groups present in the biomolecules of the extract. A broad and intense band at 3394 cm−1 is attributed to O–H and N–H stretching vibrations, indicating the presence of alcohols, phenols, and amide groups from proteins. The peaks at 2939 cm−1 and 2386 cm−1 correspond to C–H stretching of aliphatic compounds and possible stretching of C≡N or CO2, respectively. The band observed at 1658 cm−1 is assigned to the amide I group (C=O stretching of proteins), while the peak at 1411 cm−1 corresponds to C–N stretching vibrations of aromatic amines. The absorption band at 1049 cm−1 can be attributed to C–O stretching vibrations of alcohols, carboxylic acids, and polysaccharides, indicating the presence of carbohydrates. The band at 617 cm−1 represents out-of-plane bending vibrations associated with aromatic compounds. These functional groups collectively confirm the presence of proteins, carbohydrates, and other biomolecules in the extract, which likely act as reducing and stabilizing agents in the green synthesis of ZnONPs [44]. The FTIR spectrum of the synthesized ZnONPs (Figure 6B) shows both similarities and distinct differences compared to the extract, reflecting the successful formation of ZnO. The broad peak around 3541–3749 cm−1 corresponds to O–H stretching vibrations from adsorbed water molecules and surface hydroxyl groups on ZnO. The band at 2368 cm−1 is due to atmospheric CO2 absorption. A sharp band at 1620 cm−1 is assigned to O–H bending of adsorbed water, while the peaks at 1373 and 1203 cm−1 may be related to residual organic molecules from the extract, suggesting partial capping of the nanoparticles. Importantly, the strong absorption band at 455 cm−1 is attributed to Zn–O stretching vibrations, which is the characteristic fingerprint confirming the successful synthesis of ZnO nanoparticles. The FTIR spectra demonstrate that the biomolecules in pigeon droppings extract provided functional groups capable of reducing zinc ions and stabilizing the resulting ZnO nanoparticles. The disappearance or shift of several peaks in the ZnO spectrum compared to the extract indicates the involvement of these biomolecules in nanoparticle formation, while the strong Zn–O stretching band confirms the crystalline ZnO framework [45].
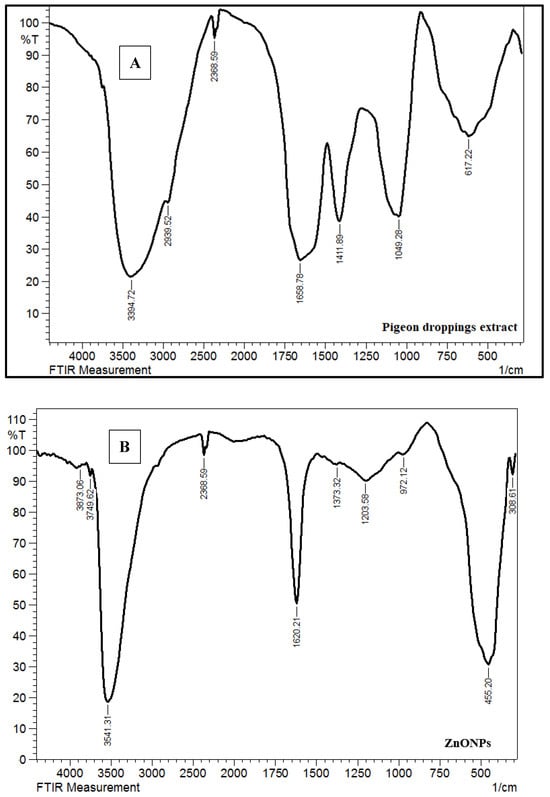
Figure 6.
Fourier-transform infrared spectroscopy (FTIR) spectra of (A) pigeon droppings extract and (B) synthesized ZnONPs.
The UV–Vis absorption spectrum of the synthesized ZnO nanoparticles (Figure 7A) exhibited a strong absorption peak at 372 nm, which is characteristic of the intrinsic band–band transition of ZnO. This sharp absorption in the near-UV region confirms the successful formation of ZnO nanoparticles with good crystallinity. Compared to the absorption edge of bulk ZnO, typically observed around 375–380 nm, a slight shift was noticed, which may be attributed to quantum confinement effects at the nanoscale. The absence of significant absorption in the visible region further indicates high purity of the synthesized ZnONPs without the presence of secondary phases. The optical band gap was further determined from the Tauc plot method by extrapolating the linear portion of (αhν)2 versus photon energy (hν), as shown in Figure 7B. The calculated band gap was found to be 2.67 eV, which is lower than that of bulk ZnO. This red-shifted band gap narrowing can be explained by the presence of defect states, oxygen vacancies, or interstitial defects that create localized energy levels near the conduction or valence bands. In addition, the biological synthesis route may contribute to surface modification of the nanoparticles due to capping by biomolecules, which could also influence the electronic structure. The observed narrowing of the band gap enhances the optical response of ZnONPs towards the visible region, thereby making them promising candidates for visible-light-driven photocatalysis [46].
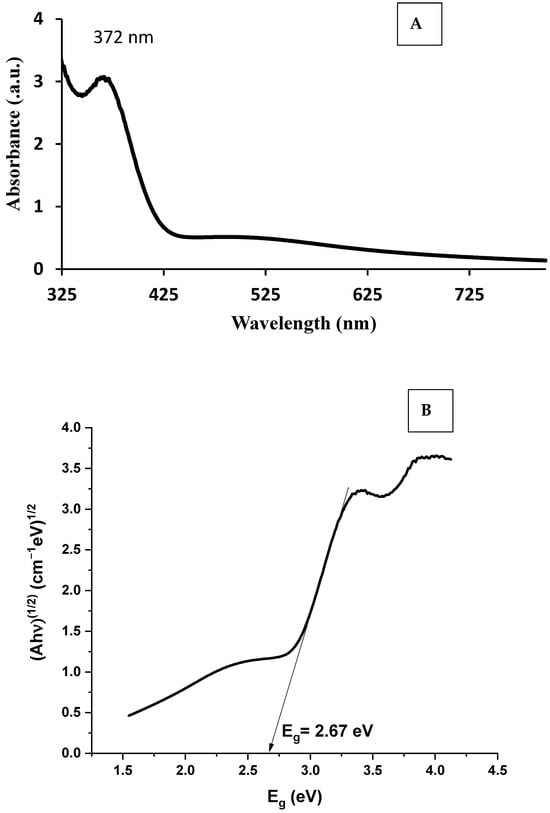
Figure 7.
(A) UV analysis spectrum, (B) band gap “Eg” analysis of synthesized ZnONPs.
The X-ray photoelectron spectroscopy (XPS) survey spectra provide valuable insights into the elemental composition and chemical states of both the raw pigeon droppings extract (Figure 8A) and the synthesized ZnO nanoparticles (Figure 8B), where the extract served as a bio-reducing and stabilizing agent. In the XPS spectrum of pigeon droppings (Figure 8A), the major peaks are assigned to O 1s (~532 eV), C 1s (~285 eV), and N 1s (~400 eV), along with Ca-related peaks (Ca 2s, Ca 2p1/2, Ca 2p3/2). The strong presence of C, N, and O confirms the abundance of organic matter such as proteins, uric acid, polysaccharides, and other biomolecules in the droppings. The Ca peaks are attributed to mineralized components commonly present in avian excreta, such as calcium phosphates and carbonates, as previously reported by Rouzet et al. and Gómez-Heras et al. [47,48]. The relatively high intensity of the O 1s and C 1s peaks suggests the predominance of oxygenated and carbonaceous groups, which play a critical role in the reduction and stabilization of metal precursors during nanoparticle synthesis, consistent with the findings of El-Belely et al. [27]. In contrast, the XPS spectrum of the synthesized ZnO nanoparticles (Figure 8B) reveals distinct peaks corresponding to Zn 2p3/2 (~1021–1022 eV) and Zn 2p1/2 (~1044–1045 eV), confirming the presence of Zn in the +2 oxidation state, characteristic of ZnO. The binding energy separation of ~23 eV between Zn 2p3/2 and Zn 2p1/2 further supports the formation of ZnO. Additionally, the O 1s peak (~530 eV) is attributed to lattice oxygen in Zn–O bonds, while a peak or broadening in this region may be related to surface hydroxyl groups or oxygen vacancies, which enhance surface reactivity and photocatalytic activity, as observed in similar studies by Rambabu et al. [49]. A weak C 1s signal is also detected, likely originating from residual organic capping agents derived from the pigeon droppings, suggesting their role in nanoparticle stabilization. Compared with the pigeon droppings extract, the disappearance or significant reduction of Ca and N signals in the ZnO NP spectrum confirms the successful transformation of the biological matrix into an inorganic ZnO phase, with only minor organic residues remaining as surface passivating agents. These surface residues may contribute to improved biocompatibility and dispersibility of the nanoparticles, in agreement with observations by Barrak et al. and Khan et al. [50,51].
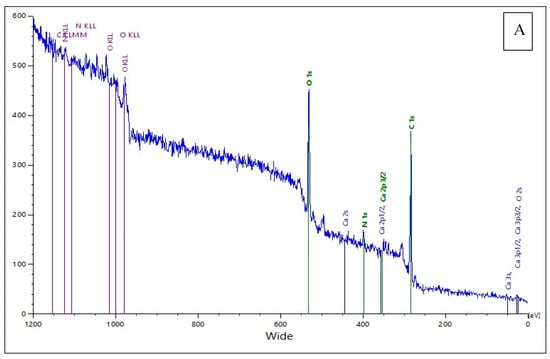
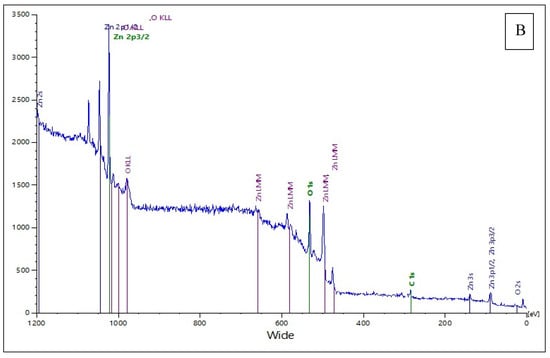
Figure 8.
X-ray photoelectron spectroscopy (XPS) analysis of (A) raw pigeon droppings and (B) synthesized ZnONPs.
Figure 9 shows that green-treated ZnONPs are a good photocatalytic material for degrading organic chemicals and colors in wastewater. While 98.8% of the crystal violet (CV) dye (Figure 9A) was destroyed after 24 h under UV and visible irradiation, the methylene blue (MB) dye (Figure 9B) was almost entirely degraded at 99.7% after 24 h under both types of radiation. In general, ZnONPs show great promise as a catalyst for the photocatalytic breakdown of harmful compounds or dyes, which might lead to their use in bioremediation and treatment [52]. The procedure for the photodegradation experiment followed the methodology previously reported by [52], with slight modifications.
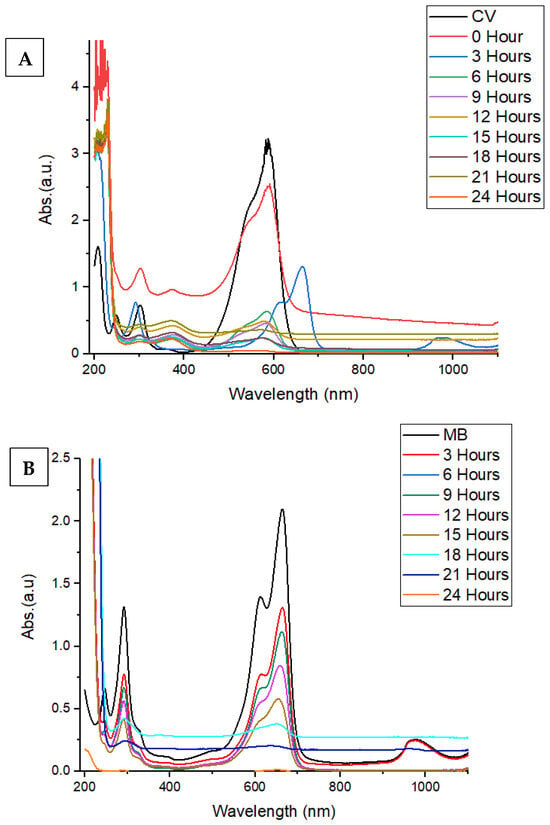
Figure 9.
UV–Vis spectral degradation of (A) CV and (B) BM dyes using green synthesized ZnONPs as a nanocatalyst.
This use of green-synthesized ZnONPs to treat sewage water demonstrated encouraging outcomes in terms of organic pollutant reduction, suspended solid improvement, and overall water quality enhancement. Previous studies have shown that using ZnO-based photocatalysis for wastewater treatment may significantly reduce the biological oxygen demand (BOD) to less than 2 mg/L and the chemical oxygen demand (COD) to 11.8 mg/L (Table 1). Our results align with these findings [53,54].

Table 1.
Physicochemical analysis of sewage water treated with green-synthesized ZnONPs.
The chloride levels remained at 368 mg/L for inorganic measures, which is higher than in other investigations that used additional post-treatment methods such as adsorption or membrane filtration [55]. Consistent with conventional disinfection methods, a chlorine residual of less than 0.20 mg/L guarantees low levels of residual chlorine without compromising antimicrobial capabilities. The overall hardness level of 288 mg/L and alkalinity level of 42 mg/L as CaCO3 indicate moderate hardness levels, which is in line with other studies reporting that ZnONPs had little influence on hardness but successfully decreased heavy metal concentrations [56]. Prior research has investigated the presence of ionic species using hybrid treatments such as ZnO combined with activated carbon or membrane technology, as shown by the electrical conductivity (1700 µS/cm) and total dissolved solids (TDS; 1090 mg/L) [57].
Furthermore, the elimination of total suspended solids (TSS < 5 mg/L) demonstrates efficient particulate filtration, in agreement with prior findings that emphasize the function of ZnO in destabilizing and aggregating suspended particles [58]. The nearly neutral pH of 6.78 is consistent with earlier research, which has shown that ZnO treatment typically maintains water pH within a safe range, preventing it from becoming overly acidic or alkaline [59].
Our results show that green-synthesized ZnONPs perform well for sewage treatment, but further research into how to maximize the breakdown of pollutants by adjusting dose, light exposure, and nanoparticle characteristics is needed. Research using hybrid ZnO systems, including ZnO-TiO2 composites or doped ZnONPs, has shown better photocatalytic effectiveness and elimination of long-lasting pollutants compared to other investigations. Hence, these adjustments, if implemented, might make ZnO-based wastewater treatment even more effective [60].
ZnONPs are an environmentally benign and economically viable method for treating wastewater. They have shown considerable photocatalytic activity when exposed to sunlight. In the presence of natural sunlight, these nanoparticles have been shown to efficiently break down organic contaminants, such as phenolic chemicals and dyes. In 2023, researchers investigated the performance of biosynthesized ZnONPs as photocatalysts in a solar environment. Within 180 min of exposure to sunlight, these nanoparticles efficiently degraded organic chemicals found in synthetic petroleum effluent. For phenol, o-cresol, and other compounds, the degradation efficiencies were 51%, 52%, 88%, and 93%, respectively [60].
In 2020, another study investigated the environmentally friendly production of ZnONPs using several plant extracts, such as cedar, beetroot, and pomegranate. These nanoparticles showed promise in degrading organic contaminants in wastewater: the research demonstrated improved photocatalytic activity under sunshine for these nanoparticles [58]. Also, in 2025, researchers used chia seed extract to coat the metal oxide NPs they generated. Under sunlight, these nanoparticles showed photocatalytic activity, suggesting they may be used for environmental cleanup [61,62].
Both Triticum aestivum (T. aestivum) (Figure 10) and Hordeum vulgare (H. vulgare) (Figure 11) demonstrated a 100% final germination percentage (FGP) when irrigated with sewage water treated using ZnONPs, significantly higher than the germination observed in chlorine-treated sewage water (65% for wheat and 68% for barley) and even the control group (89% for wheat and 91% for barley). These findings indicate that ZnO NP treatment not only detoxifies sewage water but also creates favorable conditions for seed germination.

Figure 10.
Triticum aestivum seedlings after 21 days of treatment.

Figure 11.
Hordeum vulgare seedlings after 21 days of treatment.
In terms of root development (see Table 2), T. aestivum showed a progressive increase in root length over time, reaching a maximum of 25.63 ± 0.15 cm on day 21 under ZnO NP treatment, compared to 18.00 ± 0.26 cm in the control and just 7.60 ± 0.15 cm in the chlorine-treated group. Similarly, H. vulgare exhibited the greatest root length of 27.53 ± 0.23 cm under ZnO NP treatment, outperforming the control (20.57 ± 0.18 cm) and chlorine treatment (9.13 ± 0.28 cm). A similar trend was observed in shoot length, with ZnO NP-treated plants showing the highest values at day 21: 22.53 ± 0.15 cm in T. aestivum and 24.47 ± 0.19 cm in H. vulgare (Figure 12A). Additionally, shoot growth at earlier stages (days 7 and 14) was significantly higher in the ZnO NP group than in the other treatments, indicating that nanoparticle treatment promotes early seedling vigor [63].

Table 2.
Effect of sewage water treated with nanoparticles on seed germination and seedling stages of Triticum aestivum and Hordeum vulgare after 7, 14, and 21 days of treatment.
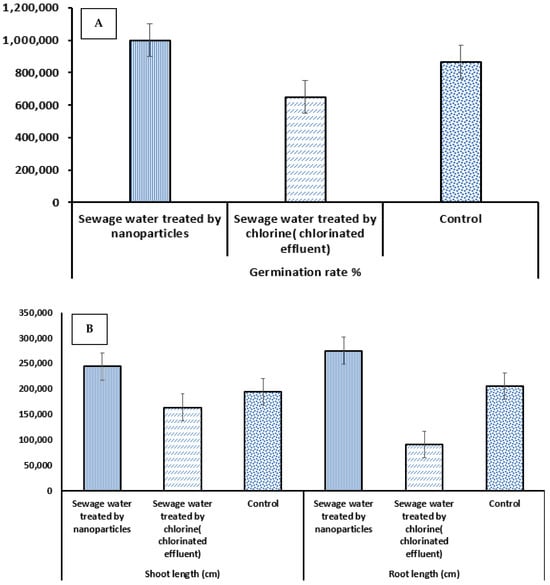
Figure 12.
Germination rates and the root and shoot lengths for all treatments under study in Hordeum vulgare seedlings after 21 days of treatment. (A) FGP (final germination percentage), (B) root length and shoot length.
These results suggest that ZnO NP-treated sewage water significantly enhances both germination and early seedling growth in wheat and barley. The 100% FGP recorded under ZnO NP treatment suggests that the nanoparticles effectively reduce the toxicity of sewage water, possibly through the photocatalytic degradation of harmful organic and inorganic contaminants [64]. This makes the treated water more suitable for supporting biological processes, such as seed germination. The observed improvements in root and shoot lengths further indicate enhanced nutrient availability and water quality. Zinc, released in ionic form from ZnONPs, may contribute to this effect by supporting key enzymatic and physiological processes essential for plant development.
In contrast, chlorine-treated sewage water resulted in the lowest germination rates and poorest seedling growth [65]. Thus, residual chlorine or its byproducts may have phytotoxic effects that inhibit root elongation and shoot development [66]. Both plant species displayed a clear upward trend in root and shoot growth over 7, 14, and 21 days (Figure 12B), with the ZnO NP group showing the most rapid and sustained development, highlighting the efficacy of nanoparticles in promoting early plant growth. While both wheat and barley responded positively to ZnO NP treatment, H. vulgare exhibited slightly greater shoot and root growth than T. aestivum, potentially due to species-specific physiological differences or variations in zinc uptake efficiency. These findings support the potential of ZnONPs not only for effective sewage water treatment but also for enhancing agricultural productivity through improved seed germination and seedling establishment [67]. The proposed mechanism of photocatalysis by ZnONPs for the treatment of sewage water is depicted in Figure 13.
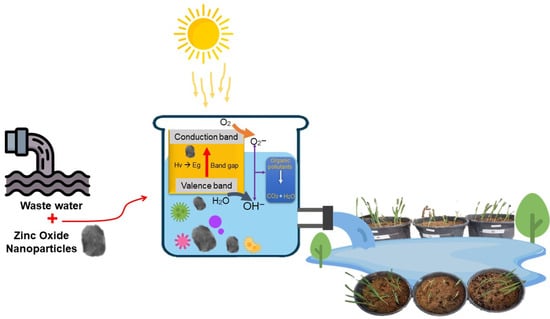
Figure 13.
Proposed mechanism of photocatalysis by ZnONPs for the treatment of sewage water.
In comparison with previous studies, Haidri et al. [22] explored the use of biologically synthesized ZnONPs as an eco-friendly and cost-effective solution for municipal wastewater treatment. ZnONPs were synthesized using the bacterium Shewanella sp. The nanoparticles demonstrated strong photocatalytic activity, significantly reducing levels of TDS, COD, sulfates, and phosphates in the wastewater by 76.5%, 57.1%, 81.1%, and 67.4%, respectively. The treated water was then used for wheat cultivation, resulting in notable increases in chlorophyll (45%), carotenoids (40.8%), and antioxidants (10.5–30.6%). Additionally, oxidative stress markers such as hydrogen peroxide (H2O2) and malondialdehyde (MDA) were reduced by 8.1% and 30.1%, respectively. These findings indicate that biosynthesized ZnONPs can effectively purify wastewater while enhancing wheat growth and stress tolerance, making them a promising tool for sustainable agriculture and water reuse.
The study in [67] investigated the effects of untreated textile effluent versus effluent treated with ZnONPs on seed germination and early seedling growth. The results showed that untreated textile wastewater significantly inhibited germination rates and suppressed root and shoot growth due to the presence of high levels of dissolved solids, dyes, and other toxic compounds that create osmotic stress and interfere with water uptake by seeds. In contrast, wastewater treated with ZnONPs demonstrated markedly improved outcomes. The seeds exposed to this treated water exhibited higher germination percentages and better-developed seedlings with enhanced root and shoot lengths. The ZnONPs likely contributed to these improvements through their strong photocatalytic activity, which effectively degrades organic dyes and reduces the toxicity of the effluent. This treatment rendered the water more suitable for plant development. The study concluded that ZnO nanoparticle treatment significantly reduced the phytotoxic effects of textile industry wastewater and restored its usability for agricultural purposes, offering a promising and efficient method for wastewater detoxification and reuse.
A study on Syzygium cumini-mediated green synthesis of ZnONPs [68] demonstrated effective and eco-friendly production of crystalline, hexagonal–spherical ZnONPs, which significantly improved Pennisetum glaucum seed germination and achieved 98% degradation of Rhodamine B dye under optimized light, temperature, and pH conditions. In ref. [69], seed priming with ZnONPs in wheat under saline irrigation was examined: primed wheat seeds (50 mg/L ZnONPs, <50 nm) showed substantial improvements in grain yield—up to 36% enhancement at moderate salinity (4.42 dS/m)—alongside increased macronutrient (N, P, K) uptake in straw and grain, although Zn uptake declined under high salinity levels. Last, a comprehensive review [70] on biosynthesis of ZnONPs using floral waste extract highlighted that floral extracts rich in polyphenols, flavonoids, and sugars produce varied ZnO nanostructures (8–90 nm) with excellent photocatalytic and adsorptive capabilities. These green-synthesized NPs have proven effective in wastewater treatment (dye removal > 95%) and agricultural enhancement (improved seed germination), as well as exhibiting antibacterial, antifungal, and anticancer properties. Together, these studies underscore the multifunctional potential of green ZnONPs in environmental remediation, crop productivity, and sustainable nanoparticle synthesis.
Previous literature has provided detailed mechanistic insights such as Zn2+ release kinetics in the rhizosphere, nanoparticle interactions with seed surfaces and root tissues, and physiological mechanisms including hormone modulation and antioxidant responses that help to enrich the understanding of ZnO NP-mediated seed germination. For example, Elhaj Baddar and Unrine [71] demonstrated that ZnONPs can serve as effective seed treatments, enhancing seedling growth and Zn nutrition more efficiently than ionic Zn sources such as ZnSO4. Similarly, Sánchez-Pérez et al. [72] reported that ZnONPs improved zinc uptake in wheat without impairing germination, whereas high concentrations of ZnSO4 reduced both germination and growth. With respect to hormone regulation and antioxidant activity, biosynthesized ZnONPs have been shown to enhance germination rates in tomato and chili seeds, likely by promoting the biosynthesis of key growth hormones such as auxins and gibberellins. In rice, seed priming with appropriate doses of ZnONPs significantly increased antioxidant enzyme activities (e.g., superoxide dismutase (SOD), catalase (CAT)) and improved germination indices (e.g., T50, early vigor), indicating their role in alleviating oxidative stress during early development [73]. Comparative studies further highlight that ZnONPs elicit distinct physiological and molecular responses compared to bulk ZnO or ionic zinc. For instance, in tomato, ZnO NP treatments were shown to enhance photosynthesis, antioxidant metabolism, and nutrient uptake more effectively than bulk forms, likely due to differences in dissolution kinetics and interactions with cellular signaling pathways [74].
3. Materials and Methods
3.1. Synthesis and Characterization of ZnONPs
The fresh droppings of French Mondain pigeons were collected from a farm in Riyadh, Saudi Arabia. The pigeons were fed with Pigeon Feed Protein 17% (Arasco, Riyadh, Saudi Arabia). An aqueous extract of the droppings was prepared by mixing 20 g of fresh droppings with 100 mL of distilled water. The mixture was first filtered through sterile gauze to remove coarse particulates, followed by filtration using Whatman No. 1 filter paper to obtain a clear filtrate (initial pH ≈ 9.3). The precipitate was used to manufacture the ZnONPs. A beige–brown paste solution was obtained by mixing 2 g of zinc nitrate hexahydrate (Zn(NO3)2·6H2O) with 100 mL of pigeon dropping extract (measured pH ≈ 12) under stirring at 60 °C. After that, the mixture was baked in an oven at 80 °C for 1 day. A white powder containing ZnONPs was produced by grinding the paste into a powder and calcining it in a muffle furnace at 400 °C for 4 h.
Several techniques were employed to characterize the synthesized ZnO nanoparticles. The optical properties were examined using a Fluorolog-3 fluorescence spectrometer (Horiba Scientific, Edison, NJ, USA) and a UV–Vis spectrophotometer (Shimadzu UV-1800 UV–Vis spectrometer, Kyoto, Japan). FTIR spectroscopy was conducted using (Perkin-Elmer FTIR Spectrum BX, Waltham, MA, USA) to identify the functional groups present in the ZnO nanoparticles. TEM was performed using (JEOL JEM-1400Plus, Tokyo, Japan) to analyze the surface morphology and particle size distribution. EDX, attached to the TEM, was used to determine the elemental composition of the sample using a system from (JEOL JSM-7610F, Tokyo, Japan). XRD analysis was carried out using a D8 Advance diffractometer (Bruker AXS GmbH, Karlsruhe, Germany), equipped with a graphite monochromator and a CuKα radiation source (λ = 1.5418 Å), operated at 45 kV and 40 mA. The crystallite sizes of the ZnO nanoparticles were estimated using the Scherrer equation. XPS (Jeol JPS-9200 photoelectron spectrometer, Tokyo, Japan) with a 500 W X-ray source (JEOL Ltd., Akishima, Tokyo, Japan) was employed to investigate the surface elemental composition and chemical states of the synthesized ZnONPs, as well as the raw pigeon droppings from which the extract was used in their synthesis.
3.2. Treatment of Sewage Water Using Synthesized ZnONPs
Following the modified method described by Haidri et al. [22], untreated sewage water was collected from the King Saud University (KSU) wastewater station. Briefly, the sewage water was continuously stirred with ZnO nanoparticles at a concentration of 0.5 g/L to ensure a homogeneous suspension. For photocatalytic degradation, the mixture was transferred to a transparent reaction vessel and exposed to direct sunlight for a fixed duration, typically 3 h. Water quality parameters, including COD and BOD, were monitored at regular intervals. Following treatment, the ZnO photocatalyst was separated via sedimentation and filtration. The treated water was then analyzed using spectroscopic and chromatographic techniques to detect any residual pollutants. Comparative analysis of water quality parameters before and after treatment was conducted to evaluate the efficiency of the photocatalytic process.
3.3. Application of Treated Wastewater for Cultivation of Wheat and Barley Crops
Wheat (T. aestivum) and barley (H. vulgare) seeds were obtained from King Saud University’s College of Agriculture, department of plant production. After being treated with 5% (w/v) sodium hypochlorite for 5 min, the barley and wheat seeds were washed and left to soak in distilled water for 1 day. After that, the seeds were split into three equal portions and placed on Petri dishes. There were three treatments: one that used nanoparticle-treated sewage water for irrigation, one that used chlorine-treated sewage water (chlorinated effluent), and a control group that used tap water. Depending on the treatment, the seedlings were watered once their roots reached a suitable length for irrigation. Seed germination, shoot length, and total root length were measured at 7, 14, and 21 days into the 3-week growth cycle of the plants.
3.4. Statistical Analysis
The statistical analysis of the data in this research was conducted using one-way analysis of variance (ANOVA) in SPSS version 26. p-values less than 0.05 were considered statistically significant. Each treatment and control group had three separate measurements taken, from which the means and standard deviations were calculated.
4. Conclusions
In conclusion, this study successfully demonstrated the green synthesis and comprehensive characterization of zinc oxide nanoparticles (ZnONPs). Techniques such as DLS, TEM, XRD, EDX, and PL confirmed their nanoscale size, crystallinity, morphology, and elemental composition. The ZnONPs exhibited a predominantly spherical morphology with an average crystallite size of ~29 nm and strong near-band-edge (NBE) emission with minimal defect-related luminescence, indicating high crystal quality and low electron–hole recombination key attributes for efficient photocatalysis. XPS analysis confirmed that pigeon droppings extract enabled the green synthesis of ZnONPs, providing biomolecules for reduction and stabilization and yielding Zn2+ species with Zn–O bonds. The photocatalytic potential of the ZnONPs was validated through the effective degradation of pollutants under visible light. Their application in sewage water treatment led to substantial reductions in TDS, COD, BOD, and TSS while maintaining water quality parameters such as pH and chloride within acceptable limits. Agronomic trials further confirmed that ZnO NP-treated sewage water significantly enhanced seed germination (100%) and early seedling growth in Triticum aestivum and Hordeum vulgare, outperforming both chlorine-treated and untreated controls. Root and shoot lengths were markedly improved by day 21, suggesting enhanced nutrient bioavailability and reduced phytotoxicity. This synthesis approach utilized minimal chemical inputs, aligning with the principles of green chemistry. The results highlight the promising potential of biosynthesized ZnONPs for dual applications in environmental remediation and sustainable agriculture.
Author Contributions
Conceptualization, M.A.A., H.F.A.-H. and K.M.O.O.; methodology, M.A.A., L.A.A.-H. and A.A.I.; software, K.M.O.O.; validation, A.A.A.-H., M.A.A.; formal analysis, M.A.A., A.A.A.-H.; investigation, H.F.A.-H.; resources, A.A.A.-H.; data curation, K.M.O.O.; writing—original draft preparation, M.A.A., H.F.A.-H., L.A.A.-H. and A.A.I.; writing—review and editing, A.A.A.-H., H.F.A.-H. and M.A.A.; visualization, H.F.A.-H.; supervision, M.A.A. and A.A.A.-H.; project administration, H.F.A.-H., M.A.A. and A.A.A.-H.; funding acquisition, A.A.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by King Saud University, Riyadh, Saudi Arabia, through Ongoing Research Funding program - Research Chairs (ORF-RC-2025-2800).
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
This work was supported by King Saud University, Riyadh, Saudi Arabia, through Ongoing Research Funding program—Research Chairs (ORF-RC-2025-2800).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Luo, Y.-L.; Pan, Y.-R.; Wang, X.; Wang, Z.-Y.; Daigger, G.; Ma, J.-X.; Tang, L.-H.; Liu, J.; Ren, N.-Q.; Butler, D. Leveraging the Water-Environment-Health Nexus to Characterize Sustainable Water Purification Solutions. Nat. Commun. 2025, 16, 1269. [Google Scholar] [CrossRef] [PubMed]
- Soltani, S.; Gacem, A.; Choudhary, N.; Yadav, V.K.; Alsaeedi, H.; Modi, S.; Patel, A.; Khan, S.H.; Cabral-Pinto, M.M.S.; Yadav, K.K.; et al. Scallion Peel Mediated Synthesis of Zinc Oxide Nanoparticles and Their Applications as Nano fertilizer and Photocatalyst for Removal of Organic Pollutants from Wastewater. Water 2023, 15, 1672. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Lin, S.-T.; Thirumavalavan, M.; Jiang, T.-Y.; Lee, J.-F. Synthesis of ZnO/Zn nano photocatalyst using modified polysaccharides for photodegradation of dyes. Carbohydr. Polym. 2014, 105, 1–9. [Google Scholar] [CrossRef]
- Ong, C.B.; Ng, L.Y.; Mohammad, A.W. A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew. Sustain. Energy Rev. 2018, 81, 536–551. [Google Scholar] [CrossRef]
- Hartley, T.W. Public perception and participation in water reuse. Desalination 2006, 187, 115–126. [Google Scholar] [CrossRef]
- Rasool, A.; Kiran, S.; Gulzar, T.; Abrar, S.; Ghaffar, A.; Shahid, M.; Nosheen, S.; Naz, S. Biogenic synthesis and characterization of ZnO nanoparticles for degradation of synthetic dyes: A sustainable environmental cleaner approach. J. Clean. Prod. 2023, 398, 136616. [Google Scholar] [CrossRef]
- Qiu, R.; Zhang, D.; Mo, Y.; Song, L.; Brewer, E.; Huang, X.; Xiong, Y. Photocatalytic activity of polymer-modified ZnO under visible light irradiation. J. Hazard. Mater. 2008, 156, 80–85. [Google Scholar] [CrossRef]
- Oliveira, A.G.; de Lara Andrade, J.; Montanha, M.C.; Ogawa, C.Y.L.; de Souza Freitas, T.K.F.; Moraes, J.C.G.; Sato, F.; Lima, S.M.; da Cunha Andrade, L.H.; Hechenleitner, A.A.W.; et al. Wastewater treatment using Mg-doped ZnO nano-semiconductors: A study of their potential use in environmental remediation. J. Photochem. Photobiol. A Chem. 2021, 407, 113078. [Google Scholar] [CrossRef]
- Wang, A.-N.; Teng, Y.; Hu, X.-F.; Wu, L.-H.; Huang, Y.-J.; Luo, Y.-M.; Christie, P. Diphenylarsinic acid contaminated soil remediation by titanium dioxide (P25) photocatalysis: Degradation pathway, optimization of operating parameters and effects of soil properties. Sci. Total Environ. 2016, 541, 348–355. [Google Scholar] [CrossRef]
- Yu, J.; Yu, X. Hydrothermal synthesis and photocatalytic activity of zinc oxide hollow spheres. Environ. Sci. Technol. 2008, 42, 4902–4907. [Google Scholar] [CrossRef] [PubMed]
- Sanjeev, N.O.; Valsan, A.E. Photocatalytic and antibacterial activity of green synthesized and immobilized zinc oxide nanoparticles for the removal of sulfadiazine and acetaminophen: Effect of operational parameters and degradation pathway. J. Environ. Chem. Eng. 2024, 12, 112649. [Google Scholar] [CrossRef]
- Dindar, B.; Içli, S. Unusual photoreactivity of zinc oxide irradiated by concentrated sunlight. J. Photochem. Photobiol. A Chem. 2001, 140, 263–268. [Google Scholar] [CrossRef]
- Sacco, O.; Vaiano, V.; Rizzo, L.; Sannino, D. Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment. J. Clean. Prod. 2018, 175, 38–49. [Google Scholar] [CrossRef]
- Mallikarjunaswamy, C.; Parameswara, P.; Pramila, S.; Nagaraju, G.; Deepakumari, H.N.; Ranganatha, V.L. Green and facile synthesis of zinc oxide nanoparticles for enhanced photocatalytic organic pollutant degradation. J. Mater. Sci. Mater. Electron. 2022, 33, 20361–20372. [Google Scholar] [CrossRef]
- Uribe-López, M.; Hidalgo-López, M.; López-González, R.; Frías-Márquez, D.; Núñez-Nogueira, G.; Hernández-Castillo, D.; Alvarez-Lemus, M. Photocatalytic activity of ZnO nanoparticles and the role of the synthesis method on their physical and chemical properties. J. Photochem. Photobiol. A Chem. 2021, 404, 112866. [Google Scholar] [CrossRef]
- Boppudi, H.B.; Rao, Y.S.; Kuchi, C.; Babu, A.R.; Govinda, V.; Jagadeesh, M.; Lavanya, M. Zinc oxide nanoparticles as an efficient antioxidant, photocatalyst, and heterogeneous catalyst in C–P bond synthesis. Results Chem. 2023, 6, 101227. [Google Scholar] [CrossRef]
- Caser, M.; Percivalle, N.M.; Cauda, V. The Application of Micro-and Nano-Sized Zinc Oxide Particles Differently Triggers Seed Germination in Ocimum basilicum L., Lactuca sativa L., and Lepidium sativum L. under Controlled Conditions. Horticulturae 2024, 10, 575. [Google Scholar] [CrossRef]
- Włodarczyk, K.; Smolińska, B. The Effect of Nano-ZnO on Seeds Germination Parameters of Different Tomatoes (Solanum lycopersicum L.) Cultivars. Molecules 2022, 27, 4963. [Google Scholar] [CrossRef]
- Khanm, H.; Vaishnavi, B.; Shankar, A. Raise of Nano-Fertilizer Era: Effect of Nano Scale Zinc Oxide Particles on the Germination, Growth and Yield of Tomato (Solanum lycopersicum). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1861–1871. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Norouzi, M.; Saeri, M. Impact of zinc and zinc oxide nanoparticles on the physiological and biochemical processes in tomato and wheat. Botany 2016, 95, 441–455. [Google Scholar] [CrossRef]
- Haidri, I.; Shahid, M.; Hussain, S.; Shahzad, T.; Mahmood, F.; Hassan, M.U.; Al-Khayri, J.M.; Aldaej, M.I.; Sattar, M.N.; Rezk, A.A.-S.; et al. Efficacy of Biogenic Zinc Oxide Nanoparticles in Treating Wastewater for Sustainable Wheat Cultivation. Plants 2023, 12, 3058. [Google Scholar] [CrossRef]
- Al-Sudani, W.K.K.; Al-Shammari, R.S.S.; Abed, M.S.; Al-Saedi, J.H.; Mernea, M.; Lungu, I.I.; Dumitrache, F.; Mihailescu, D.F. The Impact of ZnO and Fe2O3 Nanoparticles on Sunflower Seed Germination, Phenolic Content and Antiglycation Potential. Plants 2024, 13, 1724. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Waste Water Treatment; Academic Press: Cambridge, MA, USA, 2018; pp. 135–175. [Google Scholar]
- Ramaprabha, K.; Kumar, S.V. Effective photocatalytic degradation and kinetic modelling of azo dyes by zinc oxide nanoparticles from Brevibacterium casei. Desalination Water Treat. 2025, 321, 100936. [Google Scholar] [CrossRef]
- El-Belely, E.F.; Farag, M.M.S.; Said, H.A.; Amin, A.S.; Azab, E.; Gobouri, A.A.; Fouda, A. Green Synthesis of Zinc Oxide Nanoparticles (ZnO-NPs) Using Arthrospira platensis (Class: Cyanophyceae) and Evaluation of their Biomedical Activities. Nanomaterials 2021, 11, 95. [Google Scholar] [CrossRef] [PubMed]
- Soto-Robles, C.; Nava, O.; Cornejo, L.; Lugo-Medina, E.; Vilchis-Nestor, A.; Castro-Beltrán, A.; Luque, P. Biosynthesis, characterization and photocatalytic activity of ZnO nanoparticles using extracts of Justicia spicigera for the degradation of methylene blue. J. Mol. Struct. 2021, 1225, 129101. [Google Scholar] [CrossRef]
- Hussain, R.T.; Hossain, S.; Shariffuddin, J.H. Green synthesis and photocatalytic insights: A review of zinc oxide nanoparticles in wastewater treatment. Mater. Today Sustain. 2024, 26, 100764. [Google Scholar] [CrossRef]
- Yassin, M.T.; Mostafa, A.A.-F.; Al-Askar, A.A.; Al-Otibi, F.O. Facile Green Synthesis of Zinc Oxide Nanoparticles with Potential Synergistic Activity with Common Antifungal Agents against Multidrug-Resistant Candidal Strains. Crystals 2022, 12, 774. [Google Scholar] [CrossRef]
- Chang, J.S.; Saint, C.P.; Chow, C.W.K.; Bahnemann, D.W.; Chong, M.N. Recent innovations in engineering Zinc Oxide (ZnO) nanostructures for water and wastewater treatment: Pushing the boundaries of multifunctional photocatalytic and advanced biotechnological applications. Int. Mater. Rev. 2024, 69, 337–379. [Google Scholar] [CrossRef]
- Kaliraj, L.; Ahn, J.C.; Rupa, E.J.; Abid, S.; Lu, J.; Yang, D.C. Synthesis of panos extract mediated ZnO nano-flowers as photocatalyst for industrial dye degradation by UV illumination. J. Photochem. Photobiol. B Biol. 2019, 199, 111588. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Le, H.T.; Nguyen, T.T.; Nguyen, T.T.T.; Bach, L.G.; Nguyen, T.D.; Van Tran, T. Multifunctional ZnO nanoparticles bio-fabricated from Canna indica L. flowers for seed germination, adsorption, and photocatalytic degradation of organic dyes. J. Hazard. Mater. 2021, 420, 126586. [Google Scholar] [CrossRef] [PubMed]
- Kardes, M.; Yatmaz, H.C.; Öztürk, K. ZnO nanorods grown on flexible polyurethane foam surfaces for photocatalytic azo dye treatment. ACS Appl. Nano Mater. 2023, 6, 6605–6613. [Google Scholar] [CrossRef]
- Zeid, S.A.; Leprince-Wang, Y. Advancements in ZnO-Based Photocatalysts for Water Treatment: A Comprehensive Review. Crystals 2024, 14, 611. [Google Scholar] [CrossRef]
- Zeng, J.H.; Bin Jin, B.; Wang, Y.F. Facet enhanced photocatalytic effect with uniform single-crystalline zinc oxide nanodisks. Chem. Phys. Lett. 2009, 472, 90–95. [Google Scholar] [CrossRef]
- Folawewo, A.D.; Bala, M.D. Nanocomposite Zinc oxide-based photocatalysts: Recent developments in their use for the treatment of dye-polluted wastewater. Water 2022, 14, 3899. [Google Scholar] [CrossRef]
- Chérif, I.; Dkhil, Y.O.; Smaoui, S.; Elhadef, K.; Ferhi, M.; Ammar, S. X-Ray Diffraction Analysis by Modified Scherrer, Williamson–Hall and Size–Strain Plot Methods of ZnO Nanocrystals Synthesized by Oxalate Route: A Potential Antimicrobial Candidate Against Foodborne Pathogens. J. Clust. Sci. 2023, 34, 623–638. [Google Scholar] [CrossRef]
- Wang, J.; Gao, L. Hydrothermal synthesis and photoluminescence properties of ZnO nanowires. Solid State Commun. 2004, 132, 269–271. [Google Scholar] [CrossRef]
- Mädler, L.; Stark, W.J.; Pratsinis, S.E. Rapid synthesis of stable ZnO quantum dots. J. Appl. Phys. 2002, 92, 6537–6540. [Google Scholar] [CrossRef]
- Tarasenka, N.; Kornev, V.; Ramanenka, A.; Li, R.; Tarasenko, N. Photoluminescent neodymium-doped ZnO nanocrystals prepared by laser ablation in solution for NIR-II fluorescence bioimaging. Heliyon 2022, 8, e09554. [Google Scholar] [CrossRef]
- Mishra, S.K.; Srivastava, R.K.; Prakash, S. ZnO nanoparticles: Structural, optical and photoconductivity characteristics. J. Alloy. Compd. 2012, 539, 1–6. [Google Scholar] [CrossRef]
- Montero-Muñoz, M.; Ramos-Ibarra, J.; Rodríguez-Páez, J.E.; Teodoro, M.D.; Marques, G.E.; Sanabria, A.R.; Cajas, P.C.; Páez, C.A.; Heinrichs, B.; Coaquira, J.A. Role of defects on the enhancement of the photocatalytic response of ZnO nanostructures. Appl. Surf. Sci. 2018, 448, 646–654. [Google Scholar] [CrossRef]
- Karthigaimuthu, D.; Kumar, B.A.; Elangovan, T.; Ramalingam, G.; Kalluri, S.; Al Omari, S.A.B.; Sangaraju, S. Redox-active pigeon excreta mediated metal oxides nanosheets for enhancing co-catalyst for photovoltaic performance in dye-sensitized solar cells. J. Mater. Res. Technol. 2023, 27, 4440–4451. [Google Scholar] [CrossRef]
- Pasieczna-Patkowska, S.; Cichy, M.; Flieger, J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules 2025, 30, 684. [Google Scholar] [CrossRef]
- Guan, S.; Cheng, Y.; Hao, L.; Yoshida, H.; Tarashima, C.; Zhan, T.; Itoi, T.; Qiu, T.; Lu, Y. Oxygen vacancies induced band gap narrowing for efficient visible-light response in carbon-doped TiO2. Sci. Rep. 2023, 13, 14105. [Google Scholar] [CrossRef]
- Rouzet, A.; Valot, B.; Reboux, G.; Millon, L.; Roussel, S. Common proteins located in pigeon, budgerigar, and hen droppings related to bird fancier’s lung. J. Investig. Allergol. Clin. Immunol. 2018, 182–184. [Google Scholar] [CrossRef]
- Nawaz, A.; Farhan, A.; Maqbool, F.; Ahmad, H.; Qayyum, W.; Ghazy, E.; Rahdar, A.; Díez-Pascual, A.M.; Fathi-Karkan, S. Zinc oxide nanoparticles: Pathways to micropollutant adsorption, dye removal, and antibacterial actions—A study of mechanisms, challenges, and future prospects. J. Mol. Struct. 2024, 1312, 138545. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharath, G.; Banat, F.; Show, P.L. Green synthesis of zinc oxide nanoparticles using Phoenix dactylifera waste as bioreductant for effective dye degradation and antibacterial performance in wastewater treatment. J. Hazard. Mater. 2021, 402, 123560. [Google Scholar] [CrossRef]
- Barrak, H.; Saied, T.; Chevallier, P.; Laroche, G.; M’nIf, A.; Hamzaoui, A.H. Synthesis, characterization, and functionalization of ZnO nanoparticles by N-(trimethoxysilylpropyl) ethylenediamine triacetic acid (TMSEDTA): Investigation of the interactions between Phloroglucinol and ZnO@TMSEDTA. Arab. J. Chem. 2019, 12, 4340–4347. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Sadiq, H.M.; Shah, N.S.; Khan, A.U.; Muhammad, N.; Hassan, S.U.; Tahir, K.; Safi, S.Z.; Khan, F.U.; Imran, M.; et al. Greener synthesis of zinc oxide nanoparticles using Trianthema portulacastrum extract and evaluation of its photocatalytic and biological applications. J. Photochem. Photobiol. B Biol. 2019, 192, 147–157. [Google Scholar] [CrossRef]
- Awad, M.A.; Hendi, A.A.; Ortashi, K.M.O.; Alnamlah, R.A.; Alangery, A.; Ali Alshaya, E.; Alshammari, S.G. Utilizing Cymbopogon Proximus Grass Extract for Green Synthesis of Zinc Oxide Nanorod Needles in Dye Degradation Studies. Molecules 2024, 29, 355. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Yang, K.; Wei, J.; Li, Z.; Ma, C.; Yang, X.; Wang, T.; Zeng, G.; Yu, G.; et al. Removal of chloride from water and wastewater: Removal mechanisms and recent trends. Sci. Total Environ. 2022, 821, 153174. [Google Scholar] [CrossRef] [PubMed]
- Baby, R.; Hussein, M.Z.; Abdullah, A.H.; Zainal, Z. Nanomaterials for the Treatment of Heavy Metal Contaminated Water. Polymers 2022, 14, 583. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Colán, M.; Castillón, R.; Ramos, P.G.; Paria, R.; Sánchez, L.; Rodríguez, J.M. Fabrication of Activated Carbon Decorated with ZnO Nanorod-Based Electrodes for Desalination of Brackish Water Using Capacitive Deionization Technology. Int. J. Mol. Sci. 2023, 24, 1409. [Google Scholar] [CrossRef] [PubMed]
- Bouafia, A.; Meneceur, S.; Chami, S.; Laouini, S.E.; Daoudi, H.; Legmairi, S.; Mohammed Mohammed, H.A.; Aoun, N.; Menaa, F. Removal of hydrocarbons and heavy metals from petroleum water by modern green nanotechnology methods. Sci. Rep. 2023, 13, 5637. [Google Scholar] [CrossRef]
- Primo, J.D.O.; Bittencourt, C.; Acosta, S.; Sierra-Castillo, A.; Colomer, J.-F.; Jaerger, S.; Teixeira, V.C.; Anaissi, F.J. Synthesis of Zinc Oxide Nanoparticles by Ecofriendly Routes: Adsorbent for Copper Removal From Wastewater. Front. Chem. 2020, 8, 571790. [Google Scholar] [CrossRef]
- Gunasekaran, A.; Rajamani, A.K.; Masilamani, C.; Chinnappan, I.; Ramamoorthy, U.; Kaviyarasu, K. Synthesis and Characterization of ZnO Doped TiO2 Nanocomposites for Their Potential Photocatalytic and Antimicrobial Applications. Catalysts 2023, 13, 215. [Google Scholar] [CrossRef]
- El Golli, A.; Contreras, S.; Dridi, C. Bio-synthesized ZnO nanoparticles and sunlight-driven photocatalysis for environmentally-friendly and sustainable route of synthetic petroleum refinery wastewater treatment. Sci. Rep. 2023, 13, 20809. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, J. A review: Metal and metal oxide nanoparticles for environmental applications. Nanoscale 2025, 17, 15068–15085. [Google Scholar] [CrossRef]
- Forouzan, S.; Pirsa, S.; Alirezalu, A. Biodegradable photocatalytic film based on chia seed mucilage (xylose, glucose, and methyl glucuronic acid polysaccharides) containing barberry extract and SnO2 nanoparticles. Carbohydr. Polym. Technol. Appl. 2024, 8, 100592. [Google Scholar] [CrossRef]
- Ochoa-Chaparro, E.H.; Patiño-Cruz, J.J.; Anchondo-Páez, J.C.; Pérez-Álvarez, S.; Chávez-Mendoza, C.; Castruita-Esparza, L.U.; Márquez, E.M.; Sánchez, E. Seed Nanopriming with ZnO and SiO2 Enhances Germination, Seedling Vigor, and Antioxidant Defense Under Drought Stress. Plants 2025, 14, 1726. [Google Scholar] [CrossRef]
- Majumder, S.; Chatterjee, S.; Basnet, P.; Mukherjee, J. ZnO based nanomaterials for photocatalytic degradation of aqueous pharmaceutical waste solutions—A contemporary review. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100386. [Google Scholar] [CrossRef]
- Ahmed, M.; Marrez, D.A.; Rizk, R.; Zedan, M.; Abdul-Hamid, D.; Decsi, K.; Kovács, G.P.; Tóth, Z. The Influence of Zinc Oxide Nanoparticles and Salt Stress on the Morphological and Some Biochemical Characteristics of Solanum lycopersicum L. Plants. Plants 2024, 13, 1418. [Google Scholar] [CrossRef]
- Lonigro, A.; Montemurro, N.; Laera, G. Effects of residual disinfectant on soil and lettuce crop irrigated with chlorinated water. Sci. Total Environ. 2017, 584–585, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Sarkhosh, S.; Kahrizi, D.; Darvishi, E.; Tourang, M.; Haghighi-Mood, S.; Vahedi, P.; Ercisli, S. Effect of Zinc Oxide Nanoparticles (ZnO-NPs) on Seed Germination Characteristics in Two Brassicaceae Family Species: Camelina sativa and Brassica napus L. J. Nanomater. 2022, 2022, 1892759. [Google Scholar] [CrossRef]
- Yadav, R.; Meena, R. Comparative Study of Seed Germination in Textile Industry Wastewater and ZnO Nanoparticles Treated Textile Industry Wastewater. Ind. J. Pure App. Biosci. 2024, 12, 28–36. [Google Scholar] [CrossRef]
- Kumar, R.; Dhar, I.; Sharma, M.M. Zinc Oxide Nanoparticles: Applications in Photocatalysis of Dyes and Pearl Millet Seed Priming for Enhanced Agricultural Output. ACS Omega 2025, 10, 7181–7193. [Google Scholar] [CrossRef]
- Al-Salama, Y. Effect of seed priming with ZnO nanoparticles and saline irrigation water in yield and nutrients uptake by wheat plants. Environ. Sci. Proc. 2022, 16, 37. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Nguyen, N.T.T.; Nguyen, T.T.T.; Van Tran, T. Recent advances in the biosynthesis of ZnO nanoparticles using floral waste extract for water treatment, agriculture and biomedical engineering. Nanoscale Adv. 2024, 6, 4047–4061. [Google Scholar] [CrossRef]
- Baddar, Z.E.; Unrine, J.M. Functionalized-ZnO-Nanoparticle Seed Treatments to Enhance Growth and Zn Content of Wheat (Triticum aestivum) Seedlings. J. Agric. Food Chem. 2018, 66, 12166–12178. [Google Scholar] [CrossRef]
- Sánchez-Pérez, D.M.; Márquez-Guerrero, S.Y.; Ramírez-Moreno, A.; Rodríguez-Sifuentes, L.; Galindo-Guzmán, M.; Flores-Loyola, E.; Marszalek, J.E. Impact of Biologically and Chemically Synthesized Zinc Oxide Nanoparticles on Seed Germination and Seedlings’ Growth. Horticulturae 2023, 9, 1201. [Google Scholar] [CrossRef]
- Donia, D.T.; Carbone, M. Seed Priming with Zinc Oxide Nanoparticles to Enhance Crop Tolerance to Environmental Stresses. Int. J. Mol. Sci. 2023, 24, 17612. [Google Scholar] [CrossRef]
- Pejam, F.; Ardebili, Z.O.; Ladan-Moghadam, A.; Danaee, E.; Chen, J.-T. Zinc oxide nanoparticles mediated substantial physiological and molecular changes in tomato. PLoS ONE 2021, 16, e0248778. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).