Insight into the Mechanism of Ultrasonic Activation of Persulfate for Enhancing the Removal of Tetracycline Hydrochloride
Abstract
1. Introduction
2. Results and Discussions
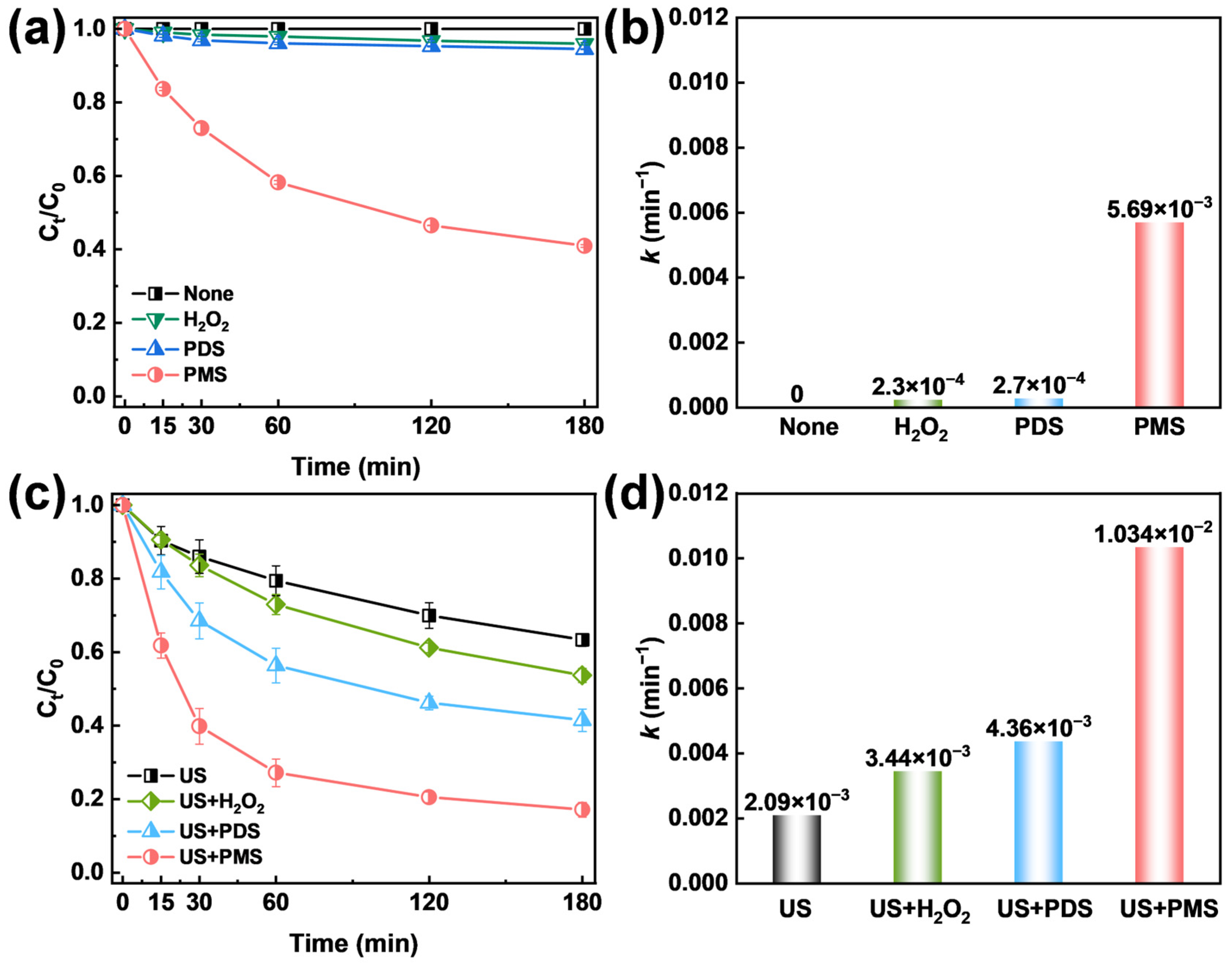
2.1. Degradation of TCH by Oxidants and US/Oxidants System
2.2. Influence of Various Experimental Parameters
2.2.1. Effects of PMS Dosage
2.2.2. Effects of Initial Solution pH and TCH Concentration
2.2.3. Effects of US Power and US Frequency
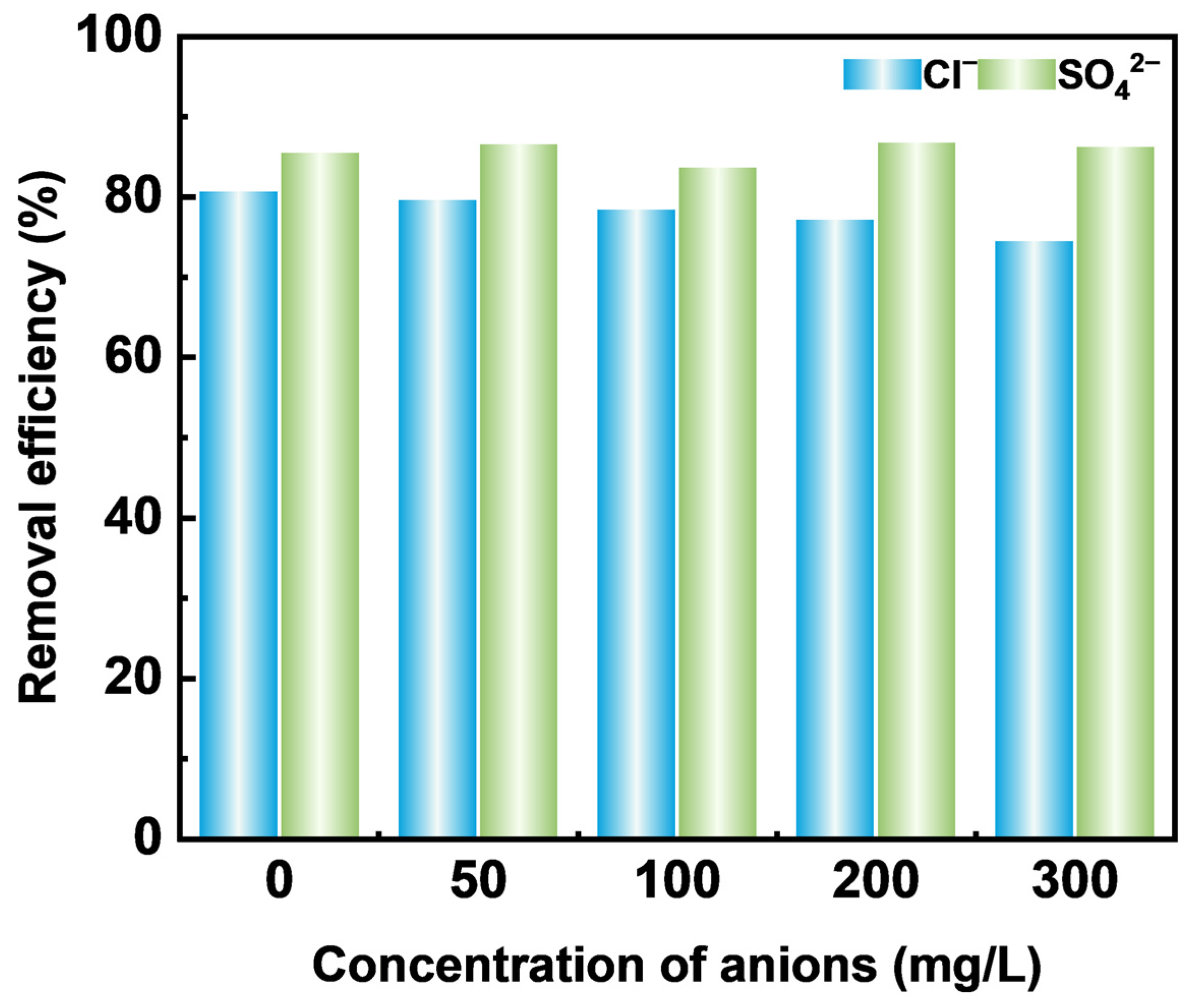
2.2.4. Effect of Inorganic Anions
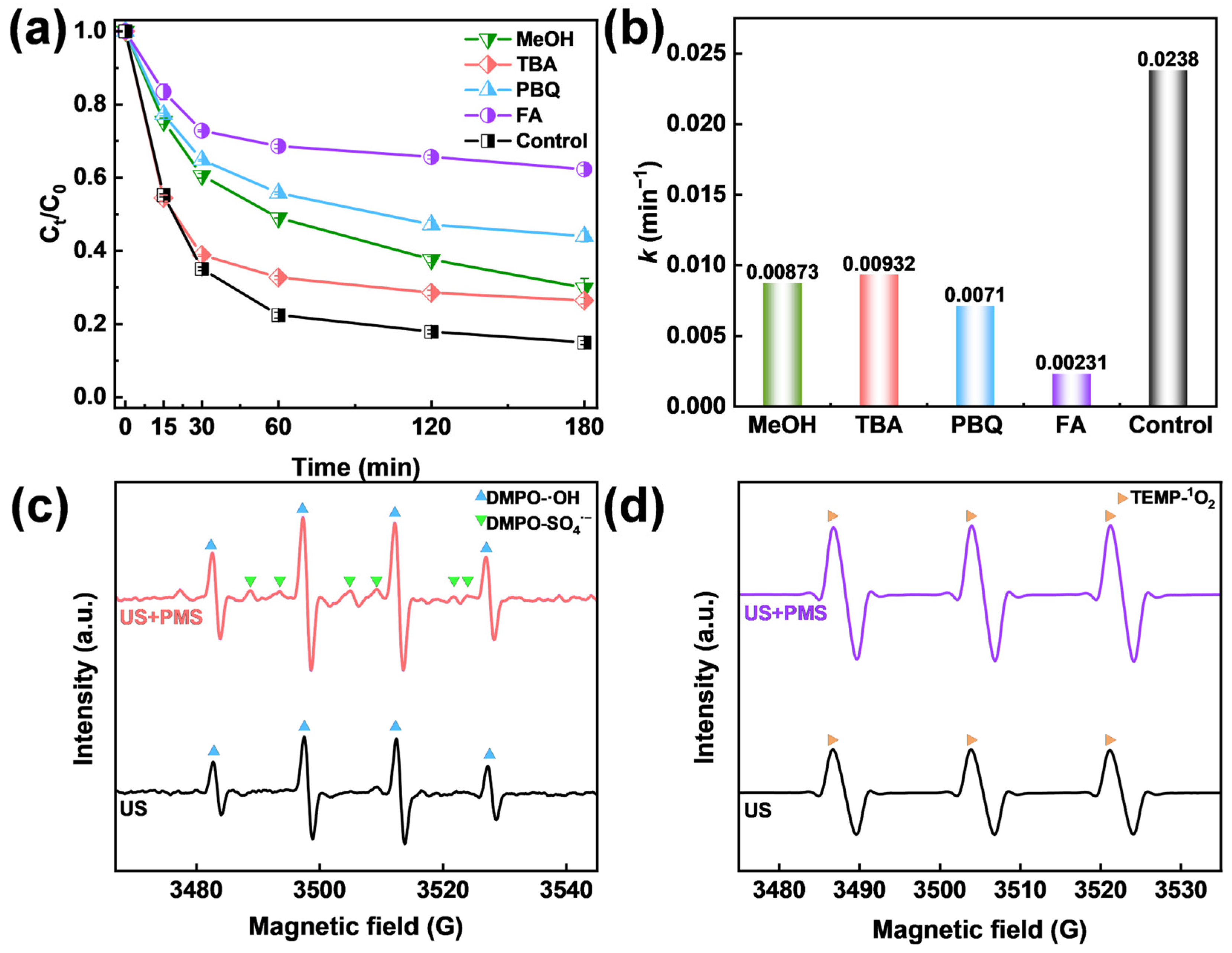
2.3. Identification of Active Species
2.4. Mechanism for Degradation of TCH by US/PMS System
3. Materials and Methods
3.1. Chemicals
3.2. Activation of PMS for Degradation of TCH
3.3. Analysis Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alduina, R. Antibiotics and Environment. Antibiotics 2020, 9, 202. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, G.; Zou, S.; Ling, Z.; Wang, G.; Yan, W. A Preliminary Investigation on the Occurrence and Distribution of Antibiotics in the Yellow River and its Tributaries, China. Water Environ. Res. 2009, 81, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, X.-R.; Sun, H.-Y.; Li, R.-P.; Fang, Y.-F.; Huang, Y.-P. The degradation of tetracycline in a photo-electro-Fenton system. Chem. Eng. J. 2013, 231, 441–448. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Zhao, Y.; Zhang, J. Performance of Traditional and Emerging Water-Treatment Technologies in the Removal of Tetracycline Antibiotics. Catalysts 2024, 14, 269. [Google Scholar] [CrossRef]
- Oh, W.-D.; Dong, Z.; Lim, T.-T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Ushani, U.; Lu, X.; Wang, J.; Zhang, Z.; Dai, J.; Tan, Y.; Wang, S.; Li, W.; Niu, C.; Cai, T.; et al. Sulfate radicals-based advanced oxidation technology in various environmental remediation: A state-of-the–art review. Chem. Eng. J. 2020, 402, 126232. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Li, S.; Tang, J.; Hua, T.; Li, F. Mechanism of the application of single-atom catalyst-activated PMS/PDS to the degradation of organic pollutants in water environment: A review. J. Clean. Prod. 2023, 397, 136468. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Giannakis, S. A review of the innovations in metal- and carbon-based catalysts explored for heterogeneous peroxymonosulfate (PMS) activation, with focus on radical vs. non-radical degradation pathways of organic contaminants. Chem. Eng. J. 2021, 411, 127957. [Google Scholar] [CrossRef]
- Lau, T.K.; Chu, W.; Graham, N.J.D. The Aqueous Degradation of Butylated Hydroxyanisole by UV/S2O82−: Study of Reaction Mechanisms via Dimerization and Mineralization. Environ. Sci. Technol. 2007, 41, 613–619. [Google Scholar] [CrossRef]
- Shen, J.; Cheng, F.; Chen, Y.; Li, Z.; Liu, Y.; Yuan, Y.; Zhou, P.; Liu, W.; Lai, B.; Zhang, Y. Vanadium trioxide mediated peroxymonosulfate for fast metronidazole oxidation: Stepwise oxidation of vanadium for donating electrons. Sep. Purif. Technol. 2022, 298, 121595. [Google Scholar] [CrossRef]
- Wacławek, S.; Lutze, H.V.; Sharma, V.K.; Xiao, R.; Dionysiou, D.D. Revisit the alkaline activation of peroxydisulfate and peroxymonosulfate. Curr. Opin. Chem. Eng. 2022, 37, 100854. [Google Scholar] [CrossRef]
- Song, K.; Liu, Y.; Umar, A.; Ma, H.; Wang, H. Ultrasonic cavitation: Tackling organic pollutants in wastewater. Chemosphere 2024, 350, 141024. [Google Scholar] [CrossRef]
- Fagan, W.P.; Villamena, F.A.; Zweier, J.L.; Weavers, L.K. In Situ EPR Spin Trapping and Competition Kinetics Demonstrate Temperature-Dependent Mechanisms of Synergistic Radical Production by Ultrasonically Activated Persulfate. Environ. Sci. Technol. 2022, 56, 3729–3738. [Google Scholar] [CrossRef]
- Cherifi, Y.; Addad, A.; Vezin, H.; Barras, A.; Ouddane, B.; Chaouchi, A.; Szunerits, S.; Boukherroub, R. PMS activation using reduced graphene oxide under sonication: Efficient metal-free catalytic system for the degradation of rhodamine B, bisphenol A, and tetracycline. Ultrason. Sonochem. 2019, 52, 164–175. [Google Scholar] [CrossRef]
- Xu, Q.; Yang, Q.; Luo, R.; Fei, Z.; You, H. Nonmetallic nitrogen doped hydrothermal carbon driven by ultrasonic mechanochemical action for activating (mono- and dual-) persulfate: Comparison of activation performance and reaction mechanism. Sep. Purif. Technol. 2025, 354, 128770. [Google Scholar] [CrossRef]
- Li, S.; Ning, X.; Hao, P.; Cao, Y.; Xie, J.; Hu, J.; Lu, Z.; Hao, A. Defect-rich MoS2 piezocatalyst: Efficient boosting piezocatalytic activation of PMS activity towards degradation organic pollutant. Dye. Pigment. 2022, 206, 110678. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, Z.; Pan, Z.; Li, F.; Gao, Z.; Li, X. Degradation of carbamazepine in piezo-activation of persulfate systems: Comparison of PDS and PMS. Chem. Eng. J. 2024, 500, 156570. [Google Scholar] [CrossRef]
- Nie, G.; Hu, K.; Ren, W.; Zhou, P.; Duan, X.; Xiao, L.; Wang, S. Mechanical agitation accelerated ultrasonication for wastewater treatment: Sustainable production of hydroxyl radicals. Water Res. 2021, 198, 117124. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, S.; Cui, M.; Ren, Y.; Park, B.; Ma, J.; Han, Z.; Khim, J. Activation of peroxodisulfate and peroxymonosulfate by ultrasound with different frequencies: Impact on ibuprofen removal efficient, cost estimation and energy analysis. Chem. Eng. J. 2021, 413, 127487. [Google Scholar] [CrossRef]
- Roy, K.; Agarkoti, C.; Malani, R.S.; Thokchom, B.; Moholkar, V.S. Mechanistic study of sulfadiazine degradation by ultrasound-assisted Fenton-persulfate system using yolk-shell Fe3O4@hollow@mSiO2 nanoparticles. Chem. Eng. Sci. 2020, 217, 115522. [Google Scholar] [CrossRef]
- Wang, B.; Li, X.; Wang, Y. Degradation of metronidazole in water using dielectric barrier discharge synergistic with sodium persulfate. Sep. Purif. Technol. 2022, 303, 122173. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Lin, Z.; Lin, W.; Chen, R.; Chen, P.; Lv, W.; Liu, G. Harnessing synergistic effects of UV/MgO2/PMS system for enhanced antibiotic degradation. Sep. Purif. Technol. 2025, 353, 128407. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S. Droplets impact on textured surfaces: Mesoscopic simulation of spreading dynamics. Appl. Surf. Sci. 2015, 327, 159–167. [Google Scholar] [CrossRef]
- Liu, S.; Kang, Y. Underwater bubbling plasma assisted with persulfate activation for the synergistic degradation of tetracycline hydrochloride. Environ. Res. 2024, 240, 117539. [Google Scholar] [CrossRef]
- Arvaniti, O.S.; Bairamis, F.; Konstantinou, I.; Mantzavinos, D.; Frontistis, Z. Degradation of antihypertensive drug valsartan in water matrices by heat and heat/ultrasound activated persulfate: Kinetics, synergy effect and transformation products. Chem. Eng. J. Adv. 2020, 4, 100062. [Google Scholar] [CrossRef]
- Monteagudo, J.M.; El-taliawy, H.; Durán, A.; Caro, G.; Bester, K. Sono-activated persulfate oxidation of diclofenac: Degradation, kinetics, pathway and contribution of the different radicals involved. J. Hazard. Mater. 2018, 357, 457–465. [Google Scholar] [CrossRef]
- Hu, Y.-b.; Lo, S.-L.; Li, Y.-F.; Lee, Y.-C.; Chen, M.-J.; Lin, J.-C. Autocatalytic degradation of perfluorooctanoic acid in a permanganate-ultrasonic system. Water Res. 2018, 140, 148–157. [Google Scholar] [CrossRef]
- Yao, K.; Fang, L.; Liao, P.; Chen, H. Ultrasound-activated peracetic acid to degrade tetracycline hydrochloride: Efficiency and mechanism. Sep. Purif. Technol. 2023, 306, 122635. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Sun, H.; Zhang, X.; Xu, L. Distribution characteristics and source analysis of sulfate in the main rivers of Heze city, China. Water Sci. Technol. 2021, 84, 2818–2829. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Effect of inorganic anions on the performance of advanced oxidation processes for degradation of organic contaminants. Chem. Eng. J. 2021, 411, 128392. [Google Scholar] [CrossRef]
- Zhan, H.; Lin, Z.; Chen, L.; Xiao, Z.; Liu, D.; Wang, Y.; Chen, Z.; Li, Z.; Fu, Z.; Liu, L.; et al. Efficient degradation of ciprofloxacin by innovative VUV/BN system: Kinetics, mechanism and toxicity assessment. J. Water Process Eng. 2024, 67, 106248. [Google Scholar] [CrossRef]
- Nejumal, K.K.; Satayev, M.I.; Rayaroth, M.P.; Arun, P.; Dineep, D.; Aravind, U.K.; Azimov, A.M.; Aravindakumar, C.T. Degradation studies of bisphenol S by ultrasound activated persulfate in aqueous medium. Ultrason. Sonochem. 2023, 101, 106700. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Zhou, C.; Li, X.; Qi, K.; Gao, L. Degradation of tetracycline by peroxymonosulfate activated with Mn0.85Fe2.15O4-CNTs: Key role of singlet oxygen. Environ. Res. 2023, 227, 115750. [Google Scholar] [CrossRef]
- Liu, S.; Yuan, X.; Shao, Z.; Xiang, K.; Huang, W.; Tian, H.; Hong, F.; Huang, Y. Investigation of singlet oxygen and superoxide radical produced from vortex-based hydrodynamic cavitation: Mechanism and its relation to cavitation intensity. Sci. Total Environ. 2024, 929, 172761. [Google Scholar] [CrossRef]
- Babu, D.S.; Srivastava, V.; Nidheesh, P.V.; Kumar, M.S. Detoxification of water and wastewater by advanced oxidation processes. Sci. Total Environ. 2019, 696, 133961. [Google Scholar] [CrossRef]
- Zhu, S.; Li, X.; Kang, J.; Duan, X.; Wang, S. Persulfate Activation on Crystallographic Manganese Oxides: Mechanism of Singlet Oxygen Evolution for Nonradical Selective Degradation of Aqueous Contaminants. Environ. Sci. Technol. 2019, 53, 307–315. [Google Scholar] [CrossRef]
- Bu, Y.; Li, H.; Yu, W.; Pan, Y.; Li, L.; Wang, Y.; Pu, L.; Ding, J.; Gao, G.; Pan, B. Peroxydisulfate Activation and Singlet Oxygen Generation by Oxygen Vacancy for Degradation of Contaminants. Environ. Sci. Technol. 2021, 55, 2110–2120. [Google Scholar] [CrossRef]
- Zhao, J.; Li, F.; Wei, H.; Ai, H.; Gu, L.; Chen, J.; Zhang, L.; Chi, M.; Zhai, J. Superior performance of ZnCoOx/peroxymonosulfate system for organic pollutants removal by enhancing singlet oxygen generation: The effect of oxygen vacancies. Chem. Eng. J. 2021, 409, 128150. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, W.; Lu, C.; Liu, X.; Nie, G.; Wang, W. Insight into the Mechanism of Ultrasonic Activation of Persulfate for Enhancing the Removal of Tetracycline Hydrochloride. Catalysts 2025, 15, 51. https://doi.org/10.3390/catal15010051
Yang W, Lu C, Liu X, Nie G, Wang W. Insight into the Mechanism of Ultrasonic Activation of Persulfate for Enhancing the Removal of Tetracycline Hydrochloride. Catalysts. 2025; 15(1):51. https://doi.org/10.3390/catal15010051
Chicago/Turabian StyleYang, Wenlong, Chun Lu, Xiaoxiao Liu, Guangze Nie, and Weiwei Wang. 2025. "Insight into the Mechanism of Ultrasonic Activation of Persulfate for Enhancing the Removal of Tetracycline Hydrochloride" Catalysts 15, no. 1: 51. https://doi.org/10.3390/catal15010051
APA StyleYang, W., Lu, C., Liu, X., Nie, G., & Wang, W. (2025). Insight into the Mechanism of Ultrasonic Activation of Persulfate for Enhancing the Removal of Tetracycline Hydrochloride. Catalysts, 15(1), 51. https://doi.org/10.3390/catal15010051







