Recent Advances in the Synthesis of Substituted Polyacetylenes
Abstract
1. Introduction
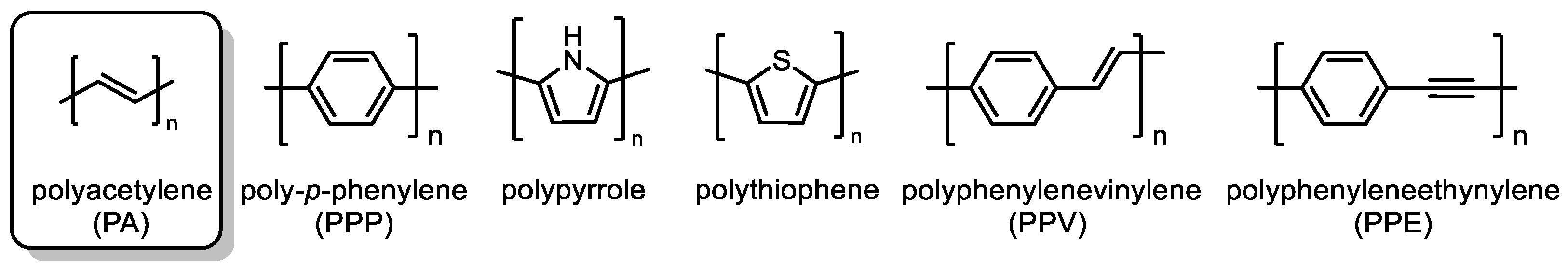
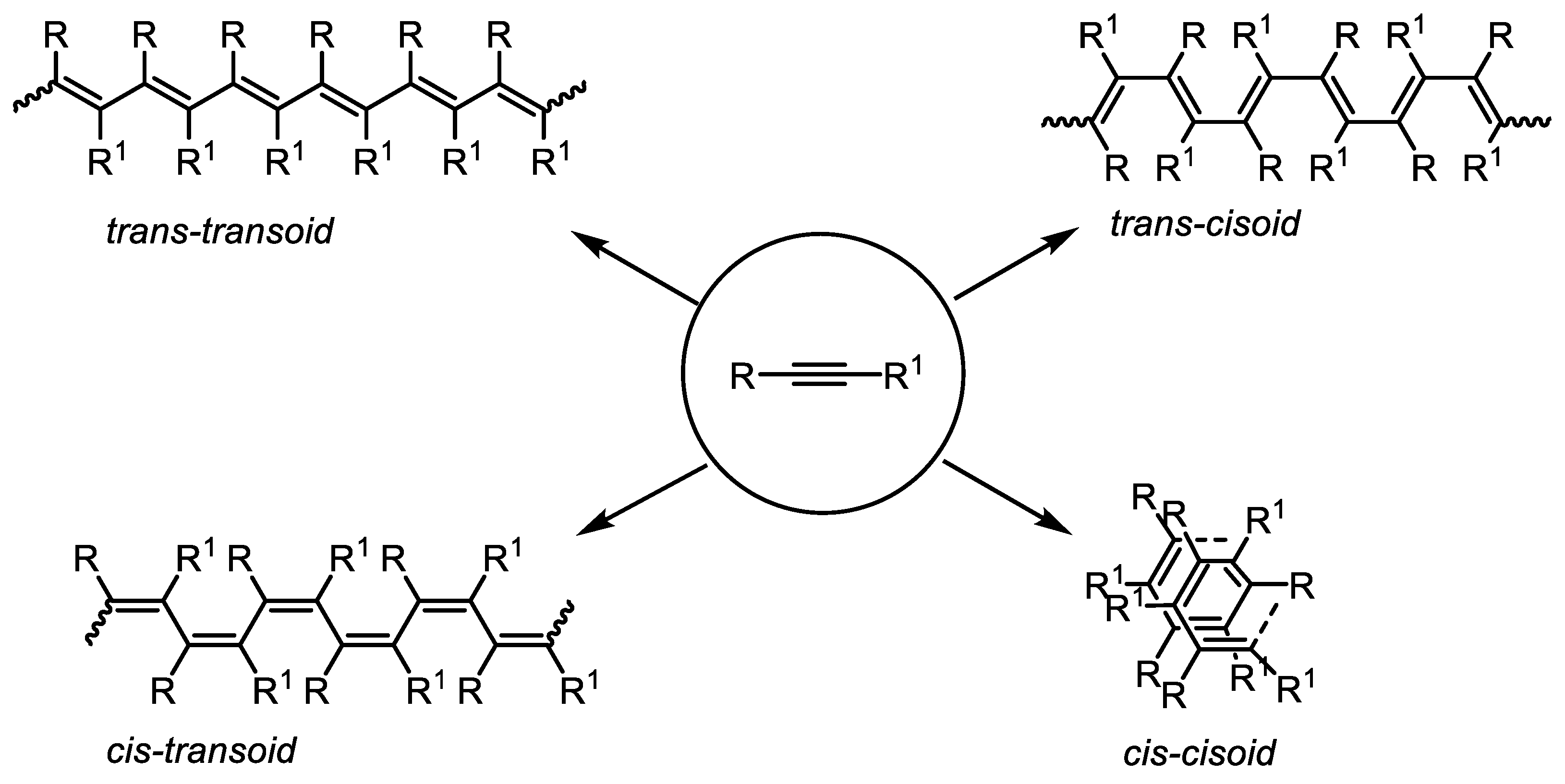
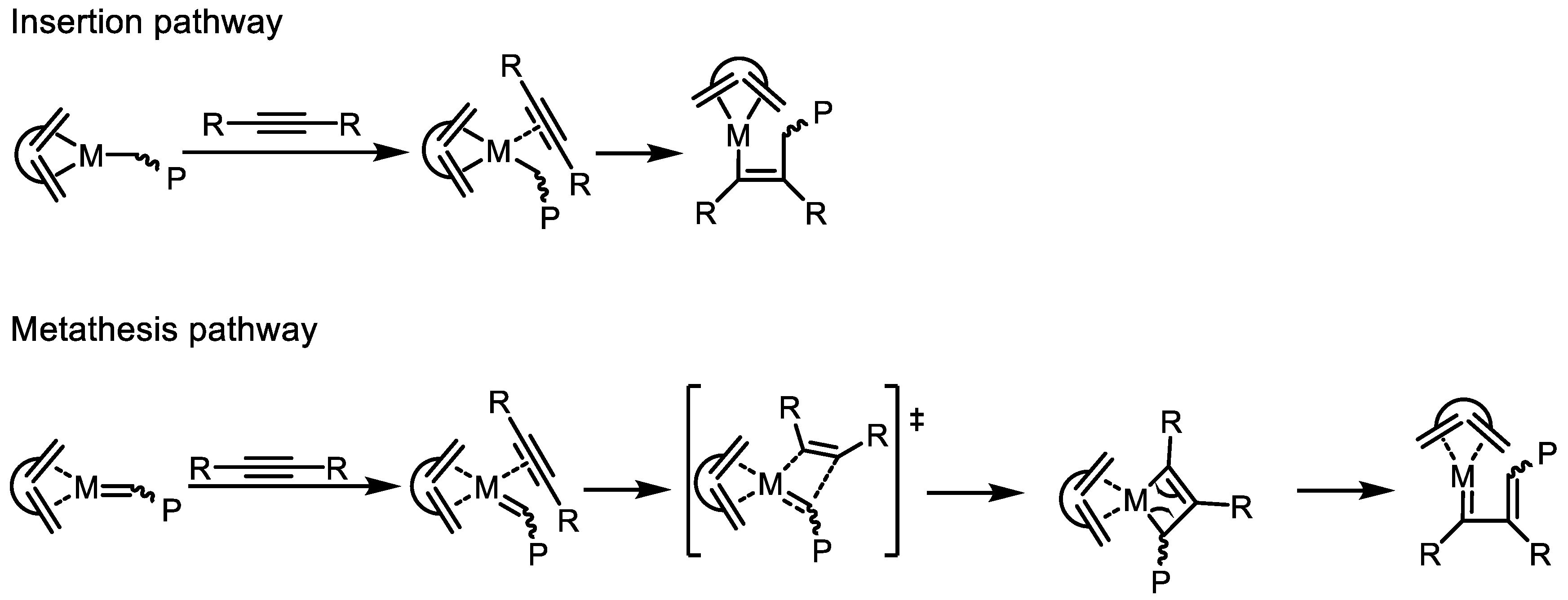
2. Structural Aspects of Substituted Polyacetylenes and Mechanistic Scenarios of Polymerization
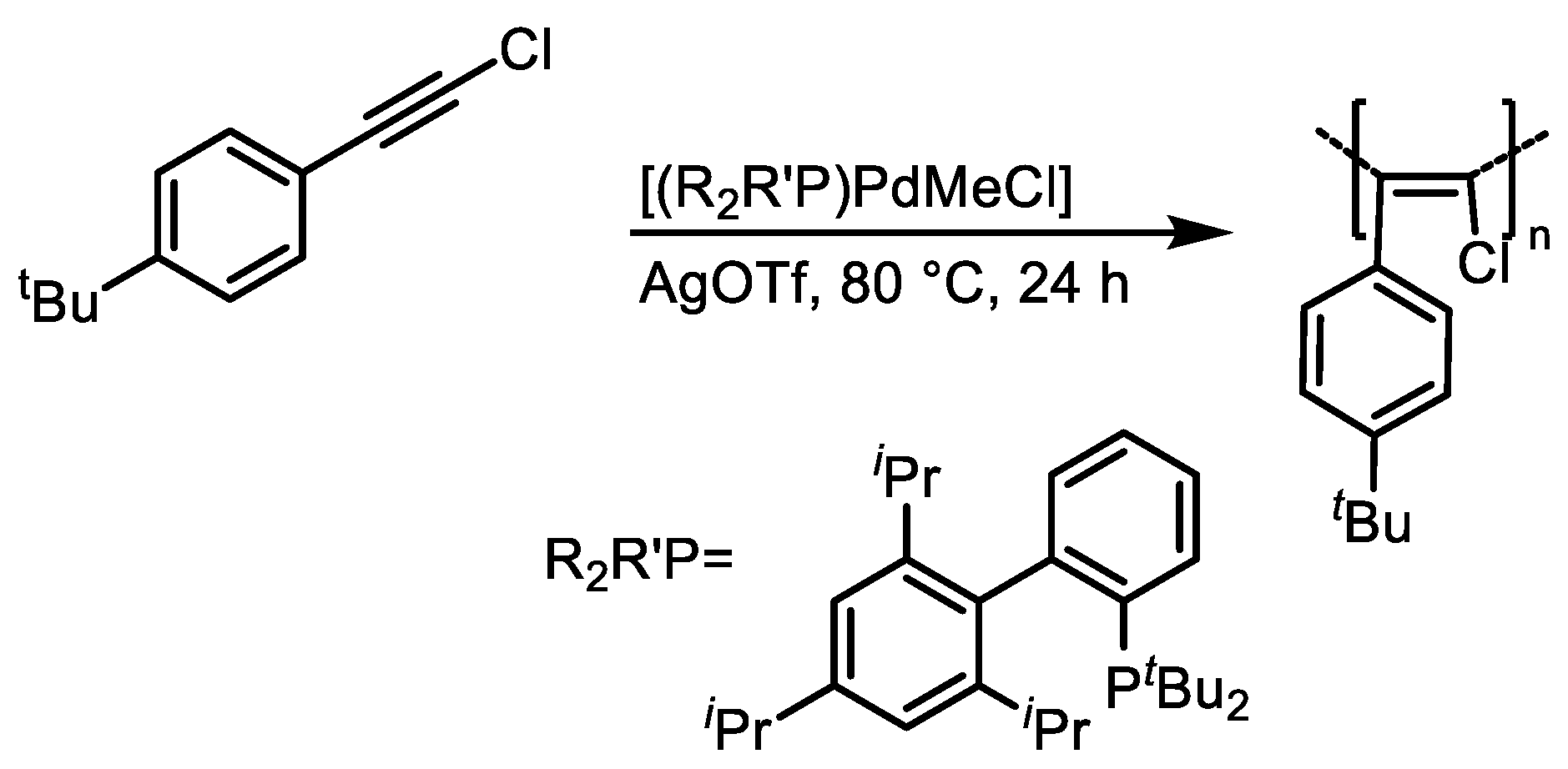
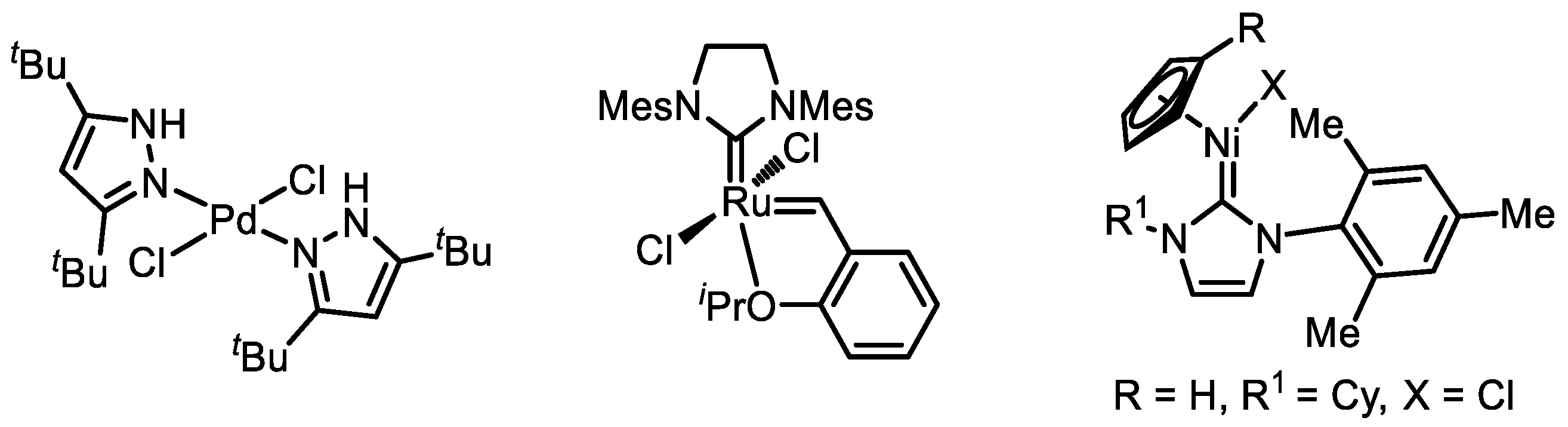
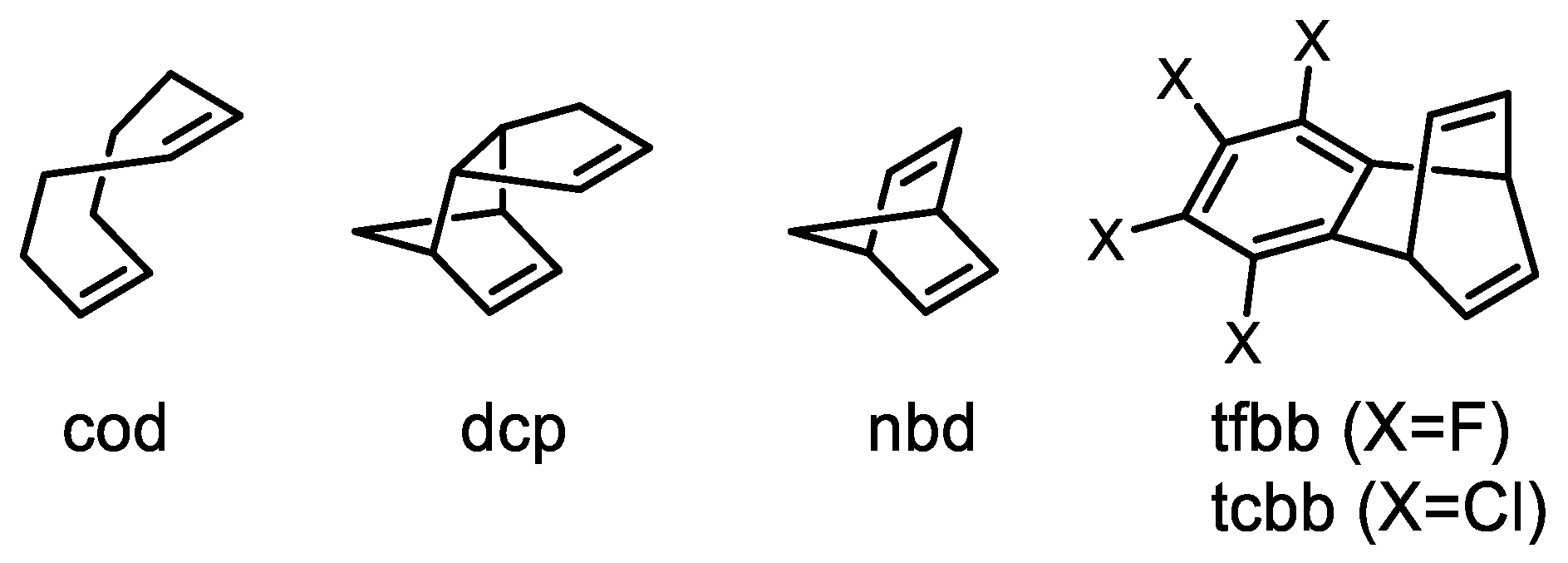
3. Catalyst Systems for the Polymerization of Substituted Acetylenes
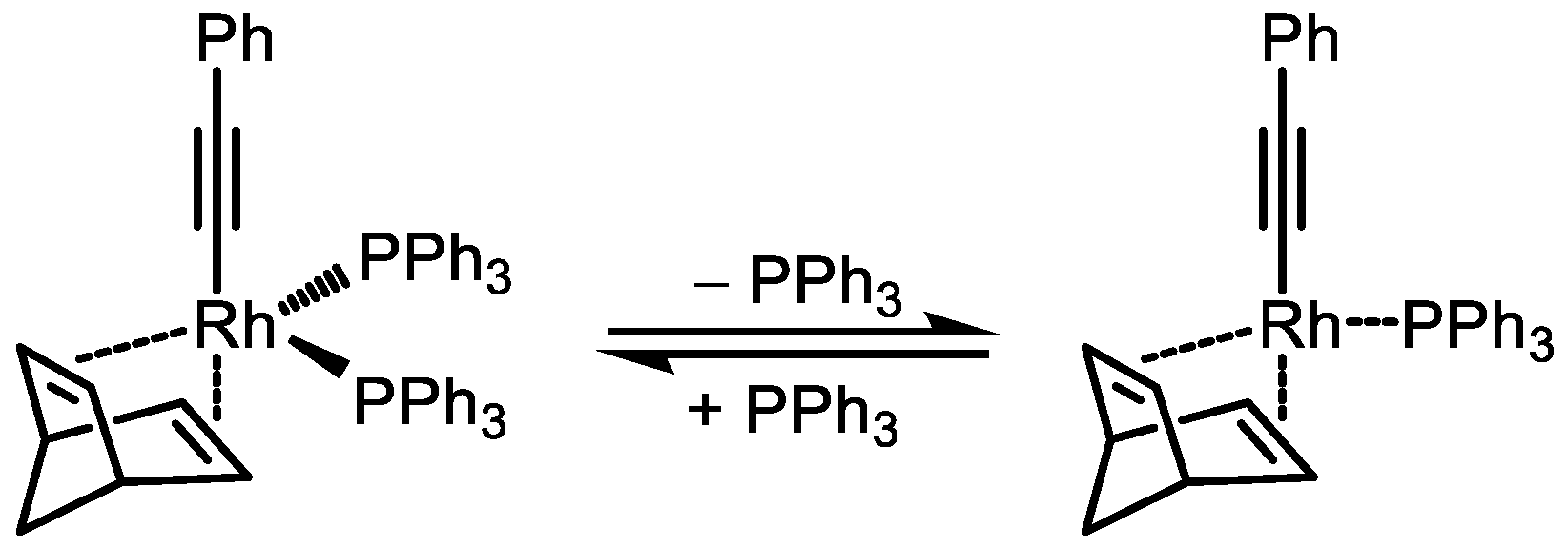
4. Rhodium-Catalyzed Polymerization of Monosubstituted Acetylenes: The Path to Living Polymerization
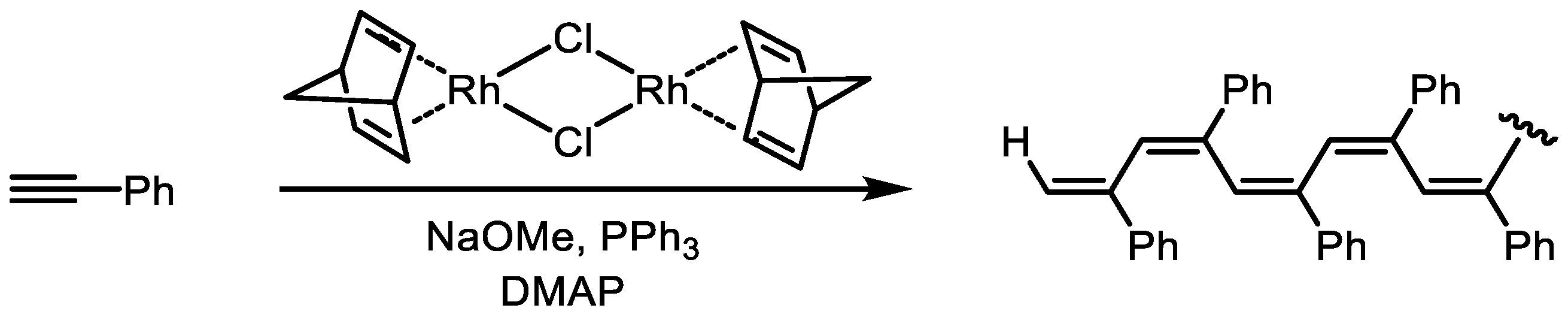
5. Syntheses and Properties of Functional Monosubstituted Arylacetylenes
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stenger-Smith, J.D. Intrinsically electrically conducting polymers. Synthesis, characterization, and their applications. Progr. Polym. Sci. 1998, 23, 57–79. [Google Scholar] [CrossRef]
- Swager, T.M. 50th Anniversary Perspective: Conducting/Semiconducting Conjugated Polymers; A Personal Perspective on the Macromolecules. Macromolecules 2017, 50, 4867–4886. [Google Scholar] [CrossRef]
- Müllen, K.; Scherf, U. Conjugated Polymers: Where We Come From, Where We Stand, and Where We Might Go. Macromol. Chem. Phys. 2023, 224, 2200337. [Google Scholar] [CrossRef]
- Friend, R.H.; Gymer, R.W.; Holmes, A.B.; Burroughes, J.H.; Marks, R.N.; Taliani, C.; Bradley, D.D.C.; Santos, D.A.D.; Brédas, J.L.; Lögdlund, M.; et al. Electroluminescence in conjugated polymers. Nature 1999, 397, 121–128. [Google Scholar] [CrossRef]
- Wang, B.-H.; Yin, J.; Xue, M.Z.; Wang, J.l.; Zhong, G.; Ding, X. Dibenzothiophene-5,5-dioxide-containing PPV based copolymer as green–blue electroluminescent material. Synth. Met. 2003, 132, 191–195. [Google Scholar] [CrossRef]
- Law, K.Y. Organic photoconductive materials: Recent trends and developments. Chem. Rev. 1993, 93, 449–486. [Google Scholar] [CrossRef]
- Günes, S.; Neugebauer, H.; Sariciftci, N.S. Conjugated Polymer-Based Organic Solar Cells. Chem. Rev. 2007, 107, 1324–1338. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, L.J.; Zhang, F.; Andersson, M.; Barrau, S.; Hellstrom, S.; Mammo, W.; Perzon, E.; Inganas, O.; Andersson, M.R. Synthesis, Characterization, and Devices of a Series of Alternating Copolymers for Solar Cells. Chem. Mater. 2009, 21, 3491–3502. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar] [CrossRef]
- Poddar, A.K.; Patel, S.S.; Patel, H.D. Synthesis, characterization and applications of conductive polymers: A brief review. Polym. Adv. Technol. 2021, 32, 4616–4641. [Google Scholar] [CrossRef]
- Sumdani, M.G.; Islam, M.R.; Yahaya, A.N.A.; Safie, S.I. Recent advancements in synthesis, properties, and applications of conductive polymers for electrochemical energy storage devices: A review. Polym. Eng. Sci. 2022, 62, 269–303. [Google Scholar] [CrossRef]
- Rasmussen, S.C. The Path to Conductive Polyacetylene. Bull. Hist. Chem. 2014, 39, 64–72. [Google Scholar]
- Rasmussen, S.C. Acetylene and Its Polymers—150+ Years of History, Springer Briefs in Molecular Science, History of Chemistry; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Rasmussen, S.C. Conjugated and Conducting Organic Polymers: The First 150 Years. ChemPlusChem 2020, 85, 1412–1429. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene, (CH). Chem. Commun. 1977, 578–580. [Google Scholar] [CrossRef]
- Chiang, C.K.; Fincher, C.R.; Park, Y.W.; Heeger, A.J.; Shirakawa, H.; Louis, E.J.; Gau, S.C.; MacDiarmid, A.G. Electrical conductivity in doped polyacetylene. Phys. Rev. Lett. 1977, 39, 1098–1101. [Google Scholar] [CrossRef]
- Durrani, M. Awards: Physicist shares chemistry Nobel prize. Phys. World 2000, 13, 6. [Google Scholar] [CrossRef]
- Hudson, B.S. Polyacetylene: Myth and Reality. Materials 2018, 11, 242. [Google Scholar] [CrossRef]
- Novak, B.M.; Risse, W.; Grubbs, R.H. The Development of Well-defined Catalysts for Ring-opening Metathesis Polymerization (ROMP). Adv. Polym. Sci. 1992, 102, 47–72. [Google Scholar] [CrossRef]
- Gibson, V.C. Metathesis Polymerization—Romping towards New Materials. Adv. Mater. 1994, 6, 37–42. [Google Scholar] [CrossRef]
- Bunz, U.H.F.; Mäker, D.; Porz, M. Alkene Metathesis—A Tool for the Synthesis of Conjugated Polymers. Macromol. Rapid Commun. 2012, 33, 886–910. [Google Scholar] [CrossRef]
- Masuda, T. Substituted polyacetylenes. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 165–180. [Google Scholar] [CrossRef]
- Masuda, T. Substituted Polyacetylenes: Synthesis, Properties, and Functions. Polym. Rev. 2017, 57, 1–14. [Google Scholar] [CrossRef]
- Sedlácek, J.; Balcar, H. Substituted Polyacetylenes Prepared with Rh Catalysts: From Linear to Network-Type Conjugated Polymers. Polym. Rev. 2017, 57, 31–51. [Google Scholar] [CrossRef]
- Yashima, E. Chiral and chirality discrimination on helical polyacetylenes. Anal. Sci. 2002, 18, 3–6. [Google Scholar] [CrossRef]
- Gong, J.; Zhang, X.J. Coordination-based circularly polarized luminescence emitters: Design strategy and application in sensing. Coord. Chem. Rev. 2022, 453, 214329. [Google Scholar] [CrossRef]
- Goh, M.; Matsushita, S.; Akagi, K. From helical polyacetylene to helical graphite: Synthesis in the chiral nematic liquid crystal field and morphology-retaining carbonization. Chem. Soc. Rev. 2010, 39, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.Z.; Chen, H.Z.; Xu, R.S.; Lam, J.W.Y.; Cheuk, K.K.L.; Wong, H.N.C.; Wang, M. Structure−Property Relationships for Photoconduction in Substituted Polyacetylenes. Chem. Mater. 2000, 12, 213–221. [Google Scholar] [CrossRef]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Synthesis and Functionality of Substituted Polyacetylenes. In Design and Synthesis of Conjugated Polymers; Mario Leclerc, M., Morin, J.F., Eds.; Wiley-VCH: Weinheim, Germany, 2010; Chapter 1; pp. 1–43. [Google Scholar] [CrossRef]
- Lu, Y.; Khan, Z.A.; Alvarez-Alvarado, M.S.; Zhang, Y.; Huang, Z.; Imran, M. A Critical Review of Sustainable Energy Policies for the Promotion of Renewable Energy Sources. Sustainability 2020, 12, 5078. [Google Scholar] [CrossRef]
- Masuda, T.; Isobe, E.; Higashimura, T.; Takada, K. Poly[1-(trimethylsilyl)-1-propyne]: A new high polymer synthesized with transition-metal catalysts and characterized by extremely high gas permeability. J. Am. Chem. Soc. 1983, 105, 7473–7474. [Google Scholar] [CrossRef]
- Chen, M.; Hu, G.; Shen, T.; Zhang, H.; Sun, J.Z.; Tang, B.Z. Applications of Polyacetylene Derivatives in Gas and Liquid Separation. Molecules 2023, 28, 2748. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lam, J.W.Y.; Tang, B.Z. Acetylenic Polymers: Syntheses, Structures, and Functions. Chem. Rev. 2009, 109, 5799–5867. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, J.Z.; Tang, B.Z. Poly(disubstituted acetylene)s: Advances in polymer preparation and materials application. Prog. Polym. Sci. 2018, 79, 98–120. [Google Scholar] [CrossRef]
- Shiotsuki, M.; Sanda, F.; Masuda, T. Polymerization of substituted acetylenes and features of the formed polymers. Polym. Chem. 2011, 2, 1044–1058. [Google Scholar] [CrossRef]
- Casado, M.A.; Fazal, A.; Oro, L.A. Rhodium-Catalyzed Polymerization of Phenylacetylene and its Derivatives. Arab. J. Sci. Eng. 2013, 38, 1631–1646. [Google Scholar] [CrossRef]
- Tanabe, Y.; Kyotani, H.; Akagi, K.; Shirakawa, H. Mechanism of Cis-Trans Thermal Isomerization of Polyacetylene. Macromolecules 1995, 28, 4173–4178. [Google Scholar] [CrossRef]
- Chien, J.C.W.; Karasz, F.E.; Wnek, G.E. Soliton formation and cis trans isomerization in polyacetylene. Nature 1980, 285, 390–392. [Google Scholar] [CrossRef]
- Mülhaupt, R. Catalytic Polymerization and Post Polymerization Catalysis Fifty Years After the Discovery of Ziegler’s Catalysts. Macromol. Chem. Phys. 2003, 204, 289–327. [Google Scholar] [CrossRef]
- Masuda, T.; Sasaki, N.; Higashimura, T. Polymerization of Phenylacetylenes. III. Structure and Properties of Poly(phenylacetylene)s Obtained by WCl6 or MoCl5. Macromolecules 1975, 8, 717–721. [Google Scholar] [CrossRef]
- Katz, T.J.; Hacker, S.M.; Kendrick, R.D.; Yannoni, C.S. Mechanisms of phenylacetylene polymerization by molybdenum and titanium initiators. J. Am. Chem. Soc. 1985, 107, 2182–2183. [Google Scholar] [CrossRef]
- Jean-Louis Hérisson, P.; Chauvin, Y. Catalyse de transformation des oléfines par les complexes du tungstène. II. Télomérisation des oléfines cycliques en présence d’oléfines acycliques. Macromol. Chem. Phys. 1971, 141, 161–176. [Google Scholar] [CrossRef]
- Lam, J.W.Y.; Tang, B.Z. Functional Polyacetylenes. Acc. Chem. Res. 2005, 38, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Luppi, B.T.; Muralidharan, A.V.; Ostermann, N.; Cheong, I.T.; Ferguson, M.J.; Siewert, I.; Rivard, E. Redox-Active Heteroatom-Functionalized Polyacetylenes. Angew. Chem. Int. Ed. 2022, 61, e202114586. [Google Scholar] [CrossRef] [PubMed]
- Gorman, C.B.; Ginsburg, E.J.; Sailor, M.J.; Moore, J.S.; Jozefiak, T.H.; Lewis, N.S.; Grubbs, R.H.; Marder, S.R.; Perry, J.W. Substituted polyacetylenes through the ring-opening metathesis polymerization (ROMP) of substituted cyclooctatetraenes: A route into soluble polyacetylene. Synth. Met. 1991, 41, 1033–1038. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, S.Y.; Canlier, A.; Hwang, T.S. Controlled Dehydrochlorination of Poly(vinyl chloride) for Fabrication of Membranes with Polyacetylene-Like Structure: XPS Analysis and Ion Exchange Membrane Discussion. Macromol. Res. 2019, 27, 33–47. [Google Scholar] [CrossRef]
- O’Rourke, G.; Hennebel, T.; Stalpaert, M.; Skorynina, A.; Bugaev, A.; Janssens, K.; Van Emelen, L.; Lemmens, V.; De Oliveira Silva, R.; Colemonts, C.; et al. Catalytic tandem dehydrochlorination–hydrogenation of PVC towards valorisation of chlorinated plastic waste. Chem. Sci. 2023, 14, 4401–4412. [Google Scholar] [CrossRef] [PubMed]
- Aldissi, M. Review of the synthesis of polyacetylene and its stabilization to ambient atmosphere. Synth. Met. 1984, 9, 131–141. [Google Scholar] [CrossRef]
- Taniguchi, T.; Yoshida, T.; Echizen, K.; Takayama, K.; Nishimura, T.; Maeda, K. Facile and Versatile Synthesis of End-Functionalized Poly(phenylacetylene)s: A Multicomponent Catalytic System for Well-Controlled Living Polymerization of Phenylacetylenes. Angew. Chem. Int. Ed. 2020, 59, 8670–8680. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, S.; Taniguchi, T.; Sakata, Y.; Akine, S.; Nishimura, T.; Maeda, K. Rhodium(I) Complexes Bearing an Aryl-Substituted 1,3,5-Hexatriene Chain: Catalysts for Living Polymerization of Phenylacetylene and Potential Helical Chirality of 1,3,5-Hexatrienes. Angew. Chem. Int. Ed. 2021, 60, 22201–22206. [Google Scholar] [CrossRef] [PubMed]
- Misumi, Y.; Masuda, T. Living Polymerization of Phenylacetylene by Novel Rhodium Catalysts. Quantitative Initiation and Introduction of Functional Groups at the Initiating Chain End. Macromolecules 1998, 31, 7572–7573. [Google Scholar] [CrossRef]
- Miyairi, M.; Taniguchi, T.; Nishimura, T.; Maeda, K. Facile Synthesis of Linear and Cyclic Poly(diphenylacetylene)s by Molybdenum and Tungsten Catalysis. Angew. Chem. Int. Ed. 2023, 62, e202302332. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Shimada, H.; Hashimoto, T. Metathesis polymerization of monomers containing two diphenylacetylene units: Synthesis and properties of poly(diphenylacetylene)s bearing diphenylacetylene units on the side chain. Polym. Chem. 2020, 11, 6471–6478. [Google Scholar] [CrossRef]
- Castanon, J.R.; Sano, N.; Shiotsuki, M.; Sanda, F. New Approach to the Polymerization of Disubstituted Acetylenes by Bulky Monophosphine-Ligated Palladium Catalysts. ACS Macro Lett. 2014, 3, 51–54. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Daugulis, O.; Brookhart, M. Polymerization of Terminal Acetylenes by a Bulky Monophosphine-Palladium Catalyst. Organometallics 2023, 42, 235–239. [Google Scholar] [CrossRef]
- Ojwach, S.O.; Guzei, I.A.; Darkwa, J.; Mapolie, S.F. Palladium complexes of multidentate pyrazolylmethyl pyridine ligands: Synthesis, structures and phenylacetylene polymerization. Polyhedron 2007, 26, 851–861. [Google Scholar] [CrossRef]
- Katsumata, T.; Shiotsuki, M.; Sanda, F.; Sauvage, X.; Delaude, L.; Masuda, T. Polymerization of ortho-Substituted Phenylacetylenes with Well-Defined Ruthenium-Alkylidene Catalysts and Related Metathesis Initiators. Macromol. Chem. Phys. 2009, 210, 1891–1902. [Google Scholar] [CrossRef]
- Buchowicz, W.; Wojtczak, W.; Pietrzykowski, A.; Lupa, A.; Jerzykiewicz, L.B.; Makal, A.; Woźniak, K. Synthesis, Structure, and Polymerization Activity of Cyclopentadienylnickel(II) N-Heterocyclic Carbene Complexes: Selective Cross-Metathesis in Metal Coordination Spheres. Eur. J. Inorg. Chem. 2010, 2010, 648–656. [Google Scholar] [CrossRef]
- Echizen, K.; Taniguchi, T.; Nishimura, T.; Maeda, K. Well-Controlled Living Polymerization of Phenylacetylenes in Water: Synthesis of Water-Soluble Stereoregular Telechelic Poly(phenylacetylene)s. Angew. Chem. Int. Ed. 2022, 61, e202202676. [Google Scholar] [CrossRef] [PubMed]
- Nikishkin, N.I.; Huskens, J.; Verboom, W. Highly active and robust rhodium(I) catalyst for the polymerization of arylacetylenes in polar and aqueous medium under air atmosphere. Polymer 2013, 54, 3175–3181. [Google Scholar] [CrossRef]
- Kern, R.J. Preparation and properties of isomeric polyphenylacetylenes. J. Polym. Sci. 1969, 7, 621–631. [Google Scholar] [CrossRef]
- Mayershofer, M.G.; Nuyken, O. Living polymerization of substituted acetylenes. J. Polym. Sci. 2005, 43, 5723–5747. [Google Scholar] [CrossRef]
- Tabata, M.; Yang, W.; Yokota, K. Polymerization of m-Chlorophenylacetylene Initiated by [Rh(norbornadiene)Cl]2-Triethylamine Catalyst Containing Long-Lived Propagation Species. Polym. J. 1990, 22, 1105–1107. [Google Scholar] [CrossRef]
- Saeed, I.; Shiotsuki, M.; Masuda, T. Effect of Diene Ligands in the Rhodium-Catalyzed Polymerization of Phenylacetylene. Macromolecules 2006, 39, 8977–8981. [Google Scholar] [CrossRef]
- Kanki, K.; Misumi, Y.; Masuda, T. Remarkable Cocatalytic Effect of Organometallics and Rate Control by Triphenylphosphine in the Rh-Catalyzed Polymerization of Phenylacetylene. Macromolecules 1999, 32, 2384–2386. [Google Scholar] [CrossRef]
- Goldberg, Y.; Alper, H. Polymerisation of phenylacetylene catalysed by a zwitterionic rhodium(I) complex under hydrosilyation conditions. J. Chem. Soc. Chem. Commun. 1994, 10, 1209–1210. [Google Scholar] [CrossRef]
- Szwarc, M.; Levy, M.; Milkovich, R. Polymerization inititiated by electron transfer to monomer: A new method of formation of block polymers. J. Am. Chem. Soc. 1956, 78, 2656–2657. [Google Scholar] [CrossRef]
- Webster, O.W. Living Polymerization Methods. Science 1991, 251, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Eckerle, P.; Miyatake, T.; Ikariya, T.; Noyori, R. Living Polymerization of Phenylacetylenes Initiated by Rh(C≡CC6H5)(2,5-norbornadiene)[P(C6H5)3]2. J. Am. Chem. Soc. 1994, 116, 12131–12132. [Google Scholar] [CrossRef]
- Misumi, Y.; Kanki, K.; Miyake, M.; Masuda, T. Living polymerization of phenylacetylene by rhodium-based ternary catalysts, (diene)Rh(I) complex/vinyllithium/phosphorus ligand. Effects of catalyst components. Macromol. Chem. Phys. 2000, 201, 2239–2244. [Google Scholar] [CrossRef]
- Echizen, K.; Taniguchi, T.; Nishimura, T.; Maeda, K. Synthesis of Stereoregular Telechelic Poly(phenylacetylene)s: Facile Terminal Chain-End Functionalization of Poly(phenylacetylene)s by Terminative Coupling with Acrylates and Acrylamides in Rhodium-Catalyzed Living Polymerization of Phenylacetylenes. J. Am. Chem. Soc. 2021, 143, 3604–3612. [Google Scholar] [CrossRef]
- Ito, K.; Taniguchi, T.; Nishimura, T.; Maeda, K. Well-Controlled Living Polymerization of N-Propargylamides and Their Derivatives by Rhodium Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202117234. [Google Scholar] [CrossRef]
- Freire, F.; Quiñoá, E.; Riguera, R. Supramolecular Assemblies from Poly(phenylacetylene)s. Chem. Rev. 2016, 116, 1242–1271. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, R.; Quiñoá, E.; Riguera, R.; Freire, F. Architecture of Chiral Poly(phenylacetylene)s: From Compressed/Highly Dynamic to Stretched/Quasi-Static Helices. J. Am. Chem. Soc. 2016, 138, 9620–9628. [Google Scholar] [CrossRef]
- Freire, F.; Quiñoá, E.; Riguera, R. Chiral nanostructure in polymers under different deposition conditions observed using atomic force microscopy of monolayers: Poly(phenylacetylene)s as a case study. Chem. Commun. 2017, 53, 481–492. [Google Scholar] [CrossRef]
- Reggelin, M.; Doerr, S.; Klussmann, M.; Schultz, M.; Holbach, M. Helically chiral polymers: A class of ligands for asymmetric catalysis. Proc. Natl. Acad. Sci. USA 2004, 101, 5461–5466. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Yashima, E. Helical Polyacetylenes Induced via Noncovalent Chiral Interactions and Their Applications as Chiral Materials. Top. Curr. Chem. 2017, 375, 72. [Google Scholar] [CrossRef] [PubMed]
- Inaba, A.; Nishimura, T.; Yamamoto, M.; Das, S.; Yurtsever, A.; Miyata, K.; Fukuma, T.; Kawaguchi, S.; Kikuchi, M.; Taniguchi, T.; et al. Synthesis of optically active star polymers consisting of helical poly(phenylacetylene) chains by the living polymerization of phenylacetylenes and their chiroptical properties. RSC Adv. 2023, 13, 30978–30984. [Google Scholar] [CrossRef] [PubMed]
- Mino, S.; Matsui, K.; Goto, M.; Ryoki, A.; Suzuki, T.; Fujimoto, K.; Sogawa, H.; Kudo, H.; Sanda, F. Star-Shaped Polymers with Helical Polyacetylene Arms. Comparison of Solution- and Solid-State Properties with Linear Helical Polyacetylenes. Macromolecules 2024, 57, 10824–10834. [Google Scholar] [CrossRef]
- Lu, X.; Ren, L.; Zhang, X.; Whittaker, A.K.; Li, W.; Zhang, A. Thermo/Light Dual-Responsive Helical Dendronized Poly(phenylacetylene)s. Macromolecules 2024, 57, 5915–5928. [Google Scholar] [CrossRef]
- Lam, J.W.Y.; Dong, Y.; Kwok, H.S.; Tang, B.Z. Light-Emitting Polyacetylenes: Synthesis and Electrooptical Properties of Poly(1-phenyl-1-alkyne)s Bearing Naphthyl Pendants. Macromolecules 2006, 39, 6997–7003. [Google Scholar] [CrossRef]
- Wang, S.; Hu, D.; Guan, X.; Cai, S.; Shi, G.; Shuai, Z.; Zhang, J.; Peng, Q.; Wan, X. Brightening up Circularly Polarized Luminescence of Monosubstituted Polyacetylene by Conformation Control: Mechanism, Switching, and Sensing. Angew. Chem. Int. Ed. 2021, 60, 21918–21926. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.Z.; Zhao, H.; Shen, X.Y.; Mahtab, F.; Lam, J.W.Y.; Sun, J.Z.; Tang, B.Z. Luminogenic Polyacetylenes and Conjugated Polyelectrolytes: Synthesis, Hybridization with Carbon Nanotubes, Aggregation-Induced Emission, Superamplification in Emission Quenching by Explosives, and Fluorescent Assay for Protein Quantitation. Macromolecules 2009, 42, 9400–9411. [Google Scholar] [CrossRef]
- Sun, J.Z.; Qin, A.J.; Tang, B.Z. Functional polyacetylenes: Hybrids with carbon nanotubes. Polym. Chem. 2013, 4, 211–223. [Google Scholar] [CrossRef]
- Tang, B.Z.; Kotera, N. Synthesis of optically active polyacetylene containing an asymmetric silicon by using organotransition-metal complexes as catalysts. Macromolecules 1989, 22, 4388–4390. [Google Scholar] [CrossRef]
- Gangadhar, P.S.; Reddy, G.; Prasanthkumar, S.; Giribabu, L. Phenothiazine functional materials for organic optoelectronic applications. Phys. Chem. Chem. Phys. 2021, 23, 14969–14996. [Google Scholar] [CrossRef]
- Otteny, F.; Desmaizieres, G.; Esser, B. Phenothiazine-based Redox Polymers for Energy Storage. In Redox Polymers for Energy and Nanomedicine; Casado, N., Mecerreyes, D., Eds.; The Royal Society of Chemistry: London, UK, 2020; Chapter 5; pp. 166–197. [Google Scholar] [CrossRef]
- Mayer, L.; Müller, T.J.J. 3,10-Diaryl Phenothiazines—One-pot Synthesis and Conformational Tuning of Ground and Excited State Electronics. Eur. J. Org. Chem. 2021, 2021, 3516–3527. [Google Scholar] [CrossRef]
- Meyer, T.; Müller, T.J.J. Consecutive Three-Component Synthesis of Donor-Substituted Merocyanines by One-pot Suzuki-Knoevenagel Condensation (SuKnoCon) Sequence. Org. Mater. 2020, 2, 64–70. [Google Scholar] [CrossRef]
- Stephan, M.; Stute, B.; von Lieres, E.; Müller, T.J.J. Consecutive Three-component Synthesis of Phenothiazine Based Merocyanines—Bayesian Optimization, Electronic properties, and DSSC Characteristics. Eur. J. Org. Chem. 2022, 2022, e202200163. [Google Scholar] [CrossRef]
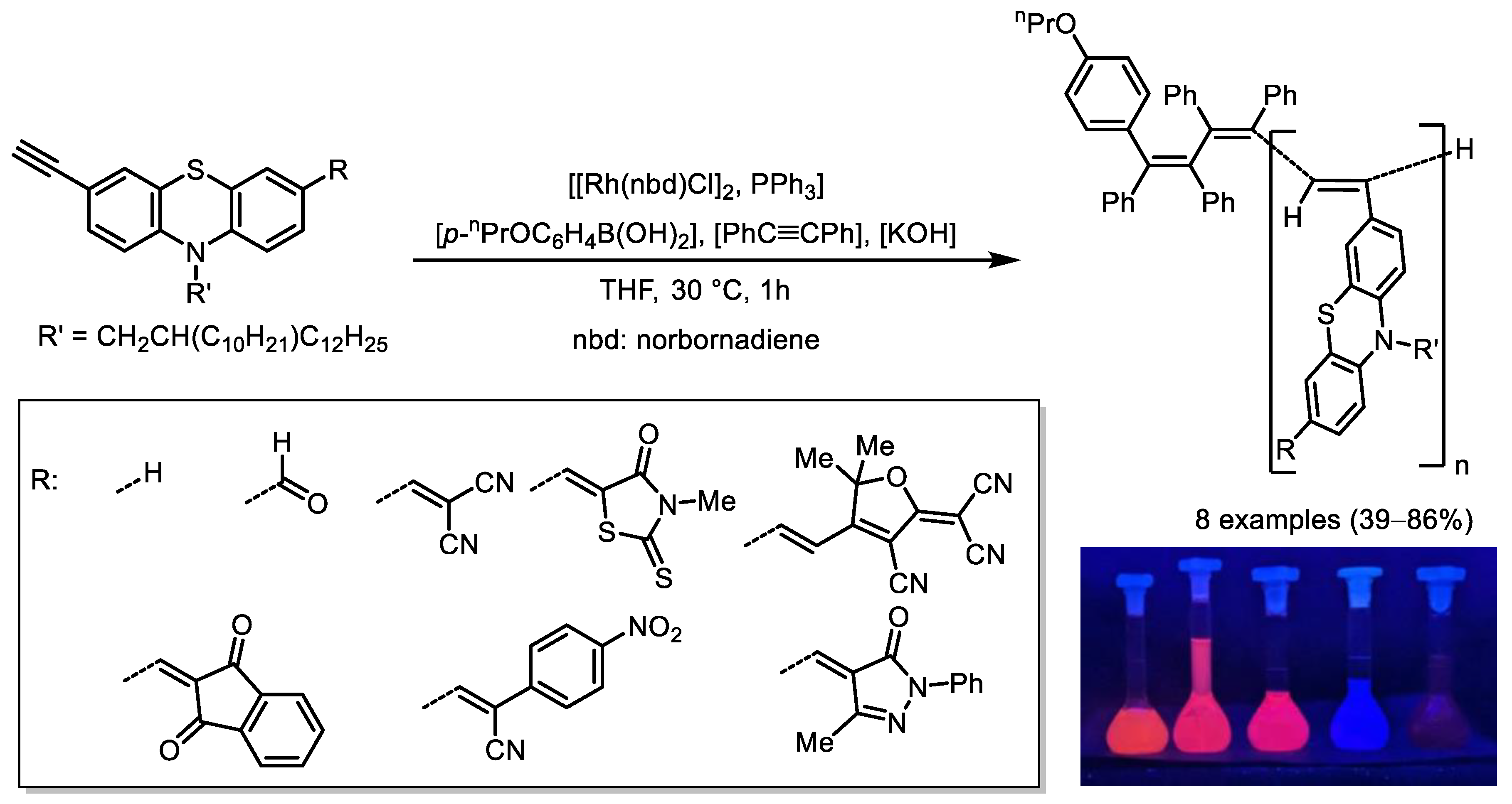
- Pisetsky, W.; Budny, P.; Müller, T.J.J. Synthesis and Photophysical Properties of Luminescent Phenothiazinyl Merocyanine Substituted Polyacetylenes. Angew. Chem. Int. Ed. 2024, 63, e202316246. [Google Scholar] [CrossRef] [PubMed]
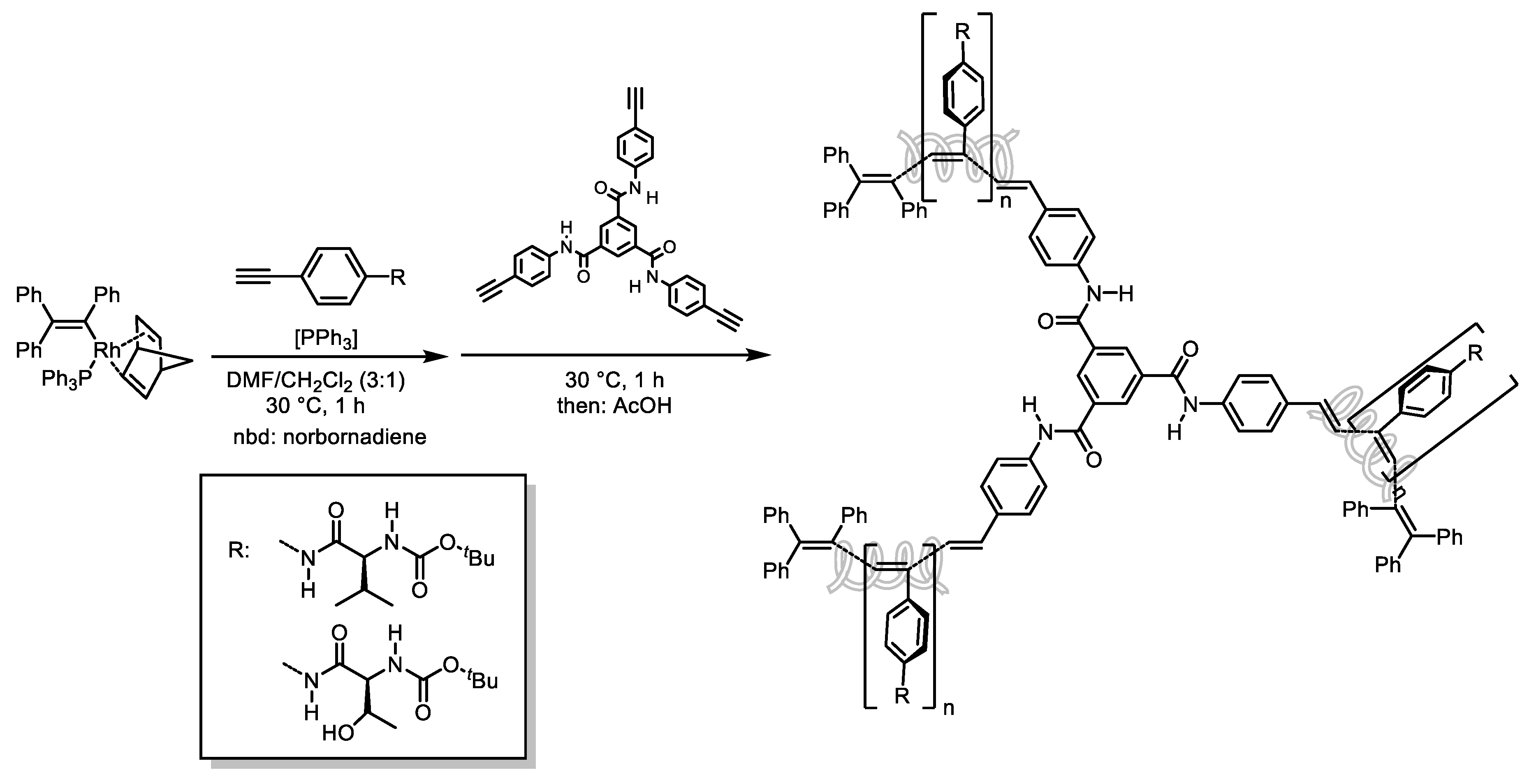
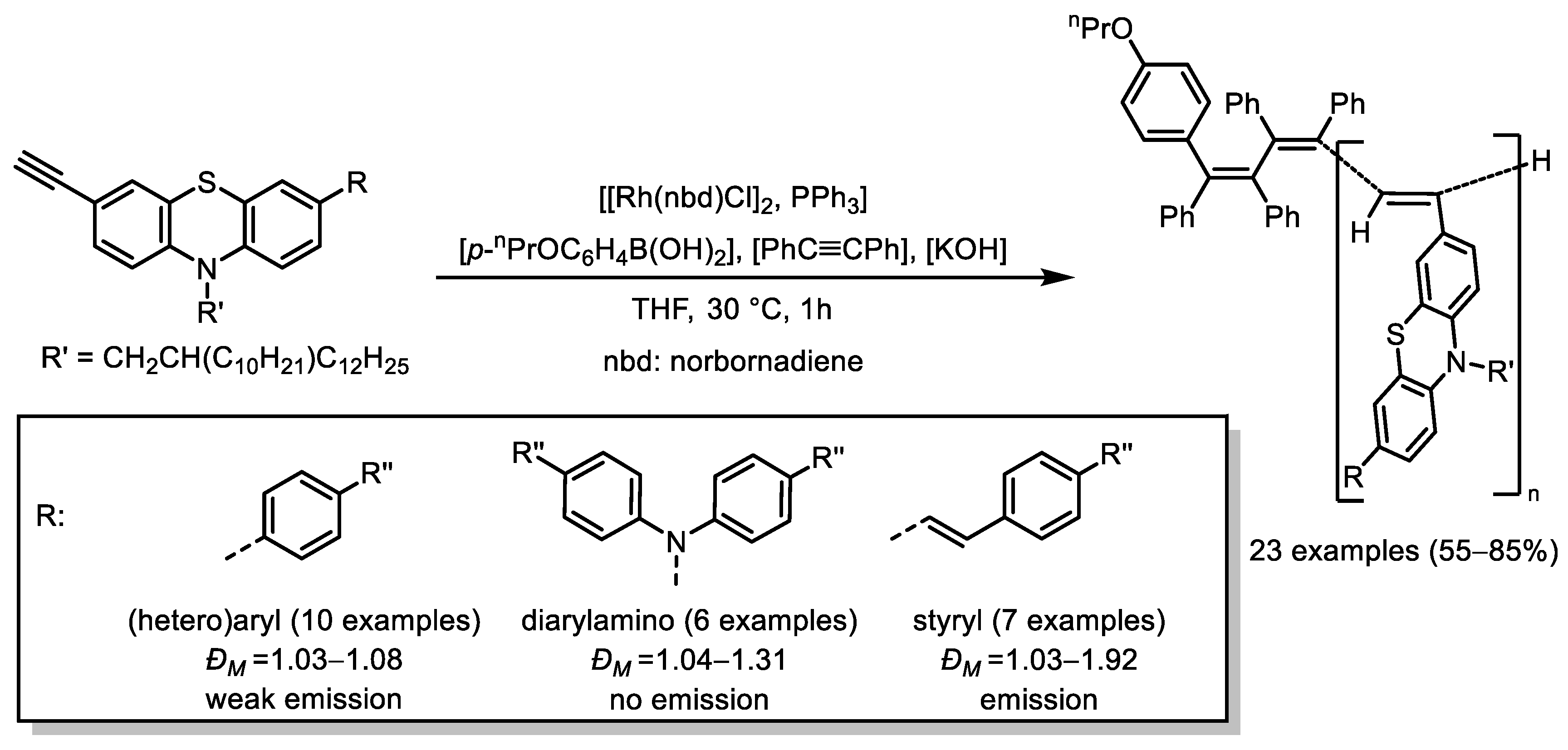
- Pisetsky, W.; Müller, T.J.J. Polyacetylenes with (Hetero)Aryl-, Styryl-, and Amino-Phenothiazinyl Sidechains—Synthesis and Photophysics. RSC Adv. 2024, 14, 10638–10643. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, T.; Nishimura, T.; Maeda, K. Development of Methods for Controlled Polymerization of Monosubstituted Acetylenes Enabling Versatile Design of Polymer End Structures. J. Synth. Org. Chem. Jpn. 2023, 81, 594–606. [Google Scholar] [CrossRef]
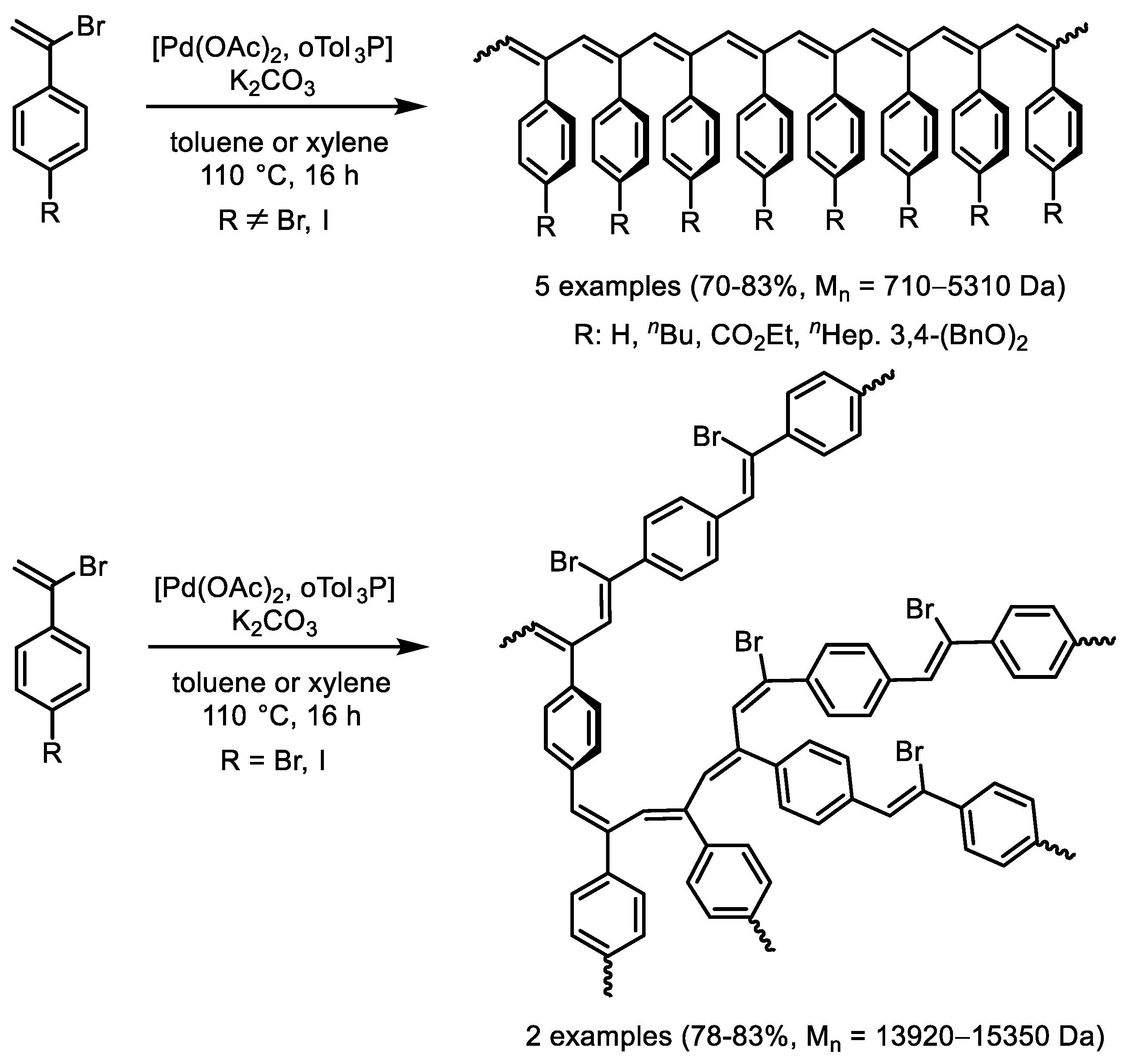
- He, L.; Yu, Y.; Liu, F.; He, J. Synthesis of linear and branched poly(phenylacetylene)s through Mizoroki-Heck coupling reaction of vinyl bromides. Eur. Polym. J. 2023, 195, 112233. [Google Scholar] [CrossRef]


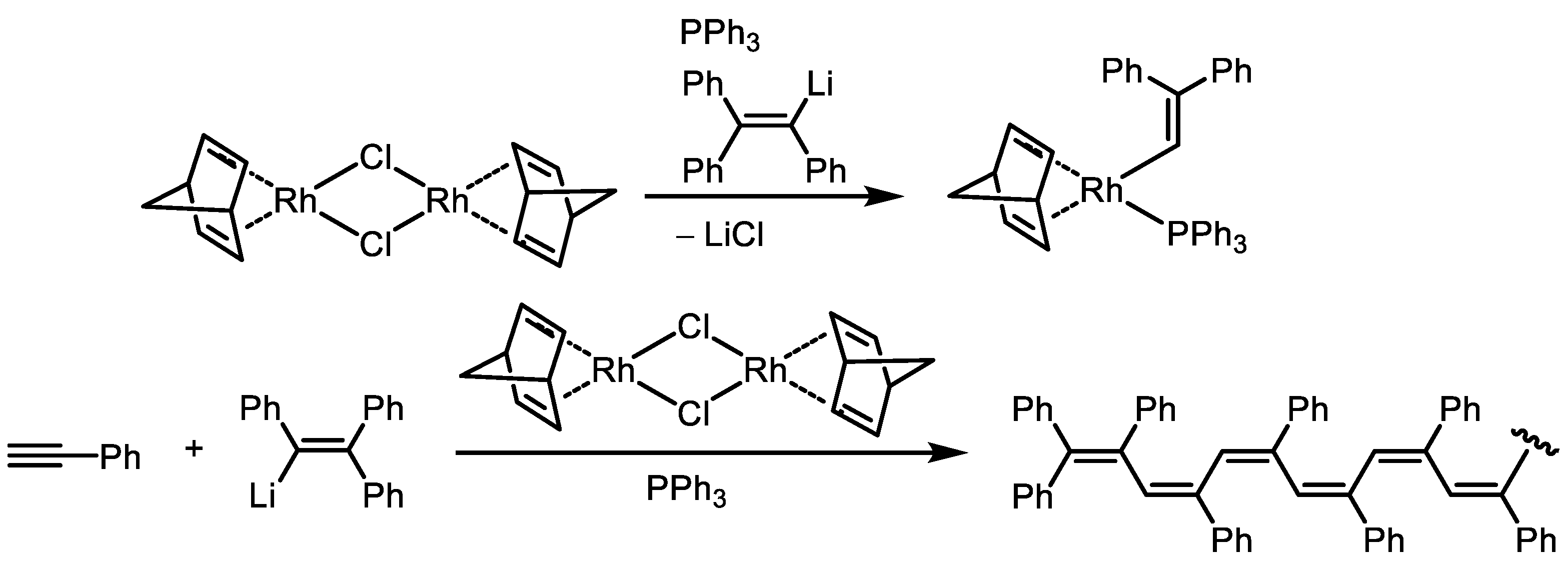
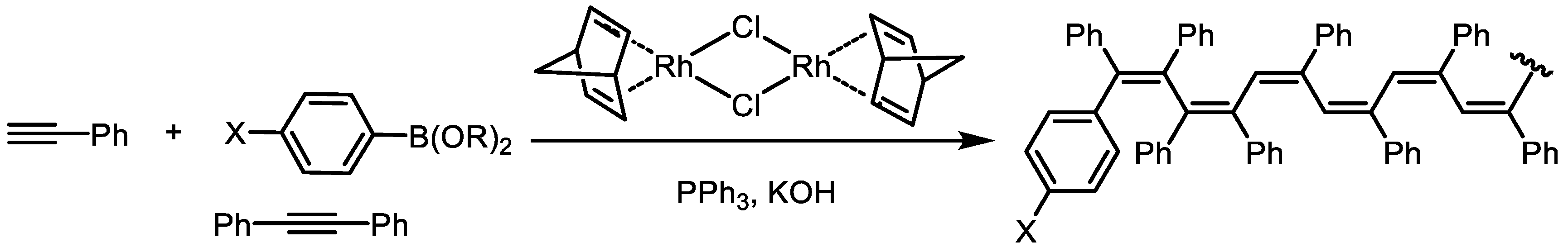
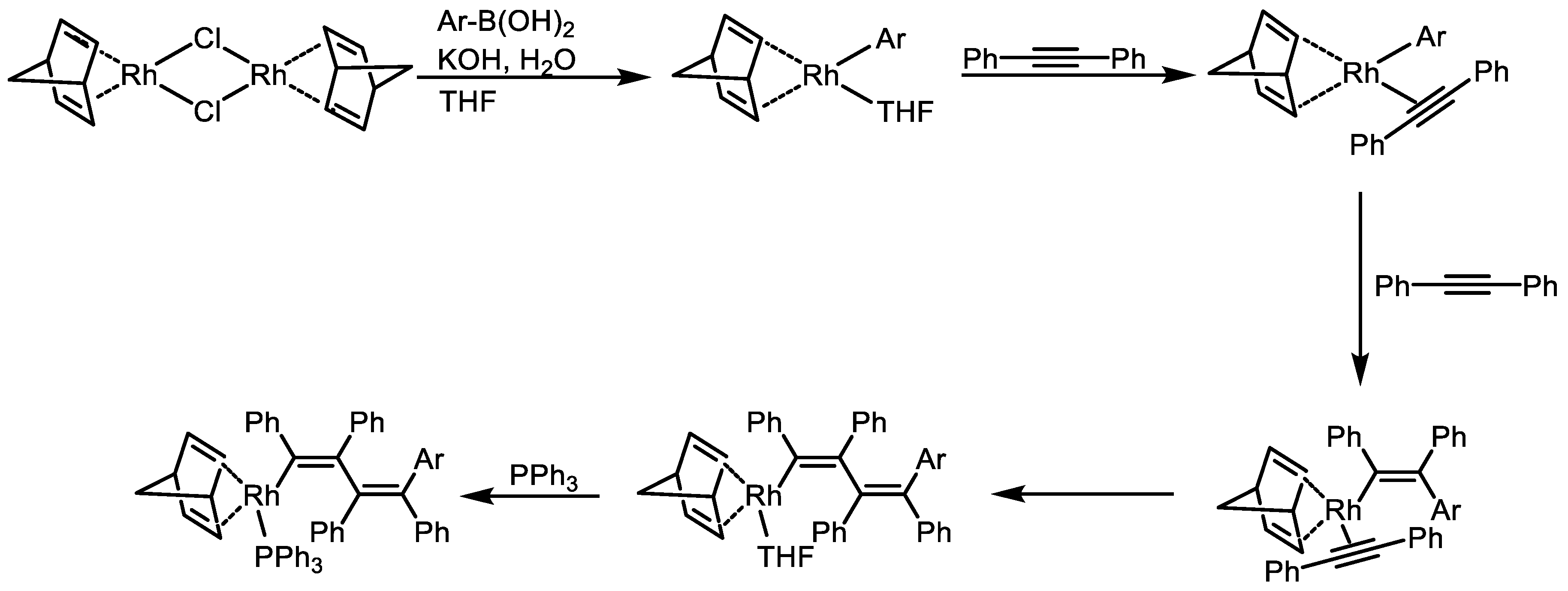

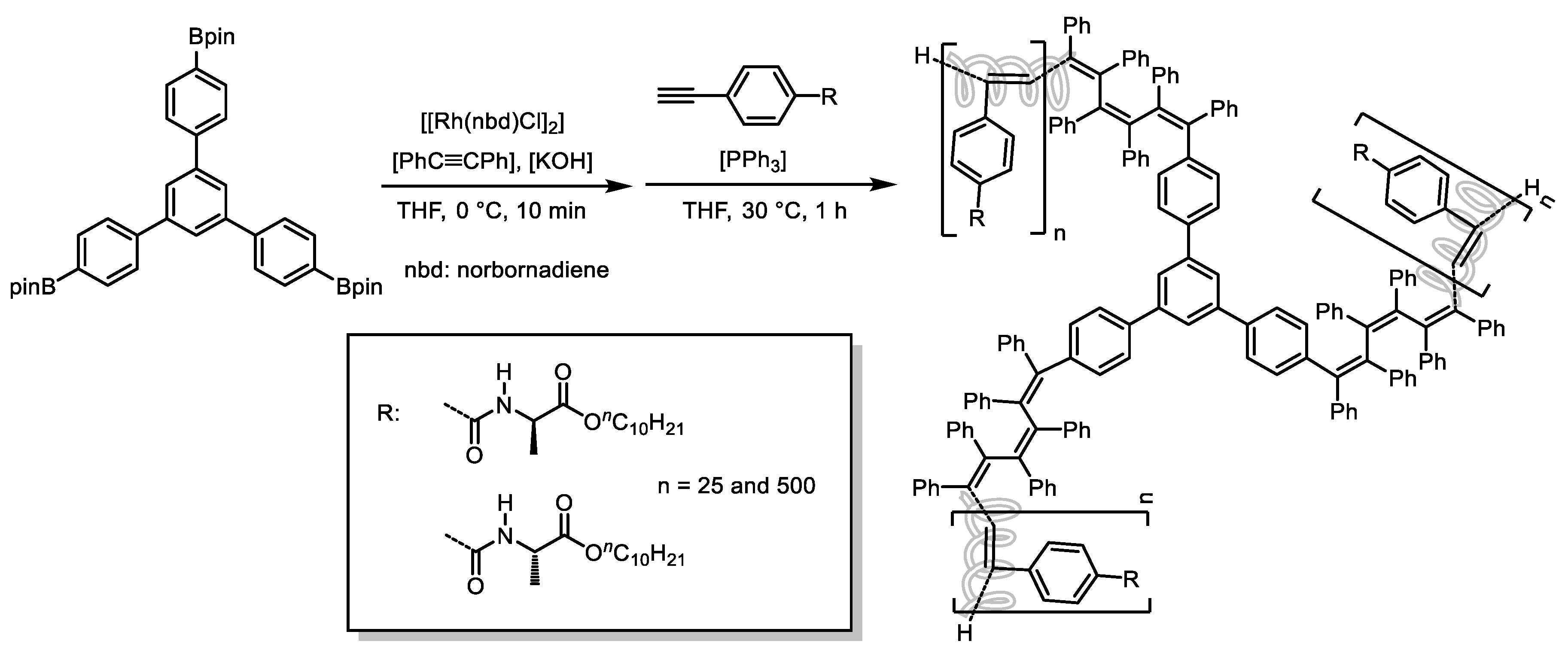

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pisetsky, W.; Müller, T.J.J. Recent Advances in the Synthesis of Substituted Polyacetylenes. Catalysts 2025, 15, 50. https://doi.org/10.3390/catal15010050
Pisetsky W, Müller TJJ. Recent Advances in the Synthesis of Substituted Polyacetylenes. Catalysts. 2025; 15(1):50. https://doi.org/10.3390/catal15010050
Chicago/Turabian StylePisetsky, Wladislaw, and Thomas J. J. Müller. 2025. "Recent Advances in the Synthesis of Substituted Polyacetylenes" Catalysts 15, no. 1: 50. https://doi.org/10.3390/catal15010050
APA StylePisetsky, W., & Müller, T. J. J. (2025). Recent Advances in the Synthesis of Substituted Polyacetylenes. Catalysts, 15(1), 50. https://doi.org/10.3390/catal15010050






