Investigation of Pore Size on the Hydrogen Evolution Reaction of 316L Stainless Steel Porous Electrodes
Abstract
1. Introduction
2. Results and Discussion
2.1. Sintering
2.2. 3D Image Analysis
2.3. Permeability Analysis
2.4. Linear Sweep Voltammetry Analysis
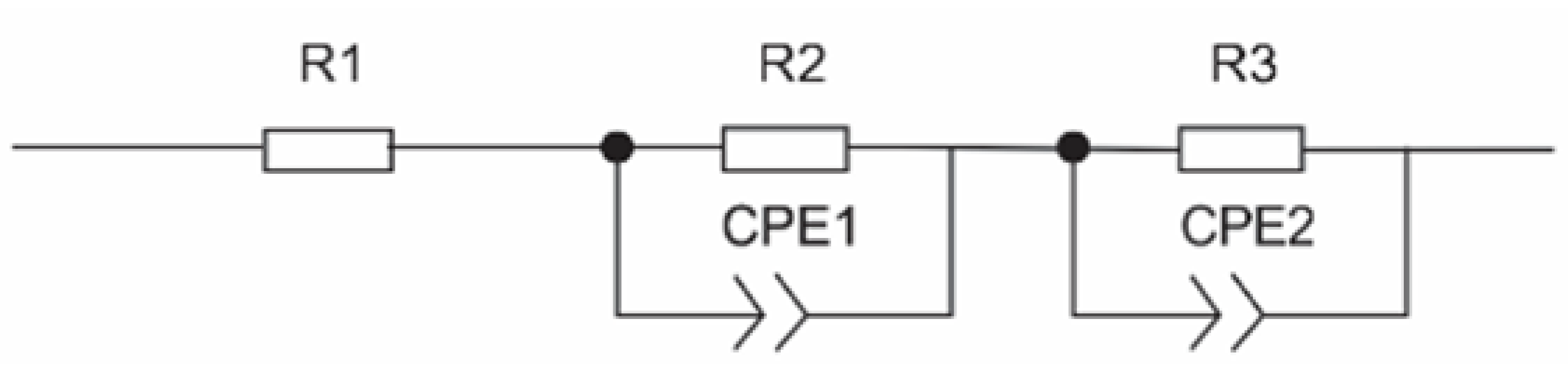
2.5. Electrochemical Impedance Spectroscopy Analysis
3. Experimental Method
3.1. Sintering 316L Stainless Steels
3.2. Electrochemical Analysis
4. Conclusions
- The increased NaOH concentration reduces the overpotential for HER, indicating a higher catalytic activity on porous electrodes.
- The S100 and S200 electrodes in 1.5 M NaOH showed a reduction in overpotential caused by an increase in the active area due to an increment in porous size; however, the S300 electrode, with the largest pores, showed a slightly increased overpotential.
- The S300 sample showed a higher impedance magnitude, indicating a higher corrosion resistance; however, a low charge transfer reduced the catalytic activity of HER.
- The S100 and WS electrodes showed a lower impedance magnitude, indicating a lower corrosion resistance; however, high charge transfer increased the catalytic activity of HER.
- The sample that showed higher Cdl and Rf values was S100, which means a higher active area favoring the HER.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ďurovič, M.; Hnát, J.; Bouzek, K. Electrocatalysts for the Hydrogen Evolution Reaction in Alkaline and Neutral Media. A Comparative Review. J. Power Sources 2021, 493, 229708. [Google Scholar] [CrossRef]
- Pletcher, D.; Li, X. Prospects for Alkaline Zero Gap Water Electrolysers for Hydrogen Production. Int. J. Hydrogen Energy 2011, 36, 15089–15104. [Google Scholar] [CrossRef]
- Safizadeh, F.; Ghali, E.; Houlachi, G. Electrocatalysis Developments for Hydrogen Evolution Reaction in Alkaline Solutions—A Review. Int. J. Hydrogen Energy 2015, 40, 256–274. [Google Scholar] [CrossRef]
- Shiva Kumar, S.; Lim, H. An Overview of Water Electrolysis Technologies for Green Hydrogen Production. Energy Rep. 2022, 8, 13793–13813. [Google Scholar] [CrossRef]
- Xie, J.; Wang, F.; Zhou, Y.; Dong, Y.; Chai, Y.; Dong, B. Internal Polarization Field Induced Hydroxyl Spillover Effect for Industrial Water Splitting Electrolyzers. Nanomicro Lett. 2024, 16, 39. [Google Scholar] [CrossRef]
- Marini, S.; Salvi, P.; Nelli, P.; Pesenti, R.; Villa, M.; Kiros, Y. Stable and Inexpensive Electrodes for the Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2013, 38, 11484–11495. [Google Scholar] [CrossRef]
- Gao, W.K.; Yang, M.; Chi, J.Q.; Zhang, X.Y.; Xie, J.Y.; Guo, B.Y.; Wang, L.; Chai, Y.M.; Dong, B. In Situ Construction of Surface Defects of Carbon-Doped Ternary Cobalt-Nickel-Iron Phosphide Nanocubes for Efficient Overall Water Splitting. Sci. China Mater. 2019, 9, 1285–1296. [Google Scholar] [CrossRef]
- Laleh, M.; Hughes, A.E.; Xu, W.; Cizek, P.; Tan, M.Y. Unanticipated Drastic Decline in Pitting Corrosion Resistance of Additively Manufactured 316L Stainless Steel after High-Temperature Post-Processing. Corros. Sci. 2020, 165, 108412. [Google Scholar] [CrossRef]
- Yu, F.; Li, F.; Sun, L. Stainless Steel as an Efficient Electrocatalyst for Water Oxidation in Alkaline Solution. Int. J. Hydrogen Energy 2016, 41, 5230–5233. [Google Scholar] [CrossRef]
- Alhakemy, A.Z.; Ahmed Amine Nassr, A.B.; Kashyout, A.E.H.; Wen, Z. Modifying the 316L Stainless Steel Surface by an Electrodeposition Technique: Towards High-Performance Electrodes for Alkaline Water Electrolysis. Sustain. Energy Fuels 2022, 6, 1382–1397. [Google Scholar] [CrossRef]
- Cabo Rios, A.; Hryha, E.; Olevsky, E.; Harlin, P. Sintering Anisotropy of Binder Jetted 316L Stainless Steel: Part I–Sintering Anisotropy. Powder Metall. 2022, 65, 273–282. [Google Scholar] [CrossRef]
- Garibay-Coria, S.; Velasco-Plascencia, M.; Villalobos, J.C.; Vázquez-Gómez, O.; Molina-Ocampo, A.; Vergara-Hernández, H.J. Chromium Effect Over the Hydrogen Evolution Reaction and Corrosion Behavior on Ni-Cr Porous Electrodes. J. Mater. Eng. Perform. 2024, 1–11. [Google Scholar] [CrossRef]
- Velasco-Plascencia, M.; Vázquez-Gómez, O.; Olmos, L.; Reyes-Calderón, F.; Vergara-Hernández, H.J.; Villalobos, J.C. Determination of Activation Energy on Hydrogen Evolution Reaction for Nickel-Based Porous Electrodes during Alkaline Electrolysis. Catalysts 2023, 13, 517. [Google Scholar] [CrossRef]
- Kan, X.; Yang, D.; Zhao, Z.; Sun, J. 316L FFF Binder Development and Debinding Optimization. Mater. Res. Express 2021, 8, 116515. [Google Scholar] [CrossRef]
- Larimian, T.; Kannan, M.; Grzesiak, D.; AlMangour, B.; Borkar, T. Effect of Energy Density and Scanning Strategy on Densification, Microstructure and Mechanical Properties of 316L Stainless Steel Processed via Selective Laser Melting. Mater. Sci. Eng. A 2020, 770, 138455. [Google Scholar] [CrossRef]
- Jamalkhani, M.; Dorula, M.; Roberts, E.; Mostafaei, A. Densification Kinetics, Microstructural Evolution and Mechanical Properties of Isothermally Sintered Binder Jetted 316L Stainless Steel. J. Manuf. Process 2024, 125, 267–282. [Google Scholar] [CrossRef]
- Nagaraju, K.V.V.; Kumaran, S.; Srinivasa Rao, T. Microwave Sintering of 316L Stainless Steel: Influence of Sintering Temperature and Time. Mater. Today Proc. 2020, 27, 2066–2071. [Google Scholar] [CrossRef]
- Chadha, K.; Tian, Y.; Spray, J.G.; Aranas, C. Effect of Annealing Heat Treatment on the Microstructural Evolution and Mechanical Properties of Hot Isostatic Pressed 316L Stainless Steel Fabricated by Laser Powder Bed Fusion. Metals 2020, 10, 753. [Google Scholar] [CrossRef]
- Tanvir, G.; Karim, M.A.; Jadhav, S.; Islam, S.; Kim, Y.M.; Ryu, H.J.; Kim, D.B. Effect of Hot Isostatic Pressing on Porosity of Wire-Arc Directed Energy Deposited TZM/NbZr1 Bimetallic Structure. Virtual Phys. Prototyp. 2024, 19, e2404989. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Z.; Wang, G.; Liu, Y.; Jing, Y. Effects of Sintering Temperature and Particle Size on Permeability of Functionally Gradient Composite Porous Materials Prepared by Hanging Slurry Process. SN Appl. Sci. 2020, 2, 1–20. [Google Scholar] [CrossRef]
- Jain, H.; Mondal, D.P.; Gupta, G.; Kumar, R.; Singh, S. Synthesis and Characterization of 316L Stainless Steel Foam Made through Two Different Removal Process of Space Holder Method. Manuf. Lett. 2020, 26, 33–36. [Google Scholar] [CrossRef]
- Jain, H.; Mondal, D.P.; Gupta, G.; Kumar, R. Effect of Compressive Strain Rate on the Deformation Behaviour of Austenitic Stainless Steel Foam Produced by Space Holder Technique. Mater. Chem. Phys. 2021, 259, 124010. [Google Scholar] [CrossRef]
- Lecis, N.; Mariani, M.; Beltrami, R.; Emanuelli, L.; Casati, R.; Vedani, M.; Molinari, A. Effects of Process Parameters, Debinding and Sintering on the Microstructure of 316L Stainless Steel Produced by Binder Jetting. Mater. Sci. Eng. A 2021, 828, 142108. [Google Scholar] [CrossRef]
- Yusuf, S.M.; Chen, Y.; Boardman, R.; Yang, S.; Gao, N. Investigation on Porosity and Microhardness of 316L Stainless Steel Fabricated by Selective Laser Melting. Metals 2017, 7, 64. [Google Scholar] [CrossRef]
- Jain, H.; Gupta, G.; Mondal, D.P.; Srivastava, A.K.; Pandey, A.; Srivastava, S.K.; Kumar, R. Effect of Particle Shape on Microstructure and Compressive Response of 316L SS Foam by Space Holder Technique. Mater. Chem. Phys. 2021, 271, 124924. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Xu, Z.; Nai, M.L.S.; Lu, S.; Ten, J.S.; Wei, J. Binder Jetting Additive Manufacturing of High Porosity 316L Stainless Steel Metal Foams. Materials 2020, 13, 3744. [Google Scholar] [CrossRef]
- Zhang, G.; Qu, Z.; Tao, W.Q.; Wang, X.; Wu, L.; Wu, S.; Xie, X.; Tongsh, C.; Huo, W.; Bao, Z.; et al. Porous Flow Field for Next-Generation Proton Exchange Membrane Fuel Cells: Materials, Characterization, Design, and Challenges. Chem. Rev. 2023, 123, 989–1039. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, Y.; Zhang, Y.; Wu, M. Numerical Study on Fluid Flow Behavior and Heat Transfer Performance of Porous Media Manufactured by a Space Holder Method. Materials 2024, 17, 2695. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.J.; Kim, H.J.; Seo, E.C.; Kim, M.J.; Park, M.; Lee, J.; Yun, J.Y. Fabrication of a Plate-Type Porous Stainless Steel 316l Powder Filter with Different Pore Structures without Use of a Binder. Arch. Metall. Mater. 2024, 69, 497–500. [Google Scholar] [CrossRef]
- Kale, A.B.; Kim, B.K.; Kim, D.I.; Castle, E.G.; Reece, M.; Choi, S.H. An Investigation of the Corrosion Behavior of 316L Stainless Steel Fabricated by SLM and SPS Techniques. Mater. Charact. 2020, 163, 110204. [Google Scholar] [CrossRef]
- Zhang, C.; Yuan, Y.; Shi, C.; Chen, J. Material Extrusion-Based Fabrication of 316L Stainless Steel: Analysis of Debinding and Sintering, Mechanical Properties, and Corrosion Resistance. Int. J. Adv. Manuf. Technol. 2023, 129, 587–599. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Barhoumi, E.M.; Mansir, I.B.; Emori, W.; Bhowmik, H. Effect of Electrode Material on the Hydrogen Production Using a Low-Cost Home-Made Alkaline Electrolyzer. Vacuum 2022, 198, 110878. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Fan, Y.; Zhou, W. Fabrication of Porous Metal by Selective Laser Melting as Catalyst Support for Hydrogen Production Microreactor. Int. J. Hydrogen Energy 2020, 45, 10–22. [Google Scholar] [CrossRef]
- Olmos, L.; Bouvard, D.; Salvo, L.; Bellet, D.; Di Michiel, M. Characterization of the Swelling during Sintering of Uniaxially Pressed Copper Powders by in Situ X-Ray Microtomography. J. Mater. Sci. 2014, 49, 4225–4235. [Google Scholar] [CrossRef]
- Manière, C.; Saccardo, E.; Lee, G.; McKittrick, J.; Molinari, A.; Olevsky, E.A. Swelling Negation during Sintering of Sterling Silver: An Experimental and Theoretical Approach. Results Phys. 2018, 11, 79–84. [Google Scholar] [CrossRef]
- Biguereau, E.; Bouvard, D.; Chaix, J.M.; Roure, S. On the Swelling of Silver Powder during Sintering. Powder Metall. 2016, 59, 394–400. [Google Scholar] [CrossRef]
- Cabezas-Villa, J.L.; Olmos, L.; Bouvard, D.; Lemus-Ruiz, J.; Jiménez, O. Processing and Properties of Highly Porous Ti6Al4V Mimicking Human Bones. J. Mater. Res. 2018, 33, 650–661. [Google Scholar] [CrossRef]
- Lin, C.L.; Miller, J.D. 3D Characterization and Analysis of Particle Shape Using X-Ray Microtomography (XMT). Powder Technol. 2005, 154, 61–69. [Google Scholar] [CrossRef]
- Olmos, L.; Bouvard, D.; Cabezas-Villa, J.L.; Lemus-Ruiz, J.; Jiménez, O.; Arteaga, D. Analysis of Compression and Permeability Behavior of Porous Ti6Al4V by Computed Microtomography. Met. Mater. Int. 2019, 25, 669–682. [Google Scholar] [CrossRef]
- Callow, B.; Falcon-Suarez, I.; Marin-Moreno, H.; Bull, J.M.; Ahmed, S. Optimal X-Ray Micro-CT Image Based Methods for Porosity and Permeability Quantification in Heterogeneous Sandstones. Geophys. J. Int. 2020, 223, 1210–1229. [Google Scholar] [CrossRef]
- Zeng, M.; Li, Y. Recent Advances in Heterogeneous Electrocatalysts for the Hydrogen Evolution Reaction. J. Mater. Chem. A Mater. 2015, 3, 14942–14962. [Google Scholar] [CrossRef]
- Fu, H.Q.; Zhou, M.; Liu, P.F.; Liu, P.; Yin, H.; Sun, K.Z.; Yang, H.G.; Al-Mamun, M.; Hu, P.; Wang, H.F.; et al. Hydrogen Spillover-Bridged Volmer/Tafel Processes Enabling Ampere-Level Current Density Alkaline Hydrogen Evolution Reaction under Low Overpotential. J. Am. Chem. Soc. 2022, 144, 6028–6039. [Google Scholar] [CrossRef]
- Takaki, S.; Nanba, S.; Imakawa, K.; Macadre, A.; Yamabe, J.; Matsunaga, H.; Matsuoka, S. Determination of Hydrogen Compatibility for Solution-Treated Austenitic Stainless Steels Based on a Newly Proposed Nickel-Equivalent Equation. Int. J. Hydrogen Energy 2016, 41, 15095–15100. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Liu, S.; Wang, L.; Xie, L.; Zhuang, Z.; Sun, C.; Wang, J.; Tang, M.; Sun, S.; et al. Tailoring Activation Sites of Metastable Distorted 1T′-Phase MoS2 by Ni Doping for Enhanced Hydrogen Evolution. Nano Res. 2022, 15, 5946–5952. [Google Scholar] [CrossRef]
- Shervedani, R.K.; Alinoori, A.H.; Madram, A.R. Electrocatalytic Activities of Nickel-Phosphorous Composite Coating Reinforced with Codeposited Graphite Carbon for Hydrogen Evolution Reaction in Alkaline Solution. J. New Mater. Electrochem. Syst. 2008, 11, 259–265. [Google Scholar]
- Olivares-Ramírez, J.M.; Campos-Cornelio, M.L.; Uribe Godínez, J.; Borja-Arco, E.; Castellanos, R.H. Studies on the Hydrogen Evolution Reaction on Different Stainless Steels. Int. J. Hydrogen Energy 2007, 32, 3170–3173. [Google Scholar] [CrossRef]
- Yin, H.; Rong, F.; Xie, Y. A Review of Typical Transition Metal Phosphides Electrocatalysts for Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2024, 52, 350–375. [Google Scholar] [CrossRef]
- Argyle, M.D.; Bartholomew, C.H. Heterogeneous Catalysts Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145–269. [Google Scholar] [CrossRef]
- Gomez, M.J.; Franceschini, E.A.; Lacconi, G.I. Ni and NixCoy Alloys Electrodeposited on Stainless Steel AISI 316L for Hydrogen Evolution Reaction. Electrocatalysis 2018, 9, 459–470. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, J.; Gong, G.; Song, R.; Yuan, Y.; Li, M.; Hu, W.; Fan, P.; Yuan, L.; Wang, L. In Situ Surface Reconstruction of Catalysts for Enhanced Hydrogen Evolution. Catalysts 2023, 13, 120. [Google Scholar] [CrossRef]
- Łosiewicz, B.; Budniok, A.; Rówiński, E.; Łagiewka, E.; Lasia, A. The Structure, Morphology and Electrochemical Impedance Study of the Hydrogen Evolution Reaction on the Modified Nickel Electrodes. Int. J. Hydrogen Energy 2004, 29, 145–157. [Google Scholar] [CrossRef]
- Tang, J.; Xu, J.L.; Li, L.L.; Ma, Y.C.; Ye, Z.G.; Luo, H.Y.; Luo, J.M. In-Situ Hydrothermal Synthesis of Ni–MoO2 Heterostructure on Porous Bulk NiMo Alloy for Efficient Hydrogen Evolution Reaction. Trans. Nonferrous Met. Soc. China 2022, 32, 1598–1608. [Google Scholar] [CrossRef]
- Xu, J.L.; Li, L.L.; Tang, J.; Dai, L.; Li, X.B.; Ye, Z.G.; Luo, J.M. Powder Metallurgy Synthesis of Porous NiMo Alloys as Efficient Electrocatalysts to Enhance the Hydrogen Evolution Reaction. J. Alloys Compd. 2021, 865, 158901. [Google Scholar] [CrossRef]
- Vesborg, P.C.K.; Seger, B.; Chorkendorff, I. Recent Development in Hydrogen Evolution Reaction Catalysts and Their Practical Implementation. J. Phys. Chem. Lett. 2015, 6, 951–957. [Google Scholar] [CrossRef] [PubMed]
- Hitz, C.; Lasia, A. Experimental Study and Modeling of Impedance of the Her on Porous Ni Electrodes. J. Electroanal. Chem. 2001, 500, 213–222. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Chong, Z.; Omanovic, S. Characterization of Ni, NiMo, NiW and NiFe Electroactive Coatings as Electrocatalysts for Hydrogen Evolution in an Acidic Medium. J. Mol. Catal. A Chem. 2005, 226, 179–197. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Xu, Y.; Xiao, Y.; Qian, J.; Xu, Y.; Guan, Z.; He, Y.; Zeng, Y. Electrocatalytic Activity of Porous Ni–Fe–Mo–C–LaNi 5 Sintered Electrodes for Hydrogen Evolution Reaction in Alkaline Solution. RSC Adv. 2017, 7, 32264–32274. [Google Scholar] [CrossRef]
- Metikoš-Huković, M.; Jukić, A. Correlation of Electronic Structure and Catalytic Activity of Zr–Ni Amorphous Alloys for the Hydrogen Evolution Reaction. Electrochim. Acta 2000, 45, 4159–4170. [Google Scholar] [CrossRef]
- Kramer, M.; Tomkiewicz, M. Porous Electrodes: I. Numerical Simulation Using. Random Network and Single-Pore Models. J. Electrochem. Soc. 1984, 131, 1283. [Google Scholar] [CrossRef]
- Navarro-Flores, E.; Omanovic, S. Hydrogen Evolution on Nickel Incorporated in Three-Dimensional Conducting Polymer Layers. J. Mol. Catal. A Chem. 2005, 242, 182–194. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of Electrode Impedances Complicated by the Presence of a Constant Phase Element. J. Electroanal. Chem. Interfacial Electrochem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Panek, J.; Serek, A.; Budniok, A.; Rówinski, E.; Łagiewka, E. Ni+Ti Composite Layers as Cathode Materials for Electrolytic Hydrogen Evolution. Int. J. Hydrogen Energy 2003, 28, 169–175. [Google Scholar] [CrossRef]
- Solmaz, R.; Döner, A.; Şahin, I.; Yüce, A.O.; Kardaş, G.; Yazici, B.; Erbil, M. The Stability of NiCoZn Electrocatalyst for Hydrogen Evolution Activity in Alkaline Solution during Long-Term Electrolysis. Int. J. Hydrogen Energy 2009, 34, 7910–7918. [Google Scholar] [CrossRef]
- Danaee, I.; Noori, S. Kinetics of the Hydrogen Evolution Reaction on NiMn Graphite Modified Electrode. Int. J. Hydrogen Energy 2011, 36, 12102–12111. [Google Scholar] [CrossRef]
- Torres, C.; Moreno, B.; Chinarro, E.; de Fraga Malfatti, C. Nickel-Polyaniline Composite Electrodes for Hydrogen Evolution Reaction in Alkaline Media. Int. J. Hydrogen Energy 2017, 42, 20410–20419. [Google Scholar] [CrossRef]
- Xu, B.; Chen, Z.; Zhang, H.; Sun, Y.; Li, C. Novel Ni(S0.49Se0.51)2 Porous Flakes Array on Carbon Fiber Cloth for Efficient Hydrogen Evolution Reaction. Int. J. Hydrogen Energy 2017, 42, 30119–30125. [Google Scholar] [CrossRef]

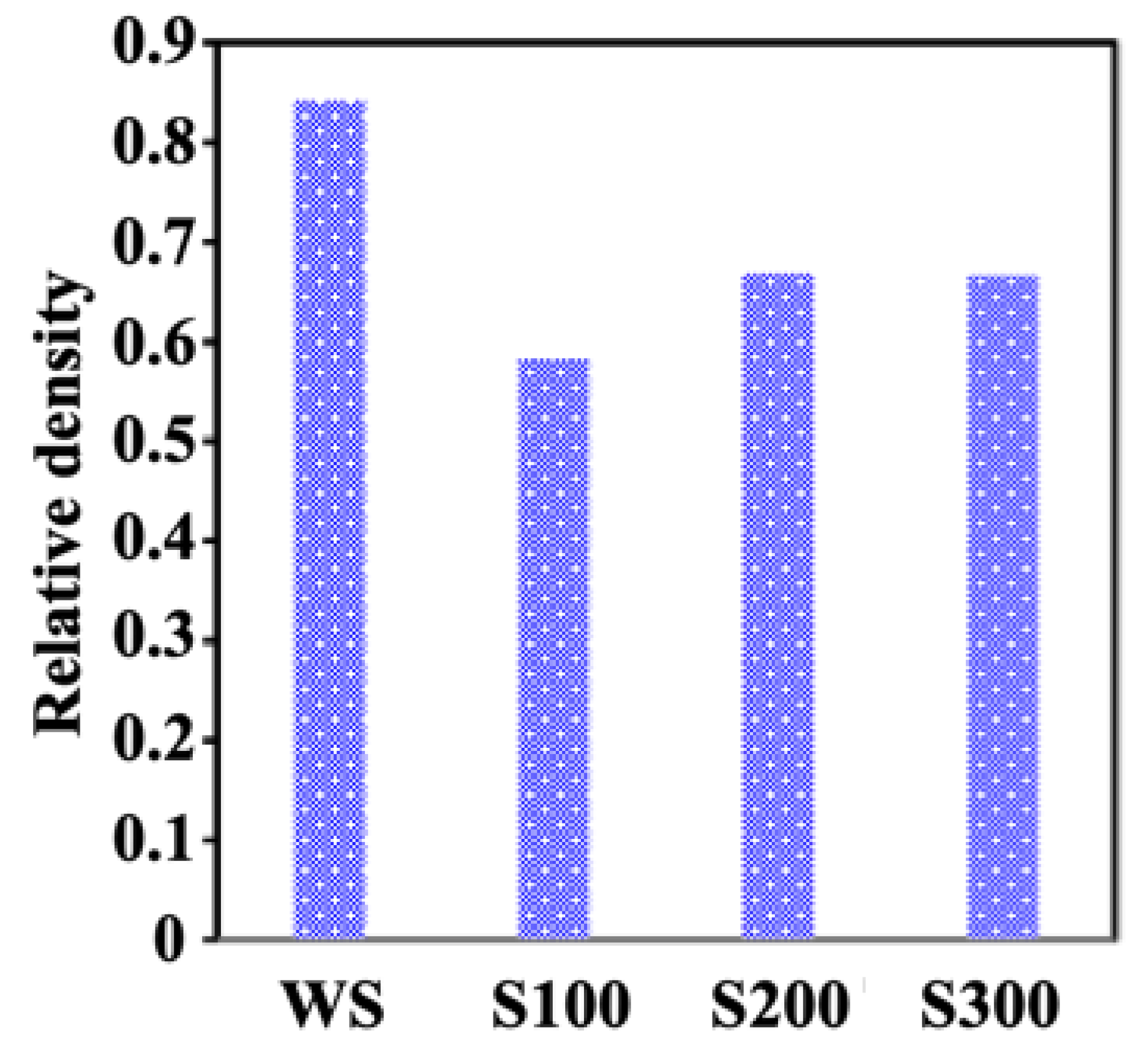

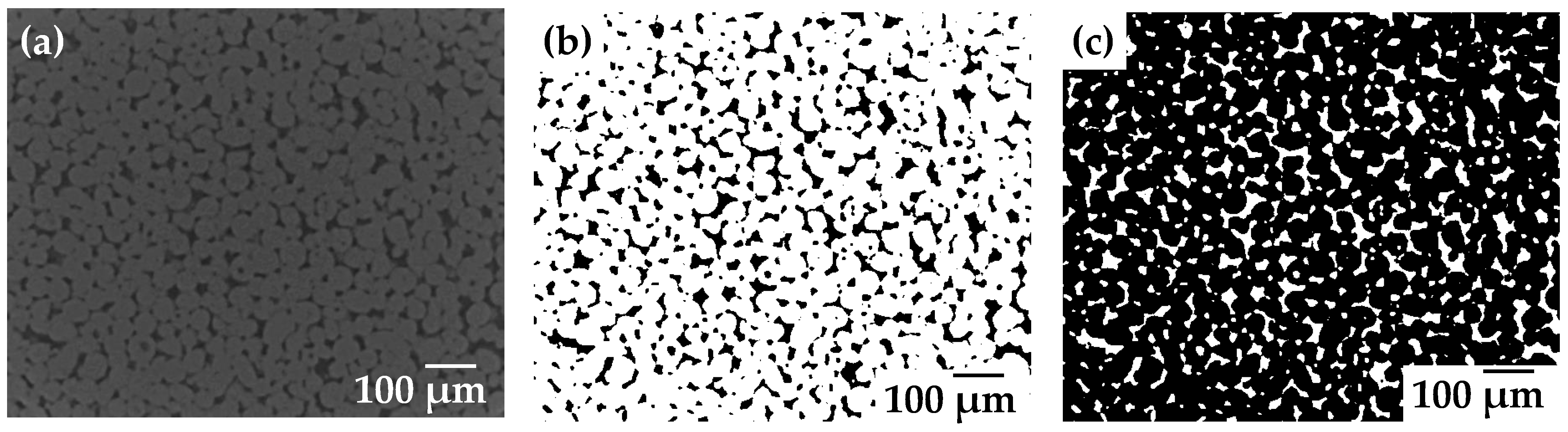
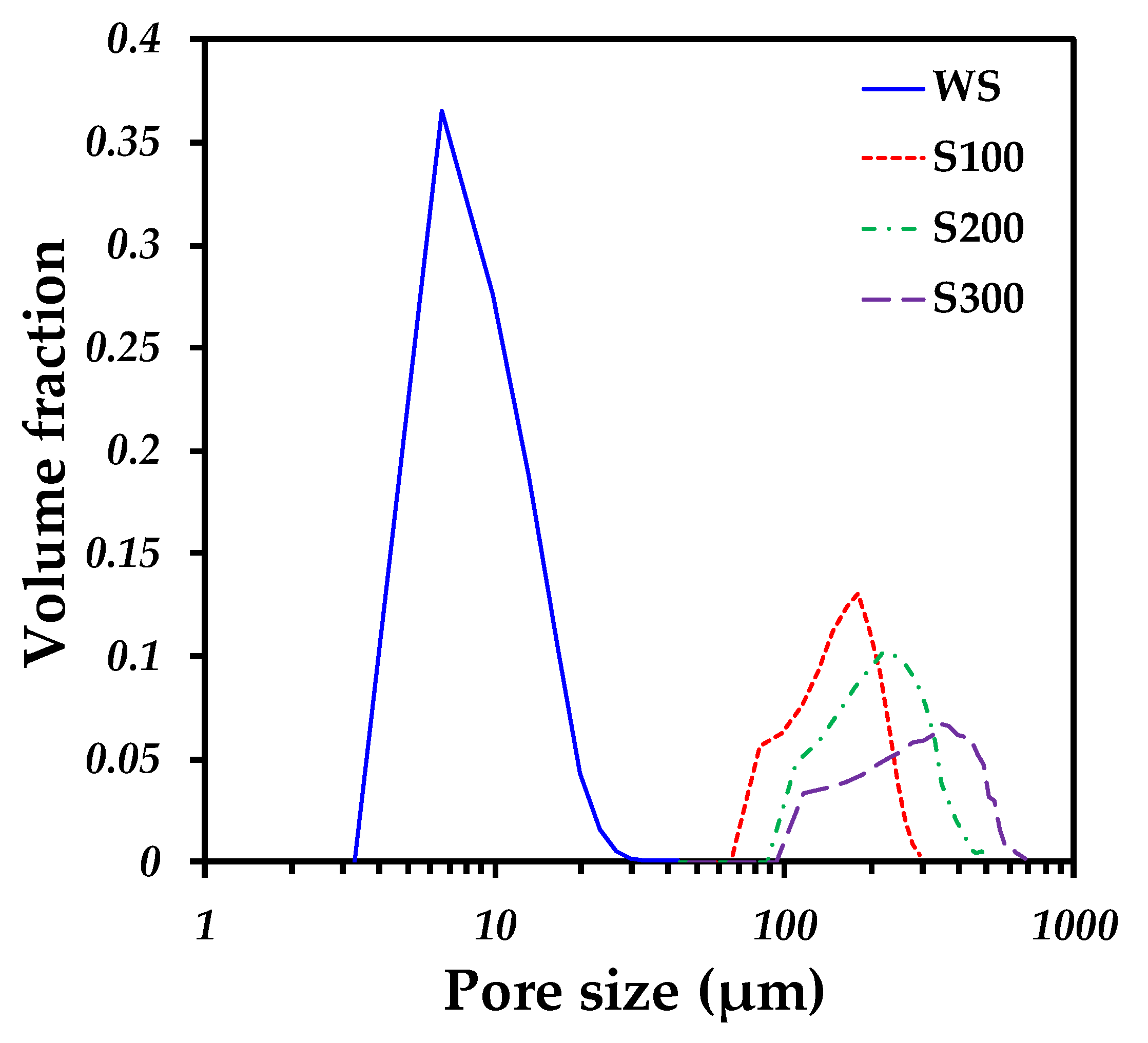

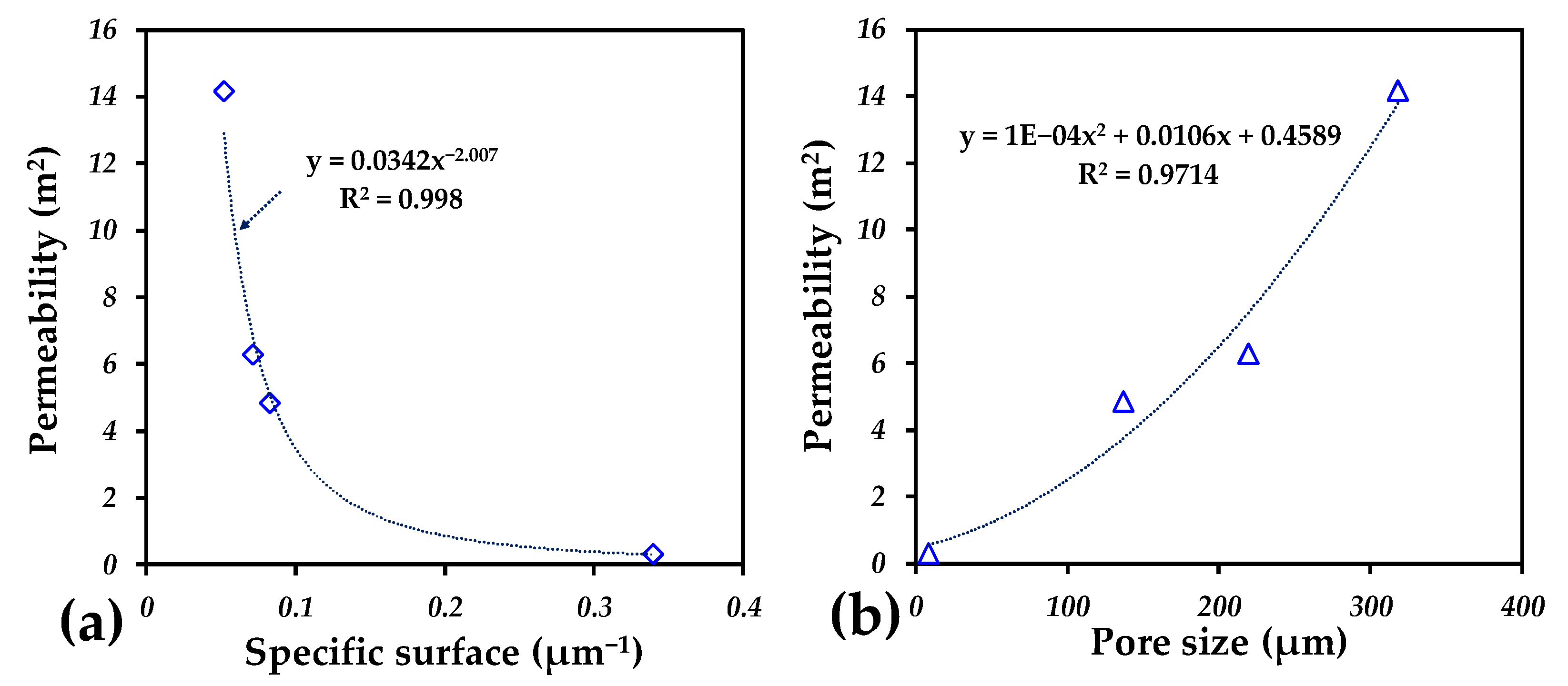


| Sample | d50 (µm) | Specific Surface (µm−1) | Pore Connectivity (%) | Permeability (µm2) |
|---|---|---|---|---|
| WS | 8.19 | 0.339564 | 90 | 0.2734 |
| S100 | 144.91 | 0.0829015 | 98 | 2.3083 |
| S200 | 228.52 | 0.071612 | 99 | 5.5710 |
| S300 | 333.05 | 0.0519835 | 99 | 9.4606 |
| Cdl (µF/cm2) | Rf | |||
|---|---|---|---|---|
| Sample | 0.5 M | 1.5 M | 0.5 M | 1.5 M |
| WS | 33.50 | 153.34 | 1.67 | 7.66 |
| S100 | 212.03 | 234.95 | 10.60 | 11.74 |
| S200 | 39.98 | 155.84 | 1.99 | 7.79 |
| S300 | 48.17 | 131.70 | 2.40 | 6.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solorio, V.M.; Olmos, L.; Velasco-Plascencia, M.; Vergara-Hernández, H.J.; Villalobos, J.C.; Machado López, M.M.; Salgado López, J.M. Investigation of Pore Size on the Hydrogen Evolution Reaction of 316L Stainless Steel Porous Electrodes. Catalysts 2025, 15, 38. https://doi.org/10.3390/catal15010038
Solorio VM, Olmos L, Velasco-Plascencia M, Vergara-Hernández HJ, Villalobos JC, Machado López MM, Salgado López JM. Investigation of Pore Size on the Hydrogen Evolution Reaction of 316L Stainless Steel Porous Electrodes. Catalysts. 2025; 15(1):38. https://doi.org/10.3390/catal15010038
Chicago/Turabian StyleSolorio, Victor Manuel, Luis Olmos, Melina Velasco-Plascencia, Héctor J. Vergara-Hernández, Julio C. Villalobos, Mario Misael Machado López, and Juan Manuel Salgado López. 2025. "Investigation of Pore Size on the Hydrogen Evolution Reaction of 316L Stainless Steel Porous Electrodes" Catalysts 15, no. 1: 38. https://doi.org/10.3390/catal15010038
APA StyleSolorio, V. M., Olmos, L., Velasco-Plascencia, M., Vergara-Hernández, H. J., Villalobos, J. C., Machado López, M. M., & Salgado López, J. M. (2025). Investigation of Pore Size on the Hydrogen Evolution Reaction of 316L Stainless Steel Porous Electrodes. Catalysts, 15(1), 38. https://doi.org/10.3390/catal15010038







