Abstract
Converting carbon dioxide (CO2) into solar fuels through photocatalysis represents an appealing approach to tackling the escalating energy crisis and mitigating the greenhouse effect. In this study, using melamine–formaldehyde (MF) nanospheres as a nitrogen source, a N element was simultaneously doped into the TiO2 nanoparticle structure supported by carbon hollow spheres using a one-step carbonization method to form a heterojunction N-CHS@N-TiO2 (marked as (N-(CHS@TiO2)). The composite showed superior photocatalytic activity in reducing CO2 compared with TiO2 and N-CHS: after 6 h of visible light irradiation, the CO yield was 4.3 times that of N-CHS and TiO2; 6 h of UV irradiation later, the CO yield reached 2.6 times that of TiO2 and 7 times that of N-CHS. The substantial enhancement in photocatalytic activity was attributed to the nitrogen simultaneously doped carbon hollow spheres and TiO2, mesoporous structure, small average TiO2 crystal size, large surface areas, and the heterostructure formed by N-CHS and N-TiO2. The UV-vis diffuse reflectance spectra (DRS) exhibit a significant improvement in light absorption, attributed to the visible-light-active carbon hollow sphere and the N element doping, thereby enhancing solar energy utilization.
1. Introduction
At present, fossil fuels remain the primary source of global energy consumption, accounting for over 85%. Yet, this excessive reliance has given rise to uncontrolled emissions of harmful greenhouse gases, notably CO2 [1,2]. The escalating levels of atmospheric CO2 pose a severe threat to global climate stability, necessitating innovative technologies for its mitigation and conversion [3,4,5]. A solar-driven semiconductor photocatalytic reduction in CO2 is a promising environmentally friendly technology [6,7,8,9]. Photocatalytic CO2 reduction outshines traditional thermal and electrocatalytic methods with benefits such as gentle reaction conditions, alignment with the tenets of green chemistry, and robust catalyst durability [10,11]. The utilization of solar energy for photocatalytic CO2 reduction to produce renewable fuels and chemicals can solve the energy crisis and form a green, low-carbon, sustainable development system [12,13]. Various efficient semiconductor-based photocatalytic systems have been evolved for CO2 reduction [14,15]. However, the narrow light absorption range, low specific surface area and high recombination of photo-induced carriers limit the photocatalytic activity [16].
TiO2, being a representative photocatalyst, is widely regarded as a promising material due to its abundant reserves and relatively superior photocatalytic performance. Alternatively, individual TiO2 exhibits a large band gap and a rapid recombination rate of photoexcited carriers, thereby impeding its viable utilization [17]. In the last few decades, extensive research efforts have been devoted to defect engineering, aiming to improve the compound’s light absorption and carrier separation characteristics. Doping with nonmetallic ions (C, N, S) represents a technique within defect engineering used to modify the band positions [18,19]. Nitrogen is the most commonly utilized dopant among all non-metal dopants [20]. This is mainly attributed to its relatively low ionization energy and its atomic size being similar to that of oxygen [21]. Nitrogen-doped TiO2 possesses strong visible light-driven photocatalytic activity [22,23,24]. Introducing N atoms into the TiO2 lattice structure results in the creation of N 2p energy levels that are situated close to the valence band (VB) of TiO2. This modification narrows the band gap of TiO2 and enables the semiconductor to absorb light in the visible spectrum [25,26,27]. Additionally, the stability of N atoms, coupled with their low ionization potential and atomic radius comparable to oxygen, allows them to form metastable centers in N-doped TiO2 [25]. However, the incorporation of nitrogen generates a significant number of recombination sites for photo-generated electrons and holes, leading to a reduction in photocatalytic activity [28]. Hence, to achieve spatial separation of electrons and holes, lightweight and conductive support, like those belonging to the carbon material family, is required [29].
Affordable carbon-based materials, possessing exceptional electron mobility, have the potential to be integrated with metal oxide semiconductors, effectively suppressing the recombination of photo-generated electron–hole pairs [30]. However, individual carbon materials lack suitable active sites. Therefore, numerous worthwhile initiatives related to doping have been carried out. Specifically, incorporating nitrogen into carbon materials is regarded as a highly effective approach to enhancing their photocatalytic performance [31]. Doped N atom carbon can regulate the work function of carbon, induce charge delocalization, further enhance electron transfer, and expand the visible light trapping region [32]. The unique nitrogen-doped carbon hollow sphere structure exhibited high surface area and remarkable performance in capturing CO2 [33]. Semiconductor heterojunction photocatalysts have been created by depositing one semiconductor material onto the surface of another, forming either supported or core–shell heterogeneous structures. These structures broaden the range of light responsiveness to longer wavelengths and inhibit the recombination of electrons and holes by spatially separating them within different components [34].
Hollow nanostructures have garnered growing interest in the field of photocatalysis due to their capability for multiple light reflections and scattering, a high concentration of active sites conducive to redox reactions, and their suitability as a platform for the effective spatial segregation of reduction and oxidation sites or cocatalysts between the inner and outer surfaces of the shell [35]. In this study, a N element simultaneously doped carbon hollow sphere-supported TiO2 (N-(CHS@TiO2)) material was synthesized by a one-step carbonization route, which demonstrated a superior photocatalytic activity under visible light compared to both pure TiO2 and N-doped carbon hollow spheres (N-CHS). Their photocatalytic properties were assessed through the conduct of the CO2 reduction reaction.
2. Results and Discussion
The N-(CHS@TiO2) composite material was fabricated through a simplified one-step carbonization process. Figure 1a presents the X-ray diffraction (XRD) spectra of N-CHS, N-(CHS@TiO2), and TiO2. In the pattern of N-(CHS@TiO2), the diffraction peak at 21° retained the original structure of N-CHS. The others were assigned to anatase and rutile-type TiO2, which showed that the composite of carbon hollow sphere and TiO2 phase was formed. Compared to N-(CHS@TiO2), the peaks of TiO2 were narrower and sharper, while the anatase phase was more obvious, which indicated that crystallinity decreased during the formation of heterojunction and the reaction environment was more favorable for anatase formation.
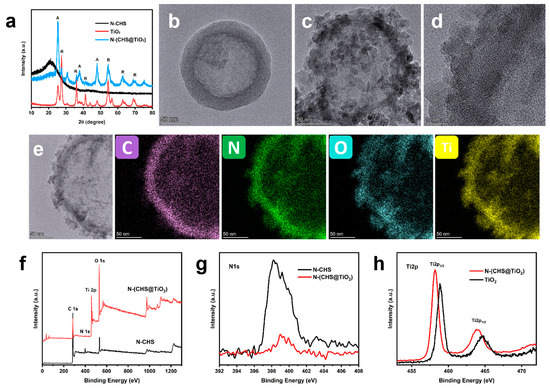
Figure 1.
XRD patterns of N-CHS, N-(CHS@TiO2), and TiO2 (A: anatase, R: rutile) (a), TEM image of N-CHS (b) and N-(CHS@TiO2) (c), HRTEM of N-(CHS@TiO2) (d), the corresponding elemental mapping of C, N, O, and Ti of single N-(CHS@TiO2) (e), XPS survey spectra of N-CHS and N-(CHS@TiO2) (f), high-resolution N1s XPS profile of N-CHS and N-(CHS@TiO2) (g), and high-resolution XPS spectra of Ti for N-(CHS@TiO2) and TiO2 (h).
The nitrogen adsorption–desorption measurements were conducted to investigate the specific surface area (BET) and pore structure of the N-(CHS@TiO2) composite, both of which are crucial factors for photocatalytic CO2 reduction. Figure S1 displays the nitrogen adsorption–desorption isotherms, along with the corresponding pore size distribution, for the representative samples N-CHS, TiO2, and N-(CHS@TiO2). The isotherm analysis of the products revealed the existence of mesoporous structures within the samples. As shown in Table S1, the BJH desorption pore size distributions exhibited values of 3.1 and 14.8 nm for N-CHS, 4.0 nm for TiO2, and 5.6 nm for N-(CHS@TiO2). The corresponding specific surface areas were 315.7 m2/g, 89.1 m2/g, and 73.1 m2/g. Compared with N-CHS, the pore size (5.6 nm) and specific surface area (73.1 m2/g) of N-(CHS@TiO2) were smaller because titanium dioxide nanoparticles were coated on the surface of N-CHS. For the TiO2 sample, we believe that the assembly of the nanoparticles created interparticle spaces, forming the porous structures. Compared with TiO2, N-(CHS@TiO2) exhibited a lower specific surface area, potentially due to the influence of CHS addition or N element doping on the nanoparticle assembly process. Nevertheless, N-(CHS@TiO2) still had a mesoporous structure and a large specific surface area, which were beneficial for the improvement of photocatalytic performance.
The TEM images of N-CHS and N-(CHS@TiO2) are depicted in Figure 1b–d. Figure 1b shows the carbon hollow sphere structure. Figure 1c exhibits the nanoparticle-coated hollow sphere structure. The nanoparticle size was about 3–10 nm (in Figure 1d and Figure S2a). Figure S2b shows the TEM image of TiO2, which is the same as in Figure 1d and Figure S2a. It proved that the nanoparticle-coated hollow sphere structure was TiO2. The corresponding elemental mapping, depicted in Figure 1e, revealed a uniform distribution of C, N, O and Ti elements, which also illustrated that the hollow sphere structure was N-CHS and the nanoparticles were TiO2. As shown in the N elemental mapping, the surface nanoparticles contained the N element, which confirmed that CHS and TiO2 were simultaneously doped with the N element.
The X-ray photoelectron spectroscopy (XPS) was utilized to analyze the chemical composition and elemental configurations of both the N-CHS and N-(CHS@TiO2) samples. The XPS survey spectra presented in Figure 1f demonstrate that the primary elements in the N-(CHS@TiO2) sample were C, O, N, and Ti. The high-resolution N1s spectra in Figure 1g show that N-(CHS@TiO2) had a weaker intensity than CHS, which seemed to be some kind of correlation with the content of the N element on the surface of the samples. From XPS analysis, the atomic percentage of nitrogen in N-CHS was 5.77, while that in N-(CHS@TiO2) was 1.12. That might be due to the nitrogen-containing gas, after MF decomposition, passed through CHS from the inside out first and then through TiO2. In addition, the CHS structure might easily be doped with more nitrogen. Figure 1h displays the XPS spectra of Ti 2p at high resolution. The peaks of Ti 2p observed in the TiO2 sample had been deconvoluted, revealing two spin–orbital components positioned approximately at 458.8 eV (2p3/2) and 464.6 eV (2p1/2). For the N-(CHS@TiO2) sample, its spectra revealed two comparable peaks associated with Ti, exhibiting a tendency to shift towards lower energy levels. This phenomenon could be ascribed to the influence exerted by nitrogen on the electronic characteristics of Ti species.
The photocatalytic performance of semiconductors was closely associated with their optical absorption characteristics. Figure 2a shows UV-vis DRS of N-CHS, TiO2, and N-(CHS@TiO2). N-CHS had extensive absorption in the visible light region. Compared with TiO2, the absorption edge of N-(CHS@TiO2) underwent a redshift. The significant absorption observed in the visible region could be ascribed to the influences induced by the introduction of nitrogen dopants and the internal N-CHS, which could effectively absorb visible light [36,37]. The above results also indicated a modification in the bandgap. Figure 2b illustrates the Tauc plots of (αhν)2 vs. photon energy (hν) for N-CHS, TiO2, and N-(CHS@TiO2). These plots were generated employing the Kubelka–Munk method [38]. The band gap energies obtained from the plots were 1.26 eV for N-CHS, 3.18 eV for TiO2, and 2.74 eV for N-(CHS@TiO2), respectively, which were mainly attributed to the nitrogen dopants.
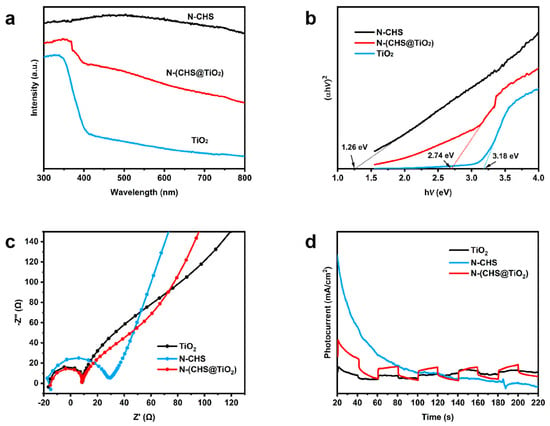
Figure 2.
(a) UV-vis DRS of N-CHS, TiO2 and N-(CHS@TiO2); (b) Tauc plots of (αhν)2 versus hν; (c) EIS spectra of TiO2, N-CHS, and N-(CHS@TiO2); and (d) photocurrent of TiO2, N-CHS, and N-(CHS@TiO2).
The photoluminescence (PL) and electrochemical impedance spectrum (EIS) were acquired aiming to delve deeper into the carrier transport properties of all samples. Figure S3 shows the PL spectra of TiO2, N-CHS, and N-(CHS@TiO2) excited at 244 nm. The intensity of the peak corresponds to the frequency of photo-induced charge recombination [39]. N-(CHS@TiO2) exhibited the lowest peak intensity, with a significant reduction in photoluminescence, suggesting that the interaction between N-CHS and N-TiO2 effectively hindered the recombination of photo-generated charges. That resulted in a much greater number of free electrons participating in the photocatalytic reactions, thereby enabling the photocatalytic process. Furthermore, the EIS (electrochemical impedance spectroscopy) was employed to delve deeper into the kinetics of charge migration pertaining to the catalysts. Figure 2c displays the semicircular Nyquist plots of the samples. It was evident that the graph of N-(CHS@TiO2) revealed a reduced semicircle diameter compared to N-CHS and pure TiO2, signifying an accelerated electron transfer rate within N-(CHS@TiO2) [40]. As depicted in Figure 2d, the N-(CHS@TiO2) heterojunction displayed the most pronounced photocurrent response signal when compared to both pure TiO2 and N-CHS. Therefore, N-(CHS@TiO2) demonstrated superior photo-induced charge separation efficiency and enhanced photocatalytic capabilities.
All samples underwent photocatalytic activity tests, with CO and CH4 emerging as the primary products. There were no other products detected during the experiments. Figure 3 illustrates the quantity of CO and CH4 generated by the photocatalysts during exposure to visible light and UV light irradiation. Negligible CH4 was obtained for all samples after 6 h of irradiation. The CO yield of N-CHS was relatively low, suggesting that the pure catalyst’s photocatalytic performance was not satisfactory, which could be attributed to the rapid recombination rate of electron–hole pairs during the reaction. The CO yield of TiO2 was also not high because of its low absorption in the visible region. When N-TiO2 nanocrystals were decorated on the N-CHS nanospheres, a significant increase in the production of CO. After 6 h of visible light irradiation, the CO yield of N-(CHS@TiO2) reached 4.3 times that of N-CHS and TiO2. As shown in Figure 3b, after 6 h of UV light irradiation, the CO yield of N-(CHS@TiO2) was also the highest, which was about 2.6 times that of TiO2 and 7 times that of N-CHS. The aforementioned results substantiated the exceptional reactivity exhibited by the N-(CHS@TiO2) material.
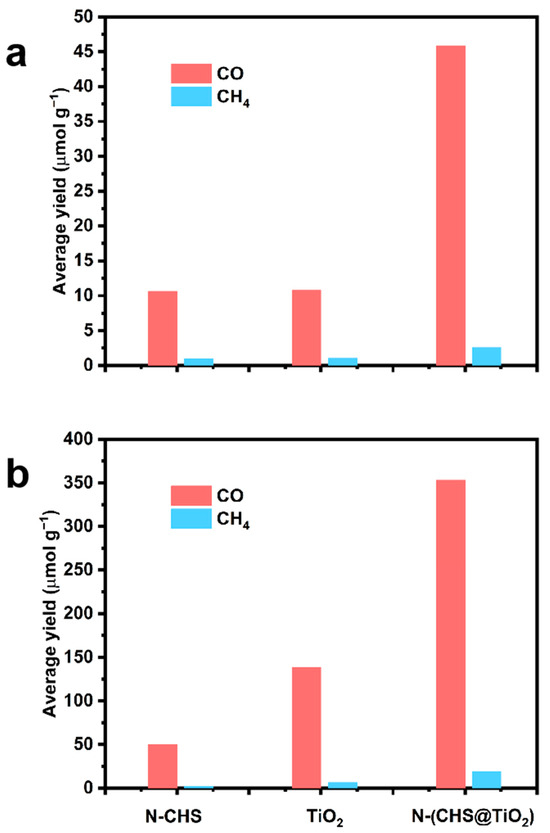
Figure 3.
The yield of CO and CH4 for N-CHS, N-(CHS@TiO2), and TiO2 under visible light (a) and under exposure to UV light (b).
Based on these findings, we elucidated why N-(CHS@TiO2) demonstrated such enhanced photocatalytic activity. It can be illuminated by taking into account various factors: (1) The contraction of the bandgap due to nitrogen doping enhances the absorption of photonic energy, which in turn makes the production of active species more feasible [41,42,43]. N doping in carbon materials can introduce structural defects into the carbon framework, enhancing electron delocalization [31]. Nitrogen-doped TiO2 narrows the band gap of TiO2 and enables the semiconductor to absorb light in the visible spectrum [25]. (2) The mesoporous structure facilitates enhanced light harvesting capabilities and efficient transportation of reactant molecules towards the active sites. Additionally, N-CHS demonstrated remarkable CO2 sorption capabilities [33], which is beneficial for photocatalytic CO2 reduction reactions. (3) The synergistic effect of N-CHS and N-TiO2, small crystal size (as shown in Figure S2a), and high surface area aid in the swift dissociation of photo-generated electron–hole pairs, thereby amplifying the photocatalytic activity.
3. Materials and Methods
3.1. Reagents Used
Chemicals Melamine and Pluronic F127 were obtained from RHAWN Reagent (Shanghai, China). Cetyltrimethylammonium bromide (CTAB), Formaldehyde aqueous solution (37~40%), NaOH, Anhydrous ethanol, Hydrochloric acid (35~38%), and ammonium solution (25~28%) were of analytical grade and purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). Resorcinol and Tetrabutyl titanate was obtained from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were used as received without any further purification.
3.2. Synthesis of MF (Melamine–Formaldehyde) Template
The spherical MF resin particles were fabricated by adhering to a marginally adjusted methodology outlined in the referenced literature [33]. The amounts of 5.0 g melamine, 0.2 mL 1M NaOH solution, 8.9 mL formaldehyde, and 40 mL deionized water were mixed and stirred at 100 °C for 60 min. After 60 min, the solution was mixed with a solution containing 7.0 g of F127 and 60 mL deionized water and stirred at room temperature for 6 h. After that, the mixture was transferred into a Teflon-lined autoclave and kept at 100 °C for 24 h. The obtained white solution was centrifuged and washed with ethanol, and the obtained deposit was donated as MF.
3.3. Synthesis of MF@RF
It was synthesized using a modified method [33,44]. The amounts of 0.4 g MF, 35 mL deionized water, and 14 mL ethanol were mixed and ultrasounded for 20 min. An amount of 1.2 g CTAB, 0.2 g resorcinol, and 0.05 mL ammonium solution was added to the above mixture and stirred at 30 °C for 60 min. After 60 min, 0.6 mL formaldehyde solution was added into the dispersion. The above mixture was kept stirring for 12 h. The obtained white solution was centrifuged and washed with ethanol. The product was dried at 80 °C for 12 h.
3.4. Synthesis of N-CHS
The obtained MF@RF was calcined under high-purity nitrogen, heated to 400 °C at a rate of 1 °C/min and kept for 5 h.
3.5. Synthesis of TiO2
It was synthesized using a modified method [45]. The amounts of 3 mL tetrabutyl titanate and 3 mL ethanol were mixed and stirred at 40 °C for 60 min. A mixed acid solution (3 mL concentrated hydrochloric acid, 3 mL ethanol, and 3 mL deionized water) was added dropwise slowly into the above solution and constantly stirred for 24 h at room temperature. The resultant product was left for aging for 12 h. The final product was dried and annealed in air at 400 °C at a ramping rate of 1 °C/min for 5 h to obtain TiO2.
3.6. Synthesis of N-(CHS@TiO2)
An amount of 0.1 g MF@RF was added to a solution containing 3 mL tetrabutyl titanate and 3 mL ethanol and stirred at 40 °C for 60 min. A mixed acid solution (3 mL concentrated hydrochloric acid, 3 mL ethanol, and 3 mL deionized water) was added dropwise slowly into the above solution and constantly stirred for 24 h at room temperature. The resultant product was left at room temperature for aging for 12 h. The obtained suspension was dried at 80 °C for 24 h and annealed in air at 400 °C at a ramping rate of 1 °C/min for 300 min to obtain N-(CHS@TiO2).
3.7. Characterization
X-ray powder diffraction (XRD) analysis was carried out using a D/Max-2550 X-ray powder diffractometer with Cu Kα radiation (Rigaku, Tokyo, Japan). The ultraviolet-visible diffuse reflectance spectra of the samples were measured on a UV-Vis-NIR spectrophotometer (Shimadzu U-4100, Kyoto, Japan), detecting absorption over the range of 200–800 nm. The X-ray photoelectron spectroscopy (XPS) spectra were acquired using a Thermofisher escalab 250xi spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). N2 adsorption–desorption isotherms were obtained at 77K on a Micromeritics ASAP 2020 sorptometer (Norcross, GA, USA). The BET (Brunauer–Emmett–Teller) surface area (BET) was calculated using the N2 adsorption isotherm data within the relative pressure ranging from 0.05 to 0.25, and the pore size distributions were calculated by the Barrett–Joyner–Halenda (BJH) method from the desorption isotherm. The morphology of the samples was determined using a FEI-Talos F200S transmission electron microscopy (TEM) (Thermo Fisher Scientific, Waltham, MA, USA). Electrochemical impedance spectroscopy (EIS) measurements were conducted using the PGSTAT 302 N electrochemical workstation (Metrohm Instruments, Herisau, Switzerland). Photocurrent was measured using a CHI660E (Shanghai Chenhua Limited, Shanghai, China) electrochemical analyzer.
3.8. Photocatalytic CO2 Reduction Test
The CO2 photoreduction experiment was conducted utilizing a 200 mL reactor within a closed-loop gas circulation system. During the reaction, illumination was provided by a 300 W Xenon lamp serving as the primary light source. The experimental procedure was designed as follows: A 10 mg sample was dispersed in a mixed solution containing 30 mg bipyridinium ruthenium, 4 mL N, N-dimethylacetamide, 1 mL deionized water and 1 mL triethanolamine, then magnetic stirring was performed at an appropriate rotation speed. In the photocatalytic reduction in CO2, bipyridinium ruthenium was the photosensitizer [46]; H2O was the electron source [47]; N, N-dimethylacetamide was the reaction solvent [48]; and triethanolamine was the sacrificial agent [49]. The reactor was vacuum-treated, and 100 kPa of 5 mL high-purity CO2 was passed into the reactor under the throttling of airflow. The gas in the reactor was obtained and analyzed by gas chromatography (GC-7920, China Education Au-light, Beijing, China) in the course of the reaction. CO and CH4 were detected by a flame ionization detector (FID) using column model TDX-01 (Haohan Chromatography (Shandong) Applied Technology Development Co., Ltd., Tengzhou, China).
4. Conclusions
In summary, a new N simultaneously doped carbon hollow sphere-supported TiO2 (N-(CHS@TiO2)) material with mesoporous structures and small crystal size was successfully synthesized, which demonstrated superior photocatalytic performance compared to N-CHS and TiO2 when exposed to visible light and UV light due to the nitrogen doping, mesoporous structure, the small average TiO2 crystal size, the large surface areas, and the heterostructure formed by N-CHS and N-TiO2. This work presents a simple and economical method for synthesizing highly efficient N simultaneously doped carbon hollow sphere-supported TiO2 (N-(CHS@TiO2)) photocatalyst, which has a wide application prospect in energy development.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/catal15010039/s1: Figure S1: N2 sorption isotherms for (a) N-CHS, (b) TiO2, and (c) N-(CHS@TiO2) samples. The BJH desorption pore size distributions for each sample (d) 3.1 and 14.8 nm, (e) 4.0 nm, (f) 5.6 nm; Figure S2: (a) The TEM image of TiO2 on CHS surface; (b) The TEM image of TiO2; Figure S3: PL spectra of TiO2, N-CHS, and N-(CHS@TiO2). Table S1: BET surface areas and pore size of all samples.
Author Contributions
W.F., methodology, supervision, data analysis, editing draft of manuscript; Z.W., data analysis; X.L., synthesized the photocatalysts; T.L., methodology and data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Jilin Education Foundation (JJKH20230904KJ, JJKH20241005KJ) and Changchun Normal University Research Fund (2021002).
Data Availability Statement
The data supporting this article have been included as part of the Supplementary Information.
Conflicts of Interest
There are no conflicts to declare.
References
- Mcnutt, M. Time’s up, CO2. Science 2019, 365, 411. [Google Scholar] [CrossRef]
- Welsby, D.; Price, J.; Pye, S.; Ekins, P. Unextractable fossil fuels in a 1.5 °C world. Nature 2021, 597, 230–234. [Google Scholar] [CrossRef]
- Lin, H.; Luo, S.; Zhang, H.; Ye, J. Toward solar-driven carbon recycling. Joule 2022, 6, 294–314. [Google Scholar] [CrossRef]
- Duffy, P.B.; Field, C.B.; Diffenbaugh, N.S.; Doney, S.C.; Dutton, Z.; Goodman, S.; Heinzerling, L.; Hsiang, S.; Lobell, D.B.; Mickley, L.J.; et al. Strengthened scientific support for the Endangerment Finding foratmospheric greenhouse gases. Science 2019, 363, 5982. [Google Scholar] [CrossRef] [PubMed]
- Strunk, J. Separating fiction from fact for photocatalytic CO2 reduction. Nat. Chem. 2023, 15, 1209–1211. [Google Scholar] [CrossRef]
- Wang, Y.J.; Zhuang, G.L.; Zhang, J.W.; Luo, F.; Cheng, X.; Sun, F.L.; Fu, S.S.; Lu, T.B.; Zhang, Z.M. Co-Dissolved Isostructural Polyoxovanadates to Construct Single-Atom-Site Catalysts for Efficient CO2 Photoreduction. Angew. Chem. Int. Ed. 2023, 62, e202216592. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Xu, S.; Wu, L.; Li, M.; Chong, Y.; Qiu, Y.; Chen, G.; Zhao, Y.; Feng, C.; Ye, D.; et al. Strain-Engineering of Mesoporous Cs3Bi2Br9/BiVO4 S-Scheme Heterojunction for Efficient CO2 Photoreduction. Small 2023, 19, 2302058. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Liang, G.; Zhu, B.; Macyk, W.; Yu, J.; Xu, F. Highly Selective Photoconversion of CO2 to CH4 over SnO2/Cs3Bi2Br9 Heterojunctions Assisted by S-Scheme Charge Separation. ACS Catal. 2023, 13, 12623–12633. [Google Scholar] [CrossRef]
- Goto, H.; Masegi, H.; Sadale, S.B.; Noda, K. Intricate behaviors of gas phase CO2 photoreduction in high vacuum using Cu2O-loaded TiO2 nanotube arrays. J. CO2 Util. 2022, 59, 101964. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, Y.; Li, C.; Zhang, Z.; Xia, C.; Pan, W.; Guo, R. Research Progress on Photocatalytic CO2 Reduction Based on Perovskite Oxides. Small 2023, 19, 2301892. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Rahaman, M.; Bharti, J.; Reisner, E.; Robert, M.; Ozin, G.A.; Hu, Y.H. Photocatalytic CO2 reduction. Nat. Rev. Method Prime 2023, 3, 61. [Google Scholar] [CrossRef]
- Vu, N.N.; Kaliaguine, S.; Do, T.O. Critical Aspects and Recent Advances in Structural Engineering of Photocatalysts for Sunlight-Driven Photocatalytic Reduction of CO2 into Fuels. Adv. Funct. Mater. 2019, 29, 1901825. [Google Scholar] [CrossRef]
- Sun, K.; Qian, Y.; Jiang, H.L. Metal-Organic Frameworks for Photocatalytic Water Splitting and CO2 Reduction. Angew. Chem. Int. Ed. 2023, 62, e202217565. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Wang, L.; Guo, H. Efficient Nb2O5@g-C3N4 heterostructures for enhanced photocatalytic CO2 reduction with highly selective conversion to CH4. Inorg. Chem. Front. 2024, 11, 123–132. [Google Scholar] [CrossRef]
- Song, W.; Qi, G.; Liu, B. Halide perovskite quantum dots for photocatalytic CO2 reduction. J. Mater. Chem. A 2023, 11, 12482–12498. [Google Scholar] [CrossRef]
- Qin, D.; Zhou, Y.; Wang, W.; Zhang, C.; Zeng, G.; Huang, D.; Wang, L.; Wang, H.; Yang, Y.; Lei, L. Recent advances in two-dimensional nanomaterials for photocatalytic reduction of CO2: Insights into performance, theories and perspective. J. Mater. Chem. A 2020, 8, 19156–19195. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Tian, J.; Sang, Y.H.; Cabot, A.; Liu, H. Structure, synthesis, and applications of tio2 nanobelts. Adv. Mater. 2015, 27, 2557–2582. [Google Scholar] [CrossRef]
- Andrade, Ó.R.; Rodríguez, V.; Camarillo, R.; Martínez, F.; Jiménez, C.; Rincón, J. Photocatalytic Reduction of CO2 with N-Doped TiO2-Based Photocatalysts Obtained in One-Pot Supercritical Synthesis. Nanomaterials 2022, 12, 1793. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-H.; Kim, T.K.; Boo, J.-H. Physical property and photo-catalytic activity of sulfur doped TiO2 catalysts responding to visible light. Catal. Today 2012, 185, 259–262. [Google Scholar] [CrossRef]
- Mittal, A.; Mari, B.; Sharma, S.; Kumari, V.; Maken, S.; Kumari, K.; Kumar, N. Non-metal Modified TiO2: A Step towards Visible Light Photocatalysis. J. Mater. Sci. Mater. Electron. 2019, 30, 3186–3207. [Google Scholar] [CrossRef]
- Bergamonti, L.; Predieri, G.; Paz, Y.; Fornasini, L.; Lottici, P.P.; Bondioli, F. Enhanced Self-cleaning Properties of N-doped TiO2 Coating for Cultural Heritage. Microchem. J. 2017, 133, 1–12. [Google Scholar] [CrossRef]
- Piᶐtkowska, A.; Janus, M.; Szymański, K.; Mozia, S. C-, N- and S-Doped TiO2 Photocatalysts: A Review. Catalysts 2021, 11, 144. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Mozhiarasi, V.; Tayade, R.J. Nitrogen Doped Titanium Dioxide (N-TiO2): Synopsis of Synthesis Methodologies, Doping Mechanisms, Property Evaluation and Visible Light Photocatalytic Applications. Photochem 2021, 1, 371–410. [Google Scholar] [CrossRef]
- Du, S.; Lian, J.; Zhang, F. Visible Light-Responsive N-Doped TiO2 Photocatalysis: Synthesis, Characterizations, and Applications. Trans. Tianjin Univ. 2022, 28, 33–52. [Google Scholar] [CrossRef]
- Divyasri, Y.V.; Reddy, N.L.; Lee, K.; Sakar, M.; Rao, V.N.; Venkatramu, V.; Shankar, M.V.; Reddy, N.C.G. Optimization of N doping in TiO2 nanotubes for the enhanced solar light mediated photocatalytic H2 production and dye degradation. Environ. Pollut. 2021, 269, 116170. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.K.; Ganguli, S.; Sabur, M.A. Nitrogen doped titanium dioxide (N-TiO2): Electronic band structure, visible light harvesting and photocatalytic applications. J. Water Process. Eng. 2023, 55, 104183. [Google Scholar] [CrossRef]
- Balapure, A.; Dutta, J.R.; Ganesan, R. Recent advances in semiconductor heterojunctions: A detailed review of the fundamentals of photocatalysis, charge transfer mechanism and materials. RSC Appl. Interfaces 2024, 1, 43–69. [Google Scholar] [CrossRef]
- Xu, H.; Ouyang, S.; Liu, L.; Reunchan, P.; Umezawa, N.; Ye, J. Recent advances in TiO2-based photocatalysis. J. Mater. Chem. A 2014, 2, 12642–12661. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. 2D materials. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Li, Z.G.; Li, K.X.; Du, P.R.; Mehmandoust, M.; Karimi, F.; Erk, N. Carbon-based photocatalysts for hydrogen production: A review. Chemosphere 2022, 308, 135998. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, Y.; Fu, D.; Chen, Y. Molybdenum carbide nanocrystal embedded N-doped carbon nanotubes as electrocatalysts for hydrogen generation. J. Mater. Chem. A 2015, 3, 5783–5788. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Benyounes, A.; Serp, P.; Faria, J.L.; Silva, C.G. Photocatalytic synthesis of vanillin using N-doped carbon nanotubes/ZnO catalysts under UV-LED irradiation. Appl. Catal. A Gen. 2018, 551, 71–78. [Google Scholar] [CrossRef]
- Wang, Y.; Zou, H.; Zeng, S.; Pan, Y.; Wang, R.; Wang, X.; Sun, Q.; Zhang, Z.; Qiu, S. One-step Carbonization Route to Nitrogen-doped Porous Carbon Hollow Spheres with Ultrahigh Nitrogen Content for CO2 Adsorption. Chem. Commun. 2015, 51, 12423–12426. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, P.; Lv, X.; Niu, X.; Lin, X.; Zhong, S.; Wang, D.; Lin, H.; Chen, J.; Bai, S. Stacking Engineering of Semiconductor Heterojunctions on Hollow Carbon Spheres for Boosting Photocatalytic CO2 Reduction. ACS Catal. 2022, 12, 2569–2580. [Google Scholar] [CrossRef]
- Zhang, P.; Lou, X.W. Design of eterostructured Hollow Photocatalysts for Solar-to-Chemical Energy Conversion. Adv. Mater. 2019, 31, 1900281. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Wang, X.; Xiao, Y.; Liu, Y.; Huo, Q. A versatile cooperative template-directed coating method to construct uniform microporous carbon shells for multifunctional core-shell nanocomposites. Nanoscale 2013, 5, 2469–2475. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Bai, X.; Guo, L.K.; Yang, S.J.; Jin, P.K.; Yang, L. Facial fabrication of carbon quantum dots (CDs)-modified N-TiO2-x nanocomposite for the efficient photoreduction of Cr(VI) under visible light. Chem. Eng. J. 2019, 357, 473–486. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D.; Cheng, B.; Yu, J.; Jiang, C. Hybrid carbon@TiO2 hollow spheres with enhanced photocatalytic CO2 reduction activity. J. Mater. Chem. A 2017, 5, 5020–5029. [Google Scholar] [CrossRef]
- Nowak, M.; Kauch, B.; Szperlich, P. Determination of energy band gap of nanocrystalline SbSI using diffuse reflectance spectroscopy. Rev. Sci. Instrum. 2009, 80, 046107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; Zhang, M.S.; Yin, Z.; Chen, Q. Photoluminescence in anatase titanium dioxide nanocrystals. Appl. Phys. B Laser Opt. 2000, 70, 261–265. [Google Scholar] [CrossRef]
- Wu, D.; Ye, L.Q.; Yip, H.Y.; Wong, P.K. Organic-free synthesis of {001} facet dominated BiOBr nanosheets for selective photoreduction of CO2 to CO. Catal. Sci. Technol. 2017, 7, 265–271. [Google Scholar] [CrossRef]
- Wang, W.; Qiang, W.; Chen, C.; Sun, D. NH2-MIL-125-Derived N-Doped TiO2@C Visible Light Catalyst for Wastewater Treatment. Polymers 2024, 16, 186. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Peng, Z.; Jiang, R.; Jia, P.; Feng, J.; Yang, P.; Chi, Q.; Ye, W.; Xu, F.; Gao, P. Nanolayered Heterostructures of N-Doped TiO2 and N-Doped Carbon for Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 1373–1381. [Google Scholar] [CrossRef]
- Jia, T.; Fu, F.; Yu, D.; Cao, J.; Sun, G. Facile synthesis and characterization of N-doped TiO2/C nanocomposites with enhanced visible-light photocatalytic performance. Appl. Surf. Sci. 2018, 430, 438–447. [Google Scholar] [CrossRef]
- Fu, W.; Li, G.; Wang, Y.; Zeng, S.; Yan, Z.; Wang, J.; Xin, S.; Zhang, L.; Wu, S.; Zhang, Z. Facile formation of mesoporous structured mixed-phase (anatase/rutile) TiO2 with enhanced visible light photocatalytic activity. Chem. Commun. 2018, 54, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Santoro, A.; Cancelliere, A.M.; Kamogawa, K.; Serroni, S.; Puntoriero, F.; Tamaki, Y.; Campagna, S.; Ishitani, O. Photocatalyzed CO2 reduction to CO by supramolecular photocatalysts made of Ru(II) photosensitizers and Re(I) catalytic subunits containing preformed CO2TEOA adducts. Sci. Rep. 2023, 13, 11320. [Google Scholar] [CrossRef]
- Kuramochi, Y.; Kamiya, M.; Ishida, H. Exploring the Impact of Water Content in Solvent Systems on Photochemical CO2 Reduction Catalyzed by Ruthenium Complexes. Molecules 2024, 29, 4960. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Kamiya, M.; Ishida, H. Photocatalytic CO2 Reduction in N,N-Dimethylacetamide/Water as an Alternative Solvent System. Inorg. Chem. 2014, 53, 3326–3332. [Google Scholar] [CrossRef]
- Liu, Z.; Li, J.; Chen, Z.; Li, M.; Wang, L.; Wu, S.; Zhang, J. Photocatalytic conversion of carbon dioxide on triethanolamine: Unheeded catalytic performance of sacrificial agent. Appl. Catal. B Environ. 2023, 326, 122338. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).