Optimization of Electrocatalytic Chlorazol Yellow Degradation Using PbO2 Nanostructure Immobilized on Stainless Steel Substrate
Abstract
1. Introduction
2. Results and Discussion
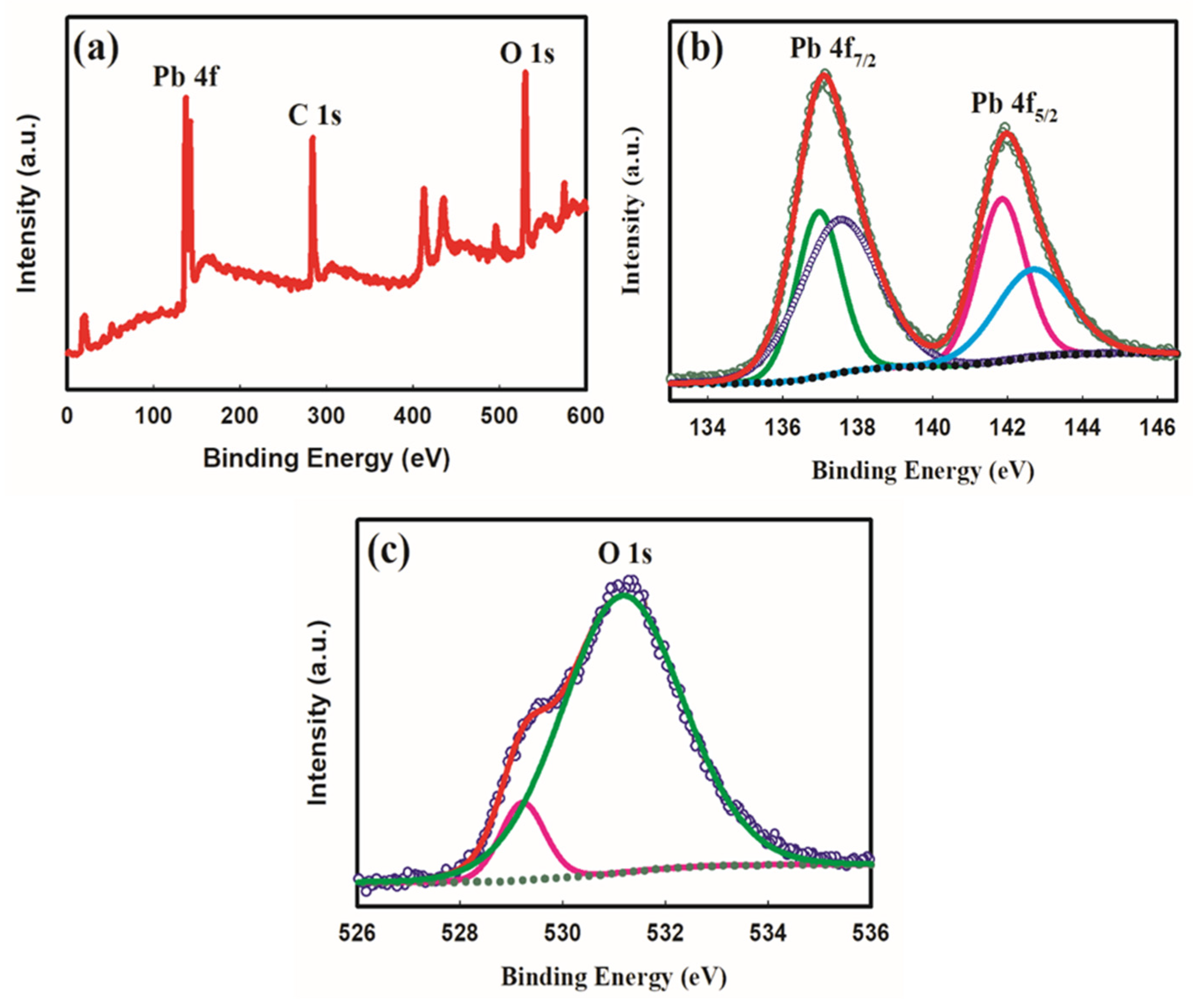
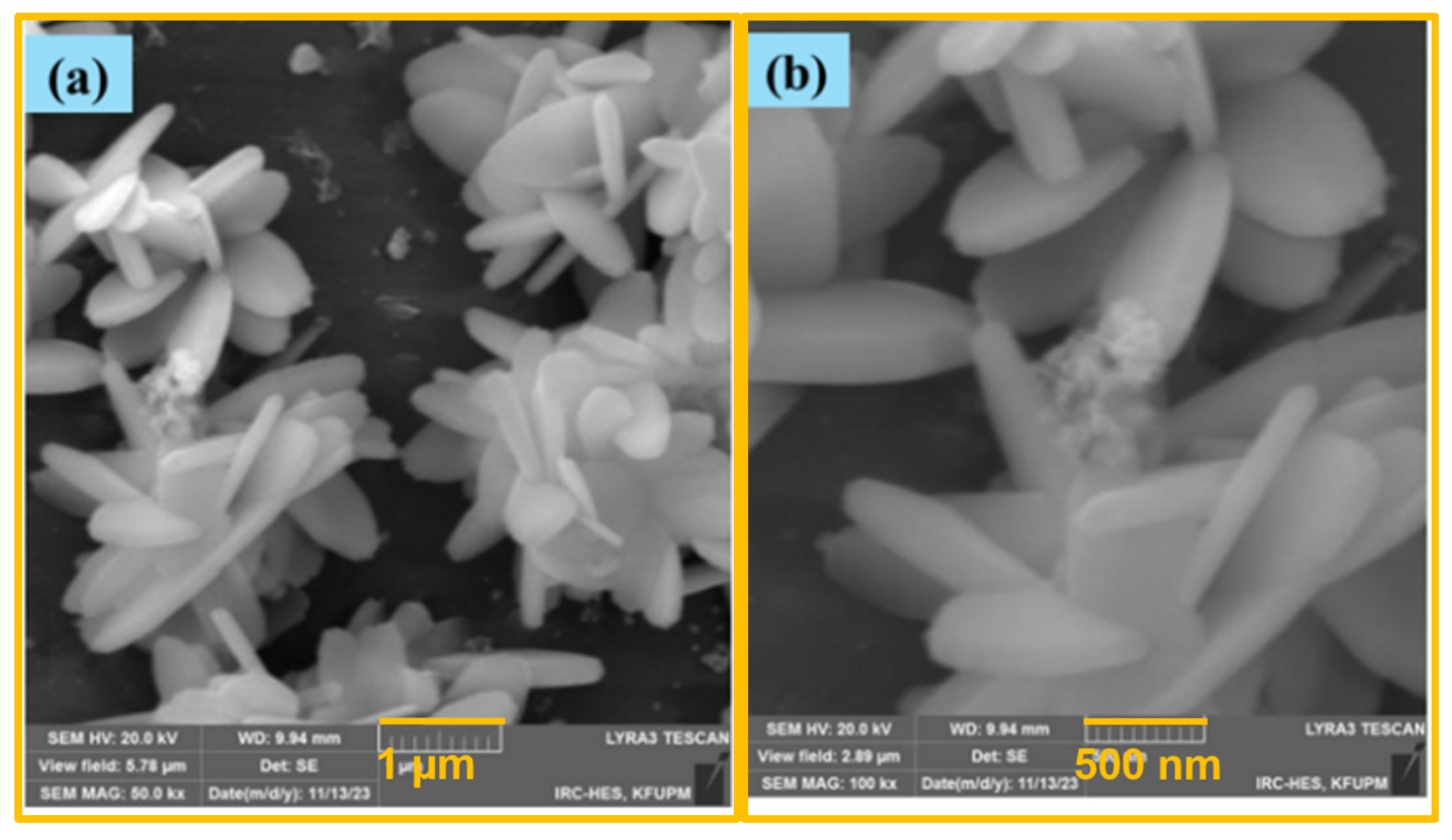
2.1. Electrode Characterization
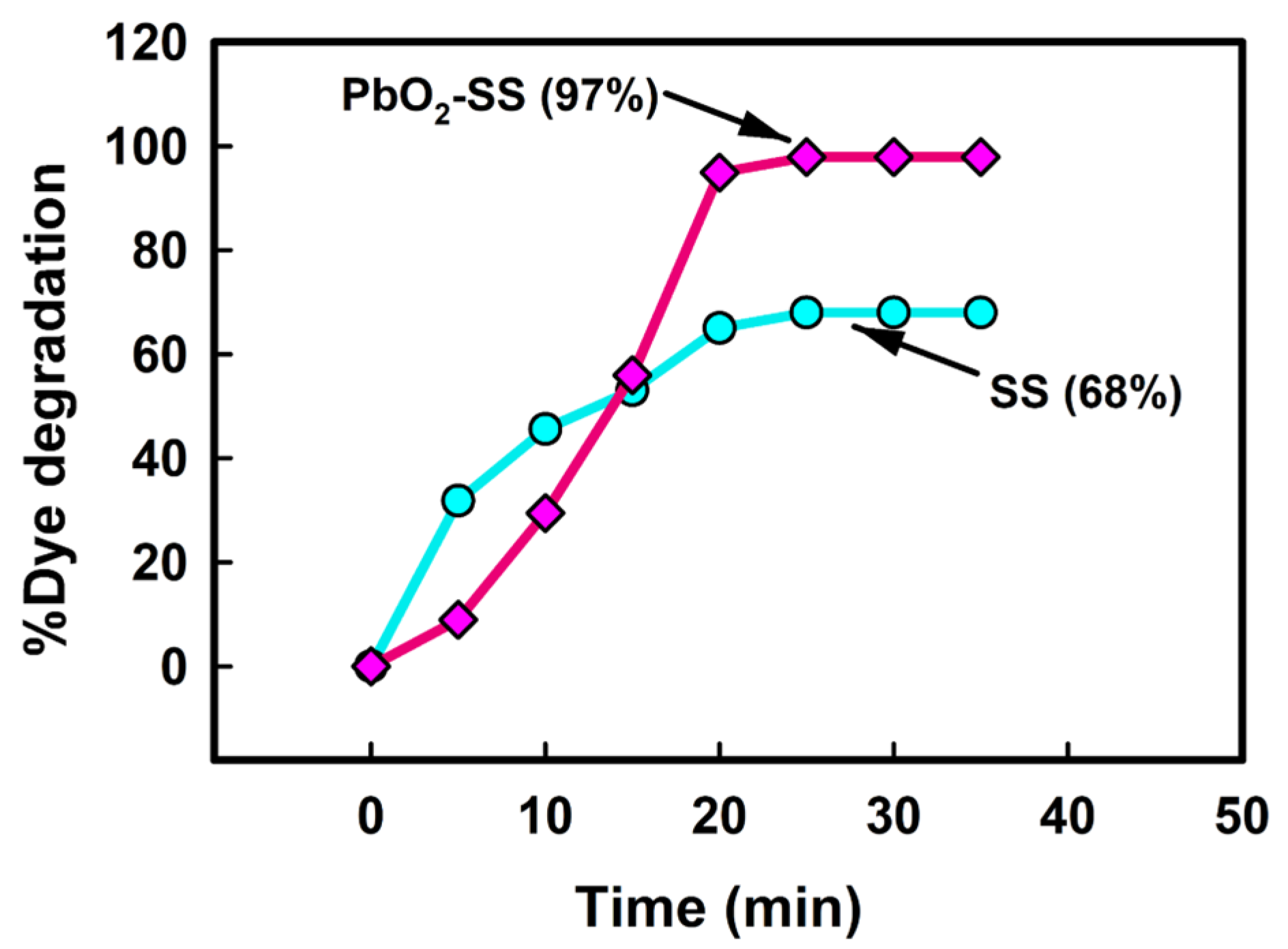
2.2. Effect of PbO2 on CY Degradation
2.3. Effect of Applied Voltage
2.4. Effect of Supporting Electrolyte
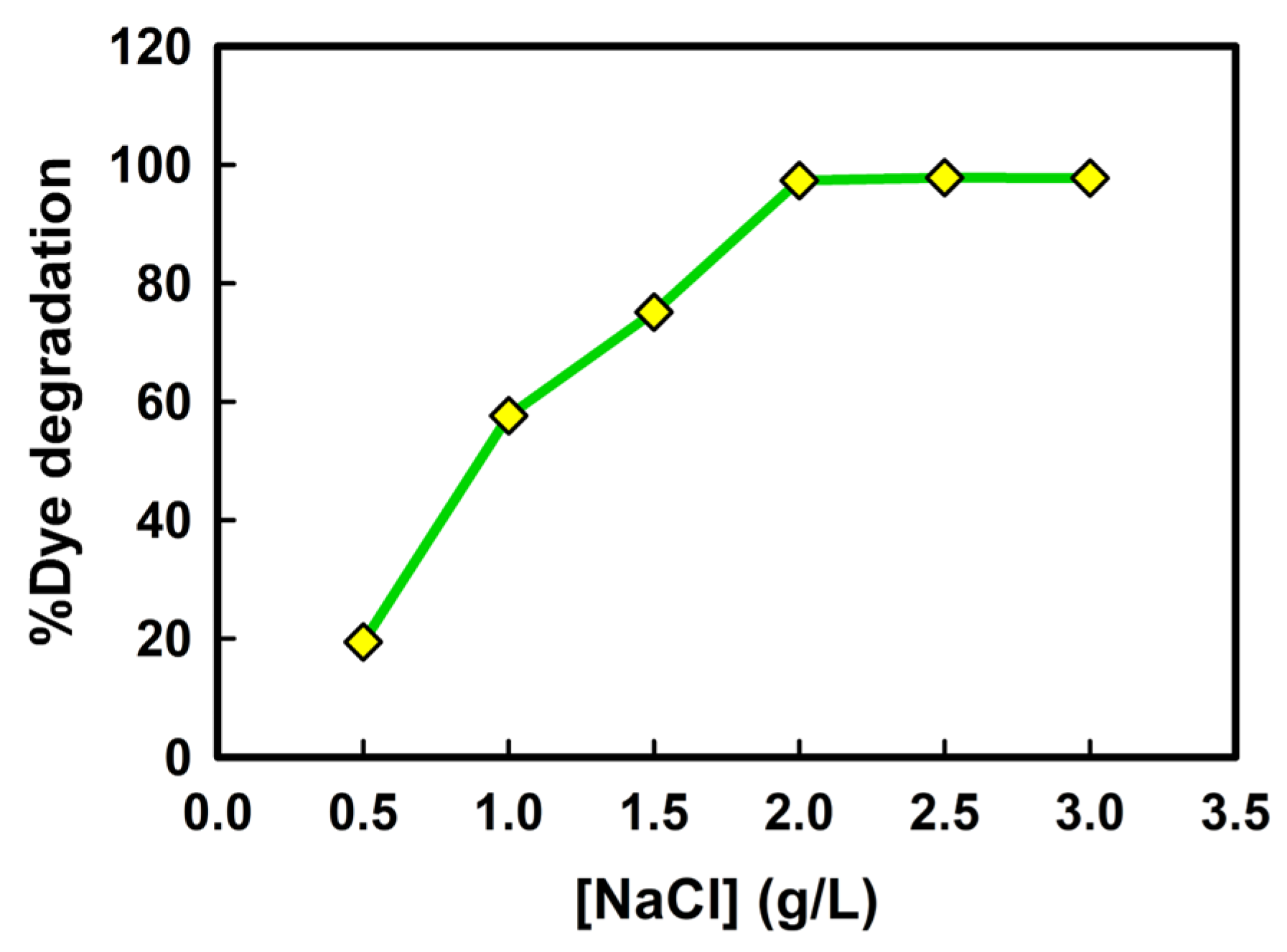
2.5. Effect of NaCl Concentration
2.6. Effect of CY Concentration
2.7. Effect of pH
2.8. Mechanism
3. Experimental
3.1. Chemicals
3.2. Instruments
3.3. Electrode Fabrication
3.4. Characterization
3.5. Dye Degradation Experiment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahamad, M.H. Impact of International Trade on Economic Growth in Bangladesh. Int. J. Sci. Res. 2016, 7, 1624–1627. [Google Scholar] [CrossRef]
- Singh, S.; Tiwari, R.K.; Singh, U.; Pandey, R.S. Water Pollution due to Discharge of Industrial Effluents with special reference to Uttar Pradesh, India—A review. Int. Arch. App. Sci. Technol. 2018, 9, 111–121. [Google Scholar] [CrossRef]
- Saeed, M.; Muneer, M.; Haq A ul Akram, N. Photocatalysis: An effective tool for photodegradation of dyes—A review. Environ. Sci. Pollut. Res. 2022, 29, 293–311. [Google Scholar] [CrossRef]
- Alamier, W.M.; Hasan, N.; Syed, I.S.; Bakry, A.M.; Ismail, K.S.; Gedda, G.; Girma, W.M. Silver Nanoparticles’ Biogenic Synthesis Using Caralluma subulata Aqueous Extract and Application for Dye Degradation and Antimicrobials Activities. Catalysts 2023, 13, 1290. [Google Scholar] [CrossRef]
- Javed, M.; Iqbal, S.; Qamar, M.A.; Shariq, M.; Ahmed, I.A.; BaQais, A.; Alzahrani, H.; Ali, S.K.; Masmali, N.A.; Althagafi, T.M.; et al. Fabrication of Effective Co-SnO2/SGCN Photocatalysts for the Removal of Organic Pollutants and Pathogen Inactivation. Crystals 2023, 13, 163. [Google Scholar] [CrossRef]
- Islam, M.; Mostafa, M. Textile Dyeing Effluents and Environment Concerns—A Review. J. Environ. Sci. Nat. Resour. 2019, 11, 131–144. [Google Scholar] [CrossRef]
- John Sundar, V.; Gnanamani, A.; Muralidharan, C.; Chandrababu, N.K.; Mandal, A.B. Recovery and utilization of proteinous wastes of leather making: A review. Rev. Environ. Sci. Biotechnol. 2011, 10, 151–163. [Google Scholar] [CrossRef]
- Vandevivere, P.C.; Bianchi, R.; Verstraete, W. Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 1998, 72, 289–302. [Google Scholar] [CrossRef]
- Parasuraman, B.; Vasudevan, V.; Kandasamy, B.; Rangaraju, H.; Thangavelu, P. Development of Bi2S3/Cu2S hetrojuction as an effective photocatalysts for the efficient degradation of antibiotic drug and organic dye. Environ. Sci. Pollut. Res. 2023, 31, 40245–40256. [Google Scholar] [CrossRef] [PubMed]
- Alamier, W.M.; Oteef, M.D.Y.; Bakry, A.M.; Hasan, N.; Ismail, K.S.; Awad, F.S. Green Synthesis of Silver Nanoparticles Using Acacia ehrenbergiana Plant Cortex Extract for Efficient Removal of Rhodamine B Cationic Dye from Wastewater and the Evaluation of Antimicrobial Activity. ACS Omega 2023, 8, 18901–18914. [Google Scholar] [CrossRef]
- Shawon, M.A.A.; Ahmed, S.; Karim, M.R. Impact of Irrigation with Polluted River Water on the Accumulation of Toxic Metals in Soil and Crops in the Region of Dhaka, Bangladesh and Potential Effects on Health. Environ. Process. 2021, 8, 219–237. [Google Scholar] [CrossRef]
- Cuervo Lumbaque, E.; Gomes, M.F.; Da Silva Carvalho, V.; de Freitas, A.M.; Tiburtius, E.R.L. Degradation and ecotoxicity of dye Reactive Black 5 after reductive-oxidative process: Environmental Science and Pollution Research. Environ. Sci. Pollut. Res. 2017, 24, 6126–6134. [Google Scholar] [CrossRef] [PubMed]
- Paz, A.; Carballo, J.; Pérez, M.J.; Domínguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Shindhal, T.; Rakholiya, P.; Varjani, S.; Pandey, A.; Ngo, H.H.; Guo, W.; Ng, H.Y.; Taherzadeh, M.J. A critical review on advances in the practices and perspectives for the treatment of dye industry wastewater. Bioengineered 2021, 12, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.; Bakhoum, E.S.; Zaher, K. Coagulation/flocculation process for textile mill effluent treatment: Experimental and numerical perspectives. Int. J. Sustain. Eng. 2021, 14, 983–995. [Google Scholar] [CrossRef]
- Sabur, M.A.; Khan, A.A.; Safiullah, S. Treatment of Textile Wastewater by Coagulation Precipitation Method. J. Sci. Res. 2012, 4, 623–633. [Google Scholar] [CrossRef]
- Bangash, F.K.; Alam, S. Adsorption of acid blue 1 on activated carbon produced from the wood of Ailanthus altissima. Braz. J. Chem. Eng. 2009, 26, 275–285. [Google Scholar] [CrossRef]
- Akter, N.; Karim, M.R.; Ahmad, N.; Rahman, M.M.; Siddiquey, I.; Bahadur, N.M.; Hasnat, M. Optimization, kinetic and thermodynamic studies for removal of Brilliant Red (X-3B) using Tannin gel. J. Environ. Chem. Eng. 2014, 2, 76–83. [Google Scholar] [CrossRef]
- Akter, N.; Hossain, M.A.; Hassan, M.J.; Amin, M.K.; Elias, M.; Rahman, M.M.; Asiri, A.M.; Siddiquey, I.A.; Hasnat, M.A. Amine modified tannin gel for adsorptive removal of Brilliant Green dye. J. Environ. Chem. Eng. 2016, 4, 1231–1241. [Google Scholar] [CrossRef]
- Alharby, N.F.; Almutairi, R.S.; Mohamed, N.A. Adsorption behavior of methylene blue dye by novel crosslinked o-cm-chitosan hydrogel in aqueous solution: Kinetics, isotherm and thermodynamics. Polymers 2021, 13, 3659. [Google Scholar] [CrossRef]
- Jain, R.; Mathur, M.; Sikarwar, S.; Mittal, A. Removal of the hazardous dye rhodamine B through photocatalytic and adsorption treatments. J. Environ. Manag. 2007, 85, 956–964. [Google Scholar] [CrossRef] [PubMed]
- Kheirabadi, M.; Samadi, M.; Asadian, E.; Zhou, Y.; Dong, C.; Zhang, J.; Moshfegh, A.Z. Well-designed Ag/ZnO/3D graphene structure for dye removal: Adsorption, photocatalysis and physical separation capabilities. J. Colloid. Interface Sci. 2019, 537, 66–78. [Google Scholar] [CrossRef] [PubMed]
- Rojviroon, O.; Rojviroon, T.; Sirivithayapakorn, S. Removal of Color and Chemical Oxygen Demand from Landfill Leachate by Photocatalytic Process with AC/TiO2. Energy Procedia 2015, 79, 536–541. [Google Scholar] [CrossRef]
- Zaleska, A. Doped-TiO2: A Review. Recent Pat. Eng. 2008, 2, 157–164. [Google Scholar] [CrossRef]
- Uddin, N.; Alam Shibly, S.U.; Ovali, R.; Mazumder, M.R.; Islam, S.; Uddin, M.J.; Gulseren, O.; Bengu, E. An experimental and first-principles study of the effect of B/N doping in TiO2 thin films for visible light photo-catalysis. J. Photochem. Photobiol. A Chem. 2013, 254, 25–34. [Google Scholar] [CrossRef]
- Hasnat, M.A.; Siddiquey, I.A.; Nuruddin, A. Comparative photocatalytic studies of degradation of a cationic and an anionic dye. Dye. Pigment. 2005, 66, 185–188. [Google Scholar] [CrossRef]
- Uddin, M.; Cesano, F.; Bonino, F.; Bordiga, S.; Spoto, G.; Scarano, D.; Zecchina, A. Photoactive TiO2 films on cellulose fibres: Synthesis and characterization. J. Photochem. Photobiol. A Chem. 2007, 189, 286–294. [Google Scholar] [CrossRef]
- Mogal, S.I.; Mishra, M.; Gandhi, V.G.; Tayade, R.J. Metal doped titanium dioxide: Synthesis and effect of metal ions on physico-chemical and photocatalytic properties. Mater. Sci. Forum 2013, 734, 364–378. [Google Scholar] [CrossRef]
- Lachheb, H.; Puzenat, E.; Houas, A.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation of various types of dyes (Alizarin S, Crocein Orange G, Methyl Red, Congo Red, Methylene Blue) in water by UV-irradiated titania. Appl. Catal. B 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Zanoni, M.V.B.; Sene, J.J.; Anderson, M.A. Photoelectrocatalytic degradation of Remazol Brilliant Orange 3R on titanium dioxide thin-film electrodes. J. Photochem. Photobiol. A Chem. 2003, 157, 55–63. [Google Scholar] [CrossRef]
- Kordouli, E.; Bourikas, K.; Lycourghiotis, A.; Kordulis, C. The mechanism of azo-dyes adsorption on the titanium dioxide surface and their photocatalytic degradation over samples with various anatase/rutile ratios. Catal. Today 2015, 252, 128–135. [Google Scholar] [CrossRef]
- Parashar, S.D.; Meshram, A.A.; Sontakke, S.M. Electro-photocatalytic degradation processes for dye/colored wastewater treatment. In Handbook of Nanomaterials for Wastewater Treatment: Fundamentals and Scale Up Issues; Elsevier: Amsterdam, The Netherlands, 2021; pp. 833–846. [Google Scholar] [CrossRef]
- Hiragond, C.B.; Kshirsagar, A.S.; Khanna, D.P.; More, P.V.; Khanna, P.K. Electro-photocatalytic degradation of methylene blue dye using various nanoparticles: A demonstration for undergraduates. J. Nanomed. Res. 2018, 7, 254–257. [Google Scholar] [CrossRef]
- Liu, Y.X.; Liao, Z.Y.; Wu, X.Y.; Zhao, C.J.; Lei, Y.X.; Bin Ji, D. Electrochemical degradation of methylene blue using electrodes of stainless steel net coated with single-walled carbon nanotubes. Desalination Water Treat. 2015, 54, 2757–2764. [Google Scholar] [CrossRef]
- Gomathi, E.; Balraj, B.; Kumaraguru, K. Electrochemical degradation of scarlet red dye from aqueous environment by titanium-based dimensionally stable anodes with SS electrodes. Appl. Biol. Chem. 2018, 61, 289–293. [Google Scholar] [CrossRef]
- Morsi, M.S.; Al-Sarawy, A.A.; El-Dein, W.A.S. Electrochemical degradation of some organic dyes by electrochemical oxidation on a pb/pbo2 electrode. Desalination Water Treat. 2011, 26, 301–308. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Shabanloo, A.; Nasab, H.Z.; Vaziri, Y.; Ansari, A. Electrochemical degradation of methylene blue dye using a graphite doped PbO2 anode: Optimization of operational parameters, degradation pathway and improving the biodegradability of textile wastewater. Arab. J. Chem. 2020, 13, 6847–6864. [Google Scholar] [CrossRef]
- Kariyajjanavar, P. Degradation of Textile Wastewater by Electrochemical Method. J. Waste Water Treat. Anal. 2011, 2, 110. [Google Scholar] [CrossRef]
- Gaber, M.; Abu Ghalwa, N.; Khedr, A.M.; Salem, M.F. Electrochemical degradation of reactive yellow 160 dye in real wastewater using C/PbO, Pb + Sn/PbO+ SnO, and Pb/PbOmodified electrodes. J. Chem. 2013, 2013, 691763. [Google Scholar] [CrossRef]
- He, H.; Zhou, Z. Electro-fenton process for water and wastewater treatment. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2100–2131. [Google Scholar] [CrossRef]
- Guivarch, E.; Trevin, S.; Lahitte, C.; Oturan, M.A. Degradation of azo dyes in water by Electro-Fenton process. Environ. Chem. Lett. 2003, 1, 38–44. [Google Scholar] [CrossRef]
- Routoula, E.; Patwardhan, S.V. Degradation of Anthraquinone Dyes from Effluents: A Review Focusing on Enzymatic Dye Degradation with Industrial Potential. Environ. Sci. Technol. 2020, 54, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Piaskowski, K.; Świderska-Dąbrowska, R.; Zarzycki, P.K. Dye removal from water and wastewater using various physical, chemical, and biological processes. J. AOAC Int. 2018, 101, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Lago, A.; Rocha, V.; Barros, O.; Costa, L.; Vipotnik, Z.; Silva, B.; Tavares, T. A review on biological processes for pharmaceuticals wastes abatement—A growing threat to modern society. Environ. Sci. Technol. 2019, 53, 7185–7202. [Google Scholar] [CrossRef] [PubMed]
- Khandegar, V.; Saroha, A.K. Electrocoagulation for the treatment of textile industry effluent—A review. J. Environ. Manag. 2013, 128, 949–963. [Google Scholar] [CrossRef]
- Cotillas, S.; Llanos, J.; Cañizares, P.; Clematis, D.; Cerisola, G.; Rodrigo, M.A.; Panizza, M. Removal of Procion Red MX-5B dye from wastewater by conductive-diamond electrochemical oxidation. Electrochim. Acta 2018, 263, 1–7. [Google Scholar] [CrossRef]
- Rafiq, A.; Ikram, M.; Ali, S.; Niaz, F.; Khan, M.; Khan, Q.; Maqbool, M. Photocatalytic degradation of dyes using semiconductor photocatalysts to clean industrial water pollution. J. Ind. Eng. Chem. 2021, 97, 111–128. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Moon, J.H.; Choi, Y.S.; Park, G.O.; Jin, M.; Jin, L.Y.; Li, D.; Lee, J.Y.; Son, S.U.; Kim, J.M. Visible-Light Driven Photocatalytic Degradation of Organic Dyes over Ordered Mesoporous CdxZn1-xS Materials. J. Phys. Chem. C 2017, 121, 5137–5144. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2 based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Ikram, M.; Umar, E.; Raza, A.; Haider, A.; Naz, S.; Ul-Hamid, A.; Haider, J.; Shahzadi, I.; Hassan, J.; Ali, S. Dye degradation performance, bactericidal behavior and molecular docking analysis of Cu-doped TiO2 nanoparticles. RSC Adv. 2020, 10, 24215–24233. [Google Scholar] [CrossRef]
- Babuponnusami, A.; Muthukumar, K. A review on Fenton and improvements to the Fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Zhou, M.; Oturan, M.A. An overview on the removal of synthetic dyes from water by electrochemical advanced oxidation processes. Chemosphere 2018, 197, 210–227. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Brillas, E.; Martínez-Huitle, C.A. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods. An updated review. Appl. Catal. B 2015, 166–167, 603–643. [Google Scholar] [CrossRef]
- Chen, G. Electrochemical technologies in wastewater treatment. Sep. Purif. Technol. 2004, 38, 11–41. [Google Scholar] [CrossRef]
- Mussa, Z.H.; Al-Qaim, F.F. Electrochemical degradation of 10,11-dihydro-10-hydroxy carbamazepine as the main metabolite of carbamazepine. Environ. Sci. Pollut. Res. 2023, 30, 50457–50470. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, Y.; Xiong, Z.; Yao, G.; Lai, B. The electrochemical advanced oxidation processes coupling of oxidants for organic pollutants degradation: A mini-review. Chin. Chem. Lett. 2019, 30, 2139–2146. [Google Scholar] [CrossRef]
- Belal, R.M.; Zayed, M.A.; El-Sherif, R.M.; Abdel Ghany, N.A. Advanced electrochemical degradation of basic yellow 28 textile dye using IrO2/Ti meshed electrode in different supporting electrolytes. J. Electroanal. Chem. 2021, 882, 114979. [Google Scholar] [CrossRef]
- Ghernaout, D.; Elboughdiri, N.; Alghamdi, A.; Ghernaout, B. Trends in Decreasing Disinfection By-Products Formation during Electrochemical Technologies. OAlib 2020, 7, 1–17. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Keller, J.; Brillas, E.; Radjenovic, J. Removal of organic contaminants from secondary effluent by anodic oxidation with a boron-doped diamond anode as tertiary treatment. J. Hazard. Mater. 2015, 283, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Abdelhay, A.; Jum’h, I.; Albsoul, A.; Arideh, D.A.; Qatanani, B. Performance of electrochemical oxidation over bdd anode for the treatment of different industrial dye-containing wastewater effluents. J. Water Reuse Desalination 2021, 11, 110–121. [Google Scholar] [CrossRef]
- Zayed, M.A.; Abo-Ayad, Z.A.; Medany, S.S. Catalytic Efficient Electro-oxidation Degradation of DO26 Textile Dye via UV/VIS, COD, and GC/MS Evaluation of By-products. Electrocatalysis 2021, 12, 731–746. [Google Scholar] [CrossRef]
- Ma, Q.; Yan, C.; Lv, W.; Mei, Y.; Peng, H.; Du, J.; Zheng, B.; Guo, Y. Coexisting Chloride Ion for Boosting the Photoelectrocatalytic Degradation Efficiency of Organic Dyes. Catal. Lett. 2023, 153, 378–387. [Google Scholar] [CrossRef]
- Pacheco-Álvarez, M.; Fuentes-Ramírez, R.; Brillas, E.; Peralta-Hernández, J.M. Assessing the electrochemical degradation of reactive orange 84 with Ti/IrO2–SnO2–Sb2O5 anode using electrochemical oxidation, electro-Fenton, and photoelectro-Fenton under UVA irradiation. Chemosphere 2023, 339, 139666. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Lesage, G.; Rivallin, M.; Cretin, M.; Hamdani, M. Electrochemical oxidation treatment of Direct Red 23 aqueous solutions: Influence of the operating conditions. Sep. Sci. Technol. 2022, 57, 1501–1520. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wu, D.; Yuang, D.; Ge, H.; Wu, D. PbO2 materials for electrochemical environmental engineering: A review on synthesis and applications. Sci. Total Environ. 2023, 855, 158880. [Google Scholar] [CrossRef]
- Sarkar, S.; Banerjee, A.; Halder, U.; Biswas, R.; Bandopadhyay, R. Degradation of Synthetic Azo Dyes of Textile Industry: A Sustainable Approach Using Microbial Enzymes. Water Conserv. Sci. Eng. 2017, 2, 121–131. [Google Scholar] [CrossRef]
- Moushumy, Z.M.; Hassan, M.J.; Ahsan, M.; Hasan, M.; Uddin, N.; Nagao, Y. Photocatalytic degradation of chlorazol yellow dye under sunlight irradiation using Ce, Bi, and N co-doped TiO2 photocatalyst in neutral medium. Environ. Sci. Pollut. Res. 2023, 30, 35153–35169. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Morales, J.; Petkova, G.; Cruz, M.; Caballero, A. Synthesis and characterization of lead dioxide active material for lead-acid batteries. J. Power Sources 2006, 158, 831–836. [Google Scholar] [CrossRef]
- Xia, Y.; Bian, X.; Xia, Y.; Zhou, W.; Wang, L.; Fan, S.; Xiong, P.; Zhan, T.; Dai, Q.; Chen, J. Effect of indium doping on the PbO2 electrode for the enhanced electrochemical oxidation of aspirin: An electrode comparative study. Sep. Purif. Technol. 2020, 237, 116321. [Google Scholar] [CrossRef]
- Lv, Z.; Chen, Z.; Yu, Q.; Zhu, W.; You, H.; Chen, B.; Zheng, Z.; Liu, Y.; Hu, Q. Micro-area investigation on electrochemical performance improvement with Co and Mn doping in PbO2 electrode materials. RSC Adv. 2021, 11, 28949–28960. [Google Scholar] [CrossRef]
- Shmychkova, O.; Luk’Yanenko, T.; Amadelli, R.; Velichenko, A. Physico-chemical properties of PbO2-anodes doped with Sn 4+and complex ions. J. Electroanal. Chem. 2014, 717–718, 196–201. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, Y.; Chen, K.; Yu, X.; Sun, F.; Wang, M.; Cheng, Z.; Zhang, J.; Niu, Q. Effects of PbO2/Pb3O4 ratio alteration for enhanced electrochemical advanced oxidation performance. J. Solid. State Chem. 2021, 301, 122277. [Google Scholar] [CrossRef]
- Xia, Y.; Feng, J.; Fan, S.; Zhou, W.; Dai, Q. Fabrication of a multi-layer CNT-PbO2 anode for the degradation of isoniazid: Kinetics and mechanism. Chemosphere 2021, 263, 128069. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Xia, Y.; Chen, J. Mechanism of enhanced electrochemical degradation of highly concentrated aspirin wastewater using a rare earth La-Y co-doped PbO2 electrode. Electrochim. Acta 2016, 188, 871–881. [Google Scholar] [CrossRef]
- Dou, W.; Hu, X.; Kong, L.; Yu, Y.; Yang, Y.; Lu, L. Removal of Cl(−I) from acidic industrial wastewater through oxidation: A review on methods and mechanisms. Environ. Sci. Water Res. Technol. 2023, 9, 38–47. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.F.; Oyshi, T.A.; Hosen, N.; Hassan, M.J.; Shah, S.S.; Rahaman, M.; Aldalbahi, A.; Maiyalagan, T.; Hasnat, M.A. Optimization of Electrocatalytic Chlorazol Yellow Degradation Using PbO2 Nanostructure Immobilized on Stainless Steel Substrate. Catalysts 2025, 15, 34. https://doi.org/10.3390/catal15010034
Islam MF, Oyshi TA, Hosen N, Hassan MJ, Shah SS, Rahaman M, Aldalbahi A, Maiyalagan T, Hasnat MA. Optimization of Electrocatalytic Chlorazol Yellow Degradation Using PbO2 Nanostructure Immobilized on Stainless Steel Substrate. Catalysts. 2025; 15(1):34. https://doi.org/10.3390/catal15010034
Chicago/Turabian StyleIslam, Md. Fahamidul, Tahamida A. Oyshi, Nazmul Hosen, Mohammad Jobaer Hassan, Syed Shaheen Shah, Mostafizur Rahaman, Ali Aldalbahi, Thandavarayan Maiyalagan, and Mohammad A. Hasnat. 2025. "Optimization of Electrocatalytic Chlorazol Yellow Degradation Using PbO2 Nanostructure Immobilized on Stainless Steel Substrate" Catalysts 15, no. 1: 34. https://doi.org/10.3390/catal15010034
APA StyleIslam, M. F., Oyshi, T. A., Hosen, N., Hassan, M. J., Shah, S. S., Rahaman, M., Aldalbahi, A., Maiyalagan, T., & Hasnat, M. A. (2025). Optimization of Electrocatalytic Chlorazol Yellow Degradation Using PbO2 Nanostructure Immobilized on Stainless Steel Substrate. Catalysts, 15(1), 34. https://doi.org/10.3390/catal15010034









