1. Introduction
The implementation of automotive catalysts is critical to mitigate the airborne emission of harmful gases exiting internal combustion engines. Catalysts based on supported platinum group metal (PGM) nanoparticles are highly effective for this purpose. However, operational temperatures in excess of 600 °C sinter both metal oxide supports and PGM particles. Particle sintering reduces the catalytically active surface area and causes performance degradation over the catalyst’s lifespan [
1,
2]. Excess platinum, palladium, and rhodium is often added to extend the period of effective performance. However, this strategy adds significantly to catalyst cost. Alternative approaches focus on design of the metal oxide support, which greatly influences catalyst activity and stability via interfaces with PGM species [
3,
4]. For instance, cerium oxide (CeO
2) was previously found to maintain atomically dispersed Pt stabilized via strong binding at surface defect sites after aging [
5]. Although Pt sintering was avoided, the resulting single atom structure was not particularly active and exhibited lower activity than the fresh catalyst. Aging also significantly reduces the mobility of reactive oxygen species within CeO
2 that are otherwise active in catalytic oxidation reactions [
6,
7]. On the other hand, alumina is commonly used as a more thermally stable oxide support. However, alumina is chemically inert and does not participate in catalysis. This oxide exhibits weak bonding with supported metals, allowing these to sinter into micron size particles upon exposure to high temperatures [
5]. Sintered Pt catalysts lose activity as a consequence of the reduced number of surface exposed metal atoms. In general, the compositional simplicity of these oxides offers relatively few parameters to improve catalyst performance.
Alternatively, numerous catalyst attributes are significantly enhanced by increasing the compositional complexity of oxide supports. The incorporation of rare earth and transition metal dopants boosts the thermal stability and reactivity of CeO
2 supports [
7,
8]. Likewise, the formation of spinels via divalent metal addition to alumina strengthens interactions with supported metal particles and mitigates thermal deactivation [
3]. Spinels alone exhibit catalytic activity for the conversion of harmful emissions, such as CO, with performance highly dependent on composition [
9]. The concept of catalyst property improvement via compositional complexity is indeed advanced by the relatively new field of high-entropy oxides (HEOs) [
10,
11]. These oxides contain five or more metal cations within a phase pure structure. The enthalpic penalties of elemental mixing are overcome by an increase in configurational entropy, which acts to stabilize HEOs, particularly at high temperatures [
12]. HEOs have been applied as catalysts and catalyst supports for PGM in the previous literature for oxidation reactions [
6,
13,
14,
15,
16,
17,
18]. In several studies, HEO supports of rock salt and fluorite phases demonstrated the ability to thermally stabilize supported gold, palladium, ruthenium, and platinum species [
13,
15,
16]. The entropy-driven incorporation of PGM atoms into HEO supports at high temperature was proposed as a mechanism for the high temperature stability [
15,
16]. Despite the retained dispersion of active elements, HEO catalysts have shown relatively low activity after aging, often due to the formation of a stable, yet less active, material structures [
6,
15].
In this study, we evaluate Pt on HEO aluminate spinels as compositionally and structurally novel catalysts for the oxidation of CO, one of the primary chemical reactions facilitated by automotive aftertreatment catalysts. Our recent work demonstrated the stabilization of stoichiometric and non-stoichiometric high-entropy aluminate spinels at 800 °C and higher temperatures, which thermally degrade conventional catalysts [
19]. Here, we add Pt to simple and high-entropy spinel supports and compare catalytic activity before and after thermal aging. Simple spinels had MAl
2O
4 stoichiometry, where M = Co, Cu, Mg, or Ni. High-entropy spinels contained all these divalent metals in nominal equimolar concentrations with the total divalent metal concentration in a stoichiometric or sub-stoichiometric ratio with aluminum. Fresh and aged samples were compared according to catalytic performance and structural characterization. Catalysts containing simple spinels all showed degradation after aging. However, Pt on HEO spinel supports showed nearly identical performance before and after heat treatment. The many cationic constituents within HEO spinels enhance catalytic performance through exsolution and alloying with supported Pt particles, as well as by promoting reactant adsorption and activation on a variety of active sites for improved CO conversion throughout the temperature regime tested. Uniquely, our work demonstrates that catalytic performance can be maintained after exposure to high temperatures, despite structural evolution.
2. Results
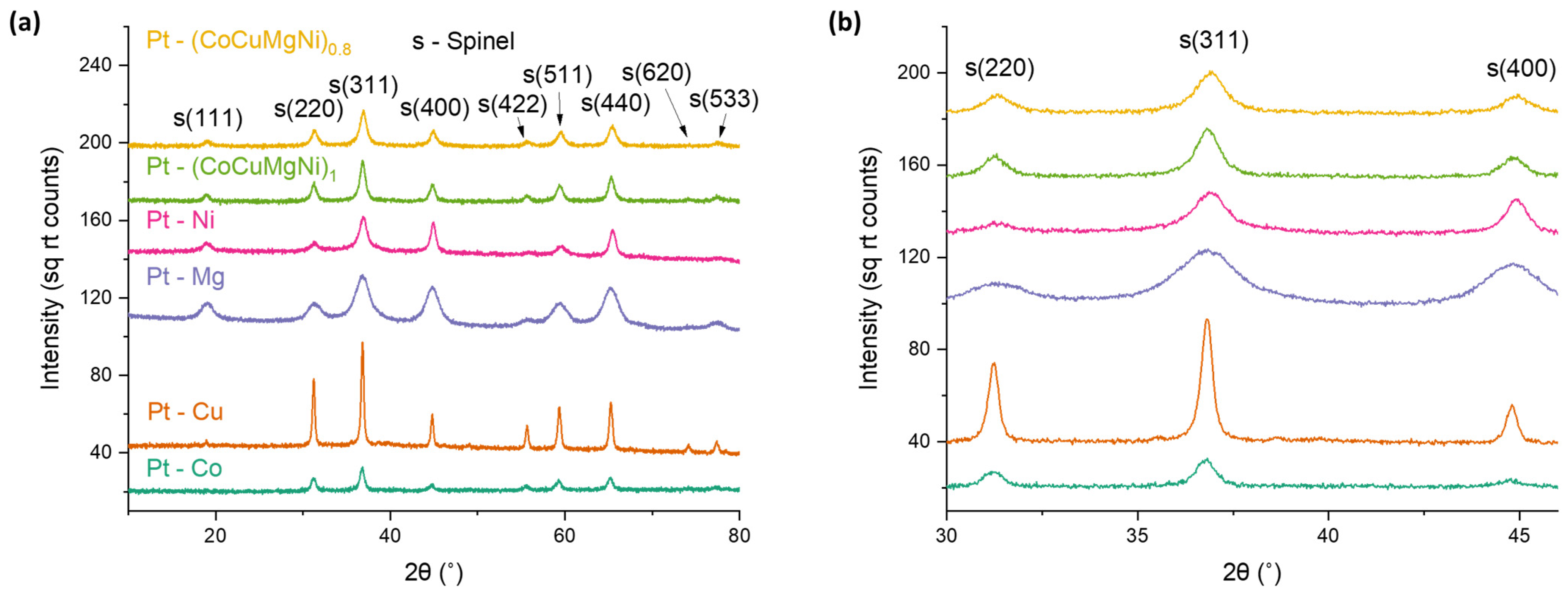
Spinel samples were first evaluated for phase purity via PXRD. The synthesis of phase pure aluminate spinels required varying calcination temperature depending on spinel composition, as discussed in our previous work [
19]. These calcination temperatures are listed in
Table 1. Given the relatively high formation enthalpies of NiAl
2O
4 and CuAl
2O
4, these samples required calcination at 950 °C and quenching in ambient air [
20]. PXRD patterns show the segregation of CuO and NiO phases, respectively, when lower calcination temperatures were used (
Figure S1). All other spinels were phase pure after initial calcination at 800 °C due to the lower formation enthalpies of CoAl
2O
4 and MgAl
2O
4, as well as the increased configurational entropy of (CoCuMgNi)Al
2O
4 and (CoCuMgNi)
0.8Al
2O
3.8 compositions [
20]. This latter effect suppresses the formation temperature of complex oxides [
12,
19]. The PXRD patterns of prepared Pt-loaded spinel samples are shown in
Figure 1. Pt peaks are not evident after 500 °C calcination, likely due to both the low platinum loading and small crystallite size. Composition and specific surface area characterization data are listed in
Table 1. Elemental analysis shows that measured spinel compositions are near nominal values. The molar ratio of total divalent metals to aluminum is near 1:2 for stoichiometric samples, and divalent metals within HEO samples are in near-equimolar concentration. Pt loading is near 1 wt% in prepared samples, as intended. Specific surface area varied appreciably, from 107 m
2/g for Pt–Mg to 5 m
2/g for Pt–Cu. The higher calcination temperatures required to form CuAl
2O
4 caused the significant sintering of the spinel support, which is also evidenced from the relatively narrow PXRD peaks. The lattice parameter of the aluminate spinels was largely influenced by the size of the divalent cation and the relative site occupation of aluminum and divalent cations between tetrahedral and octahedral lattice sites.
As prepared Pt-loaded samples were tested in a CO oxidation reaction with results shown in
Figure 2. Catalytic activity varies widely depending on the spinel support. Samples with copper-containing spinels demonstrate significantly higher activity throughout the low temperature regime. The characteristic S-shaped light-off curve of these samples indicates first-order reaction kinetics, in which relatively weak CO binding enables high turnover rates when CO conversion is low. However, the depletion of CO reactant limits the reaction at high conversions [
21]. Similar performance was observed from catalysts containing ionic active sites and metal oxide supports that play an active role in oxidation catalysis [
7,
22]. Here, synergistic effects between copper spinels and supported platinum particles are apparent given that these spinels alone catalyze CO oxidation but demonstrate higher activity upon platinum addition, as shown in
Figure S2. Intriguingly, Pt–Cu, Pt–(CoCuMgNi)
1, and Pt–(CoCuMgNi)
0.8 samples showed very similar activity, despite having different support compositions. Copper content in these samples was much higher than is typically added as either an active metal or a support dopant and likely exceeds the amount required to induce beneficial catalytic effects.
On the other hand, Pt–Mg, Pt–Co, and Pt–Ni samples gave U-shaped light-off curves indicative of negative first-order reaction kinetics [
21]. This behavior indicates strong CO adsorption on catalytic sites, which impedes O
2 adsorption and reaction progress at low CO conversions. Once strongly bound CO begins to convert and desorb, the reaction proceeds very rapidly (lighting off), particularly in the high temperature regime [
21]. This behavior is characteristic of metallic platinum particles on inert supports, such as alumina, which do not facilitate the reaction by weakening CO adsorption or by generating reactive oxygen species. Pt–Co and Pt–Ni samples had higher activity than Pt–Mg, further suggesting that transition metals play a non-negligible catalytic role, even as components of an oxidized support. Despite diminished activity over much of the temperature range tested, the light-off behavior of Pt–Co and Pt–Ni enabled complete CO conversion at temperatures comparable to copper-containing samples, as indicated by T
100 values in
Table 2. The apparent activation energies of CO oxidation over these samples were calculated from Arrhenius plots (
Figure 2) and are 41, 61, and 36 kJ/mol, respectively. Overall, the composition of the spinel support clearly influenced oxidation activity, with the most to least active components being copper, followed by other transition metals, followed by magnesium.
We next compared catalyst structural features and activity before and after aging at 800 °C. PXRD and BET characterization results showed similar crystallite sizes and specific surface areas for most samples before and after aging (
Table S1). Similar interplanar spacing and preferentially exposed spinel facets were imaged via STEM and are shown in
Figure S3. However, notable structural differences were seen for copper-containing samples. For instance, whole pattern fitting results for Pt–Cu suggest that CuAl
2O
4 crystallite size increases significantly during aging. Specific surface areas of Pt–(CoCuMgNi)
1 and Pt–(CoCuMgNi)
0.8 decrease during aging. Thermal quenching and the inclusion of many divalent metals in these samples likely increase lattice distortions, which promote structural evolution during aging. The light-off curves of aged samples are shown in
Figure 3. The temperatures needed to achieve 50, 90, and 100% CO conversion are provided in
Table 2 as T
50, T
90, and T
100. Samples containing compositionally simple spinels deactivated significantly during aging, with corresponding increases in light-off curve temperature of up to 77 °C. Pt–Cu displayed similar activity before and after aging at low temperatures with a small increase in T
50 of only 11 °C, but activity diminished severely at higher temperatures after aging. On the other hand, Pt supported on HEO spinels demonstrated exceptional thermal stability. T
100 increased by only 12 °C for Pt–(CoCuMgNi)
1 after aging, and T
50 and T
90 values even decreased by 10 and 3 °C, respectively. Light-off curves were nearly identical for the Pt–(CoCuMgNi)
0.8 sample, increasing only as much as 6 °C for the aged sample. Since platinum catalysts are highly prone to thermal deactivation, the stability of platinum supported on HEO spinels is remarkable.
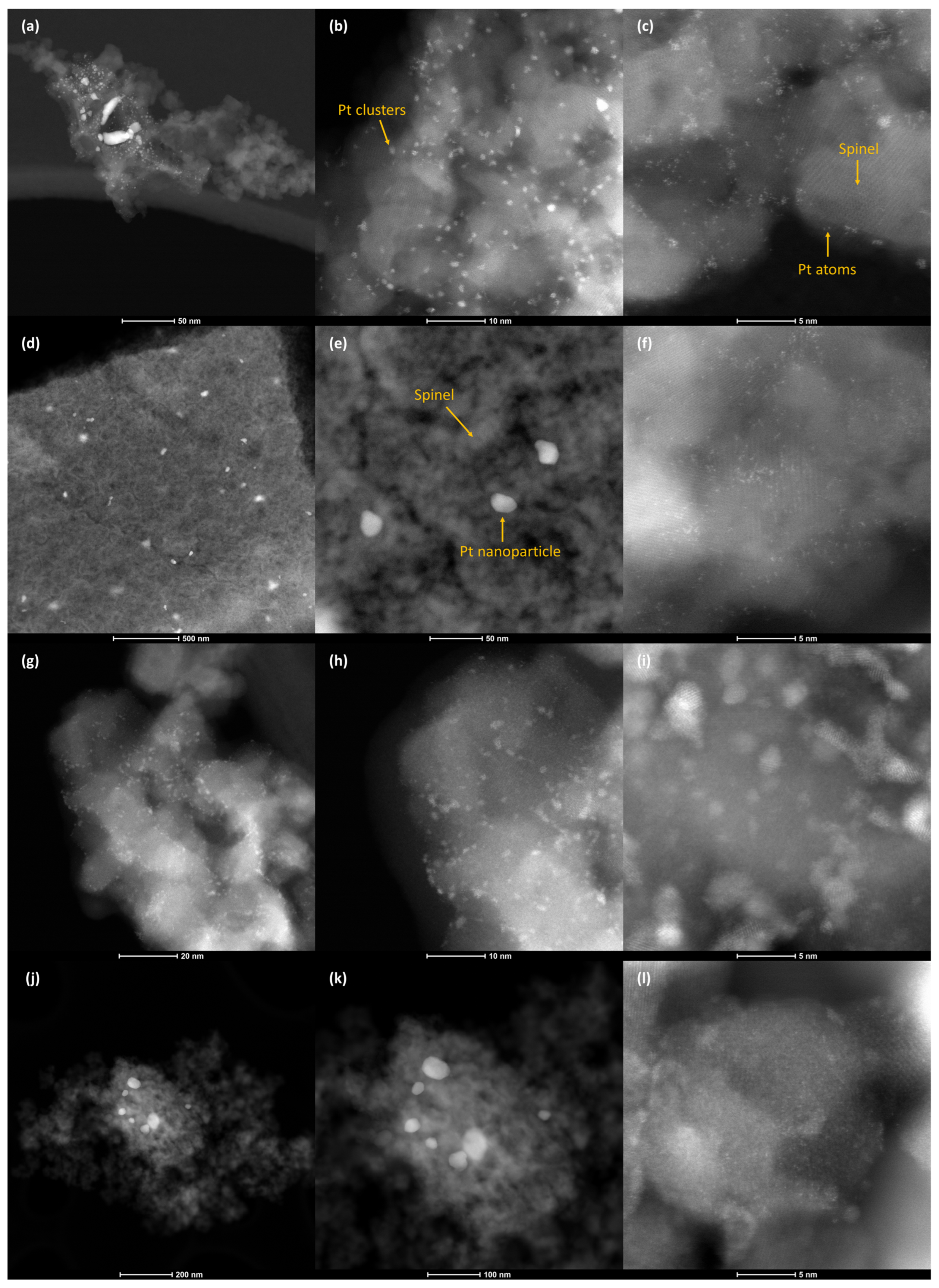
Catalyst samples were further evaluated through STEM to better understand the evolution of platinum particle size. Since PGM particle sintering is linked to catalyst deactivation, STEM images were collected in high-angle annular dark-field (HAADF) mode to identify changes in the relative platinum size distributions of Pt–Mg and Pt–(CoCuMgNi)
1 in fresh and aged states. Pt species ranged from relatively few particles 10s of nanometers in size to more numerous clusters 1–2 nanometers and isolated Pt atoms within the prepared Pt–Mg sample (
Figure 4a–c). After aging, a bimodal distribution became apparent, which included an increased proportion of Pt particles >10 nm in size and retained atomically dispersed Pt species (
Figure 4d–f). An estimated particle size distribution for Pt–Mg before and after aging is provided in
Figure S4. These results agree with previous studies of thermally aged Pt supported on aluminate spinels [
3]. Similar Pt size distributions were observed in Pt–(CoCuMgNi)
1, wherein the prepared sample primarily contained Pt clusters up to several nanometers in size and Pt in the aged sample existed primarily as particles 10s of nanometers in size and highly dispersed Pt atoms and clusters. Previous work demonstrated the ability of magnesium aluminate spinels to maintain the high dispersion of platinum particles after aging, owed to the strong binding of Pt to spinel (111) facets. Here, both compositionally simple and high-entropy spinels maintained relatively small platinum particles, which were shown to sinter into micron-size particles on common inert supports like alumina [
5].
The PXRD characterization of aged samples was also performed to characterize platinum particle size on bulk scales. The average crystallite size of platinum in aged samples was calculated from the Pt(111) peak in PXRD patterns provided in
Figure 5. Platinum crystallite sizes listed in
Table 3 were comparable among the aged samples, ranging from 34–53 nm, except in Pt–Co, which contained appreciably larger crystallites of 67 nm, respectively. PXRD average crystallite sizes are similar to the larger particles observed in STEM images. This result shows that, regardless of composition, aluminate spinels maintain high platinum dispersions after thermal aging in comparison to other common supports, such as alumina and silica. Platinum supported on these oxides sinters into much larger particles under similar thermal aging treatments [
5]. The significant loss of surface-exposed platinum sites is a well-understood mode of deactivation in these catalysts. On the other hand, the lack of severe platinum sintering and comparable crystallite size among all aged samples suggests that the deactivation of platinum supported on simple spinels is caused by other structural or chemical changes. PXRD results were further analyzed via Rietveld refinement to determine the lattice parameter of platinum particles. Particles within aged Pt–Cu, Pt–(CoCuMgNi)
1, and Pt–(CoCuMgNi)
0.8 had lattice parameters that were notably smaller than in the other samples and in bulk platinum (3.9242 Å) as listed in
Table 3. The Pt(111) peak shifts to higher 2θ values in these samples (
Figure 5b), suggesting a compositional change in the platinum phase.
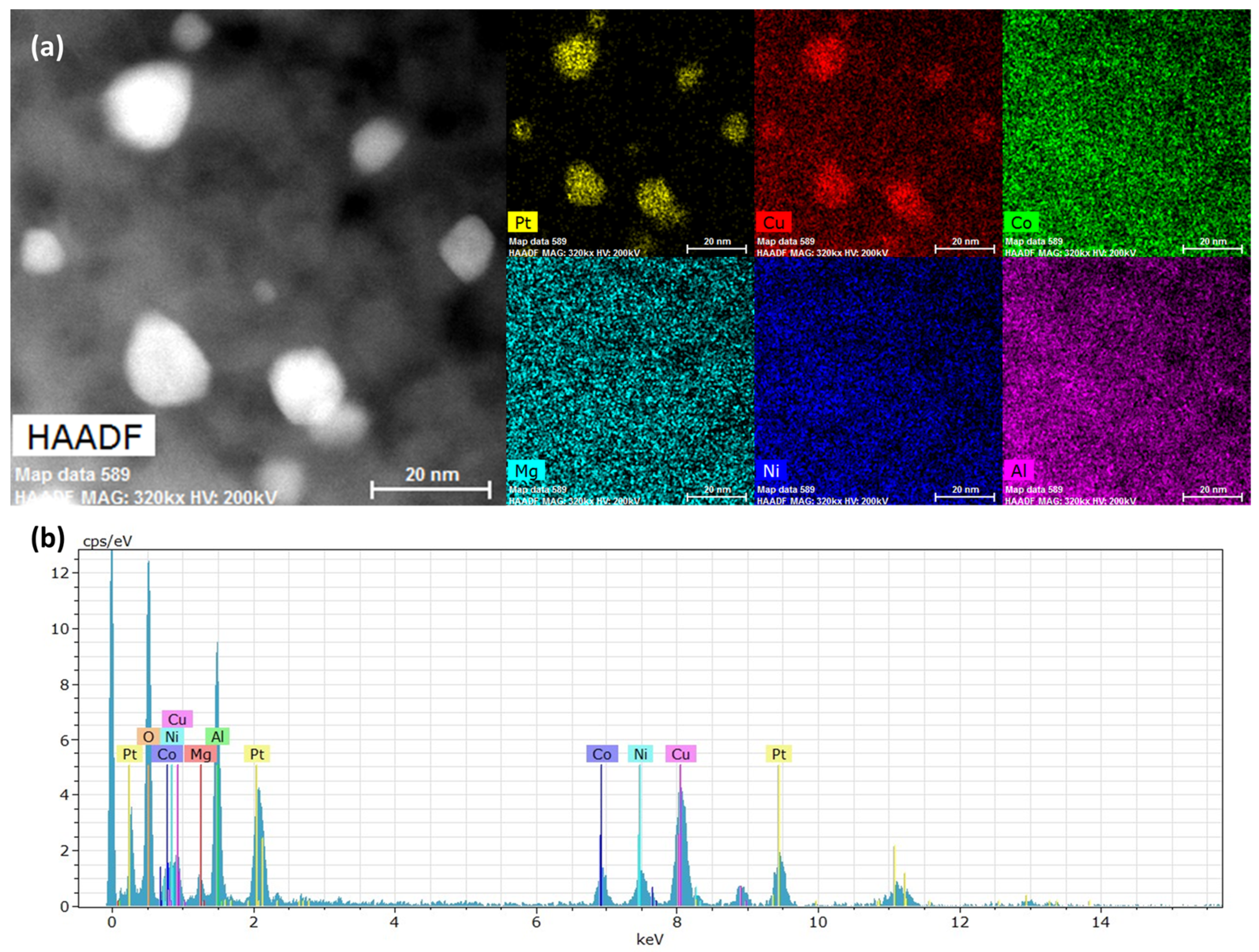
Aged Pt–(CoCuMgNi)
0.8 was imaged through STEM-EDS to evaluate the composition of the metallic phase. Elemental maps in
Figure 6 clearly show copper alloyed with the platinum particles upon aging. The EDS spectra confirmed the presence of all expected elements without impurities. The higher intensity of the copper peak may be caused by copper exsolution and surface enrichment. However, the accurate quantification of these data is not provided due to use of a copper TEM grid, which may have also elevated the apparent concentration of this metal. For comparison, the elemental maps of aged Pt–Mg are provided in
Figure S5 and show only Pt as present in the metallic phase. Due to its smaller atomic radius, copper alloying reduces the platinum lattice parameter in agreement with PXRD lattice parameter refinements. Using Vegard’s law, we estimated that the metallic particles in aged Pt–Cu and Pt–(CoCuMgNi)
1 contain 5 and 3% copper, respectively. From copper mass balances, as well as EDS mapping, most copper remained dispersed within the bulk of the spinel lattice after aging. However, the XPS spectra of the aged Pt–(CoCuMgNi)
0.8 sample (
Figure S6) shows that a majority of near-surface copper in the spinel surface is predominantly in metallic form.
From initial sample characterization, CuO was shown to segregate from the CuAl
2O
4 sample during 800 °C calcination. Although the pure phase spinel was prepared through calcination at 950 °C and subsequent quenching, a fraction of contained copper resegregated as CuO from the spinel during aging at 800 °C and formed metallic Cu during reduction prior to CO oxidation reaction (
Figure S7). During thermal aging and CO reduction treatments, CuO reacted with platinum to form alloyed particles. Platinum-copper alloys were previously studied as CO oxidation catalysts and demonstrated cyclic redox stability. Copper oxide segregated from bimetallic particles under oxidizing conditions, but platinum-copper alloys reformed under reducing conditions with similar activity after repeated redox cycles. In a previous study, palladium oxide was further shown to trap mobile Pt atoms at high temperatures, which was then reduced to form stable Pt-Pd alloys [
23]. Here, we propose that CuO may have reacted with Pt through similar redox mechanisms.
Platinum–copper alloying clearly improved catalyst activity. However, these interactions do not fully explain the exceptional thermal stability of platinum on HEO spinel samples, which maintained better performance after aging than the Pt–Cu sample. CO chemisorption analysis was next performed to evaluate the relative number of active sites for CO adsorption before and after aging. Because both platinum and transition metal cations within the spinels are known to adsorb CO, this analysis informed changes in overall CO capacity, rather than changes in platinum particle size specifically. From the results (
Table 3), CO uptake decreased in nearly all aged samples. Platinum binds CO strongly and can further increase catalyst CO capacity through spillover to alumina-based supports [
24]. Thus, platinum sintering is expected to decrease the CO uptake of aged samples. Aged Pt on simple spinel samples retained no better than about 16% of original adsorption capacity, in the case of aged Pt–Co. Active redox chemistry and polycarbonyl formation previously observed over CoAl
2O
4 may explain the enhanced CO adsorption on this sample compared to other simple spinels, and may be evidenced through a reduction in some near-surface Co seen in XPS spectra (
Table S3) [
25]. Pt–Cu shows significantly lower CO capacity than other spinels both before and after aging. Since CO is known to bind to ionic copper, the significantly lower surface area of this sample following 950 °C calcination is expected to decrease the number of exposed copper sites. This effect, coupled with platinum sintering and weakened CO binding to copper–platinum alloy surfaces, may further explain the deactivation of the aged Pt–Cu sample.
On the other hand, Pt on high-entropy spinel samples retained much higher CO adsorption capacity after aging. Pt–(CoCuMgNi)
1 retained about 52% of its original CO capacity after aging, and the capacity of Pt–(CoCuMgNi)
0.8 even increased. These results agree with the finding that Pt on high-entropy spinels showed little deactivation after aging. We further note that the high-entropy spinels exhibited excellent CO adsorption capacity, even without added platinum. Thus, these samples retained active sites despite the sintering of platinum during aging. Transition metal aluminate spinels were previously observed to be effective CO adsorbents, in which the strength of interaction is heavily influenced by the divalent cation [
25,
26,
27,
28]. The previous literature further observed that CO adsorption on aluminate spinels was significantly enhanced through the incorporation of multiple transition metal elements, as well as under-coordinated defect sites [
29,
30,
31]. Indeed, CO adsorption capacity after aging was significantly higher for Pt–(CoCuMgNi)
0.8, containing a non-stoichiometric spinel. Our previous work demonstrated that non-stoichiometric HEO spinels contain high concentrations of both cationic and anionic vacancies and thus undercoordinated sites, which appear to promote CO adsorption herein [
19].
As reported in our previous work, high-entropy spinels show complex site occupation in the crystal lattice caused by varying degrees of inversion (divalent metals occupying octahedral sites and Al
3+ occupying tetrahedral sites) among the contained cations [
19]. Both stoichiometric and non-stoichiometric high-entropy spinels contain high concentrations of cationic and anionic vacancies generated by structural complexity and charge compensation affects. Because these defect sites activate O
2 and other reactants, vacancies are considered essential to the activity of many catalysts in CO oxidation and other reactions, particularly when vacancies act in conjunction with neighboring metallic sites [
32,
33,
34]. These under-coordinated defect sites promote CO adsorption and conversion to CO
2. CO adsorption was even previously observed on undercoordinated sites in magnesium aluminates, which are known to be relatively inactive catalysts [
31]. XPS 1s oxygen spectra (
Figure S6) of MgAl
2O
4, Mg
0.8Al
2O
3.8, and (CoCuMgNi)
0.8Al
2O
3.8 spinel supports show a peak shift toward a lower binding energy with the incorporation of multiple divalent metals and vacancies induced through non-stoichiometric cation concentrations. The complex compositional and structural features of HEO spinels appear to alter the energetics of near-surface oxygen atoms, suggesting the increased reactivity of these species. Cooperative effects between spinel supports and loaded platinum is again evidenced by CO oxidation activity for spinels with and without platinum (
Figure S2). The conversion of CO on the HEO spinels confirms the capability of these supports to adsorb and activate CO and O
2 reactants. However, activity is significantly improved through active metal addition suggesting that metal–support interfacial effects, such as reactant spillover, play a significant role in oxidation catalysis [
35].
3. Materials and Methods
Spinel samples were synthesized according to our previously reported sol-gel synthesis [
19]. A total of 5 g of polyvinylpyrrolidone (PVP, Sigma Aldrich, Milwaukee, WI, USA, average molecular weight = 40,000) was dissolved into 100 mL of deionized water while stirring. A total of 14 mmol of aluminum (III) nitrate nonahydrate (J.T. Baker Inc., Baker analyzed reagent, Phillipsburg, NJ, USA) and appropriate amounts of cobalt (II) nitrate hexahydrate (Sigma-Aldrich, reagent grade 98%), copper (II) nitrate hemipentahydrate (Sigma Aldrich, Milwaukee, WI, USA, ≥99.99%), nickel (II) nitrate hexahydrate (Alfa Aesar, Ward Hill, MA, USA, puratronic, 99.99985% metals basis), and magnesium (II) nitrate hexahydrate (Sigma-Aldrich, ACS reagent 99%) were added to the solution and stirred for 1 h. Excess water was evaporated through drying in an oven at 110 °C overnight. The resulting hard gels were coarsely ground and combusted in a furnace preheated to 800 °C for 2 h within a fume hood. To achieve phase purity, several spinel samples were calcined in a muffle furnace at temperatures of up to 950 °C for 2 h. These samples were removed from the furnace and quenched in ambient air to prevent phase segregation. Platinum was added to the resulting oxides through incipient wetness impregnation. Tetraammine platinum (II) nitrate (Sigma Aldrich 99.995% trace metals basis) was dissolved in deionized water, added dropwise to powdered oxide supports, and dried at 110 °C upon saturation. Platinum-loaded samples were calcined at 500 °C in a tube furnace under a 50 mL/min flow of air. Samples were further aged at 800 °C for 8 h under a 50 mL/min air flow. Heating rates of 10 °C/min were used during calcination and aging of Pt-loaded samples. For simplicity, Pt-loaded samples are labeled as “Pt–M”, where “M” indicates the divalent metal composition within the spinel support. As examples, the 1wt% Pt–MgAl
2O
4 sample is labeled as “Pt–Mg” and the 1wt% Pt–(CoCuMgNi)
0.8Al
2O
3.8 as “Pt–(CoCuMgNi)
0.8”.
Samples were characterized by powder X-ray diffraction (PXRD), Brunauer–Emmett–Teller (BET) specific surface area analysis, electron probe microanalysis (EPMA), scanning electron microscopy (SEM), scanning transmission electron microscopy (STEM), X-ray energy dispersive spectroscopy (EDS), X-ray photoelectron spectroscopy (XPS), and CO chemisorption. PXRD measurements were performed using a Bruker D2 Phaser system equipped with a sealed tube X-ray source (Cu Kα radiation) and a LynxEye silicon strip detector. Power settings for X-ray generation were 30 kV and 10 mA. The scan range was 10–80° with a step size of 0.02° 2θ and a dwell time of 0.2 s. PXRD data were analyzed with MDI Jade Pro version 8.7. Crystallites sizes were estimated through peak broadening, and lattice parameters were calculated using whole pattern fitting. BET analysis was performed by outgassing samples overnight at 120 °C under nitrogen flow. A Micromeritics Gemini 2360 surface area analyzer was used to measure outgassed samples. The EPMA compositional analysis of pelletized samples was used to determine platinum loading in terms of weight percent (wt%) and Al, Co, Cu, Mg, and Ni composition in atomic percent (at%). An SNE-4500 Plus SEM microscope was used for compositional analysis, as well as for the imaging of select samples. Compositional measurements were taken with a JEOL JXA-8530F HyperProbe Microanalyzer. Divalent metal stoichiometry was calculated relative to an assumed Al stoichiometry of 2. An FEI Titan
TM G2 80–200 STEM with a Cs probe corrector and ChemiSTEM
TM technology (X-FEG
TM and SuperX
TM EDS with four windowless silicon drift detectors) was operated at 200 kV for sample imaging. High-angle annular dark-field (HAADF) modes were used for imaging and compositional analysis, respectively. XPS spectra were measured using a Kratos AXIS DLD Ultra photoelectron spectrometer with monochromatic Al K
α source at 150 W. Spectra were evaluated according to previous literature [
36,
37].
Samples were tested in CO oxidation reaction before and after aging at 800 °C for 8 h in 50 mL/min of flowing air. A total of 20 mg of catalyst was loaded into a stainless steel reactor and reduced in a 77.5 mL/min 3% H2 flow (balance He) at 275 °C for 1 h. Feed gas consisting of 1.5 mL/min CO, 1 mL/min O2, and 75 mL/min He was used, giving a space velocity of 232,500 mL g−1 h−1. The temperature of the reactor was raised to a maximum of 300 °C or until 100% CO conversion was achieved at a heating rate of 2 °C/min. Three consecutive runs were performed on each sample, and data from the third run are plotted herein. A Varian CP-4900 micro-gas chromatograph was used to analyze gas composition.