1. Introduction
Nitriles can be easily converted into amines, aldehydes, esters, carboxylic acids, and other chemical functionalities (
Figure 1) [
1]. This high synthetic diversity can potentially enable new synthesis routes, which may be of particular interest in the development of new, sustainable processes. On a laboratory scale, nitriles can be synthesized by nucleophilic substitution reactions of an alkyl halide and an alkali cyanide in polar solvents. Nevertheless, the formation of alkali halides as byproducts excludes this method for industrial-scale synthesis of such important nitriles. These are economically, as well as ecologically, disadvantageous and difficult to handle. Hydrocyanation, the catalytic hydrogen cyanide (HCN) addition to C=C-double-bonds, provides selective and 100% atom-economical access to nitriles [
1,
2].
Large parts of the academic literature focus on HCN surrogates like Trimethylsilyl cyanide-MeOH [
3], acetone cyanohydrin (ACH) [
4,
5,
6,
7,
8,
9], or zinc cyanide and water [
10] for the in situ formation of HCN. More recent research focuses on transfer hydrocyanation at increased temperatures, utilizing the equilibrium established by the nickel catalyst. The remaining low boiling alkene can be evaporated from the reaction mixture (
Figure 1) [
11,
12,
13].
Synthetic routes employing HCN surrogates are usually safer to be performed on a lab scale. However, they are of little interest for larger-scale industrial application. The formation of coupled products or byproducts severely diminishes the economic value of a process and is therefore highly undesirable. Pure hydrogen cyanide, produced via the Andrussow process or the BMA process, is readily used for large-scale hydrocyanation of butadiene [
1]. Unfortunately, the high toxicity of HCN requires severe safety standards, making this reasonably unattractive in purely academic research—the academic literature regarding hydrocyanation using pure HCN is limited. To fill this gap, this work explicitly focuses on the use of pure HCN and optimization of the reaction procedure in order to improve the activity of the Ni-based catalyst.
In typical lab-scale hydrocyanation experiments, the catalyst is first preformed and, after all substrates are added, hydrogen cyanide, if used in pure form, is usually added via Eppendorf pipette, either neat or in solution [
14,
15,
16]. Early academic studies on hydrocyanation mainly used HCN surrogates like acetone cyanohydrin, which require elevated temperatures of 60 to 90 °C to liberate HCN. For reasons of comparability, these conditions were mostly kept in successive work, even when pure HCN was used. So far, there is limited research focused on optimizing the experimental methodology.
Phosphine, phosphinite, phosphonite, and phosphite ligands have been applied in Ni-catalyzed hydrocyanation [
17]. The best yields and selectivities were achieved for chelating ligands with large bite angles between 105° and 120°. Rigid, large-bite-angle ligands stabilize tetragonal and trigonal bipyramidal active species and disfavor the formation of inactive square planar nickel cyanide species [
17].
Electron-withdrawing
π-acceptor ligands like phosphites and phosphonites improve yield and selectivity by accelerating the rate-limiting reductive elimination step [
14]. Early studies with tri-
o-tolylphosphite already show the positive effects of this ligand class [
18,
19].
Furthermore, it was shown that chelating ligands tend to form catalytically inactive Ni(bischelate) complexes. While this is reversible for diphosphines, the Ni(bischelates) formed with chelating π-acceptor ligands tend to be quite stable and may lead to complete catalyst deactivation over time, especially at elevated temperatures [
14]. However, the formation of bischelates can be counteracted by sterically hindered chelating ligands [
17].
Combining the mentioned characteristics, bulky and rigid diphosphites with large bite angles appear to be highly potent ligands for Ni-catalyzed hydrocyanation. Applying a diphosphonite ligand in the Ni-catalyzed hydrocyanation of styrene with pure HCN, Hofmann et al. were able to reduce the catalyst concentration to 1 mol%, still reaching a conversion of more than 90% [
20]. This translates to a maximum Turnover Number (TON) of 92. These findings emphasize the need for further optimization in this particular field.
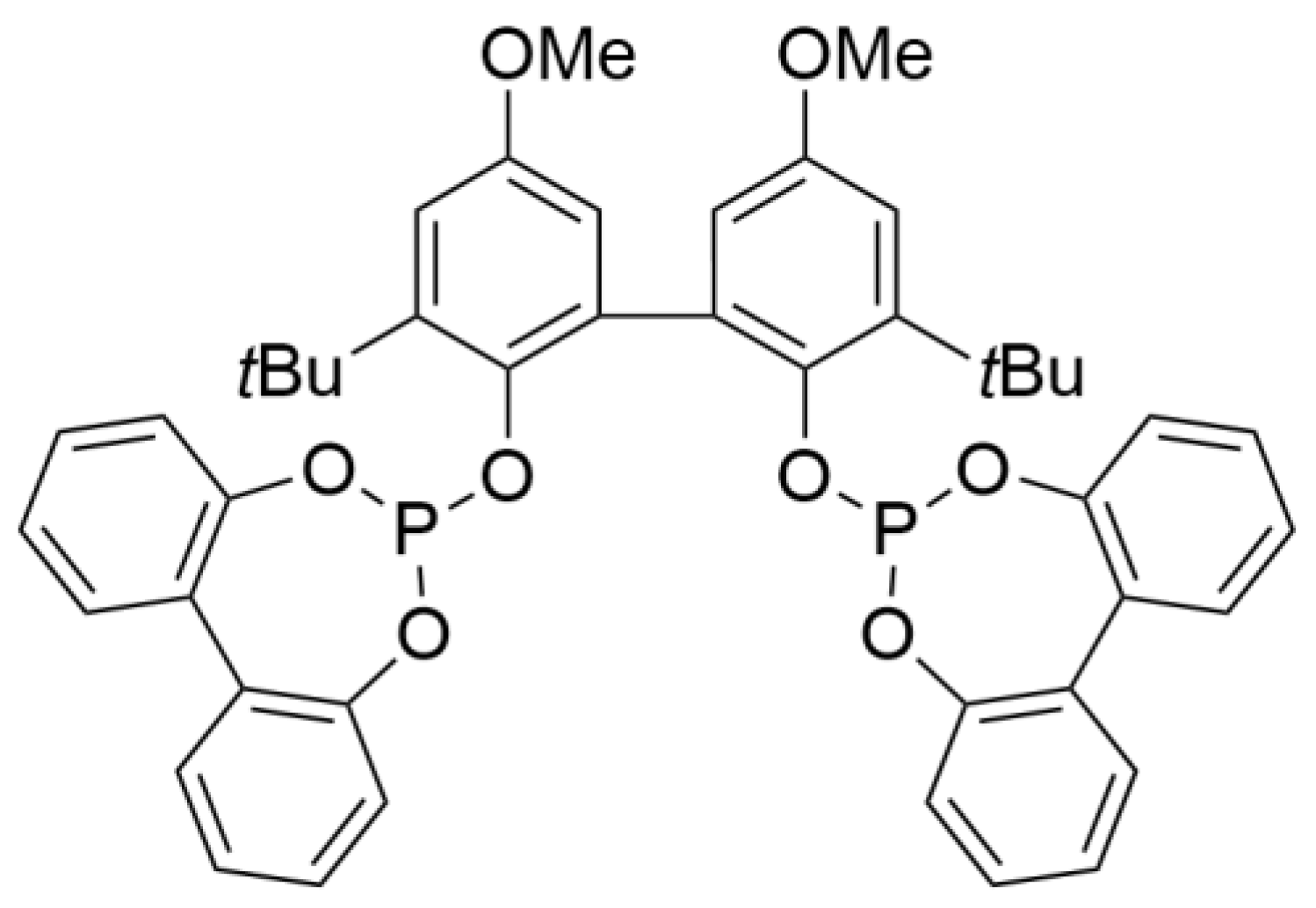
As there is a significant knowledge gap regarding the true activity of nickel complexes in hydrocyanation in terms of TOF at low conversion, in this study, we focused on the optimization of experimental procedures in order to maximize catalytic activity. For our study, we decided to apply BiPhePhos (
Figure 2) as the ligand, first described in patents by Union Carbide [
21]. Surprisingly, to the best of our knowledge, BiPhePhos has never been reported in the literature on Ni-catalyzed hydrocyanation.
2. Results and Discussion
For the intended optimizations, styrene was used as a model substrate. Due to its structural properties, it is an ideal model substrate for studies on vinyl arene hydrocyanation (
Figure 3).
Under the chosen conditions, styrene is almost exclusively converted to the branched product 2-phenylpropionitrile (
Figure 3). This is caused by the thermodynamically favored transition state between the
π-complex, which is necessary for the branched product formation, and the reductive eliminated product. This crucial step is therefore kinetically favored over the reductive elimination of the linear product to an extent where no linear product is being formed [
15].
2.1. Optimizations of Operational Procedure
2.1.1. Catalyst Preformation
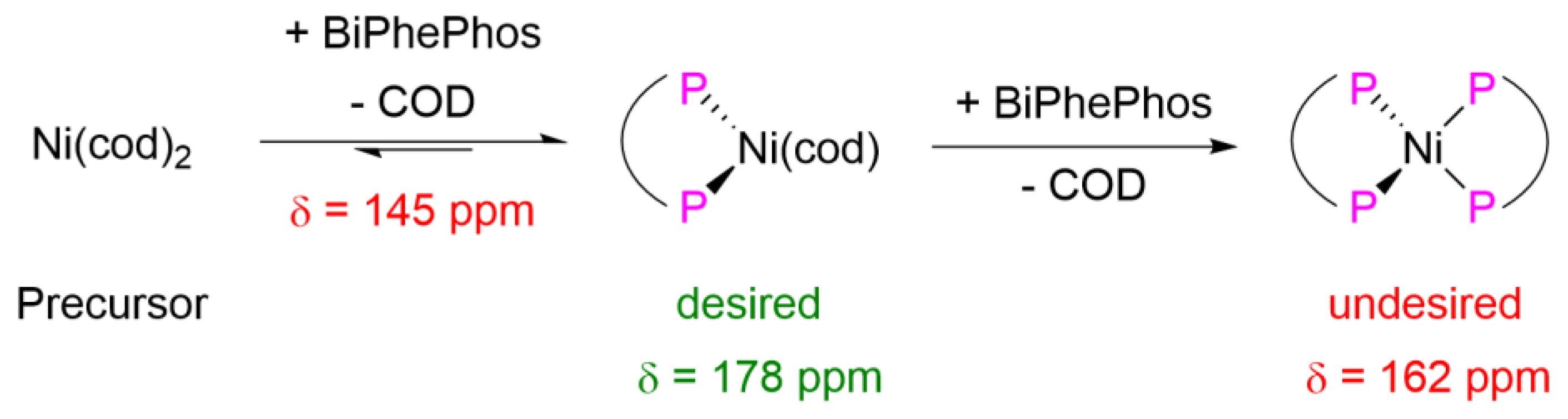
At first, the preforming procedure was investigated. It was found that dissolving the ligand BiPhePhos first, before the addition of Ni(cod)2, helped to achieve reproducible results. Dissolving Ni(cod)2 first, instead of BiPhePhos, often results in a cloudy solution, giving rise to non-reproducible results.
The preforming solutions were analyzed by
31P-NMR. After catalyst preformation for only 15 min at 22 °C, the characteristic signal for the (BiPhePhos)Ni(cod) complex was observed at δ = 178 ppm [
22]. Due to the 1.2 equivalents of ligand applied, the signal of the free ligand also remained at δ = 145 ppm. Even stirring the catalyst solution over night at 22 °C did not show the characteristic signal of the highly undesired (BiPhePhos)
2Ni bischelate complex at δ = 162 ppm. This complex was stable and catalytically inactive, as shown earlier by Fazekas (
Figure 4) [
22].
Our systematic study of catalyst preformation by
31P-NMR showed fast formation of the catalytically inactive bischelate complex at elevated temperatures (
Figure S8). This explains why hydrocyanation at 60 or 90 °C results in a lower Turn Over Number (TON). Here, we present a new and reliable protocol for hydrocyanation reactions at an ambient temperature of 22 °C.
2.1.2. Reaction Procedure
In order to optimize the reaction protocol, operational procedures were systematically varied while maintaining identical stoichiometry (see
Table 1 and the conditions given there). Initially,
n-decane was used as internal standard in the reaction mixture for analysis by gas chromatography (GC) (entry 1). Hydrogen cyanide (HCN) was added neat by Eppendorf pipette at −50 °C, resulting in a mere 5% conversion of styrene, and no product was observed. Adding the GC standard (
n-decane) after the reaction to mitigate the potential for oxygen contamination (entry 2), a modest enhancement in performance was achieved, with 19% conversion and 14% yield. Introducing hydrogen cyanide as a solution in toluene at 22 °C markedly elevated both conversion and yield to 96% (
Table 1, entry 3). This clearly shows that lowering the temperature to −50 °C while adding pure HCN via Eppendorf pipette has a negative effect on the effectiveness of the catalyst systems tested. Utilizing a rubber septum notably streamlines the operational procedure, minimizing the risk of contamination. This results in a conversion and yield exceeding 99% (
Table 1, entry 4).
To the best of our knowledge, no literature has been found that demonstrates the significant influence of the HCN addition technique and its temperature dependency. With these optimizations, the operational procedure has been established. Further investigations are carried out on the catalytic activity.
2.2. Studies on Activity
The reductive elimination, as the rate-determining step, in nickel-catalyzed hydrocyanations, is generally accelerated by electron-withdrawing ligands like the commercially available diphosphite BiPhePhos [
15].
Generally, the reaction rate is strongly temperature dependent. The protocol presented above (
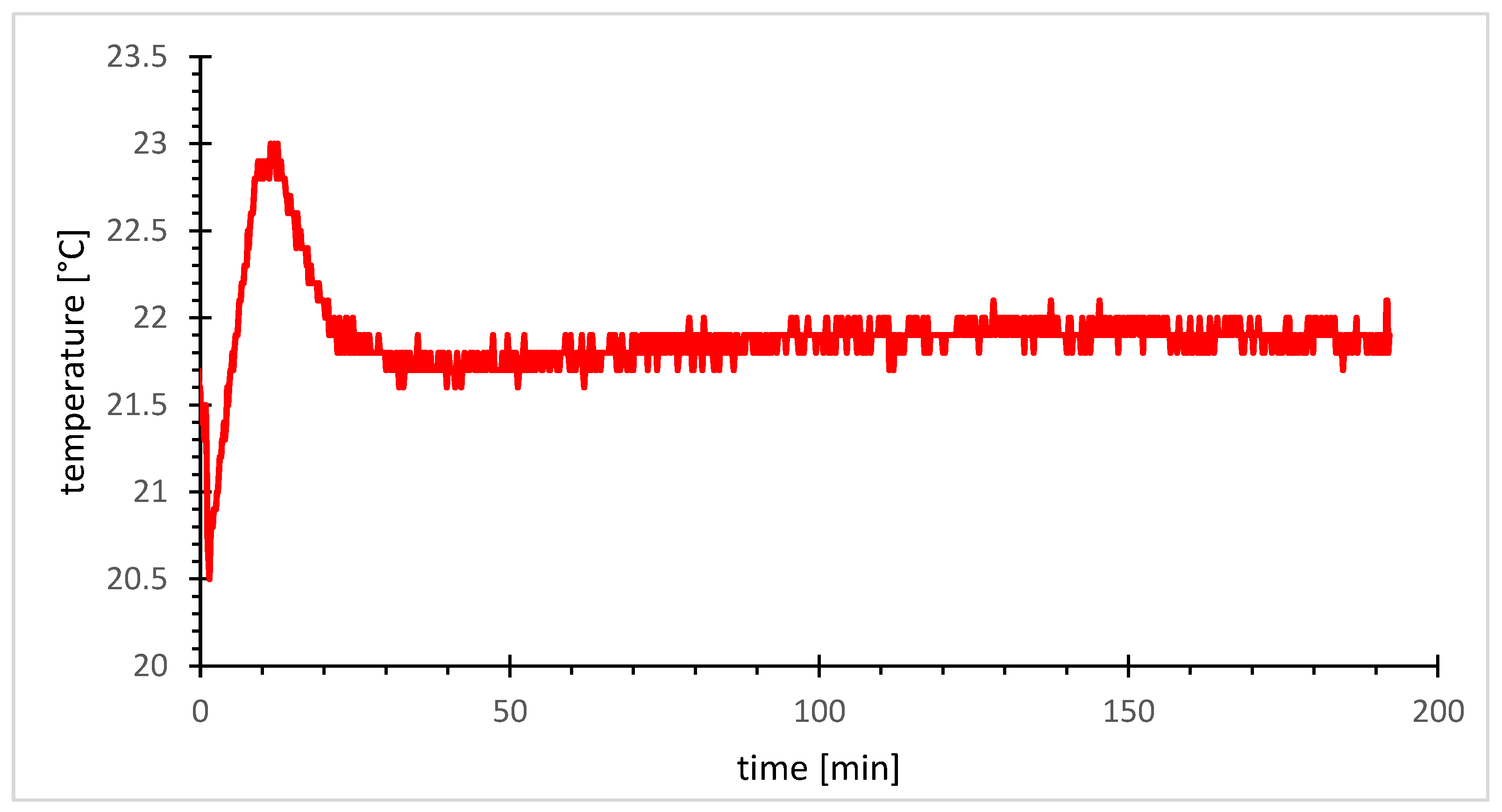
Section 2.1.2) involves catalyst solution preformation in a glovebox, followed by attaching a rubber septum lid. In a fume hood, styrene is added via syringe through the septum. Hydrogen cyanide (HCN) is added to one-third of the solvent in a separate Schlenk tube under cold inert conditions, causing HCN to freeze out. The solution is further warmed to the HCN melting point for syringe withdrawal and then injected into the reaction vessel via the septum to start the reaction. It is to be expected that this procedure will lead to a temperature profile that can strongly influence the reaction rate. The temperature is monitored by an internal thermometer (see
Figure 5).
Following the addition of the cold hydrogen cyanide (HCN) solution to the catalyst/styrene mixture, an immediate temperature drop of 1.3 °C below 22 °C (21.8 °C) is observed. Subsequently, a rapid temperature rise to 23 °C occurs within 11 min, attributed to the exothermic nature of the addition reaction. After 11 min, a noticeable temperature decline indicates the nearly complete conversion of styrene to 2-phenylpropionitrile.
Essentially, the reaction displays minimal temperature fluctuations during this phase, potentially attributed to the use of a syringe with a cannula. This configuration exposes the HCN solution in the cannula to a high surface-to-volume ratio, enabling an efficient heat exchange. However, repeating the experiment without pre-cooling the toluene eliminates the 1.5 °C temperature drop. Nevertheless, cooling of the HCN was maintained to ensure precise stoichiometry and compliance with safety parameters, thus ensuring controlled and safe execution of the reaction protocol.
The reaction progress was monitored by GC analysis of the samples withdrawn from the reaction mixture (see reaction profile
Figure S9). For this study, a catalyst concentration of 0.5 mol% was selected. The investigation revealed a remarkably fast reaction, achieving total styrene conversion in just 10 min. Notably, 20% conversion was observed already after 2.5 min, resulting in a turnover frequency (TOF
20) of 817 h
−1. This activity can likely be further increased by reducing the solvent amount, validated by stepwise solvent reduction from 3 mL (
Table 2, entry 1) to 0.5 mL (
Table 2, entry 4).
Under all reaction conditions, the conversion and yield exceeded 90%, which excludes the possibility of catalyst deactivation due to an increased HCN concentration. Another reaction profile was recorded for the minimum amount of solvent (entry 4), which reduced the total reaction volume from the previous 8.1 mL to 1.4 mL (
Figure 6).
It turns out (
Figure 6) that complete conversion is reached after 6 min, at which point no remaining styrene is detected. A yield of 70% is achieved after just one minute, which can be interpolated to 20% conversion within approximately 7.5 s, relating to a TOF
20 of 19,000 h
−1. Compared to the previous experiment, this remarkable increase in performance exceeds all values previously documented in the scientific literature. Further reducing the quantity of solvent employed in the experiment appears impractical due to the limited solubility of BiPhePhos. Alternatively, reducing the solvent amount for dilution of the highly volatile HCN risks inaccurate stoichiometry. By increasing the temperature during activity studies, the balance between deactivation and activity is investigated. The slower, gradual deactivation of the catalyst might still allow completion of the hydrocyanation reaction before full catalyst deactivation. At 60 °C, TOF
20 increases to 191,000 h
−1, more than ten times the results at 22 °C (
Table 3, entries 1 and 2). Further raising the temperature to 90 °C boosts activity even further to 309,000 h
−1 (
Table 3, entry 3).
It can be concluded that the hydrocyanation reaction rate shows a higher temperature dependency compared to catalyst deactivation by bischelate formation.
2.3. Scale-Up and HCN-Dosing
A notable constraint within the present reaction setup is the required minimum catalyst concentration of 0.5 mol% to achieve full conversion and yield (
Table 4, entry 1). Lowering the catalyst concentration to 0.1 mol% only yields 8% of the 2-phenyl propionitrile (
Table 4, entry 2). Again, one potential reason is traces of oxygen contamination leading to catalyst deactivation considering the low amount of catalyst, 0.001 mmol, applied in the reaction. Despite the optimized reaction protocol, contamination might occur during the preparation of the HCN solution. In this case, running the reaction at a larger scale is expected to minimize that risk.
The reaction (entry 3) was scaled up by a factor of 5, maintaining the total catalyst amount (0.005 mmol Ni(cod)2) used in entry 1. The result is a conversion and yield exceeding 99% (entry 3).
Another experiment analogous to entry 2 was performed but this time HCN was added slowly over 5 min. A conversion of 59% instead of 8% was achieved at >99% selectivity (
Table 4, entry 4). Both observations can be attributed to catalyst deactivation by traces of oxygen present in the hydrogen cyanide solution. If applied in a sufficient amount, i.e., exceeding the amount of the traces of oxygen, the remaining catalyst stays active and results in product formation. In the case of the fivefold preparation (entry 3), where 0.005 mmol of Ni(cod)
2 are present (similar to the entry 1 conditions), this catalyst amount appears sufficient to achieve a full yield/conversion despite partial deactivation by oxygen. In entry 4, assuming the same total amount of contaminant in the HCN solution as in entry 2, the catalyst deactivation is sustained due to the slow addition. Ultimately, however, when the full amount of solution has been added, complete catalyst deactivation occurs, preventing full styrene conversion.
Excessive HCN concentrations are another potential cause of catalyst deactivation, as well. In this case, the dosage experiment (entry 4) would have been expected to show full conversion/yield due to the HCN concentration being kept low. Furthermore, the fivefold preparation (entry 3) would have been expected to show only minimal reactivity, maintaining the same Ni:HCN ratio from entry 2, but neither could be observed. This clearly suggests that traces of oxygen are still the leading cause for the low conversion/yields in entry 2.
In order to further push to the limits, an experiment at 20 mmol scale and with a reduced catalyst amount of 0.025 mol% was performed, maintaining the total catalyst amount of 0.005 mmol (entry 5). Under these conditions, 23% conversion and 94% selectivity 21% was observed. This indicates the potential for further improvement by scaling up the batch size, a possibility not explored further in this study.
These findings emphasize the potential of the BiPhePhos-nickel system for hydrocyanation applications, showcasing a notable breakthrough in catalytic activity not reported before. However, elevated temperature still leads to catalyst deactivation over time. Thus, a balanced approach is crucial for achieving optimal activity while maintaining catalyst stability.
3. Materials and Methods
All experiments were performed using standard Schlenk techniques. Working steps in the glovebox were performed in a Labmasterpro ECO from mBRAUN under an argon atmosphere. Hydrocyanations were performed in Schlenk tubes (height: 124.5 mm, inner diameter: 23.4 mm, glass thickness: 2.6 mm) with a GL-32 closure and Young stopcock connection.
3.1. Purification of Solvents and Substrates
Toluene, n-decane, and di-n-butyl ether are purified by inert distillation over sodium. Styrene is dried over P2O5 and, afterwards, carefully distilled under argon with reduced pressure. Temperatures should not exceed 50 °C to prevent polymerization. Before use, all liquids are degassed by freeze-pump thaw method. BiPhePhos, donated by Evonik AG, was applied without further purification.
3.2. Synthesis and Handling of Ni(cod)2
Ni(cod)
2 is synthesized as a precipitate from dry Ni(acac)
2 and
n-Bu
2Mg in dry and degassed tetrahydrofuran. For purification, it is washed with dry and degassed acetone based on the work of Baker et. al. [
23]. The yielded yellow Ni(cod)
2 is highly sensitive towards oxygen, sunlight, and increased temperatures. Therefore, it must be handled with care exclusively in an inert atmosphere. If stored in the glovebox’s freezer, it is stable for several months. Ni(cod)
2 can only be handled using glass spatulas. Usage of stainless-steel spatulas results in the formation of metallic nickel black.
3.3. Handling of Hydrogen Cyanide and Safety Instructions
Hydrogen cyanide is produced from potassium cyanide and sulphuric acid in the presence of iron(III)-sulphate under a steam of argon. The yielded gaseous hydrogen cyanide is then dried by calcium chloride and condensed in a cooling trap. To remove traces of HCN from the argon stream, it is guided through two gas washing bottles containing a basic hydrogen peroxide solution.
For further purification, HCN is additionally degassed using the Freeze-Pump-Thaw method. Caution: Hydrogen cyanide is highly toxic and volatile. It must only be handled in a well-ventilated fume hood and with at least two instructed persons present. In addition to the general personal protective equipment for safe working in the laboratory (lab coat and safety goggles), a hydrogen cyanide gas detector (Honeywell BW™ Solo HCN, Poole, UK) and butyl rubber gloves (Honeywell Butoject™ 898) are required. Furthermore, HCN containers are stored at −26 °C in a lockable ATEX-certified freezer, located in a fume hood, which only instructed persons can access. The internal temperature is monitored via a battery-powered sensor connected to the Internet. For safe handling of single containers, the volume in a single storage container should not exceed 5 mL.
3.4. Gas Chromatography
GC samples were taken after removing the remaining hydrogen cyanide by a steam of argon. n-decane was added as an internal standard. About 0.7 mL of the reaction mixture is filtered through a syringe filter, placed in a GC vial, and diluted with toluene.
3.5. Calculation of Conversion (X), Yield (Y), and Selectivity (S)
First, the calibration factors for the used gas chromatography instrument (Agilent 7890A equipped with an Agilent HP-5 column equipped with an FID-detector) is determined with known masses of the analytes and
n-decane or di-
n-butyl ether (DBE) using the following Formula (1):
The resulting slope
Rf is used for further calculation of analytes masses. The mass of styrene
mstyrene within a GC sample is calculated with the weighed mass of the
n-decane
mdecane, the areas A of the chromatogram, and the calibration factor
Rf using Formula (2):
The mass is then converted into the amount of substance
n via Formula (3) to calculate the conversion
X, the yield
Y, and the selectivity
S of the hydrocyanation according to Formulas (4)–(6):