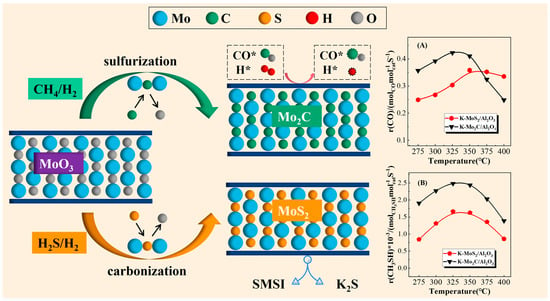
The Influence of Sulfurization and Carbonization on Mo-Based Catalysts for CH3SH Synthesis
Abstract
1. Introduction
2. Results and Discussion
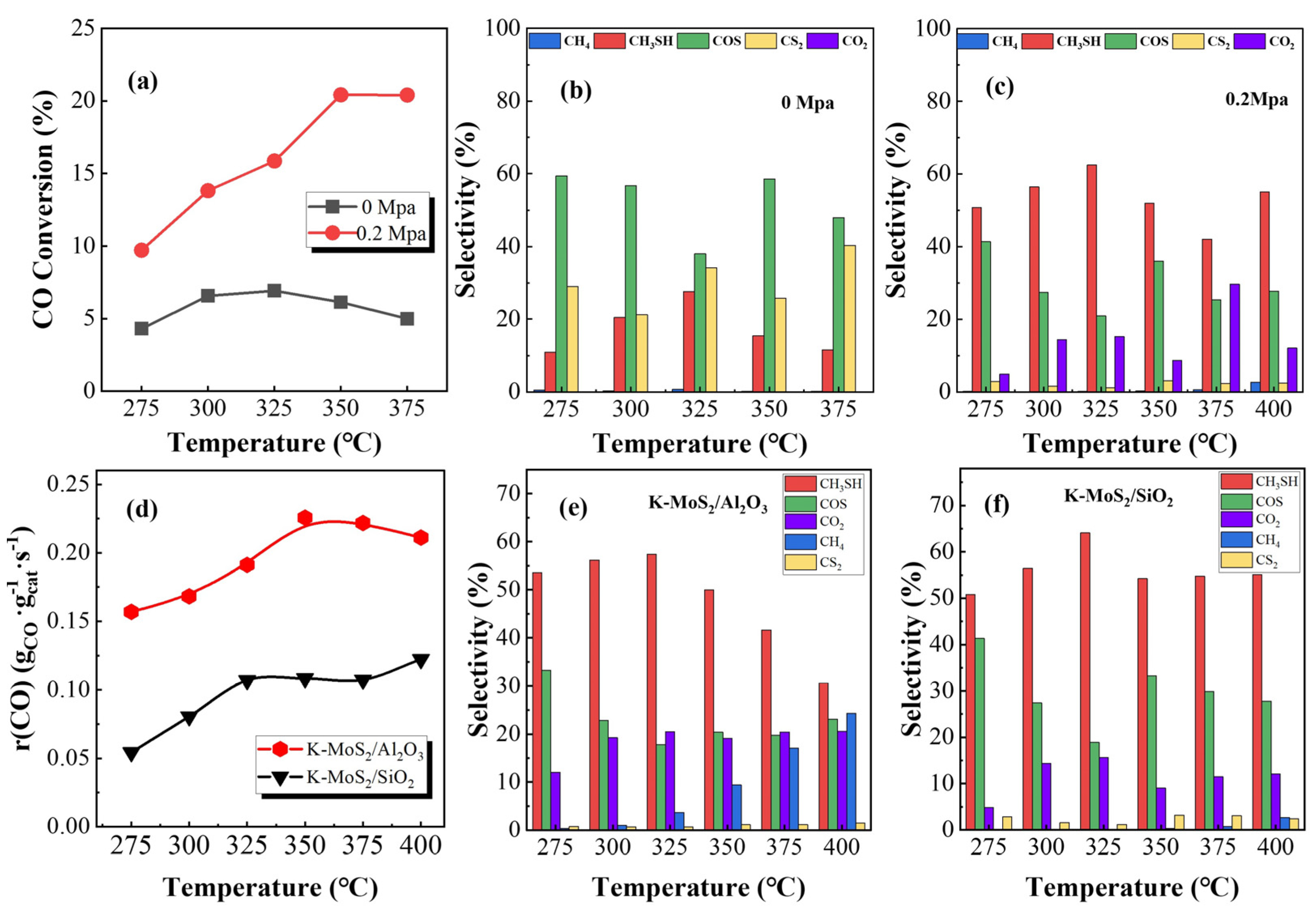
2.1. Effect of Reaction Pressure and Support
2.2. Performance of the Sulfided and Carbonized Catalysts
2.2.1. Characterization of the Phase Structure
2.2.2. Redox Properties of K-Mo Catalysts
2.2.3. Temperature-Programmed Desorption of Reactants (CO/H2/H2S-TPD)
2.2.4. Catalytic Performance of Sulfided and Carbonized Catalysts
2.3. Discussion
3. Experimental Section
3.1. Catalyst Preparation
3.1.1. Preparation of K-MoO3/Al2O3 and K-MoO3/SiO2
3.1.2. Preparation of K-MoS2/Al2O3 and K-MoS2/SiO2
3.1.3. Preparation of K-Mo2C/Al2O3
3.2. Catalyst Characterization
3.3. Catalytic Performance Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Liu, P.; Xu, Z.; He, S.; Luo, Y. Investigation of the reaction pathway for synthesizing methyl mercaptan (CH3SH) from H2S-containing syngas over K-Mo-type materials. RSC Adv. 2018, 8, 21340–21353. [Google Scholar] [CrossRef]
- Lu, J.C.; Luo, Y.M.; He, D.D.; Xu, Z.Z.; He, S.F.; Xie, D.L.; Mei, Y. An exploration into potassium (K) containing MoS2 active phases and its transformation process over MoS2 based materials for producing methanethiol. Catal. Today 2020, 339, 93–104. [Google Scholar] [CrossRef]
- Pérez, H.A.; López, C.A.; Cadús, L.E.; Agüero, F.N. Catalytic feasibility of Ce-doped LaCoO3 systems for chlorobenzene oxidation: An analysis of synthesis method. J. Rare Earths 2022, 40, 897–905. [Google Scholar] [CrossRef]
- Clark, P.D.; Dowling, N.I.; Huang, M. Conversion of CS2 and COS over alumina and titania under Claus process conditions: Reaction with H2O and SO2. Appl. Catal. B Environ. 2001, 31, 107–112. [Google Scholar] [CrossRef]
- Yao, X.-Q.; Li, Y.-W. Density functional theory study on the hydrodesulfurization reactions of COS and CS2 with Mo3S9 model catalyst. J. Mol. Struct. 2009, 899, 32–41. [Google Scholar] [CrossRef]
- Park, N.-K.; Han, D.C.; Lee, T.J.; Ryu, S.O. A study on the reactivity of Ce-based Claus catalysts and the mechanism of its catalysis for removal of H2S contained in coal gas. Fuel 2011, 90, 288–293. [Google Scholar] [CrossRef]
- Yao, S.Y.; Mudiyanselage, K.; Xu, W.Q.; Johnston-Peck, A.C.; Hanson, J.C.; Wu, T.P.; Stacchiola, D.; Rodriguez, J.A.; Zhao, H.Y.; Beyer, K.A.; et al. Unraveling the Dynamic Nature of a CuO/CeO2 Catalyst for CO Oxidation in Operando: A Combined Study of XANES (Fluorescence) and DRIFTS. ACS Catal. 2014, 4, 1650–1661. [Google Scholar] [CrossRef]
- Xie, Y.; Wu, J.F.; Jing, G.J.; Zhang, H.; Zeng, S.H.; Tan, X.P.; Zou, X.Y.; Wen, J.; Su, H.Q.; Zhong, C.J.; et al. Structural origin of high catalytic activity for preferential CO oxidation over CuO/CeO2 nanocatalysts with different shapes. Appl. Catal. B Environ. 2018, 239, 665–676. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Lercher, J.A. Influence of Potassium on the Synthesis of Methanethiol from Carbonyl Sulfide on Sulfided Mo/Al2O3 Catalyst. ChemCatChem 2011, 3, 1480–1490. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Kaufmann, C.; Hrabar, A.; Zhu, Y.; Lercher, J.A. Synthesis of methyl mercaptan from carbonyl sulfide over sulfide K2MoO4/SiO2. J. Catal. 2011, 280, 264–273. [Google Scholar] [CrossRef]
- Lamonier, C.; Lamonier, J.-F.; Aellach, B.; Ezzamarty, A.; Leglise, J. Specific tuning of acid/base sites in apatite materials to enhance their methanol thiolation catalytic performances. Catal. Today 2011, 164, 124–130. [Google Scholar] [CrossRef]
- Pashigreva, A.V.; Kondratieva, E.; Bermejo-Deval, R.; Gutiérrez, O.Y.; Lercher, J.A. Methanol thiolation over Al2O3 and WS2 catalysts modified with cesium. J. Catal. 2017, 345, 308–318. [Google Scholar] [CrossRef]
- Lu, J.; Li, X.; He, S.; Han, C.; Wan, G.; Lei, Y.; Chen, R.; Liu, P.; Chen, K.; Zhang, L.; et al. Hydrogen production via methanol steam reforming over Ni-based catalysts: Influences of Lanthanum (La) addition and supports. Int. J. Hydrog. Energy 2017, 42, 3647–3657. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Klimova, T. Effect of the support on the high activity of the (Ni)Mo/ZrO2–SBA-15 catalyst in the simultaneous hydrodesulfurization of DBT and 4,6-DMDBT. J. Catal. 2011, 281, 50–62. [Google Scholar] [CrossRef]
- Enyashin, A.N.; Yadgarov, L.; Houben, L.; Popov, I.; Weidenbach, M.; Tenne, R.; Bar-Sadan, M.; Seifert, G. New Route for Stabilization of 1T-WS2 and MoS2 Phases. J. Phys. Chem. C 2011, 115, 24586–24591. [Google Scholar] [CrossRef]
- Andersen, A.; Kathmann, S.M.; Lilga, M.A.; Albrecht, K.O.; Hallen, R.T.; Mei, D. First-Principles Characterization of Potassium Intercalation in Hexagonal 2H-MoS2. J. Phys. Chem. C 2012, 116, 1826–1832. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Zhong, L.; Zhu, Y.; Lercher, J.A. Synthesis of Methanethiol from CS2 on Ni-, Co-, and K-Doped MoS2/SiO2Catalysts. ChemCatChem 2013, 5, 3249–3259. [Google Scholar] [CrossRef]
- Lu, J.C.; Fang, J.; Xu, Z.Z.; He, D.D.; Feng, S.Y.; Li, Y.B.; Wan, G.P.; He, S.F.; Wu, H.; Luo, Y.M. Facile synthesis of few-layer and ordered K-promoted MoS2 nanosheets supported on SBA-15 and their potential application for heterogeneous catalysis. J. Catal. 2020, 385, 107–119. [Google Scholar] [CrossRef]
- Liu, P.; Lu, J.; Xu, Z.; Liu, F.; Chen, D.; Yu, J.; Liu, J.; He, S.; Wan, G.; Luo, Y. The effect of alkali metals on the synthesis of methanethiol from CO/H2/H2S mixtures on the SBA-15 supported Mo-based catalysts. Mol. Catal. 2017, 442, 39–48. [Google Scholar] [CrossRef]
- Fang, J.; Lu, J.C.; Xu, Z.Z.; Feng, S.Y.; Li, Y.B.; He, B.H.; Luo, M.; Wang, H.; Luo, Y.M. Modulation the metal-support interactions of potassium molybdenum-based catalysts for tuned catalytic performance of synthesizing CH3SH. Sep. Purif. Technol. 2023, 316, 123815. [Google Scholar] [CrossRef]
- El-Bahy, Z.M.; Alotaibi, M.T.; El-Bahy, S.M. CO oxidation and 4-nitrophenol reduction over ceria-promoted platinum nanoparticles impregnated with ZSM-5 zeolite. J. Rare Earths 2022, 40, 1247–1254. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Z.; Fang, T.; Gu, H.; Guo, Y.; Zhan, W.; Guo, Y.; Wang, L. Understanding the role of tungsten on Pt/CeO2 for vinyl chloride catalytic combustion. J. Rare Earths 2022, 40, 1462–1470. [Google Scholar] [CrossRef]
- Marquart, W.; Raseale, S.; Prieto, G.; Zimina, A.; Sarma, B.B.; Grunwaldt, J.D.; Claeys, M.; Fischer, N. CO2 Reduction over Mo2C-Based Catalysts. ACS Catal. 2021, 11, 1624–1639. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Z.; Kountoupi, E.; Tsoukalou, A.; Abdala, P.M.; Florian, P.; Fedorov, A.; Müller, C.R. Two-dimensional molybdenum carbide 2D-Mo2C as a superior catalyst for CO2 hydrogenation. Nat. Commun. 2021, 12, 5510. [Google Scholar] [CrossRef]
- Xiong, K.; Zhou, G.; Zhang, H.; Shen, Y.; Zhang, X.; Zhang, Y.; Li, J. Bridging Mo2C-C and highly dispersed copper by incorporating N-functional groups to greatly enhance the catalytic activity and durability for carbon dioxide hydrogenation. J. Mater. Chem. A 2018, 6, 15510–15516. [Google Scholar] [CrossRef]
- Calais, J.-L. Band structure of transition metal compounds. Adv. Phys. 1977, 26, 847–885. [Google Scholar] [CrossRef]
- Duan, D.; Hao, C.; He, G.; Wen, Y.; Sun, Z. Rh/CeO2 composites prepared by combining dealloying with calcination as an efficient catalyst for CO oxidation and CH4 combustion. J. Rare Earths 2022, 40, 636–644. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Z.; Zhao, N.; Teng, Y. One-pot synthesis of Cu–Ce co-doped SAPO-5/34 hybrid crystal structure catalysts for NH3-SCR reaction with SO2 resistance. J. Rare Earths 2021, 39, 1217–1223. [Google Scholar] [CrossRef]
- Liu, W.; Cao, H.; Wang, Z.; Cui, C.; Gan, L.; Liu, W.; Wang, L. A novel ceria hollow nanosphere catalyst for low temperature NOx storage. J. Rare Earths 2022, 40, 626–635. [Google Scholar] [CrossRef]
- Carrier, X.; Lambert, J.F.; Che, M. Ligand-Promoted Alumina Dissolution in the Preparation of MoOX/γ-Al2O3 Catalysts: Evidence for the Formation and Deposition of an Anderson-type Alumino Heteropolymolybdate. J. Am. Chem. Soc 1997, 119, 10137–10146. [Google Scholar] [CrossRef]
- Pérez-Martínez, D.J.; Eloy, P.; Gaigneaux, E.M.; Giraldo, S.A.; Centeno, A. Study of the selectivity in FCC naphtha hydrotreating by modifying the acid–base balance of CoMo/γ-Al2O3 catalysts. Appl. Catal. A Gen. 2010, 390, 59–70. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, Y.; Chen, A.; Fang, W.; Yang, Y. Study on Methanethiol Synthesis from H2S-Rich Syngas Over K2MoO4 Catalyst Supported on Electrolessly Ni-Plated SiO2. Catal Lett. 2009, 129, 486–492. [Google Scholar] [CrossRef]
- Hussain, S.; Zaidi, S.A.; Vikraman, D.; Kim, H.-S.; Jung, J. Facile preparation of molybdenum carbide (Mo2C) nanoparticles and its effective utilization in electrochemical sensing of folic acid via imprinting. Biosens. Bioelectron. 2019, 140, 111330. [Google Scholar] [CrossRef]
- Pinilla, J.L.; Purón, H.; Torres, D.; de Llobet, S.; Moliner, R.; Suelves, I.; Millan, M. Carbon nanofibres coated with Ni decorated MoS2 nanosheets as catalyst for vacuum residue hydroprocessing. Appl. Catal. B Environ. 2014, 148–149, 357–365. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, X.; Shi, Z.; Zhou, R. Transition metal doping effect and high catalytic activity of CeO2–TiO2 for chlorinated VOCs degradation. J. Rare Earths 2022, 40, 745–752. [Google Scholar] [CrossRef]
- Chang, S.; Jia, Y.; Zeng, Y.; Qian, F.; Guo, L.; Wu, S.; Lu, J.; Han, Y. Effect of interaction between different CeO2 plane and platinum nanoparticles on catalytic activity of Pt/CeO2 in toluene oxidation. J. Rare Earths 2022, 40, 1743–1750. [Google Scholar] [CrossRef]
- Pang, M.; Wang, X.; Xia, W.; Muhler, M.; Liang, C. Mo(VI)–Melamine Hybrid As Single-Source Precursor to Pure-Phase β-Mo2C for the Selective Hydrogenation of Naphthalene to Tetralin. Ind. Eng. Chem. Res. 2013, 52, 4564–4571. [Google Scholar] [CrossRef]
- Malaibari, Z.O.; Croiset, E.; Amin, A.; Epling, W. Effect of interactions between Ni and Mo on catalytic properties of a bimetallic Ni-Mo/Al2O3 propane reforming catalyst. Appl. Catal. A Gen. 2015, 490, 80–92. [Google Scholar] [CrossRef]
- Wang, G.; Schaidle, J.A.; Katz, M.B.; Li, Y.; Pan, X.; Thompson, L.T. Alumina supported Pt-Mo2C catalysts for the water–gas shift reaction. J. Catal. 2013, 304, 92–99. [Google Scholar] [CrossRef]
- Chen, A.; Wang, Q.; Li, Q.; Hao, Y.; Fang, W.; Yang, Y. Direct synthesis of methanethiol from H2S-rich syngas over sulfided Mo-based catalysts. J. Mol. Catal. A Chem. 2008, 283, 69–76. [Google Scholar] [CrossRef]
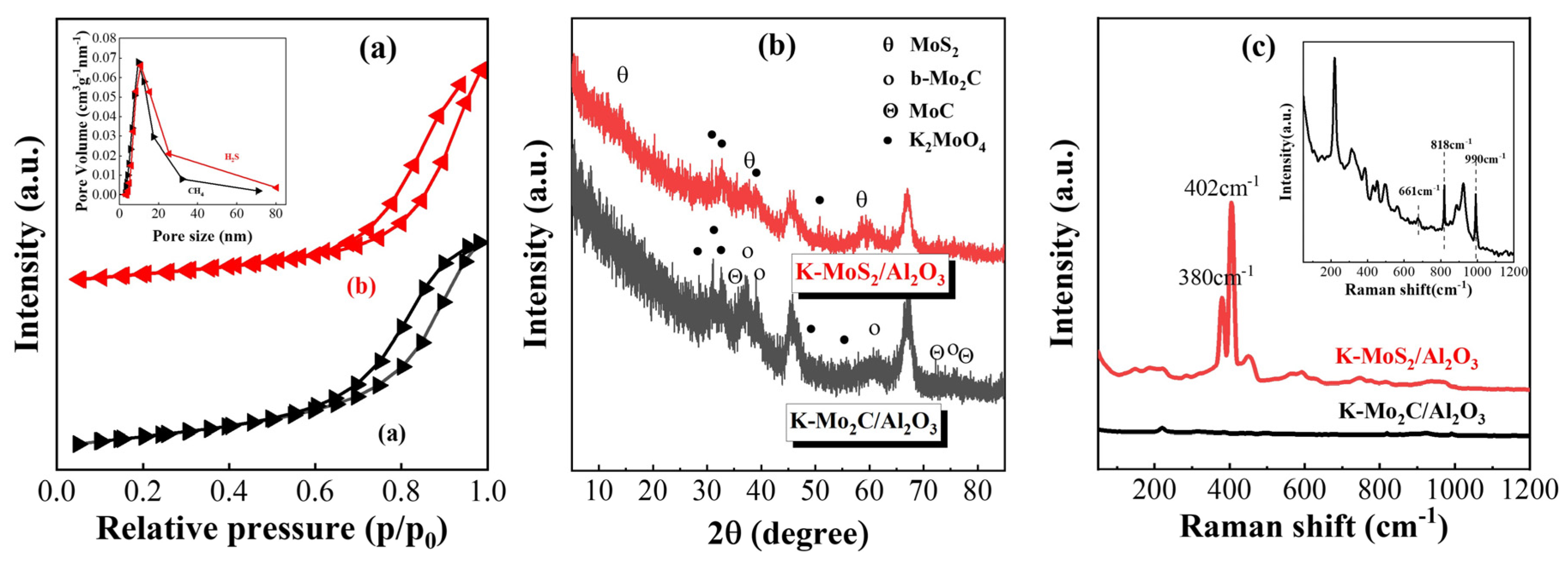



| Sample | Surface Area (m2/g) | Pore Volume (cc/g) | Pore Diameter Dv (d) (nm) |
|---|---|---|---|
| K-Mo2C/Al2O3 | 103.9 | 0.321 | 9.618 |
| K-MoS2/Al2O3 | 82.2 | 0.326 | 8.615 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhang, W.; Zheng, D.; Li, Y.; Fang, J.; Luo, M.; Lu, J.; Luo, Y. The Influence of Sulfurization and Carbonization on Mo-Based Catalysts for CH3SH Synthesis. Catalysts 2024, 14, 190. https://doi.org/10.3390/catal14030190
Wang H, Zhang W, Zheng D, Li Y, Fang J, Luo M, Lu J, Luo Y. The Influence of Sulfurization and Carbonization on Mo-Based Catalysts for CH3SH Synthesis. Catalysts. 2024; 14(3):190. https://doi.org/10.3390/catal14030190
Chicago/Turabian StyleWang, Hao, Wenjun Zhang, Dalong Zheng, Yubei Li, Jian Fang, Min Luo, Jichang Lu, and Yongming Luo. 2024. "The Influence of Sulfurization and Carbonization on Mo-Based Catalysts for CH3SH Synthesis" Catalysts 14, no. 3: 190. https://doi.org/10.3390/catal14030190
APA StyleWang, H., Zhang, W., Zheng, D., Li, Y., Fang, J., Luo, M., Lu, J., & Luo, Y. (2024). The Influence of Sulfurization and Carbonization on Mo-Based Catalysts for CH3SH Synthesis. Catalysts, 14(3), 190. https://doi.org/10.3390/catal14030190