CuSnBi Catalyst Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate
Abstract
1. Introduction
2. Results and Discussion
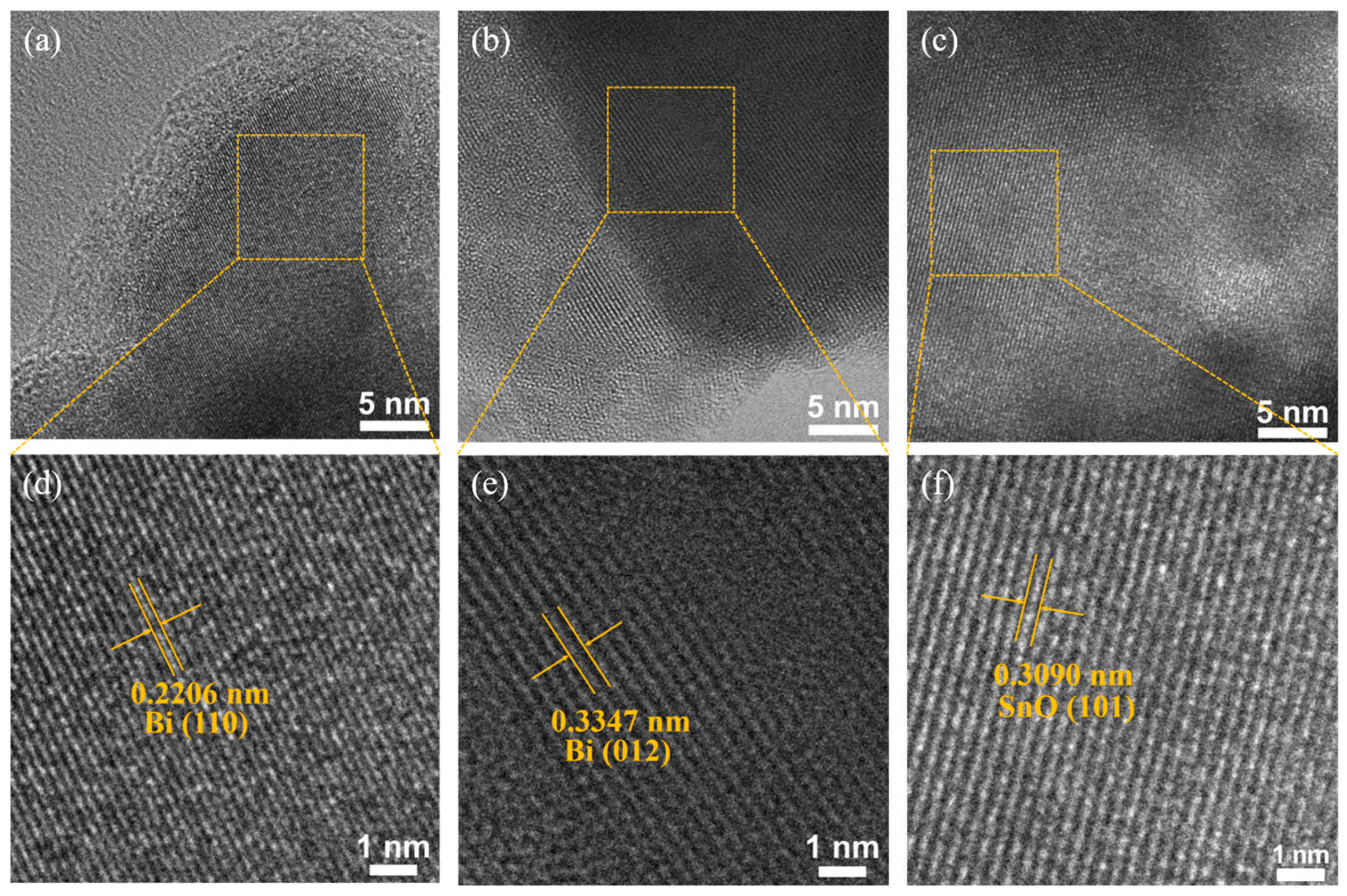
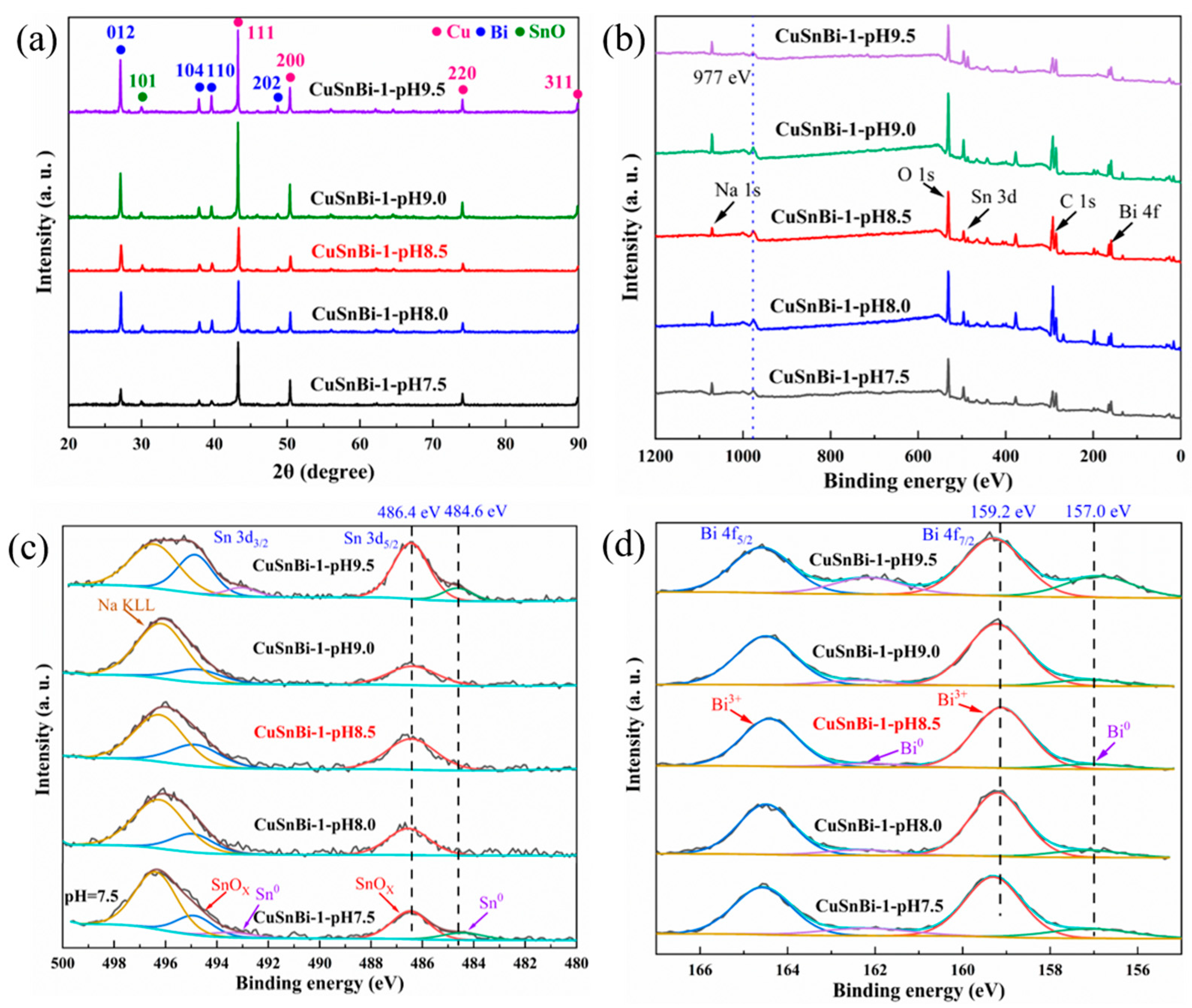
2.1. Morphological and Structural Characterization
2.2. Electrochemical Performance
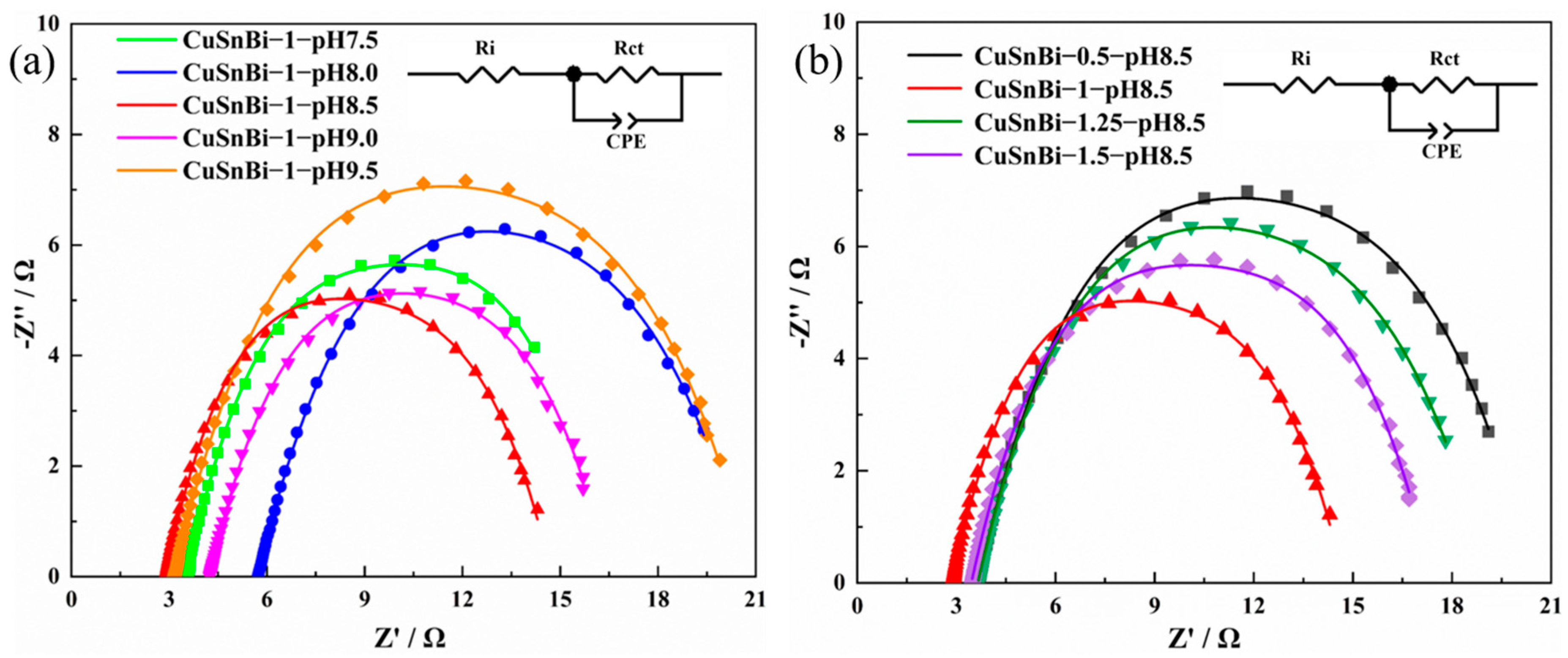
2.2.1. Effect of pH of the Electrodeposition Solution on the Catalyst Activity
2.2.2. Effects of the Cu2+:Sn2+:Bi3+ Molar Ratio in the Electrodeposition Solutions on the Catalyst Activity
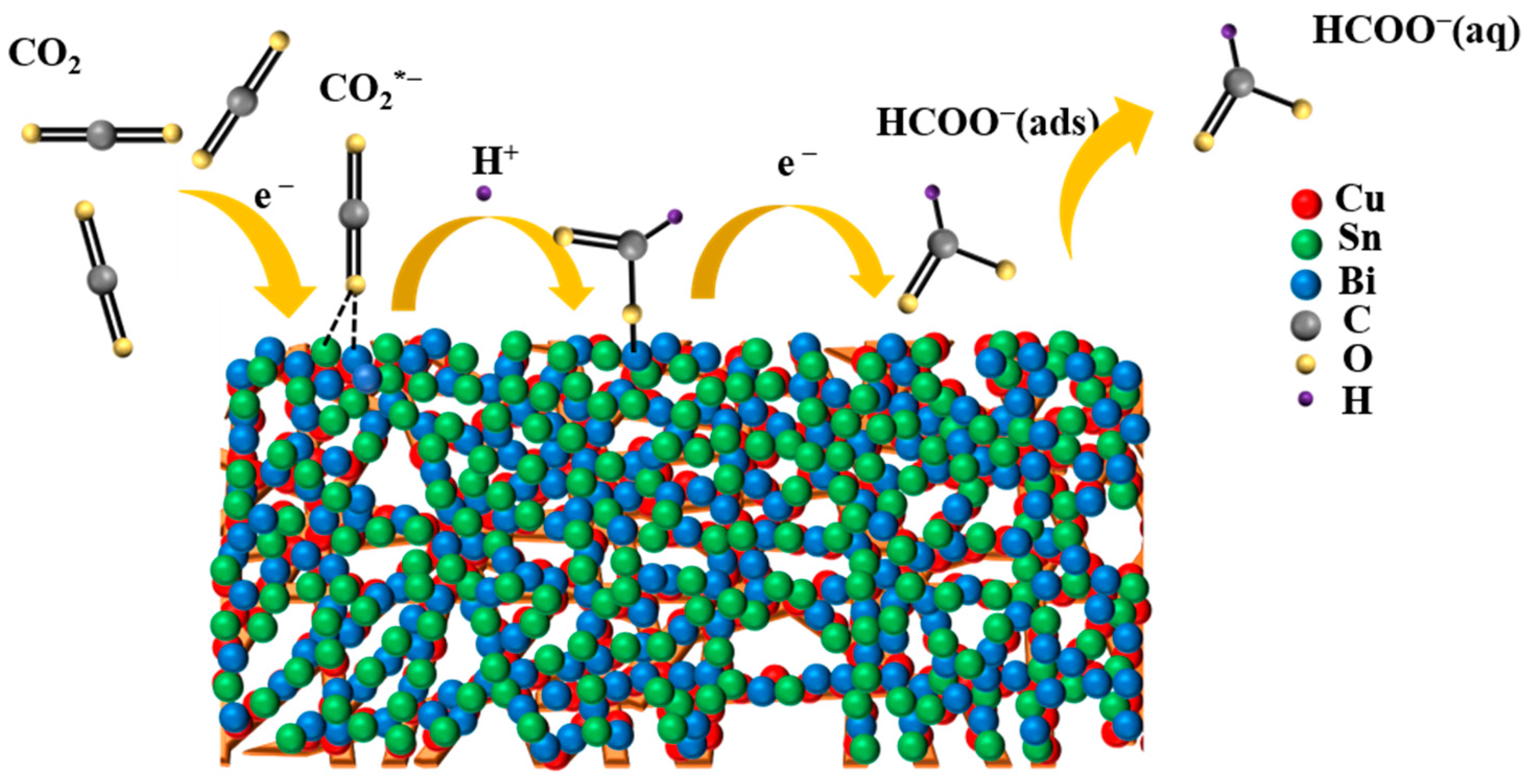
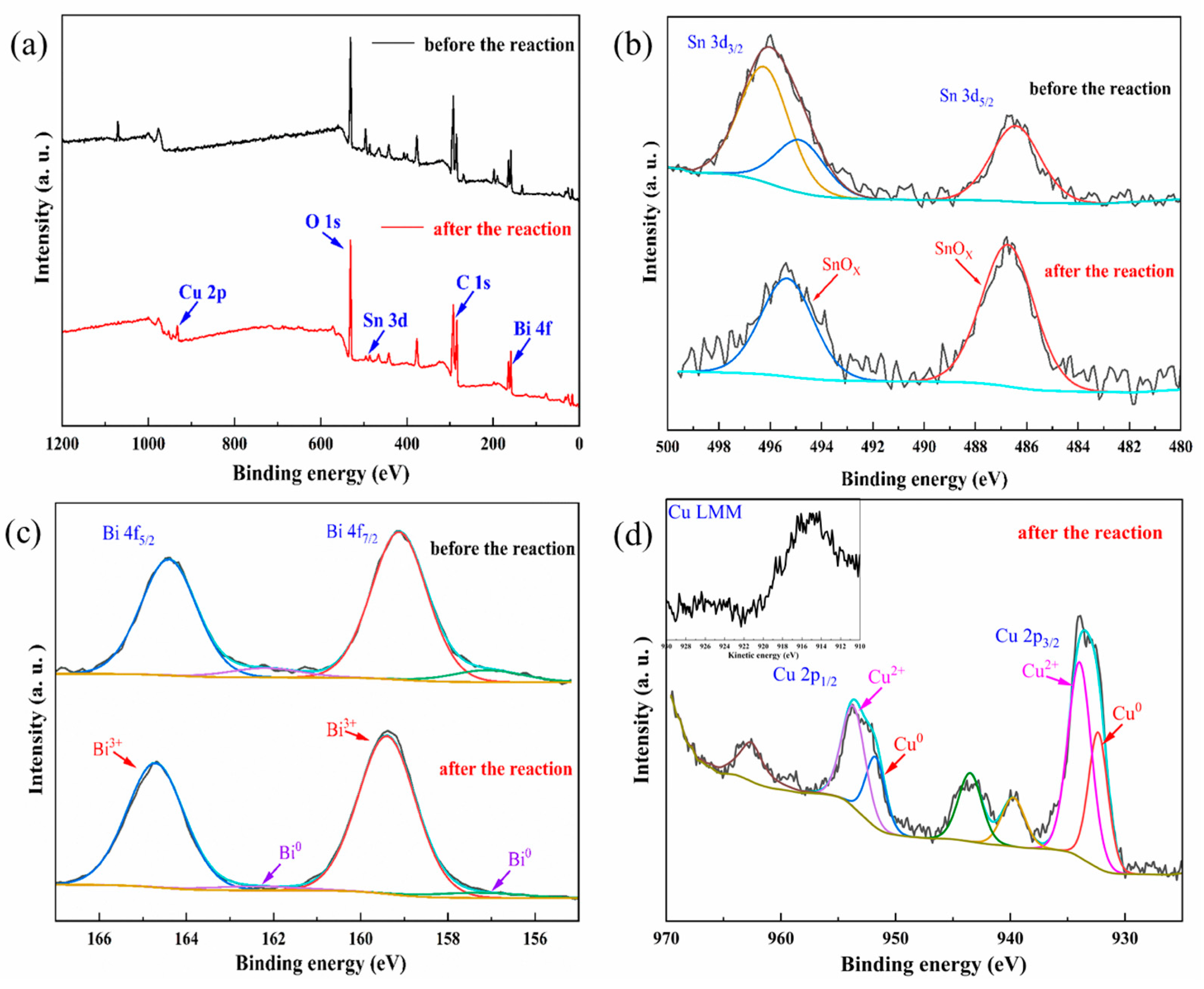
2.3. Mechanism of CO2 Electroreduction on the CuSnBi Electrodes
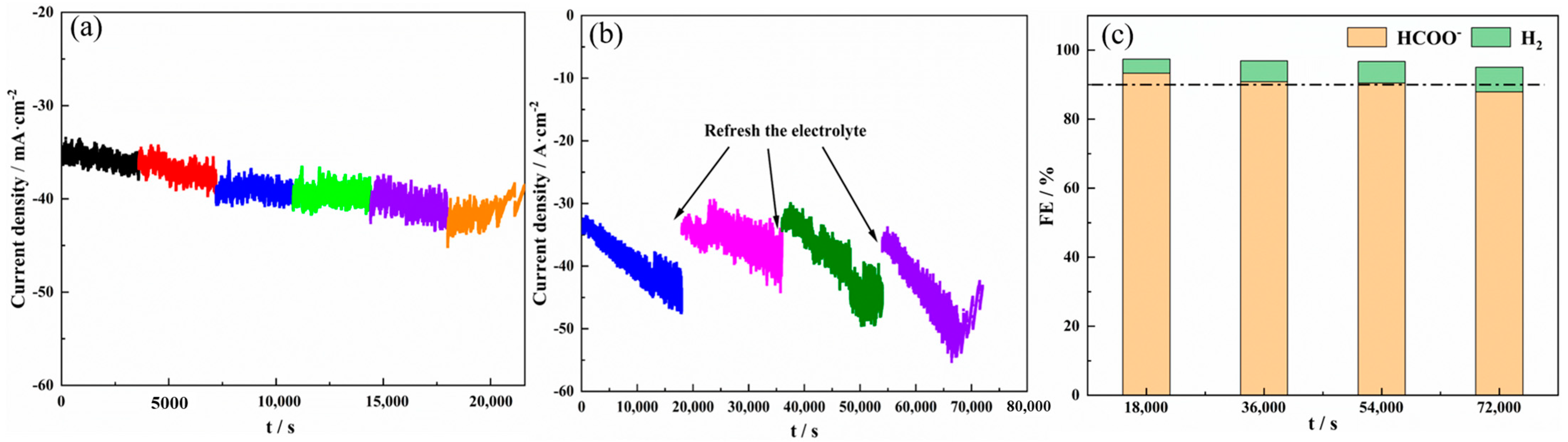
2.4. Electrode Stability in CO2 Electroreduction
3. Materials and Methods
3.1. Materials
3.2. Preparation of the Pretreated Copper Foam Substrate
3.3. Fabrication of the CuSnBi Trimetallic Electrode
3.4. Material Characterization
3.5. Electrochemical Measurements
3.6. Product Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gulzar, A.; Gulzar, A. Carbon Dioxide Utilization: A Paradigm Shift with CO2 Economy. Chem. Eng. J. Adv. 2020, 3, 100013. [Google Scholar] [CrossRef]
- Bulushev, D.A.; Ross, J.R. Towards Sustainable Production of Formic Acid. ChemSusChem 2018, 11, 821–836. [Google Scholar] [CrossRef] [PubMed]
- Duarah, P.; Haldar, D. Progress in the Electrochemical Reduction of CO2 to Formic Acid: A Review on Current Trends and Future Prospects. J. Environ. Chem. Eng. 2021, 9, 106394. [Google Scholar] [CrossRef]
- Ma, Z.; Legrand, U. From CO2 to Formic Acid Fuel Cells. Ind. Eng. Chem. Res. 2021, 60, 803–815. [Google Scholar] [CrossRef]
- Onishi, N.; Iguchi, M. Development of Effective Catalysts for Hydrogen Storage Technology Using Formic Acid. Adv. Energy Mater. 2019, 9, 1801275. [Google Scholar] [CrossRef]
- Jouny, M.; Luc, W. General Techno-Economic Analysis of CO2 Electrolysis Systems. Ind. Eng. Chem. Res. 2018, 57, 2165–2177. [Google Scholar] [CrossRef]
- Kim, S.; Shin, H. Electrochemical Reduction of Captured CO2: A Route toward the Integrated Carbon Capture and Utilization. Curr. Opin. Electrochem. 2023, 40, 101321. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, W. Advances in Electrochemical Reduction of Carbon Dioxide to Formate over Bismuth-Based Catalysts. Rare Met. 2021, 40, 2327–2353. [Google Scholar] [CrossRef]
- Liang, S.; Altaf, N. Electrolytic Cell Design for Electrochemical CO2 Reduction. J. CO2 Util. 2020, 35, 90–105. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, F. Recent Advances in Designing Efficient Electrocatalysts for Electrochemical Carbon Dioxide Reduction to Formic Acid/Formate. J. Electroanal. Chem. 2023, 928, 117018. [Google Scholar] [CrossRef]
- Gawel, A.; Jaster, T. Electrochemical CO2 Reduction—The Macroscopic World of Electrode Design, Reactor Concepts & Economic Aspects. iScience 2022, 25, 104011. [Google Scholar]
- Proietto, F.; Patel, U. Electrochemical Conversion of CO2 to Formic Acid Using a Sn Based Electrode: A Critical Review on the State-of-the-Art Technologies and Their Potential. Electrochim. Acta 2021, 389, 138753. [Google Scholar] [CrossRef]
- Yang, Z.; Oropeza, F. P-Block Metal-Based (Sn, In, Bi, Pb) Electrocatalysts for Selective Reduction of CO2 to Formate. APL Mater. 2020, 8, 060901. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S. Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction. Nano-Micro Lett. 2019, 11, 62. [Google Scholar] [CrossRef]
- Li, P.; Yang, F. Nanoscale Engineering of P-Block Metal-Based Catalysts Toward Industrial-Scale Electrochemical Reduction of CO2. Adv. Energy Mater. 2023, 13, 2301597. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y. Electrocatalytic CO2 Reduction over Bimetallic Bi-Based Catalysts: A Review. CCS Chem. 2023, 5, 544–567. [Google Scholar] [CrossRef]
- Deng, P.; Wang, H. Bismuth Oxides with Enhanced Bismuth–Oxygen Structure for Efficient Electrochemical Reduction of Carbon Dioxide to Formate. ACS Catal. 2020, 10, 743–750. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X. Bi-Based Metal-Organic Framework Derived Leafy Bismuth Nanosheets for Carbon Dioxide Electroreduction. Adv. Energy Mater. 2020, 10, 2001709. [Google Scholar] [CrossRef]
- Van Daele, K.; De Mot, B.; Kortlever, R.; Breugelmans, T. Sn-Based Electrocatalyst Stability: A Crucial Piece to the Puzzle for the Electrochemical CO2 Reduction toward Formic Acid. ACS Energy Lett. 2021, 6, 4317–4327. [Google Scholar] [CrossRef]
- Wu, J.; Bai, X. Multivalent Sn Species Synergistically Favours the CO2-into-HCOOH Conversion. Nano Res. 2021, 14, 1053–1060. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y. Separated Growth of Bi-Cu Bimetallic Electrocatalysts on Defective Copper Foam for Highly Converting CO2 to Formate with Alkaline Anion-Exchange Membrane beyond KHCO3 Electrolyte. Appl. Catal. B Environ. 2021, 288, 120003. [Google Scholar] [CrossRef]
- Lou, W.; Peng, L. CuBi Electrocatalysts Modulated to Grow on Derived Copper Foam for Efficient CO2-to-Formate Conversion. J. Colloid Interface Sci. 2022, 606, 994–1003. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zou, J. Heterostructured Intermetallic CuSn Catalysts: High Performance towards the Electrochemical Reduction of CO2 to Formate. J. Mater. Chem. A 2019, 7, 27514–27521. [Google Scholar] [CrossRef]
- Wang, J.; Ji, Y. Phase and Structure Modulating of Bimetallic CuSn Nanowires Boosts Electrocatalytic Conversion of CO2. Nano Energy 2019, 59, 138–145. [Google Scholar] [CrossRef]
- Yang, S.; Sun, Y. One-Step Co-Electrodeposition of SnBi for Efficient Electrochemical Reduction of Carbon Dioxide to Formic Acid. Catal. Sci. Technol. 2023, 13, 758–766. [Google Scholar] [CrossRef]
- Zhu, C.; Wang, Q. Rapid and Scalable Synthesis of Bismuth Dendrites on Copper Mesh as a High-Performance Cathode for Electroreduction of CO2 to Formate. J. CO2 Util. 2020, 36, 96–104. [Google Scholar] [CrossRef]
- Zheng, W.; Liu, M. Best Practices in Using Foam-Type Electrodes for Electrocatalytic Performance Benchmark. ACS Energy Lett. 2020, 5, 3260–3264. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Li, X.; Duan, G. Regulating Electrochemical CO2RR Selectivity at Industrial Current Densities by Structuring Copper@poly(Ionic Liquid) Interface. Appl. Catal. B Environ. 2021, 297, 120471. [Google Scholar] [CrossRef]
- Cai, R.; Sun, M.; Huang, B.; Yang, S. Engineering Cu(I)/Cu(0) Interfaces for Efficient Ethanol Production from CO2 Electroreduction. Chem 2024, 10, 211–233. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, Q.; Wang, M. Interfacial Engineering of Bismuth with Reduced Graphene Oxide Hybrid for Improving CO2 Electroreduction Performance. Electrochim. Acta 2020, 357, 136840. [Google Scholar] [CrossRef]
- Chen, P.; Liu, H. Bi Metal Prevents the Deactivation of Oxygen Vacancies in Bi2O2CO3 for Stable and Efficient Photocatalytic NO Abatement. Appl. Catal. B Environ. 2020, 264, 118545. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, J. In Situ Growth of Bi/Ag Double Quantum Dots on Hollow Bi2MoO6 Microspheres: Enhancement of the Surface Plasmon Resonance Effect on PMS Activation. Appl. Catal. B Environ. 2023, 338, 123041. [Google Scholar] [CrossRef]
- Peng, C.; Yang, S. Ampere-Level CO2-to-Formate Electrosynthesis Using Highly Exposed Bismuth(110) Facets Modified with Sulfur-Anchored Sodium Cations. Chem 2023, 9, 2830–2840. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, T. Facet Engineering to Regulate Surface States of Topological Crystalline Insulator Bismuth Rhombic Dodecahedrons for Highly Energy Efficient Electrochemical CO2 Reduction. Adv. Mater. 2021, 33, 2008373. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, R. Mass-Transfer-Enhanced Hydrophobic Bi Microsheets for Highly Efficient Electroreduction of CO2 to Pure Formate in a Wide Potential Window. Appl. Catal. B Environ. 2023, 322, 122127. [Google Scholar] [CrossRef]
- Chen, Z.; Löber, M. Increased Photocurrent of CuWO4 Photoanodes by Modification with the Oxide Carbodiimide Sn2O(NCN). Dalton Trans. 2020, 49, 3450–3456. [Google Scholar] [CrossRef]
- Dong, W.; Li, R. Long-Life and High Volumetric Capacity Anode with Interpenetrating Bi–O and Sn–O Networks. Cell Rep. Phys. Sci. 2022, 3, 101109. [Google Scholar] [CrossRef]
- Wen, G.; Lee, D. Orbital Interactions in Bi-Sn Bimetallic Electrocatalysts for Highly Selective Electrochemical CO2 Reduction toward Formate Production. Adv. Energy Mater. 2018, 8, 1802427. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y. Superscalar Phase Boundaries Derived Multiple Active Sites in SnO2/Cu6Sn5/CuO for Tandem Electroreduction of CO2 to Formic Acid. Adv. Energy Mater. 2023, 13, 2203506. [Google Scholar] [CrossRef]
- Liu, S.; Fan, Y. Surface-Oxygen-Rich Bi@C Nanoparticles for High-Efficiency Electroreduction of CO2 to Formate. Nano Lett. 2022, 22, 9107–9114. [Google Scholar] [CrossRef]
- Wang, W.; Ruan, G. Simultaneous Facilitation of CO2 Adsorption and Proton Feeding in Bi/Bi2O3 Heterostructure Nanosheets for Enhanced Electroreduction of CO2 to Formate in a Wide Potential Window. New J. Chem. 2023, 47, 8894–8905. [Google Scholar] [CrossRef]
- Wang, X.; Wang, W. Carbon Sustained SnO2-Bi2O3 Hollow Nanofibers as Janus Catalyst for High-Efficiency CO2 Electroreduction. Chem. Eng. J. 2021, 426, 131867. [Google Scholar] [CrossRef]
- Tian, Y.; Li, D. Electroreduction of CO2 to Formate with Excellent Selectivity and Stability on Nano-Dendrite Bi Film Electrode. J. CO2 Util. 2021, 43, 101360. [Google Scholar] [CrossRef]
- Wei, C.; Sun, S. Approaches for Measuring the Surface Areas of Metal Oxide Electrocatalysts for Determining Their Intrinsic Electrocatalytic Activity. Chem. Soc. Rev. 2019, 48, 2518–2534. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, L. Effects of the Catalyst Dynamic Changes and Influence of the Reaction Environment on the Performance of Electrochemical CO2 Reduction. Adv. Mater. 2022, 34, 2103900. [Google Scholar] [CrossRef] [PubMed]
- Kumawat, S.; Sarkar, A. Electrochemical reduction of CO2 on Pb–Bi–Sn metal mixtures: Importance of eutectics. SN Appl. Sci. 2019, 1, 317. [Google Scholar] [CrossRef]
- Hosseini, P.; Gyenge, C. The carbon dioxide redox flow battery: Bifunctional CO2 reduction/formate oxidation electrocatalysis on binary and ternary catalysts. J. Power Sources 2021, 495, 229752. [Google Scholar] [CrossRef]
- Ávila, B.; Montiel, V. Electrochemical Reduction of CO2 to Formate on Nanoparticulated Bi−Sn−Sb Electrodes. ChemElectroChem 2022, 9, e202200272. [Google Scholar] [CrossRef]
- Hussain, N.; Abdelkareem, A. Novel ternary CuO–ZnO–MoS2 composite material for electrochemical CO2 reduction to alcohols. J. Power Sources 2022, 549, 232128. [Google Scholar] [CrossRef]
- He, J.; Dettelbach, E. Brass and Bronze as Effective CO2 Reduction Electrocatalysts. Angew. Chem. Int. Ed. 2017, 56, 16579–16582. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Liu, T. On ZnAlCe-THs Nanocomposites Electrocatalysts for Electrocatalytic Carbon Dioxide Reduction to Carbon Monoxide. Catal. Lett. 2024, 154, 11–22. [Google Scholar] [CrossRef]
- Guzmán, H.; Roldán, D. CuZnAl-Oxide Nanopyramidal Mesoporous Materials for the Electrocatalytic CO2 Reduction to Syngas: Tuning of H2/CO Ratio. Nanomaterials 2021, 11, 3052. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, H.; Lv, L.; Sun, Y.; Wang, C.; Xu, J.; Tang, M. CuSnBi Catalyst Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate. Catalysts 2024, 14, 191. https://doi.org/10.3390/catal14030191
Xie H, Lv L, Sun Y, Wang C, Xu J, Tang M. CuSnBi Catalyst Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate. Catalysts. 2024; 14(3):191. https://doi.org/10.3390/catal14030191
Chicago/Turabian StyleXie, Hangxin, Li Lv, Yuan Sun, Chunlai Wang, Jialin Xu, and Min Tang. 2024. "CuSnBi Catalyst Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate" Catalysts 14, no. 3: 191. https://doi.org/10.3390/catal14030191
APA StyleXie, H., Lv, L., Sun, Y., Wang, C., Xu, J., & Tang, M. (2024). CuSnBi Catalyst Grown on Copper Foam by Co-Electrodeposition for Efficient Electrochemical Reduction of CO2 to Formate. Catalysts, 14(3), 191. https://doi.org/10.3390/catal14030191





