Quinoline Hydroxyalkylations from Iron-Catalyzed, Visible-Light-Driven Decarboxylations
Abstract
1. Introduction
2. Results and Discussion
3. Experimental Method
- General procedure of synthesis of 4-hydroxyalkyl quinolines
- 1-Quinolin-4-yl-ethanol (2)
- 3-Quinolin-4-yl-heptan-3-ol (3)
- 1-Quinolin-4-yl-nonan-1-ol (4)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stephenson, C.R.J.; Yoon, T.P.; MacMillan, D.W.C. Visible Light Photocatalysis in Organic Chemistry; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Reischauer, S.; Pieber, B. Emerging concepts in photocatalytic organic synthesis. iScience 2021, 24, 102209. [Google Scholar] [CrossRef] [PubMed]
- Marzo, L.; Pagire, S.K.; Reiser, O.; König, B. Visible-Light Photocatalysis: Does It Make a Difference in Organic Synthesis? Angew. Chem. Int. Ed. 2018, 57, 10034–10072. [Google Scholar] [CrossRef]
- König, B. Photocatalysis in Organic Synthesis—Past, Present, and Future. Eur. J. Org. Chem. 2017, 2017, 1979–1981. [Google Scholar] [CrossRef]
- Friedmann, D.; Hakki, A.; Kim, H.; Choi, W.; Bahnemann, D. Heterogeneous photocatalytic organic synthesis: State-of-the-art and future perspectives. Green Chem. 2016, 18, 5391–5411. [Google Scholar] [CrossRef]
- Laursen, S.; Poudyal, S. Photo- and Electro-Catalysis: CO2 Mitigation Technologies. In Novel Materials for Carbon Dioxide Mitigation Technology; Shi, F., Morreale, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 233–268. [Google Scholar]
- Abderrazak, Y.; Bhattacharyya, A.; Reiser, O. Visible-Light-Induced Homolysis of Earth-Abundant Metal-Substrate Complexes: A Complementary Activation Strategy in Photoredox Catalysis. Angew. Chem. Int. Ed. 2021, 60, 21100–21115. [Google Scholar] [CrossRef] [PubMed]
- Traub, L.; Reiser, O. Homogeneous visible light mediated transition metal catalysis other than Ruthenium and Iridium. Phys. Sci. Rev. 2019, 4, 20170172. [Google Scholar] [CrossRef]
- Larsen, C.B.; Wenger, O.S. Photoredox Catalysis with Metal Complexes Made from Earth-Abundant Elements. Chem. Eur. J. 2018, 24, 2039–2058. [Google Scholar] [CrossRef]
- Yamen AlSalkaa, Y.; Granonea, L.I.; Ramadan, W.; Hakki, A.; Dillert, R.; Bahnemann, D.W. Iron-based photocatalytic and photoelectrocatalytic nano-structures: Facts, perspectives, and expectations. Appl. Catal. B Environ. 2019, 244, 1065–1095. [Google Scholar] [CrossRef]
- Jack, R.S.; Ayoko, G.A.; Adebajo, M.O.; Frost, R.L. A review of iron species for visible-light photocatalytic water purification. Environ. Sci. Pollut. Res. 2015, 22, 7439–7449. [Google Scholar] [CrossRef]
- Wang, C.J.; Li, H.X. Synthesis of iron-based metal organic framework and its visible light-driven photocatalytic degradation of dye pollutants. Appl. Organomet. Chem. 2019, 33, e4642. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Lei, J.; Zhang, Y.; Meng, C.; Duan, C.; Jin, Y. Iron-Catalyzed Photoredox Functionalization of Methane and Heavier Gaseous Alkanes: Scope, Kinetics, and Computational Studies. Org. Lett. 2022, 24, 1901–1906. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Meng, Y.; Gong, X.; Wu, J.; Zhang, Y.; Ye, L.W.; Zhu, C. Facile Synthesis of α-Haloketones by Aerobic Oxidation of Olefins Using KX as Nonhazardous Halogen Source. Chin. J. Chem. 2020, 38, 173–177. [Google Scholar] [CrossRef]
- Chen, J.; Stepanovic, S.; Draksharapu, A.; Gruden, M.; Browne, W.R. A Non-Heme Iron Photocatalyst for Light-Driven Aerobic Oxidation of Methanol. Angew. Chem. Int. Ed. 2018, 57, 3207–3211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, Q.; Tang, Y.; Cai, Y. Recent trends in metal-doped carbon nitride-catalyzed heterogeneous light-driven organic transformations. Nano Trends 2023, 4, 100019. [Google Scholar] [CrossRef]
- Ye, J.H.; Miao, M.; Huang, H.; Yan, S.S.; Yin, Z.B.; Zhou, W.J.; Yu, D.G. Visible-Light-Driven Iron-Promoted Thiocarboxylation of Styrenes and Acrylates with CO2. Angew. Chem. Int. Ed. 2017, 56, 15416–15420. [Google Scholar] [CrossRef] [PubMed]
- Dadashi-Silab, S.; Pan, X.; Matyjaszewski, K. Photoinduced Iron-Catalyzed Atom Transfer Radical Polymerization with ppm Levels of Iron Catalyst under Blue Light Irradiation. Macromolecules 2017, 50, 7967–7977. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevee, J. Iron Complexes in Visible-Light-Sensitive Photoredox Catalysis: Effect of Ligands on Their Photoinitiation Efficiencies. ChemCatChem 2016, 8, 2227–2233. [Google Scholar] [CrossRef]
- Zhang, J.; Campolo, D.; Dumur, F.; Xiao, P.; Fouassier, J.P.; Gigmes, D.; Lalevee, J. Iron Complexes as Photoinitiators for Radical and Cationic Polymerization Through Photoredox Catalysis Processes. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 42–49. [Google Scholar] [CrossRef]
- Ding, L.; Niu, K.; Liu, Y.; Wang, Q. Visible Light-Induced Hydrosilylation of Electron-Deficient Alkenes by Iron Catalysis. ChemSusChem 2022, 15, e202200367. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Xia, S.; Jin, J. Ligand-Accelerated Iron Photocatalysis Enabling Decarboxylative Alkylation of Heteroarenes. Org. Lett. 2019, 21, 4259–4265. [Google Scholar]
- Feng, G.; Wang, X.; Jian Jin, J. Decarboxylative C–C and C–N Bond Formation by Ligand-Accelerated Iron Photocatalysis. Eur. J. Org. Chem. 2019, 2019, 6728–6732. [Google Scholar] [CrossRef]
- Parisien-Collette, S.; Hernandez-Perez, A.C.; Collins, S.K. Photochemical Synthesis of Carbazoles Using an [Fe(phen)3](NTf2)2/O2 Catalyst System: Catalysis toward Sustainability. Org. Lett. 2016, 18, 4994–4997. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Hu, K.; Lei, C.; Jin, J. Intramolecular Aromatic C−H Acyloxylation Enabled by Iron Photocatalysis. Org. Lett. 2020, 22, 1385–1389. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, A.; Marchini, M.; Mengozzi, L.; Natali, M.; Lucarini, M.; Ceroni, P.; Cozzi, P.G. Organocatalytic Enantioselective Alkylation of Aldehydes with [Fe(bpy)3]Br2 Catalyst and Visible Light. ACS Catal. 2015, 5, 5927–5931. [Google Scholar] [CrossRef]
- Sugimori, A.; Yamada, T. Visible Light- and Radiation-Induced Alkylation of Pyridine Ring with Alkanoic Acid. Effective Alkylation in the Presence of Iron(III) Sulfate. Bull. Chem. Soc. Jpn. 1986, 59, 3911–3915. [Google Scholar] [CrossRef]
- Batra, A.; Singh, P.; Singh, K.N. Recent Advances in Functionalization of α-C(sp3)–H Centres in Inactivated Ethers through Cross Dehydrogenative Coupling. Eur. J. Org. Chem. 2017, 2017, 3739–3762. [Google Scholar] [CrossRef]
- Simon, P.; Lorinczi, B.; Szatmári, I. Alkoxyalkylation of Electron-Rich Aromatic Compounds. Int. J. Mol. Sci. 2024, 25, 6966. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Yan, P.; Török, B.; Olah, G.A. Superacid Catalyzed Hydroxyalkylation of Aromatics with Ethyl Trifluoro-pyruvate: A New Synthetic Route to Mosher’s Acid Analogs. Synlett 2003, 2003, 527–531. [Google Scholar] [CrossRef]
- Barthel, N.; Finiels, A.; Moreau, C.; Jacquot, R.; Spagnol, M. Hydroxyalkylation of aromatic compounds over protonic zeolites. Top. Catal. 2000, 13, 269–274. [Google Scholar] [CrossRef]
- Louvar, J.J.; Francoy, A. Hydroalkylation of Aromatic Compounds. J. Catal. 1970, 16, 62–68. [Google Scholar] [CrossRef]
- Kulkarni, P.; Padhye, S.; Sinn, E. The first well characterized Fe(phen)Cl3 complex: Structure of aquo mono(1,10-phenanthroline)iron(III) trichloride: [Fe(phen)Cl3(H2O)]. Polyhedron 1998, 17, 2623–2626. [Google Scholar] [CrossRef]
- Gandhamsetty, N.; Seewon, S.; Park, S.W.; Park, S.; Chang, S. Boron-Catalyzed Silylative Reduction of Quinolines: Selective sp3 C−Si Bond Formation. J. Am. Chem. Soc. 2014, 136, 16780–16783. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, T.; Ino, A.; Isobe, M. Synthetic Studies on Antibiotic Dynemicin A. Synthesis of Cyclic Enediyne Model Compound of Dynemicin A. Tetrahedron 1994, 50, 1449–1468. [Google Scholar] [CrossRef]
- Hu, P.; Tan, M.; Cheng, l.; Zhao, H.; Feng, R.; Gu, W.J.; Han, W. Bio-inspired iron-catalyzed oxidation of alkylarenes enables late-stage oxidation of complex methylarenes to arylaldehydes. Nat. Commun. 2019, 10, 2425. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Xiong, B.; Zhang, Z.; Zhang, J.; He, Y.; Feng, Z. Thiyl radical promoted iron-catalyzed-selective oxidation of benzylic sp3 C–H bonds with molecular oxygen. Chem. Commun. 2019, 55, 12699–12702. [Google Scholar] [CrossRef]
- Hadrys, B.W.; Phipps, R.J. Acid and Solvent Effects on the Regioselectivity of Minisci-Type Addition to Quinolines Using Amino Acid Derived Redox Active Esters. Synlett 2021, 32, 179–184. [Google Scholar]
- Sugimori, A.; Yamada, T. Visible light- and gamma ray-induced alkylation in pyridine ring. Effective alkylation with visible light in the presence of iron(III) sulfate. Chem. Lett. 1986, 15, 409–412. [Google Scholar] [CrossRef]
- Ríos-Malváez, Z.G.; Cedillo-Cruz, A.; García-Bassoco, D.; Martínez-Otero, D.; Hernández-Balderas, U.; García-Eleno, M.A.; Unnamatla, M.V.B.; Frontana-Uribe, B.A.; Cuevas-Yañez, E. [Fe(bpy)Cl3X][bpy H] complexes: Synthesis, characterization and theoretical studies towards visible-light photocatalytic hydroxyethylation of quinoline. J. Coord. Chem. 2023, 76, 2071–2090. [Google Scholar] [CrossRef]
- Shehab, W.S.; Amer MM, K.; Elsayed, D.A.; Yadav, K.K.; Abdellattif, M.H. Current progress toward synthetic routes and medicinal significance of quinoline. Med. Chem. Res. 2023, 32, 2443–2457. [Google Scholar] [CrossRef]
- Oluwadunni FElebiju, O.F.; Ajani, O.O.; Oduselu, G.O.; Ogunnupebi, T.A.; Adebiyi, E. Recent advances in functionalized quinoline scaffolds and hybrids—Exceptional pharmacophore in therapeutic medicine. Front. Chem. 2023, 10, 1074331. [Google Scholar]
- Ajani, O.O.; Iyaye, K.T.; Ademosun, O.T. Recent advances in chemistry and therapeutic potential of functionalized quinoline motifs—A review. RSC Adv. 2022, 12, 18594–18614. [Google Scholar] [CrossRef] [PubMed]
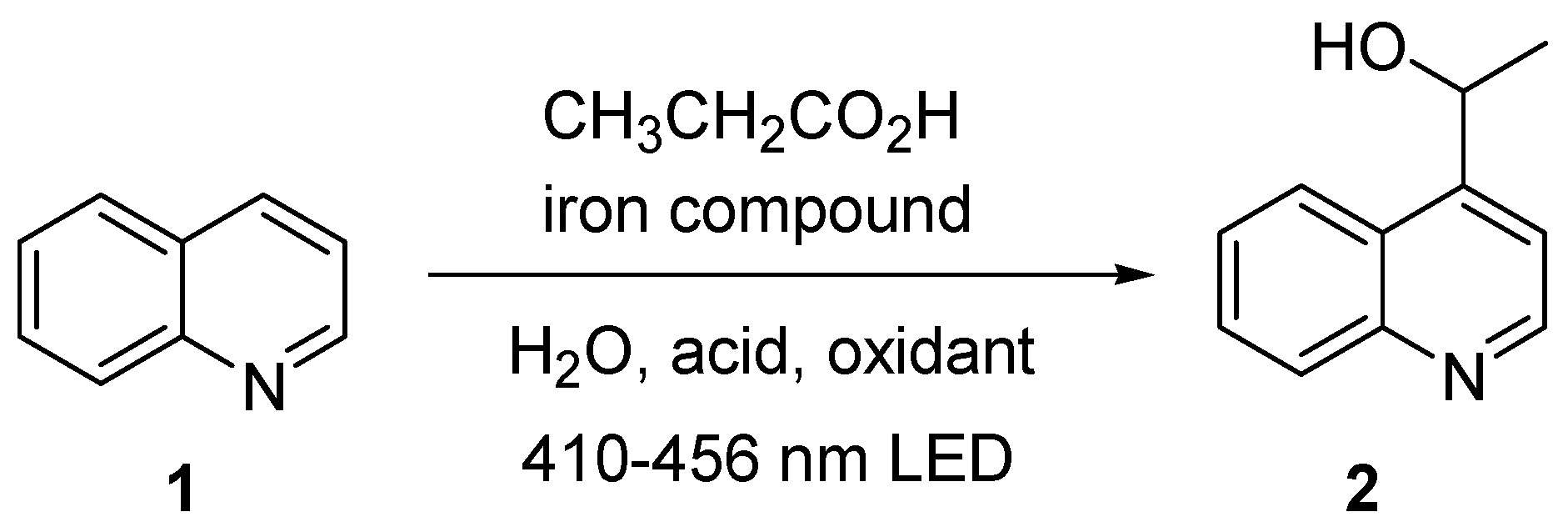
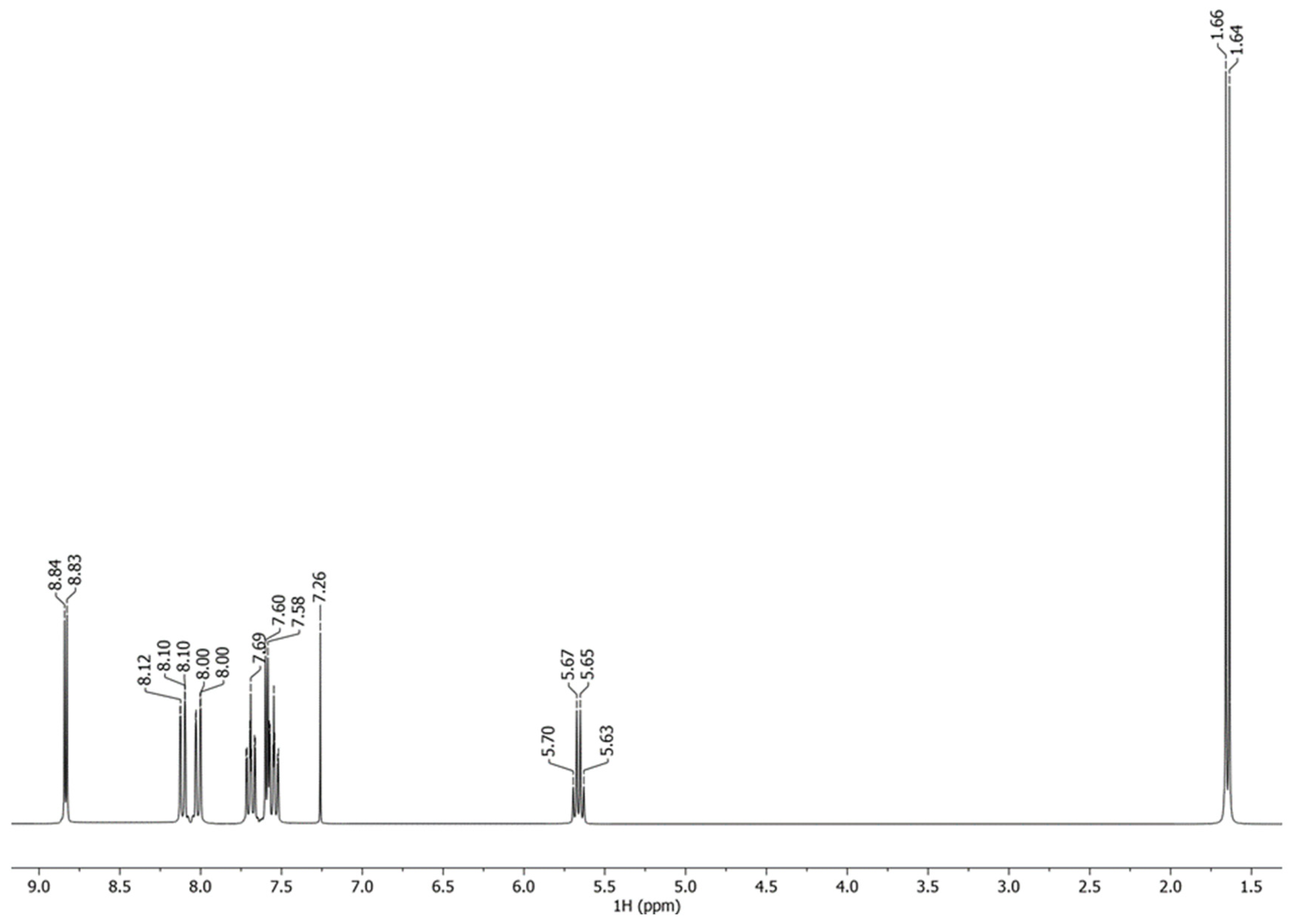

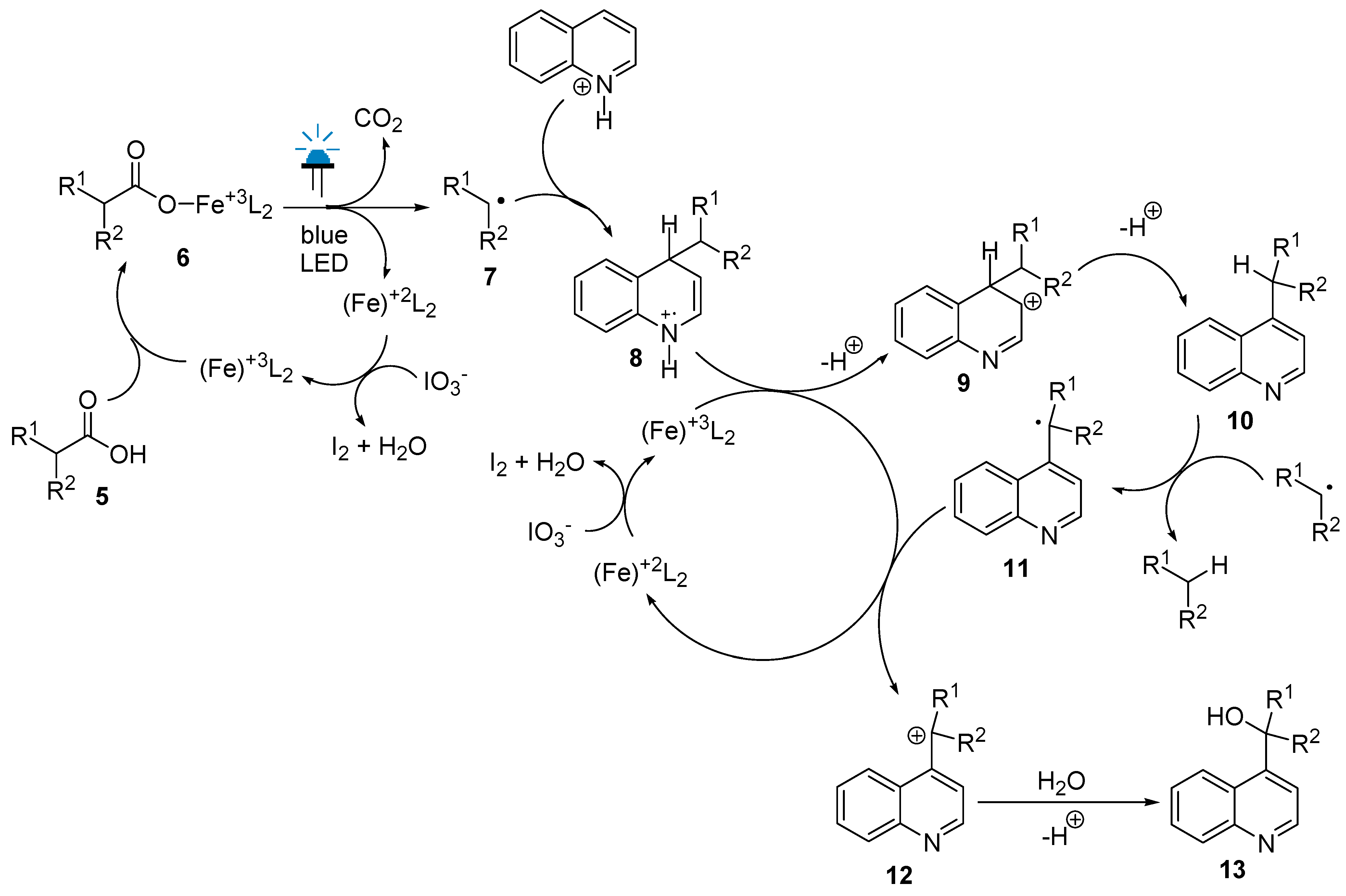
| Entry | Iron Compound | Acid Source | Sacrificial Oxidant | Reaction Time, h | % Yield |
|---|---|---|---|---|---|
| 1 | FeCl3·6H2O | none | none | 12 | 0 |
| 2 | FeCl3·6H2O | none | KIO3 | 12 | 0 |
| 3 | FeCl3·6H2O | H2SO4 | none | 12 | 0 |
| 4 | FeCl3·6H2O | H2SO4 | KIO3 | 12 | 0 |
| 5 | Fe(phen)Cl3·H2O | none | none | 12 | 0 |
| 6 | Fe(phen)Cl3·H2O | none | KIO3 | 12 | 0 |
| 7 | Fe(phen)Cl3·H2O | TFA | KIO3 | 12 | 0 |
| 8 | Fe(phen)Cl3·H2O | H2SO4 | NaIO4 | 12 | 5 |
| 9 | Fe(phen)Cl3·H2O | H2SO4 | KIO3 | 12 | 27 |
| 10 | Fe(phen)Cl3·H2O | H2SO4 | KIO3 | 20 | 37 |
| 11 | Fe(phen)Cl3·H2O | H2SO4 | KIO3 | 48 | 50 |
| 12 | Fe(phen)Cl3·H2O | H2SO4 | KIO3 | 72 | 78 |
| 13 | Fe(phen)Cl3·H2O | H2SO4 | KIO3 | 96 | 81 |
| Compound | R1 | R2 | % Yield |
|---|---|---|---|
| 2 | CH3 | H | 50 |
| 3 | CH3(CH2)3 | CH3CH2 | 31 |
| 4 | CH3(CH2)7 | H | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ríos-Malváez, Z.G.; González-Rivas, N.; Cuevas-Yañez, E. Quinoline Hydroxyalkylations from Iron-Catalyzed, Visible-Light-Driven Decarboxylations. Catalysts 2024, 14, 916. https://doi.org/10.3390/catal14120916
Ríos-Malváez ZG, González-Rivas N, Cuevas-Yañez E. Quinoline Hydroxyalkylations from Iron-Catalyzed, Visible-Light-Driven Decarboxylations. Catalysts. 2024; 14(12):916. https://doi.org/10.3390/catal14120916
Chicago/Turabian StyleRíos-Malváez, Zita G., Nelly González-Rivas, and Erick Cuevas-Yañez. 2024. "Quinoline Hydroxyalkylations from Iron-Catalyzed, Visible-Light-Driven Decarboxylations" Catalysts 14, no. 12: 916. https://doi.org/10.3390/catal14120916
APA StyleRíos-Malváez, Z. G., González-Rivas, N., & Cuevas-Yañez, E. (2024). Quinoline Hydroxyalkylations from Iron-Catalyzed, Visible-Light-Driven Decarboxylations. Catalysts, 14(12), 916. https://doi.org/10.3390/catal14120916







