Demonstrating Effectual Catalysis of Corncob with Solid Acid Sn-NUS-BH in Cyclopentyl Methyl Ether–Water for Co-Producing Reducing Sugar, Furfural, and Xylooligosaccharides
Abstract
1. Introduction
2. Results and Discussion
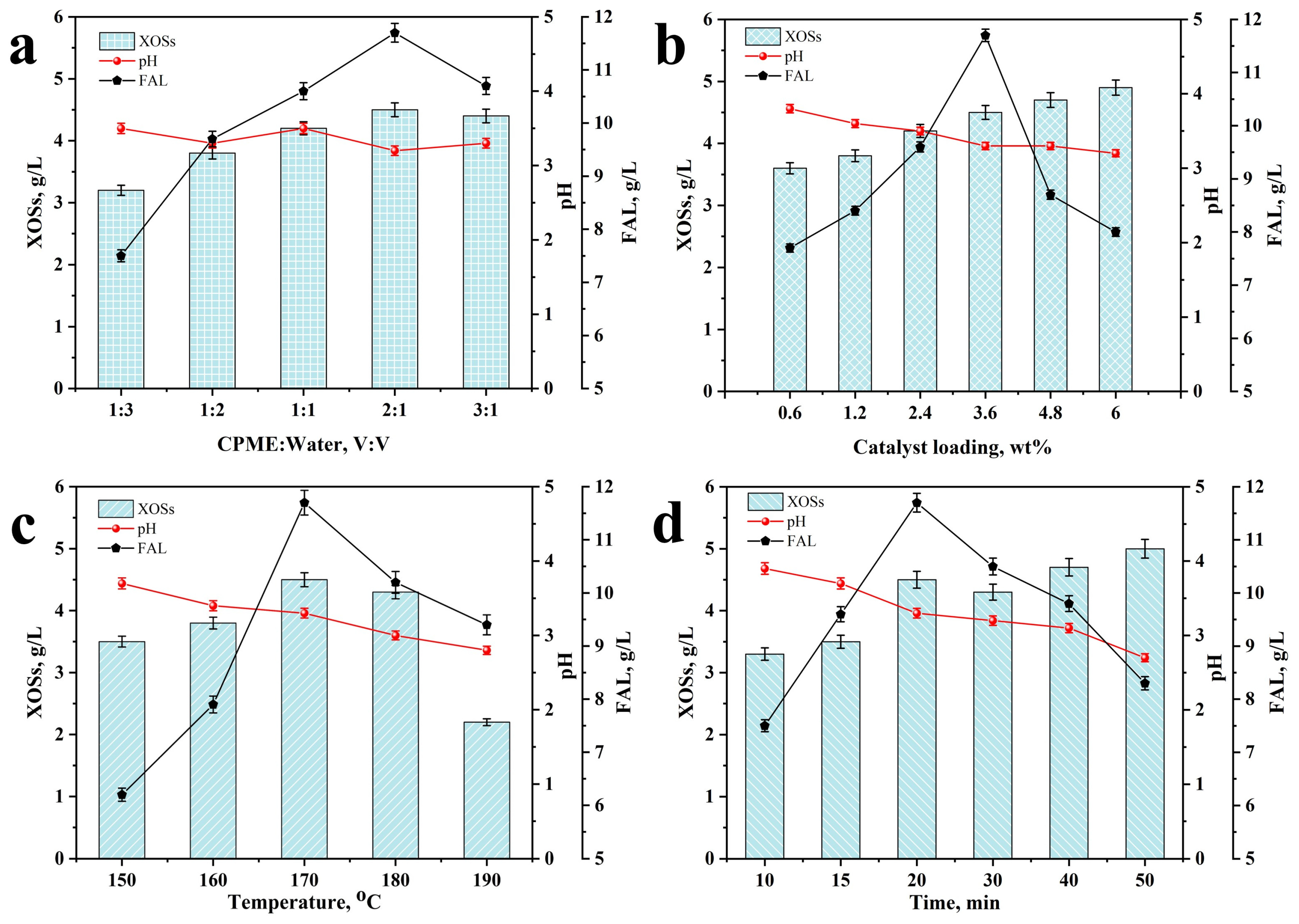
2.1. Effect of Reaction Conditions on XOSs and FAL Formation
2.2. Relationship of Enzymolysis Efficacy and Delignification After Pretreatment
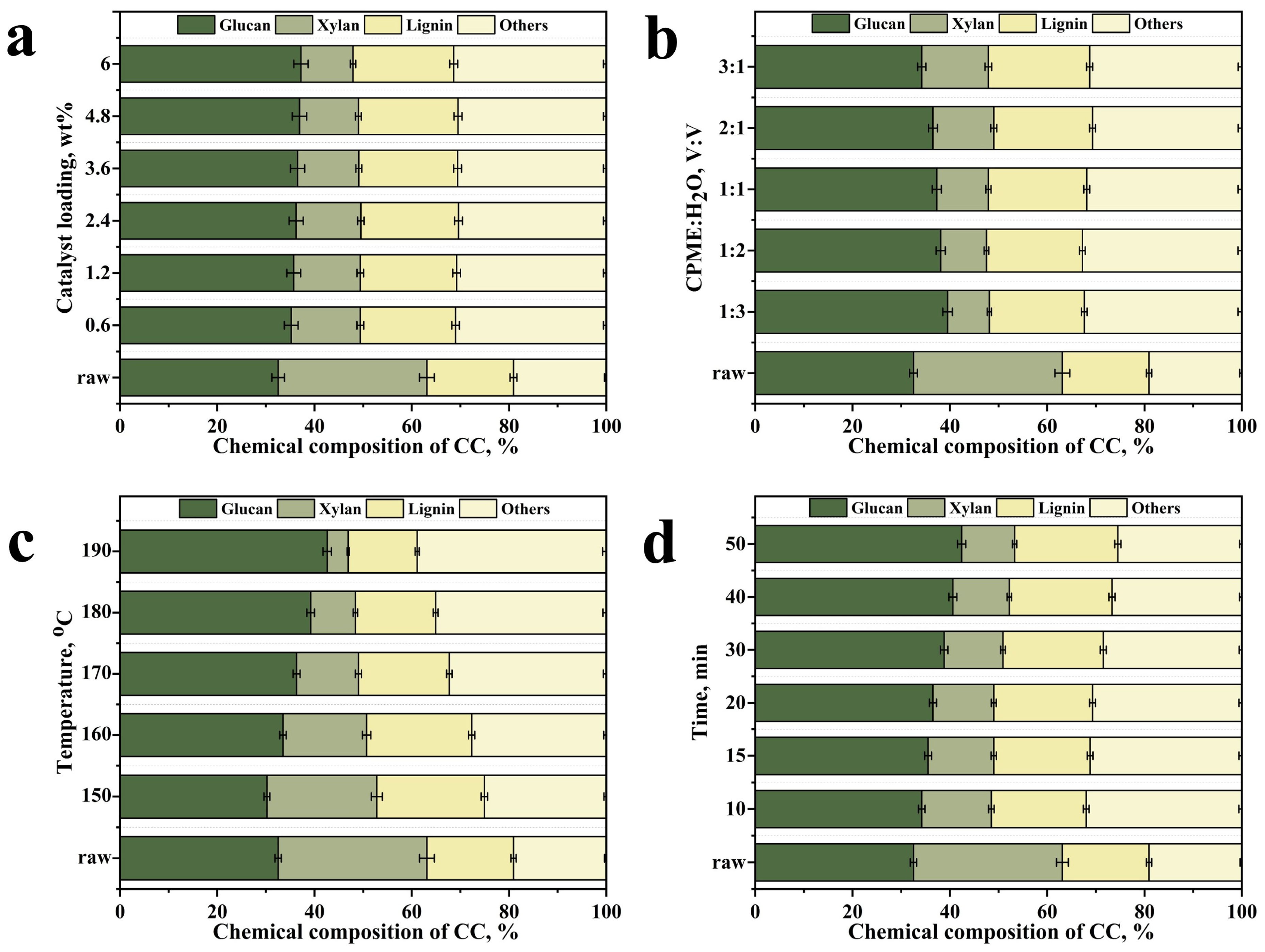
2.3. Investigation of CC Chemical Composition Alterations After Pretreatment
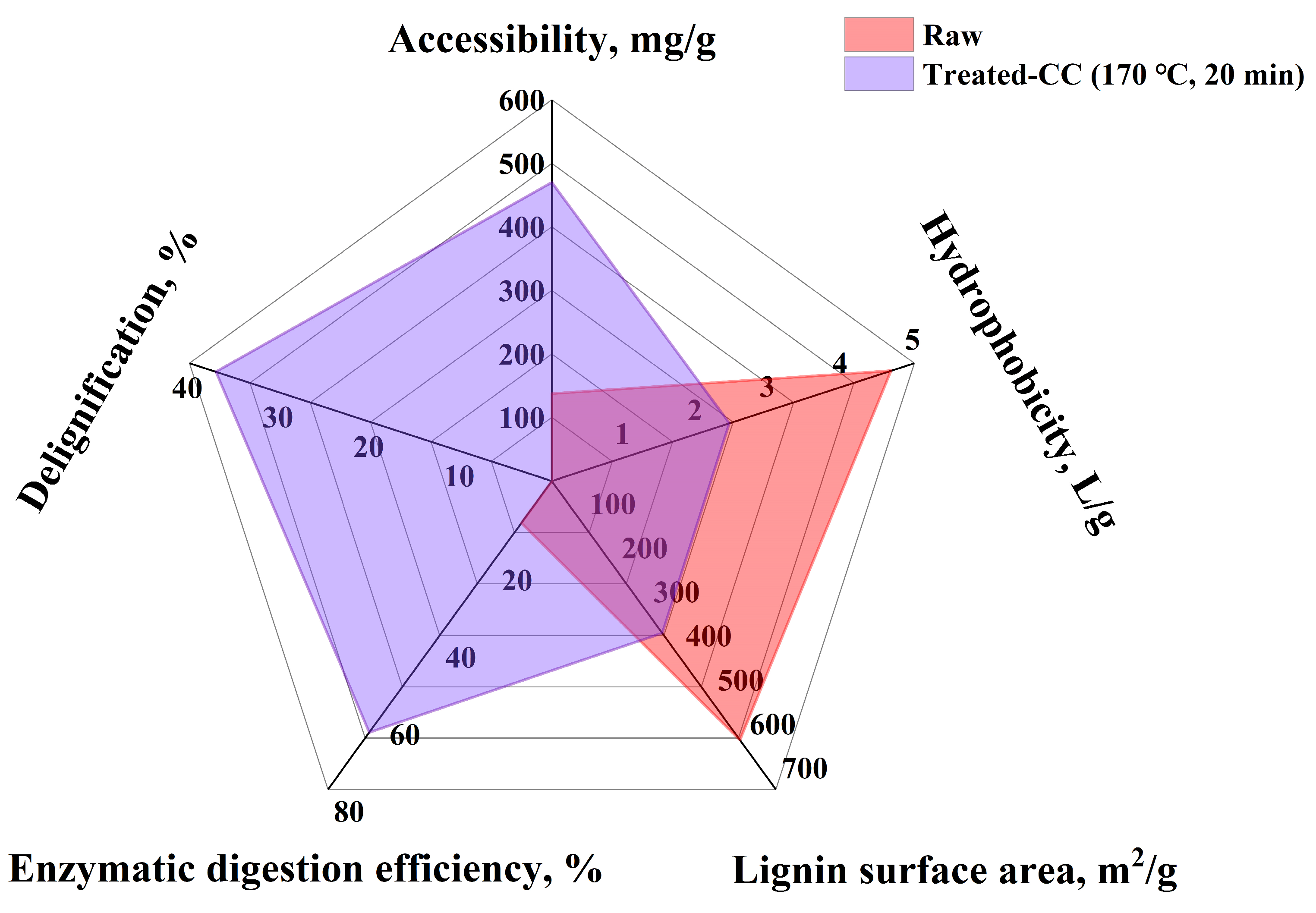
2.4. Investigation of Accessibility, Lignin Surface Area, and Hydrophobicity Change After Pretreatment
2.4.1. Accessibility
2.4.2. Lignin Surface Area
2.4.3. Hydrophobicity
2.5. Comprehensive Evaluation of Sn-NUS-BH-Catalyzed CC in CPME-H2O
2.6. Proposed Catalytic Mechanism for Catalyzing CC into FAL with Sn-NUS-BH in CPME-H2O
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Sn-NUS-BH
3.3. Production of XOSs and FAL from CC Through Catalysis with Sn-NUS-BH in CPME-H2O
3.4. Chemical Component Analysis of CC
3.5. Enzymatic Hydrolysis of CC into Reducing Sugars
3.6. Structural Features of Raw and Treated CC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shah, S.A.R.; Naqvi, S.A.A.; Riaz, S.; Anwar, S.; Abbas, N. Nexus of biomass energy, key determinants of economic development and environment: A fresh evidence from Asia. Renew. Sustain. Energy Rev. 2020, 133, 110244. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y. Assessing the interrelationship between fossil fuels resources and the biomass energy market for achieving a sustainable and green economy. Resour. Policy 2024, 88, 104397. [Google Scholar] [CrossRef]
- Saravanan, A.; Yaashikaa, P.R.; Kumar, P.S.; Thamarai, P.; Deivayanai, V.C.; Rangasamy, G. A comprehensive review on techno-economic analysis of biomass valorization and conversional technologies of lignocellulosic residues. Ind. Crops Prod. 2023, 200, 116822. [Google Scholar] [CrossRef]
- Fan, B.; Kong, L.; He, Y. Highly efficient production of furfural from corncob by Barley hull biochar-based solid acid in cyclopentyl methyl ether–water system. Catalysts 2024, 14, 583. [Google Scholar] [CrossRef]
- Li, Q.; Ma, C.-L.; He, Y.-C. Effective one-pot chemoenzymatic cascade catalysis of biobased feedstock for synthesizing 2,5-diformylfuran in a sustainable reaction system. Bioresour. Technol. 2023, 378, 128965. [Google Scholar] [CrossRef]
- Kaur, G.; Kaur, P.; Kaur, J.; Singla, D.; Taggar, M.S. Xylanase, xylooligosaccharide and xylitol production from lignocellulosic biomass: Exploring biovalorization of xylan from a sustainable biorefinery perspective. Ind. Crops Prod. 2024, 215, 118610. [Google Scholar] [CrossRef]
- Xu, D.; Ma, C.; Wu, M.; Deng, Y.; He, Y.-C. Improved production of adipic acid from a high loading of corn stover via an efficient and mild combination pretreatment. Bioresour. Technol. 2023, 382, 129196. [Google Scholar] [CrossRef]
- Singh, S.; Kaur, G.; Singh, D.P.; Arya, S.K.; Krishania, M. Exploring rice straw’s potential from a sustainable biorefinery standpoint: Towards valorization and diverse product production. Process Saf. Environ. Prot. 2024, 184, 314–331. [Google Scholar] [CrossRef]
- Deng, W.; Feng, Y.; Fu, J.; Guo, H.; Guo, Y.; Han, B.; Jiang, Z.; Kong, L.; Li, C.; Liu, H.; et al. Catalytic conversion of lignocellulosic biomass into chemicals and fuels. Green Energy Environ. 2023, 8, 10–114. [Google Scholar] [CrossRef]
- Li, X.; Zhao, J.; Sun, B.; Wang, C.; Zhang, X.; Mu, X. Preparation of furanyl primary amines from biobased furanyl derivatives over heterogeneous catalysts. ACS Sustain. Chem. Eng. 2023, 11, 17951–17978. [Google Scholar] [CrossRef]
- Ntimbani, R.N.; Farzad, S.; Görgens, J.F. Furfural production from sugarcane bagasse along with co-production of ethanol from furfural residues. Biomass Convers. Biorefinery 2022, 12, 5257–5267. [Google Scholar] [CrossRef]
- Wang, A.; Balsara, N.P.; Bell, A.T. Pervaporation-assisted catalytic conversion of xylose to furfural. Green Chem. 2016, 18, 4073–4085. [Google Scholar] [CrossRef]
- Fan, X.; Ren, M.; Zhou, C.; Kong, F.; Hua, C.; Fakayode, O.A.; Okonkwo, C.E.; Li, H.; Liang, J.; Wang, X. Total utilization of lignocellulosic biomass with xylooligosaccharides production priority: A review. Biomass Bioenergy 2024, 181, 107038. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Du, X.; Qu, Y. Selective removal of lignin to enhance the process of preparing fermentable sugars and platform chemicals from lignocellulosic biomass. Bioresour. Technol. 2020, 303, 122846. [Google Scholar] [CrossRef]
- Wang, Q.; Qi, W.; Wang, W.; Zhang, Y.; Leksawasdi, N.; Zhuang, X.; Yu, Q.; Yuan, Z. Production of furfural with high yields from corncob under extremely low water/solid ratios. Renew. Energy 2019, 144, 139–146. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A Promising Platform Compound for Sustainable Production of C4 and C5 Chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Millán, G.G.; Phiri, J.; Mäkelä, M.; Maloney, T.; Balu, A.M.; Pineda, A.; Llorca, J.; Sixta, H. Furfural production in a biphasic system using a carbonaceous solid acid catalyst. Appl. Catal. A Gen. 2019, 585, 117180. [Google Scholar] [CrossRef]
- Qi, Z.; Wang, Q.; Liang, C.; Yue, J.; Liu, S.; Ma, S.; Wang, X.; Wang, Z.; Li, Z.; Qi, W. Highly efficient conversion of xylose to furfural in a water–MIBK system catalyzed by magnetic carbon-based solid acid. Ind. Eng. Chem. Res. 2020, 59, 17046–17056. [Google Scholar] [CrossRef]
- Yang, T.; Li, W.; Su, M.; Liu, Y.; Liu, M. Production of furfural from xylose catalyzed by a novel calcium gluconate derived carbon solid acid in 1,4-dioxane. New J. Chem. 2020, 44, 7968–7975. [Google Scholar] [CrossRef]
- Lehmann, J. Bio-energy in the black. Front. Ecol. Environ. 2007, 5, 381–387. [Google Scholar] [CrossRef]
- Cao, X.; Sun, S.; Sun, R. Application of biochar-based catalysts in biomass upgrading: A review. RSC Adv. 2017, 7, 48793–48805. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.U.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 26, 1–27. [Google Scholar] [CrossRef]
- Parchami, M.; Ferreira, J.A.; Taherzadeh, M.J. Starch and protein recovery from brewer’s spent grain using hydrothermal pretreatment and their conversion to edible filamentous fungi—A brewery biorefinery concept. Bioresour. Technol. 2021, 337, 125409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, W.; Xu, Z.; Liu, Q.; Ma, Q.; Jameel, H.; Chang, H.-m.; Ma, L. Catalytic conversion of xylose and corn stalk into furfural over carbon solid acid catalyst in γ-valerolactone. Bioresour. Technol. 2016, 209, 108–114. [Google Scholar] [CrossRef]
- Wang, W.; Li, H.; Ren, J.; Sun, R.; Zheng, J.; Sun, G.; Liu, S. An efficient process for dehydration of xylose to furfural catalyzed by inorganic salts in water/dimethyl sulfoxide system. Chin. J. Catal. 2014, 35, 741–747. [Google Scholar] [CrossRef]
- García-Sancho, C.; Rubio-Caballero, J.M.; Mérida-Robles, J.M.; Moreno-Tost, R.; Santamaría-González, J.; Maireles-Torres, P. Mesoporous Nb2O5 as solid acid catalyst for dehydration of d-xylose into furfural. Catal. Today 2014, 234, 119–124. [Google Scholar] [CrossRef]
- Deng, A.; Lin, Q.; Yan, Y.; Li, H.; Ren, J.; Liu, C.; Sun, R. A feasible process for furfural production from the pre-hydrolysis liquor of corncob via biochar catalysts in a new biphasic system. Bioresour. Technol. 2016, 216, 754–760. [Google Scholar] [CrossRef]
- Li, Y.-J.; Cao, X.-F.; Sun, S.-N.; Yuan, T.-Q.; Wen, J.-L.; Wang, X.-L.; Xiao, L.-P.; Sun, R.-C. An integrated biorefinery process to comprehensively utilize corn stalk in a MIBK/water/Al(NO3)3·9H2O biphasic system: Chemical and morphological changes. Ind. Crops Prod. 2020, 147, 112173. [Google Scholar] [CrossRef]
- Watanabe, K. The Toxicological Assessment of Cyclopentyl Methyl Ether (CPME) as a Green Solvent. Molecules 2013, 18, 3183–3194. [Google Scholar] [CrossRef]
- Sun, L.-L.; Sun, S.-N.; Cao, X.-F.; Yao, S.-Q. An integrated biorefinery strategy for Eucalyptus fractionation and co-producing glucose, furfural, and lignin based on deep eutectic solvent/cyclopentyl methyl ether system. Carbohydr. Polym. 2024, 343, 122420. [Google Scholar] [CrossRef]
- Gómez Millán, G.; Hellsten, S.; King, A.W.T.; Pokki, J.-P.; Llorca, J.; Sixta, H. A comparative study of water-immiscible organic solvents in the production of furfural from xylose and birch hydrolysate. J. Ind. Eng. Chem. 2019, 72, 354–363. [Google Scholar] [CrossRef]
- Romo, J.E.; Bollar, N.V.; Zimmermann, C.J.; Wettstein, S.G. Conversion of sugars and biomass to furans using heterogeneous catalysts in biphasic solvent systems. ChemCatChem 2018, 10, 4805–4816. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, C.; Huang, C.; Fu, Y.; Chang, J. Efficient conversion of xylose into furfural using sulfonic acid-functionalized metal–organic frameworks in a biphasic system. Ind. Eng. Chem. Res. 2018, 57, 16628–16634. [Google Scholar] [CrossRef]
- Zhu, X.; Ma, C.; Xu, J.; Xu, J.; He, Y.-C. Sulfonated vermiculite-mediated catalysis of Reed (Phragmites communis) into furfural for enhancing the biosynthesis of 2-furoic acid with a dehydrogenase biocatalyst in a one-pot manner. Energy Fuels 2020, 34, 14573–14580. [Google Scholar] [CrossRef]
- Ni, J.; Li, Q.; Gong, L.; Liao, X.-L.; Zhang, Z.-J.; Ma, C.; He, Y. Highly efficient chemoenzymatic cascade catalysis of biomass into furfurylamine by a heterogeneous shrimp shell-based chemocatalyst and an ω-transaminase biocatalyst in deep eutectic solvent–water. ACS Sustain. Chem. Eng. 2021, 9, 13084–13095. [Google Scholar] [CrossRef]
- Khaleghipour, L.; Linares-Pastén, J.A.; Rashedi, H.; Ranaei Siadat, S.O.; Jasilionis, A.; Al-Hamimi, S.; Sardari, R.R.R.; Karlsson, E.N. Extraction of sugarcane bagasse arabinoxylan, integrated with enzymatic production of xylo-oligosaccharides and separation of cellulose. Biotechnol. Biofuels 2021, 14, 153. [Google Scholar] [CrossRef]
- Oliveira, D.M.; Mota, T.R.; Grandis, A.; de Morais, G.R.; de Lucas, R.C.; Polizeli, M.L.T.M.; Marchiosi, R.; Buckeridge, M.S.; Ferrarese-Filho, O.; dos Santos, W.D. Lignin plays a key role in determining biomass recalcitrance in forage grasses. Renew. Energy 2020, 147, 2206–2217. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, W.; Li, L.; Huang, M.; Ma, C.; He, Y.-C. Enhancing enzymatic hydrolysis of waste sunflower straw by clean hydrothermal pretreatment. Bioresour. Technol. 2023, 383, 129236. [Google Scholar] [CrossRef]
- Shen, X.-J.; Chen, T.; Wang, H.-M.; Mei, Q.; Yue, F.; Sun, S.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural and morphological transformations of lignin macromolecules during bio-based deep eutectic solvent (DES) pretreatment. ACS Sustain. Chem. Eng. 2020, 8, 2130–2137. [Google Scholar] [CrossRef]
- Tang, W.; Tang, Z.; Qian, H.; Huang, C.; He, Y.-C. Implementing dilute acid pretreatment coupled with solid acid catalysis and enzymatic hydrolysis to improve bioconversion of bamboo shoot shells. Bioresour. Technol. 2023, 381, 129167. [Google Scholar] [CrossRef]
- Morales, A.; Hernández-Ramos, F.; Sillero, L.; Fernández-Marín, R.; Dávila, I.; Gullón, P.; Erdocia, X.; Labidi, J. Multiproduct biorefinery based on almond shells: Impact of the delignification stage on the manufacture of valuable products. Bioresour. Technol. 2020, 315, 123896. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Sun, S.; Cao, X.; Sun, R. The role of pretreatment in improving the enzymatic hydrolysis of lignocellulosic materials. Bioresour. Technol. 2016, 199, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Wu, C.; Tang, W.; Ma, C.; He, Y.-C. A novel cetyltrimethylammonium bromide-based deep eutectic solvent pretreatment of rice husk to efficiently enhance its enzymatic hydrolysis. Bioresour. Technol. 2023, 376, 128806. [Google Scholar] [CrossRef] [PubMed]
- Nordin, N.; Md Illias, R.; Manas, N.H.A.; Ramli, A.N.M.; Selvasembian, R.; Azelee, N.I.W.; Rajagopal, R.; Thirupathi, A.; Chang, S.W.; Ravindran, B. Highly sustainable cascade pretreatment of low-pressure steam heating and organic acid on pineapple waste biomass for efficient delignification. Fuel 2022, 321, 124061. [Google Scholar] [CrossRef]
- Ladeira Ázar, R.I.S.; Bordignon-Junior, S.E.; Laufer, C.; Specht, J.; Ferrier, D.; Kim, D. Effect of lignin content on cellulolytic saccharification of liquid hot water pretreated sugarcane bagasse. Molecules 2020, 25, 623. [Google Scholar] [CrossRef]
- Qian, E.W.; Sukma, L.P.P.; Li, S.; Higashi, A. Saccharification of cellulosic biomass using sulfonated mesoporous carbon-based catalysts. Environ. Prog. Sustain. Energy 2016, 35, 574–581. [Google Scholar] [CrossRef]
- Chen, S.S.; Maneerung, T.; Tsang, D.C.W.; Ok, Y.S.; Wang, C.-H. Valorization of biomass to hydroxymethylfurfural, levulinic acid, and fatty acid methyl ester by heterogeneous catalysts. Chem. Eng. J. 2017, 328, 246–273. [Google Scholar] [CrossRef]
- Cousin, E.; Namhaed, K.; Pérès, Y.; Cognet, P.; Delmas, M.; Hermansyah, H.; Gozan, M.; Alaba, P.A.; Aroua, M.K. Towards efficient and greener processes for furfural production from biomass: A review of the recent trends. Sci. Total Environ. 2022, 847, 157599. [Google Scholar] [CrossRef]
- Parchami, M.; Agnihotri, S.; Taherzadeh, M.J. Aqueous ethanol organosolv process for the valorization of Brewer’s spent grain (BSG). Bioresour. Technol. 2022, 362, 127764. [Google Scholar] [CrossRef]
- Oh, S.-J.; Jung, S.-H.; Kim, J.-S. Co-production of furfural and acetic acid from corncob using ZnCl2 through fast pyrolysis in a fluidized bed reactor. Bioresour. Technol. 2013, 144, 172–178. [Google Scholar] [CrossRef]
- Yang, L.; Li, Y.; Wu, Y.; He, Y.; Ma, C. Efficient synthesis of furfural from corncob by a novel biochar-based heterogeneous chemocatalyst in Choline chloride: Maleic acid–water. Catalysts 2023, 13, 1277. [Google Scholar] [CrossRef]
- Yang, Q.; Tang, Z.; Xiong, J.; He, Y. Sustainable chemoenzymatic cascade transformation of corncob to furfuryl alcohol with rice husk-based heterogeneous catalyst UST-Sn-RH. Catalysts 2023, 13, 37. [Google Scholar] [CrossRef]
- Yang, Q.; Fan, B.; He, Y.-C. Combination of solid acid and solvent pretreatment for co-production of furfural, xylooligosaccharide and reducing sugars from Phyllostachys edulis. Bioresour. Technol. 2024, 395, 130398. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Teng, X.; Yu, S.; Si, Z.; Li, G.; Zhou, M.; Cai, D.; Qin, P.; Chen, B. Production of furfural from xylose and hemicelluloses using tin-loaded sulfonated diatomite as solid acid catalyst in biphasic system. Bioresour. Technol. Rep. 2019, 6, 145–151. [Google Scholar] [CrossRef]
- Shen, J.; Gao, R.; He, Y.-C.; Ma, C. Efficient synthesis of furfural from waste biomasses by sulfonated crab shell-based solid acid in a sustainable approach. Ind. Crops Prod. 2023, 202, 116989. [Google Scholar] [CrossRef]
- Lee, C.B.T.L.; Wu, T.Y. A review on solvent systems for furfural production from lignocellulosic biomass. Renew. Sustain. Energy Rev. 2021, 137, 110172. [Google Scholar] [CrossRef]
- Mangione, R.; Simões, R.; Pereira, H.; Catarino, S.; Ricardo-da-Silva, J.; Miranda, I.; Ferreira-Dias, S. Potential use of grape stems and pomaces from two red grapevine cultivars as source of oligosaccharides. Processes 2022, 10, 1896. [Google Scholar] [CrossRef]
- Chen, Y.; Ma, C.; Tang, W.; He, Y.-C. Comprehensive understanding of enzymatic saccharification of Betaine:Lactic acid-pretreated sugarcane bagasse. Bioresour. Technol. 2023, 386, 129485. [Google Scholar] [CrossRef]
- Tang, W.; Wu, X.; Huang, C.; Huang, C.; Lai, C.; Yong, Q. Humic acid-assisted autohydrolysis of waste wheat straw to sustainably improve enzymatic hydrolysis. Bioresour. Technol. 2020, 306, 123103. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Pihlajaniemi, V.; Littunen, K.; Pastinen, O.; Laakso, S. Determination of surface-accessible acidic hydroxyls and surface area of lignin by cationic dye adsorption. Bioresour. Technol. 2014, 169, 80–87. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.; Kong, L.; He, Y.-C. Demonstrating Effectual Catalysis of Corncob with Solid Acid Sn-NUS-BH in Cyclopentyl Methyl Ether–Water for Co-Producing Reducing Sugar, Furfural, and Xylooligosaccharides. Catalysts 2024, 14, 821. https://doi.org/10.3390/catal14110821
Yang D, Kong L, He Y-C. Demonstrating Effectual Catalysis of Corncob with Solid Acid Sn-NUS-BH in Cyclopentyl Methyl Ether–Water for Co-Producing Reducing Sugar, Furfural, and Xylooligosaccharides. Catalysts. 2024; 14(11):821. https://doi.org/10.3390/catal14110821
Chicago/Turabian StyleYang, Dan, Linghui Kong, and Yu-Cai He. 2024. "Demonstrating Effectual Catalysis of Corncob with Solid Acid Sn-NUS-BH in Cyclopentyl Methyl Ether–Water for Co-Producing Reducing Sugar, Furfural, and Xylooligosaccharides" Catalysts 14, no. 11: 821. https://doi.org/10.3390/catal14110821
APA StyleYang, D., Kong, L., & He, Y.-C. (2024). Demonstrating Effectual Catalysis of Corncob with Solid Acid Sn-NUS-BH in Cyclopentyl Methyl Ether–Water for Co-Producing Reducing Sugar, Furfural, and Xylooligosaccharides. Catalysts, 14(11), 821. https://doi.org/10.3390/catal14110821






