Coupling Carbon Dioxide and Cyclohexane Oxide Using Metal-Free Catalyst with Tunable Selectivity of Product Under Mild Conditions
Abstract
1. Introduction
2. Results and Discussion
3. Materials
3.1. Instruments
3.2. Experimental Procedures
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hepburn, C.; Adlen, E.; Beddington, J.; Carter, E.A.; Fuss, S.; Mac Dowell, N.; Minx, J.C.; Smith, P.; Williams, C.K. The technological and economic prospects for CO2 utilization and removal. Nature 2019, 575, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Styring, P.; McCord, S.; Rackley, S. Chapter 17—Carbon dioxide utilization. In Negative Emissions Technologies for Climate Change Mitigation; Rackley, S., Andrews, G., Clery, D., De Richter, R., Dowson, G., Knops, P., Li, W., McCord, S., Ming, T., Sewel, A., et al., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 391–413. [Google Scholar]
- Sakakura, T.; Choi, J.-C.; Yasuda, H. Transformation of Carbon Dioxide. Chem. Rev. 2007, 107, 2365–2387. [Google Scholar] [CrossRef] [PubMed]
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A.W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef] [PubMed]
- Morgan, K.M.; Ellis, J.A.; Lee, J.; Fulton, A.; Wilson, S.L.; Dupart, P.S.; Dastoori, R. Thermochemical Studies of Epoxides and Related Compounds. J. Org. Chem. 2013, 78, 4303–4311. [Google Scholar] [CrossRef] [PubMed]
- Martín, C.; Fiorani, G.; Kleij, A.W. Recent Advances in the Catalytic Preparation of Cyclic Organic Carbonates. ACS Catal. 2015, 5, 1353–1370. [Google Scholar] [CrossRef]
- Wu, X.; Chen, C.; Guo, Z.; North, M.; Whitwood, A.C. Metal- and Halide-Free Catalyst for the Synthesis of Cyclic Carbonates from Epoxides and Carbon Dioxide. ACS Catal. 2019, 9, 1895–1906. [Google Scholar] [CrossRef]
- Kim, C.; Yoo, C.-J.; Oh, H.-S.; Min, B.K.; Lee, U. Review of carbon dioxide utilization technologies and their potential for industrial application. J. CO2 Util. 2022, 65, 102239. [Google Scholar] [CrossRef]
- Hazari, N.; Iwasawa, N.; Hopmann, K.H. Organometallic Chemistry for Enabling Carbon Dioxide Utilization. Organometallics 2020, 39, 1457–1460. [Google Scholar] [CrossRef]
- Gonsalvi, L.; Mordini, A.; Peruzzini, M. Organometallic Chemistry and Challenges in CO2 Activation and Utilization. Chem. Int. 2019, 41, 46–48. [Google Scholar] [CrossRef]
- Xie, W.; Xu, J.; Idros, U.M.; Katsuhira, J.; Fuki, M.; Hayashi, M.; Yamanaka, M.; Kobori, Y.; Matsubara, R. Metal-free reduction of CO2 to formate using a photochemical organohydride-catalyst recycling strategy. Nat. Chem. 2023, 15, 794–802. [Google Scholar] [CrossRef]
- Sreejyothi, P.; Mandal, S.K. From CO2 activation to catalytic reduction: A metal-free approach. Chem. Sci. 2020, 11, 10571–10593. [Google Scholar] [CrossRef]
- Singh, G.; Nagaraja, C. Highly efficient metal/solvent-free chemical fixation of CO2 at atmospheric pressure conditions using functionalized porous covalent organic frameworks. J. CO2 Util. 2021, 53, 101716. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic carbonates synthesised from CO2: Applications, challenges and recent research trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Yan, T.; Liu, H.; Zeng, Z.; Pan, W. Recent progress of catalysts for synthesis of cyclic carbonates from CO2 and epoxides. J. CO2 Util. 2023, 68, 102355. [Google Scholar] [CrossRef]
- Vagnoni, M.; Samorì, C.; Galletti, P. Choline-based eutectic mixtures as catalysts for effective synthesis of cyclic carbonates from epoxides and CO2. J. CO2 Util. 2020, 42, 101302. [Google Scholar] [CrossRef]
- Zhang, Z.; Fan, F.; Xing, H.; Yang, Q.; Bao, Z.; Ren, Q. Efficient Synthesis of Cyclic Carbonates from Atmospheric CO2 Using a Positive Charge Delocalized Ionic Liquid Catalyst. ACS Sustain. Chem. Eng. 2017, 5, 2841–2846. [Google Scholar] [CrossRef]
- Seong, Y.; Lee, S.; Cho, S.; Kim, Y.; Kim, Y. Organocatalysts for the Synthesis of Cyclic Carbonates under the Conditions of Ambient Temperature and Atmospheric CO2 Pressure. Catalysts 2024, 14, 90. [Google Scholar] [CrossRef]
- Deacy, A.C.; Kilpatrick, A.F.R.; Regoutz, A.; Williams, C.K. Understanding metal synergy in heterodinuclear catalysts for the copolymerization of CO2 and epoxides. Nat. Chem. 2020, 12, 372–380. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2016, 374, 20150085. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.-Y.; Hu, L.-F.; Zhang, X.-H.; Du, B.-Y.; Xu, J.-T. Carbon dioxide-based copolymers with various architectures. Prog. Polym. Sci. 2018, 82, 120–157. [Google Scholar] [CrossRef]
- Darensbourg, D.J. Chain transfer agents utilized in epoxide and CO2 copolymerization processes. Green Chem. 2019, 21, 2214–2223. [Google Scholar] [CrossRef]
- Zhang, D.; Boopathi, S.K.; Hadjichristidis, N.; Gnanou, Y.; Feng, X. Metal-Free Alternating Copolymerization of CO2 with Epoxides: Fulfilling “Green” Synthesis and Activity. J. Am. Chem. Soc. 2016, 138, 11117–11120. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Z.; Guo, W.; Zhang, C.; Zhang, X. Phosphine-Borane Frustrated Lewis Pairs for Metal-Free CO2/Epoxide Copolymerization. Macromolecules 2023, 56, 4901–4909. [Google Scholar] [CrossRef]
- Yang, G.-W.; Xu, C.-K.; Xie, R.; Zhang, Y.-Y.; Lu, C.; Qi, H.; Yang, L.; Wang, Y.; Wu, G.-P. Precision copolymerization of CO2 and epoxides enabled by organoboron catalysts. Nat. Synth. 2022, 1, 892–901. [Google Scholar] [CrossRef]
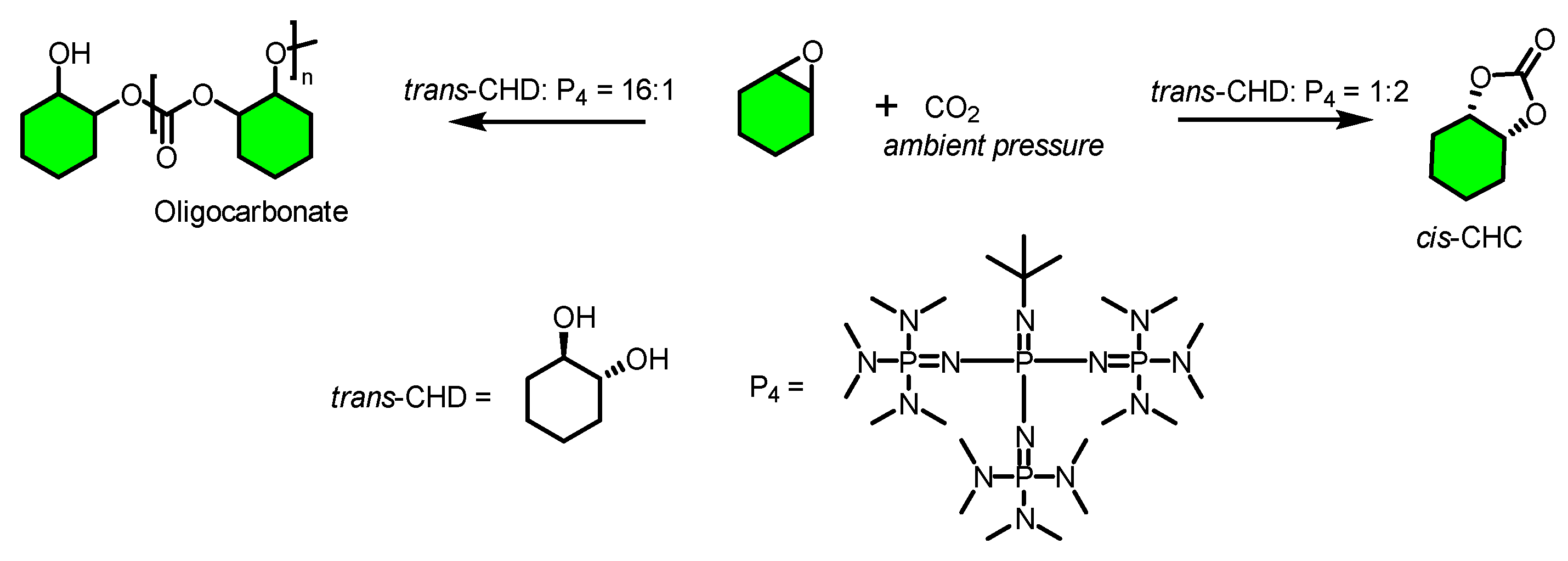
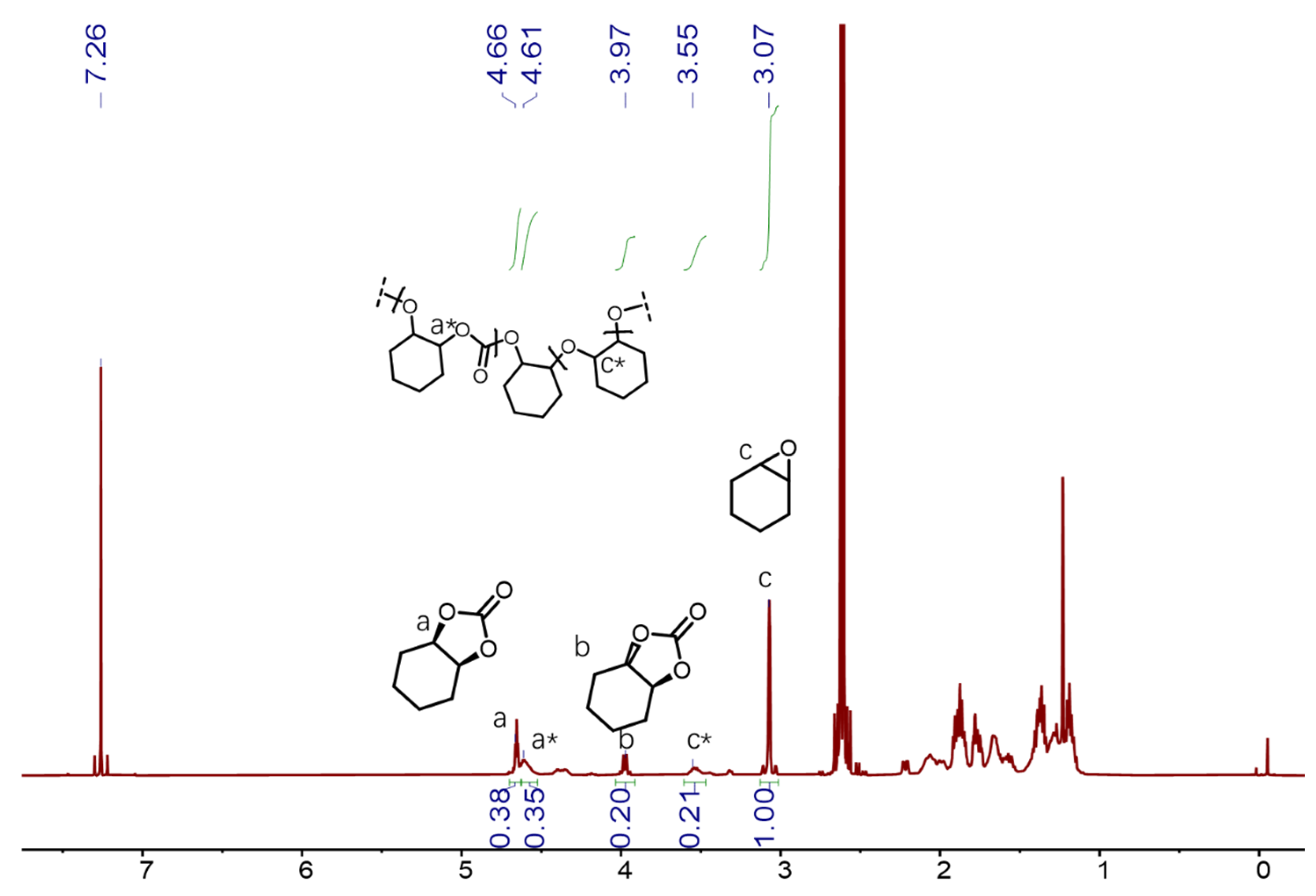
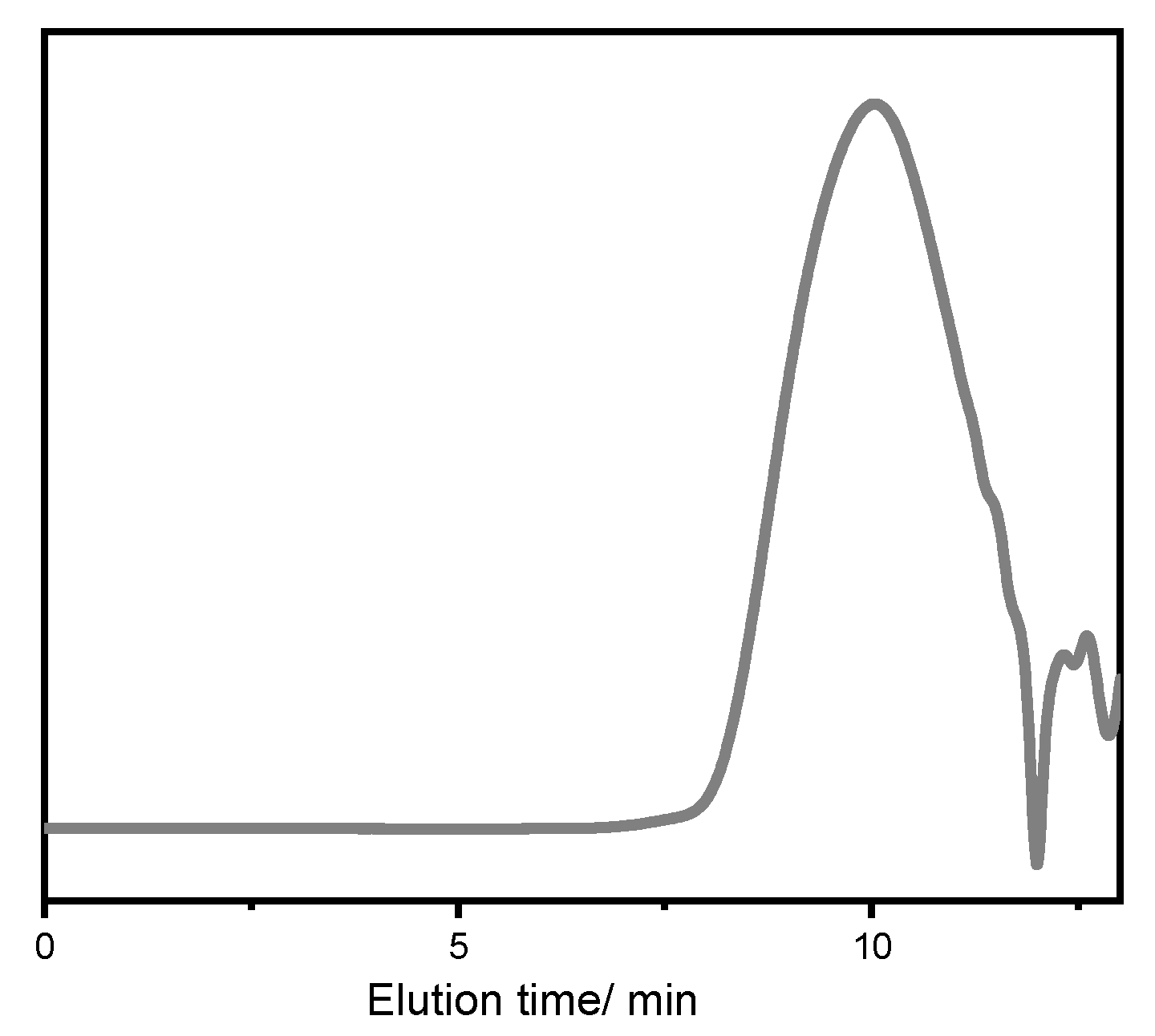
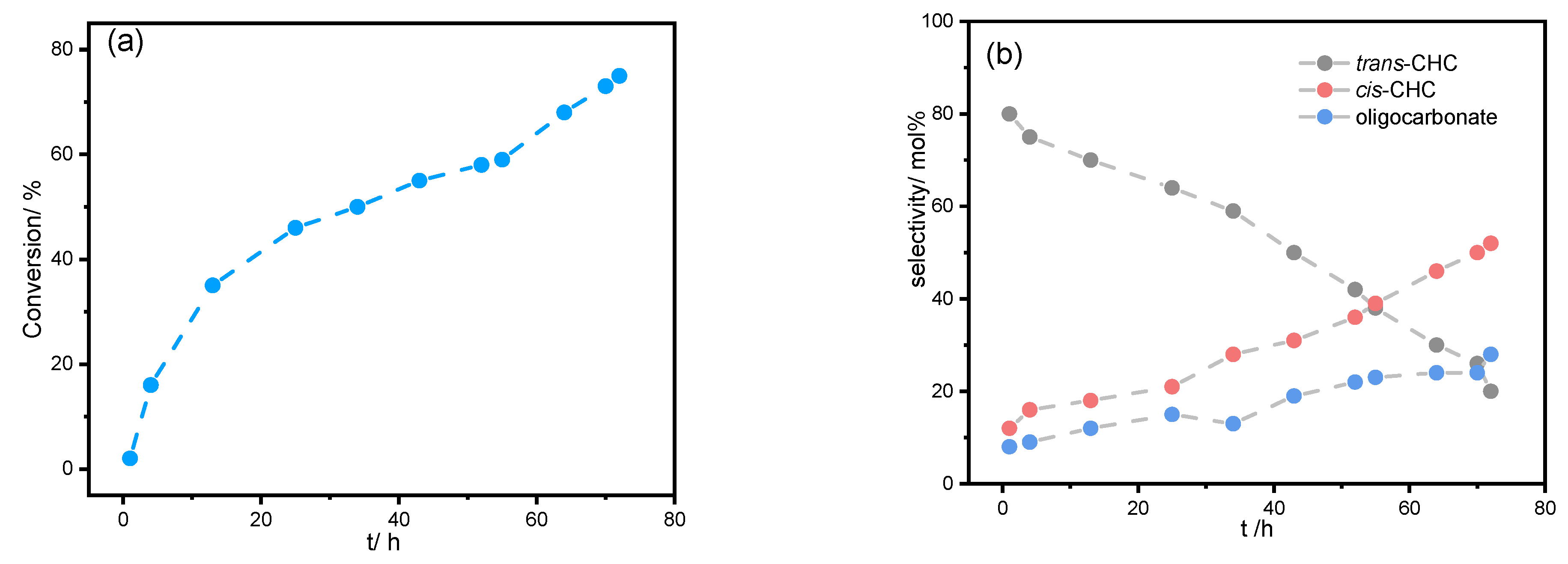
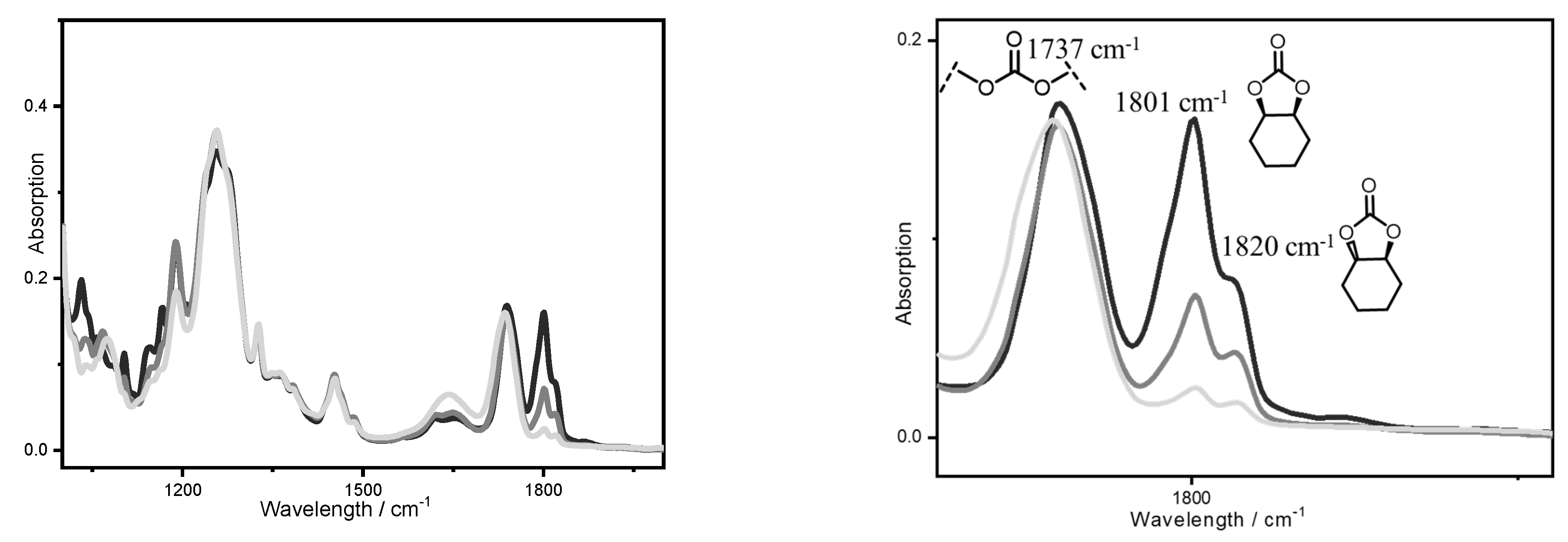
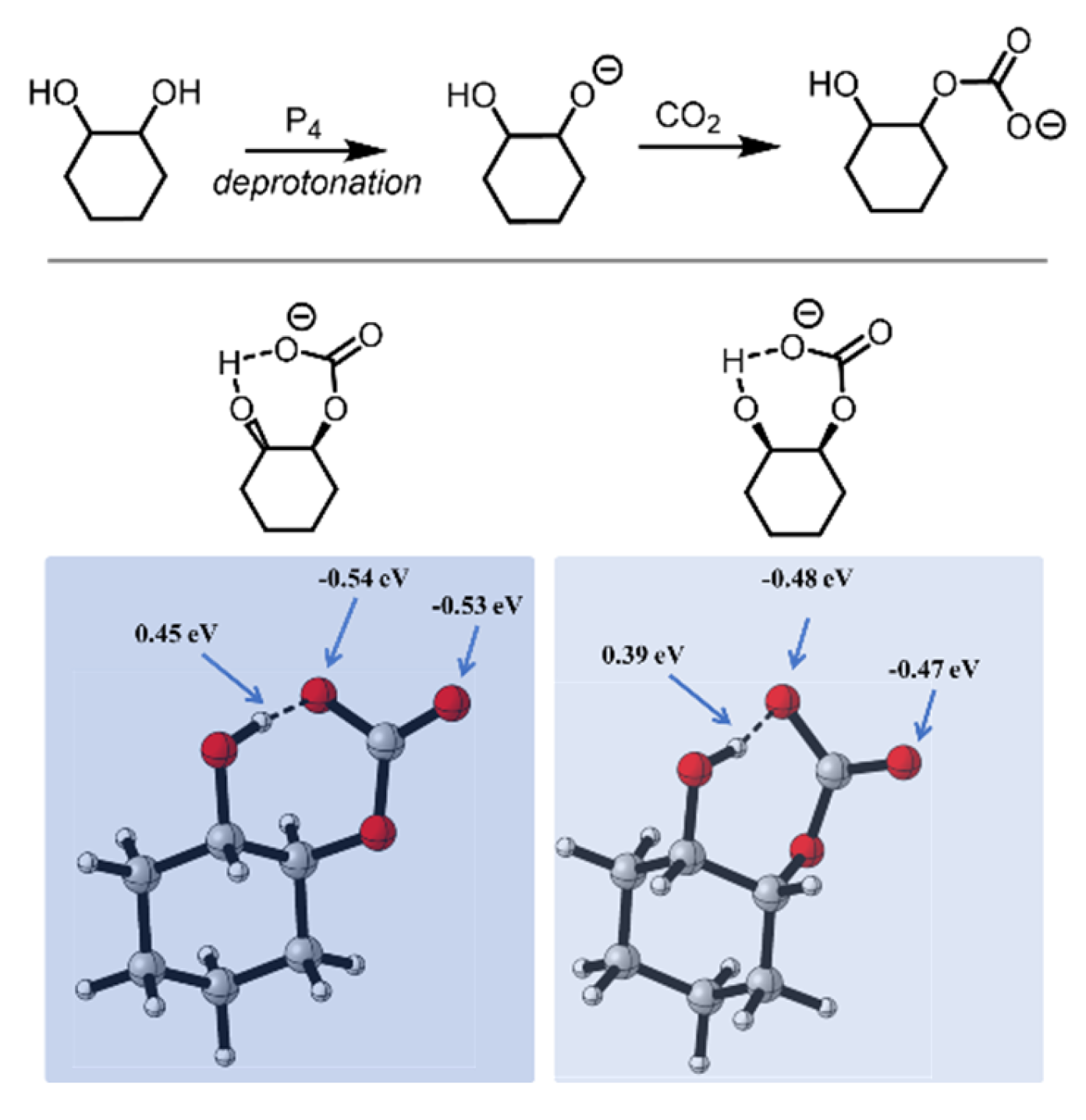

| Entry | [trans-CHD]:[P4] | Time/h | Conversion/mol% | trans-CHC/ mol% | cis-CHC/ mol% | Oligocarbonate | Mn SEC g/mol | Đ |
|---|---|---|---|---|---|---|---|---|
| 1 | 1:1 | 24 | 53 | 18 | 33 | 56 | 600 | 1.45 |
| 2 | 1:0 | 24 | N.A. f | N.A. | N.A. | N.A. | N.A. | N.A. |
| 3 | 0:1 | 24 | N.A. | N.A. | N.A. | N.A. | N.A. | N.A. |
| 4 b | 1:1 | 24 | 91 | <1 | 90 | <1 | N.A. | N.A. |
| 5 c | 1:1 | 24 | 32 | 46 | 28 | 26 | 300 | 1.26 |
| 6 | 1:1 | 48 | 59 | 42 | 42 | 16 | 450 | 1.30 |
| 7 | 1:1 | 72 | 80 | 18 | 50 | 32 | 390 | 1.35 |
| 8 d | 1:1 | 24 | 80 | 10 | 62 | 28 | 330 | 1.42 |
| 9 e | 1:1 | 24 | 92 | 10 | 85 | 5 | N.A. | N.A. |
| 10 | 1:2 | 24 | 60 | <1 | >99 | <1 | N.A. | N.A. |
| 11 | 16:1 | 24 | 54 | <1 | <1 | >99 | 670 | 1.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Pan, W. Coupling Carbon Dioxide and Cyclohexane Oxide Using Metal-Free Catalyst with Tunable Selectivity of Product Under Mild Conditions. Catalysts 2024, 14, 822. https://doi.org/10.3390/catal14110822
Ma X, Pan W. Coupling Carbon Dioxide and Cyclohexane Oxide Using Metal-Free Catalyst with Tunable Selectivity of Product Under Mild Conditions. Catalysts. 2024; 14(11):822. https://doi.org/10.3390/catal14110822
Chicago/Turabian StyleMa, Xuesuo, and Weiqing Pan. 2024. "Coupling Carbon Dioxide and Cyclohexane Oxide Using Metal-Free Catalyst with Tunable Selectivity of Product Under Mild Conditions" Catalysts 14, no. 11: 822. https://doi.org/10.3390/catal14110822
APA StyleMa, X., & Pan, W. (2024). Coupling Carbon Dioxide and Cyclohexane Oxide Using Metal-Free Catalyst with Tunable Selectivity of Product Under Mild Conditions. Catalysts, 14(11), 822. https://doi.org/10.3390/catal14110822





