Performance of Dye-Containing Wastewater Treatment Using MnxOy-Catalyzed Persulfate Oxidation
Abstract
1. Introduction
2. Results and Discussion
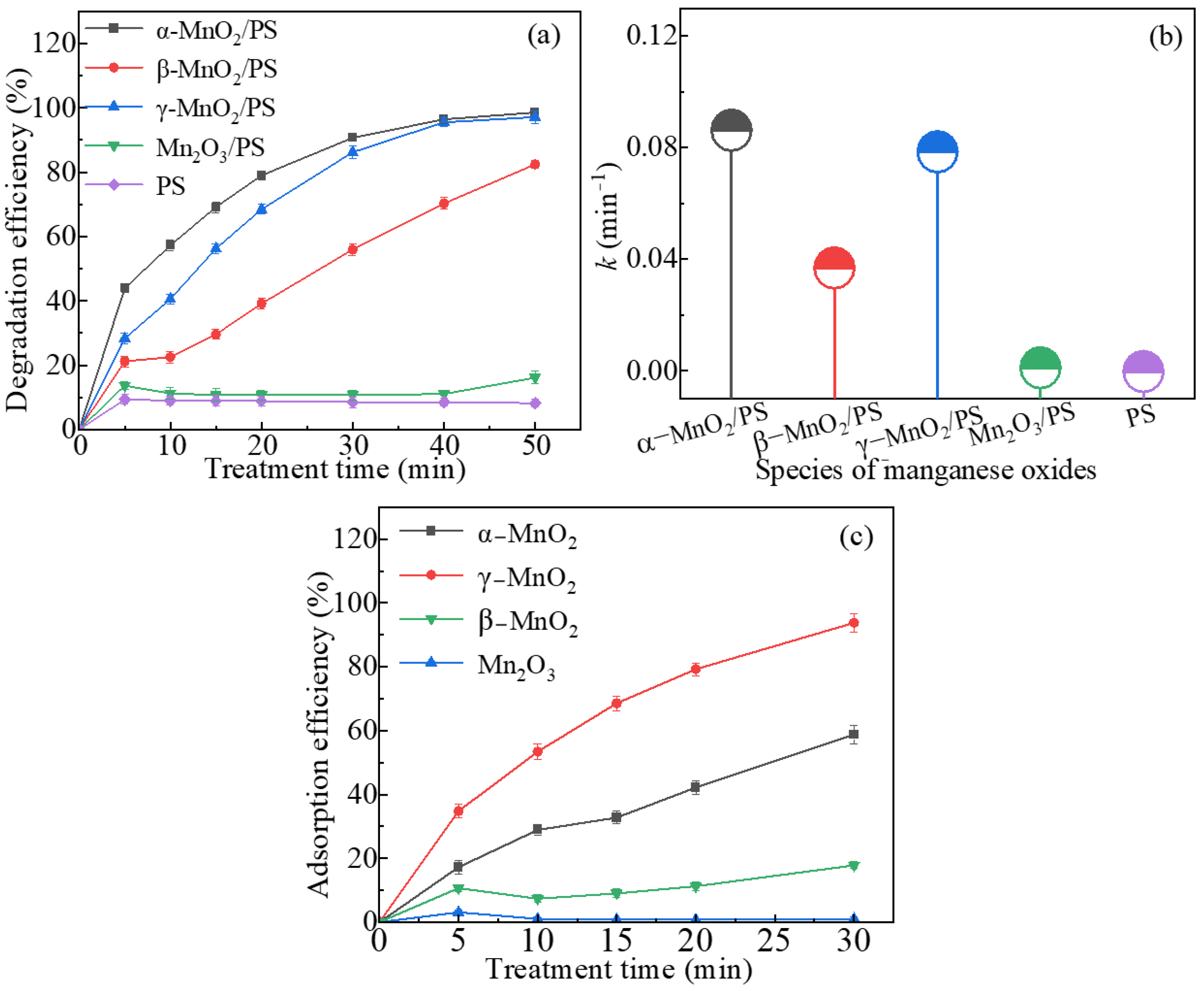
2.1. Degradation Performance of AO7 at Various MnxOy Polymorphs
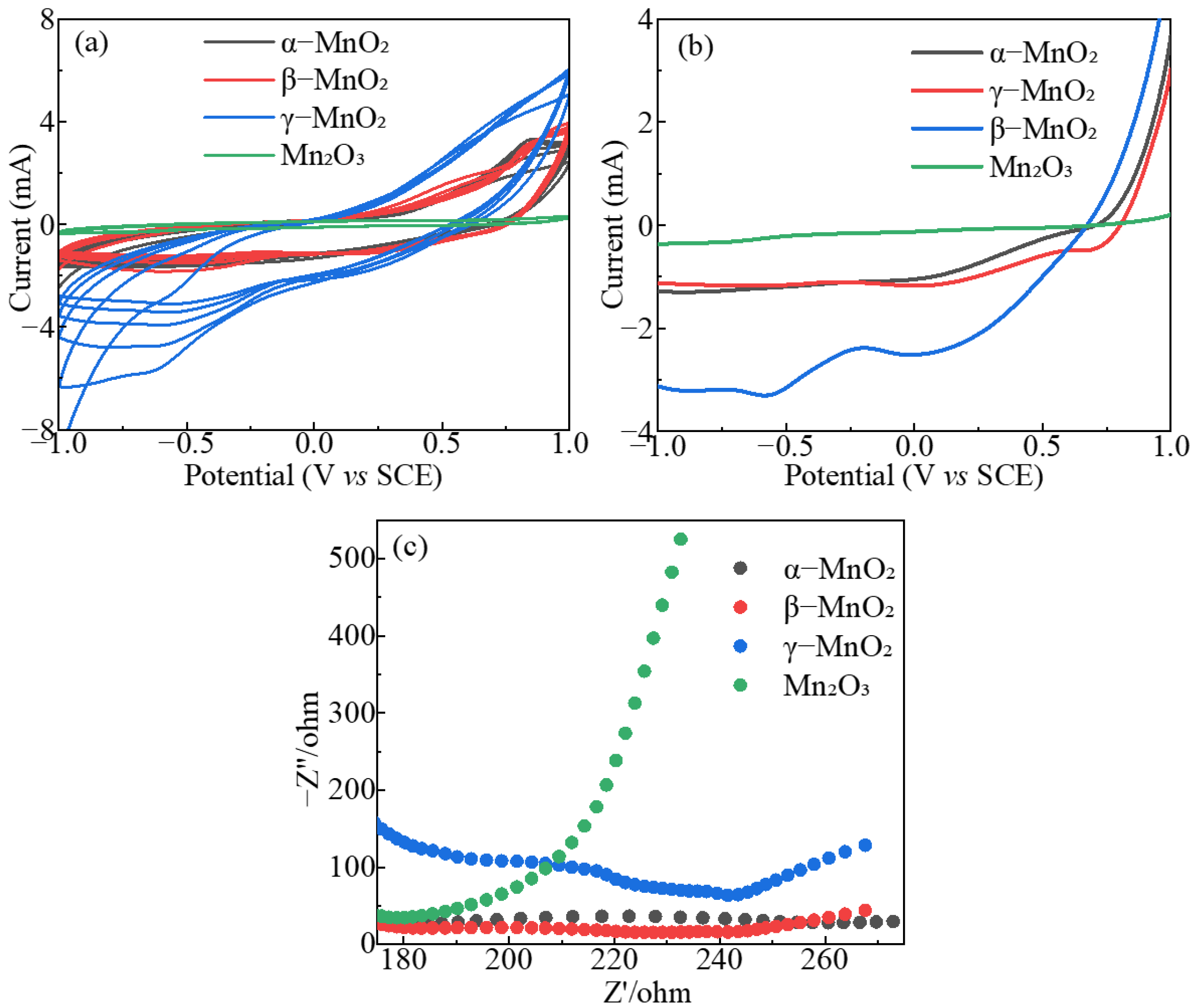
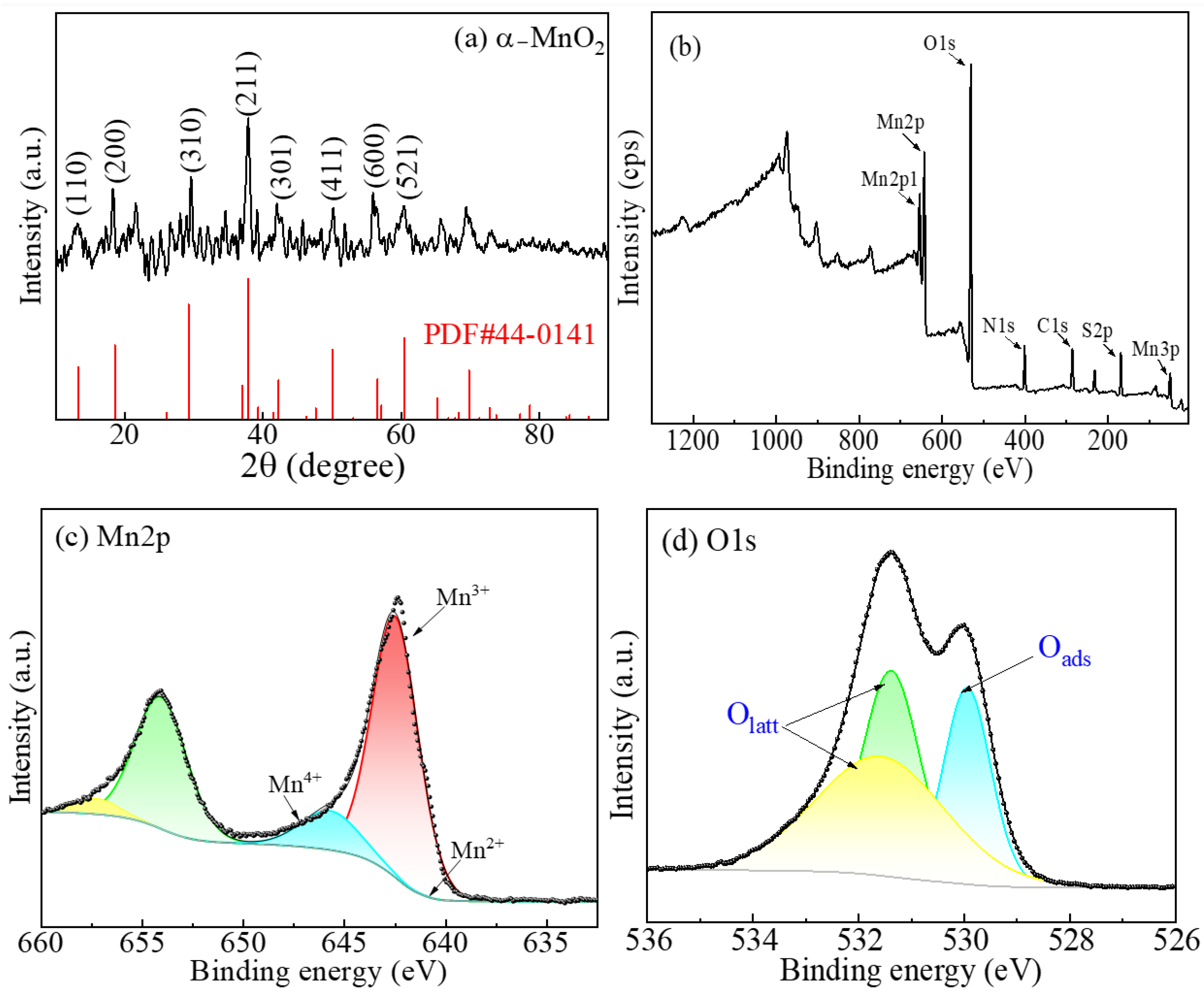
2.2. Characterization of the MnxOy Polymorphs
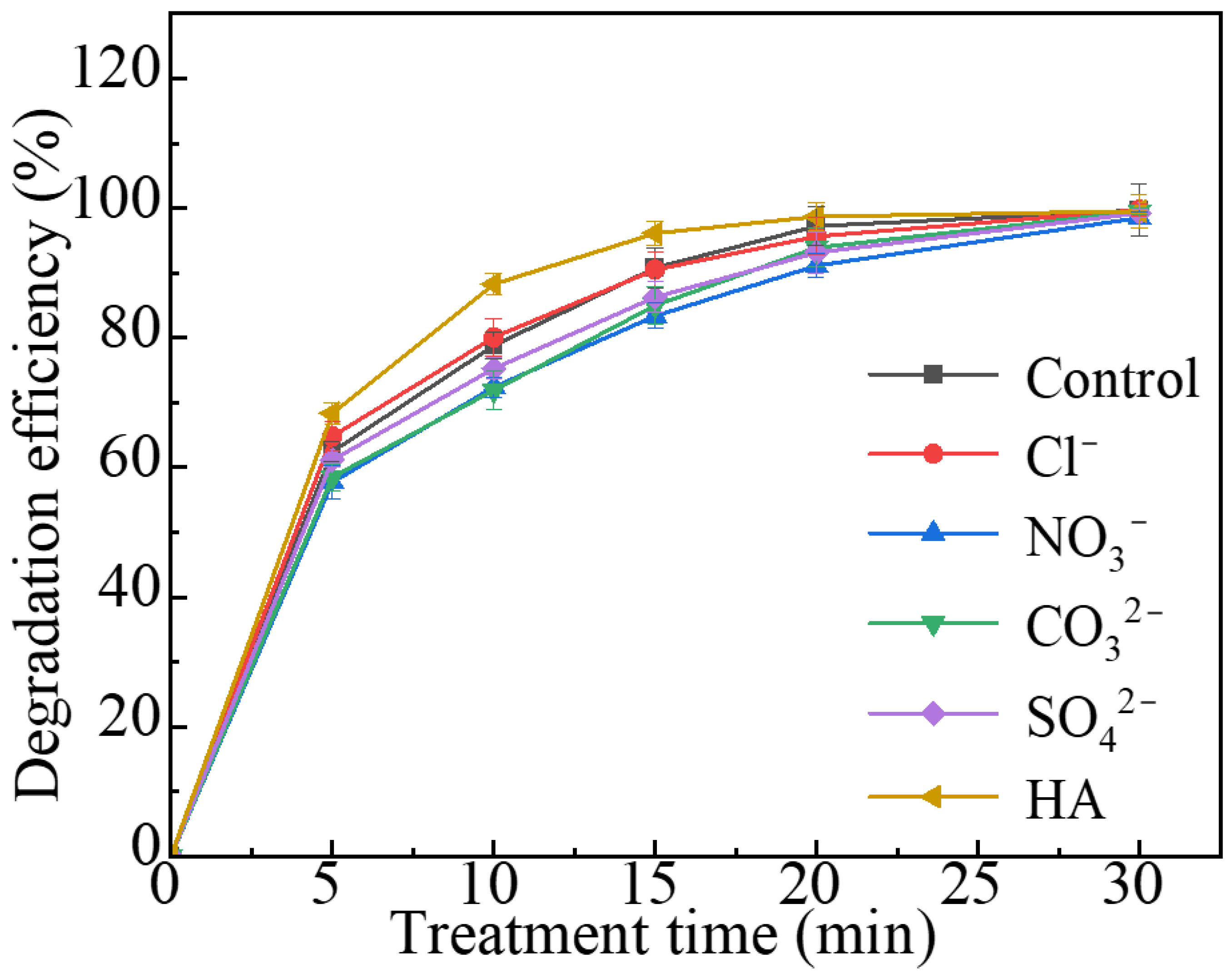
2.3. AO7 Degradation in Different Conditions
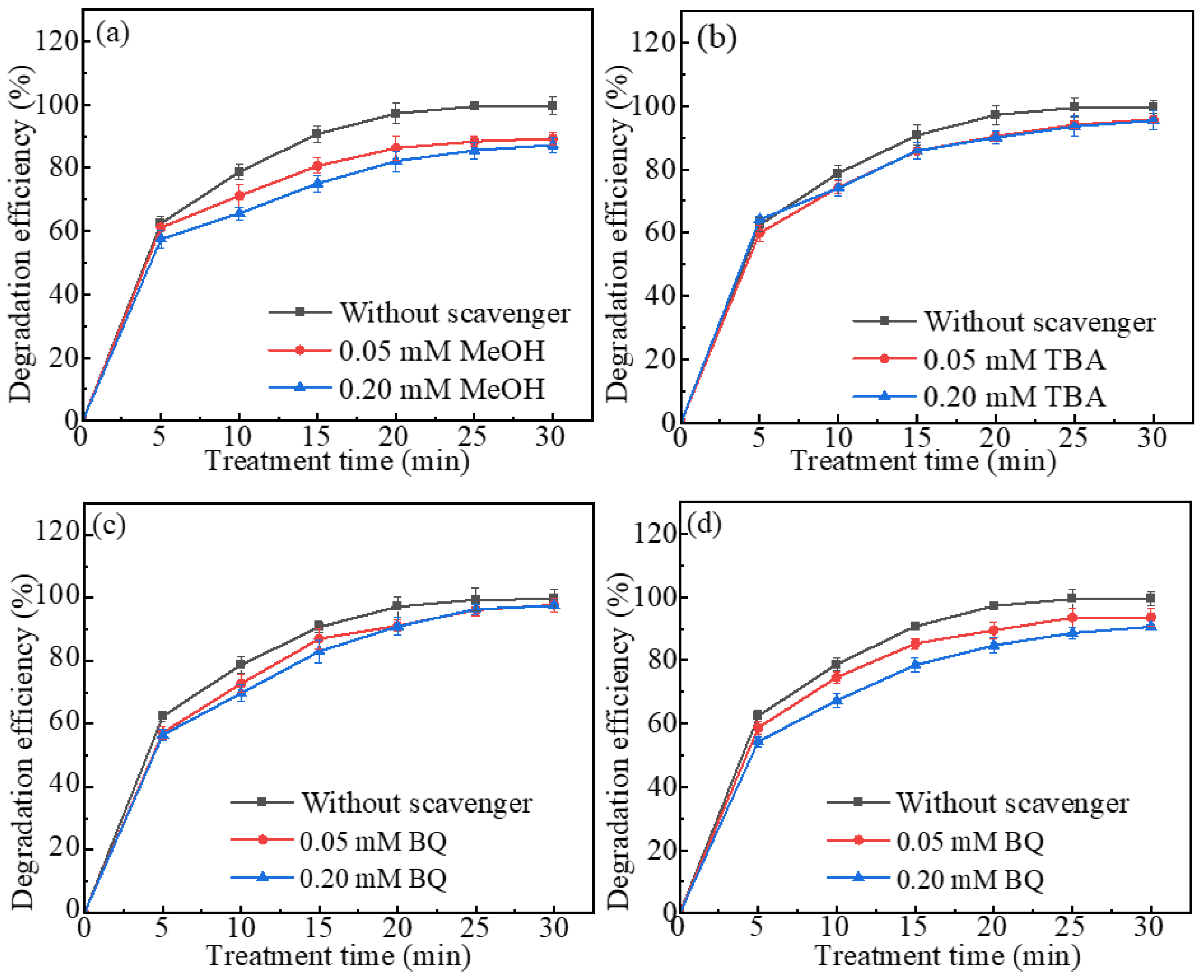
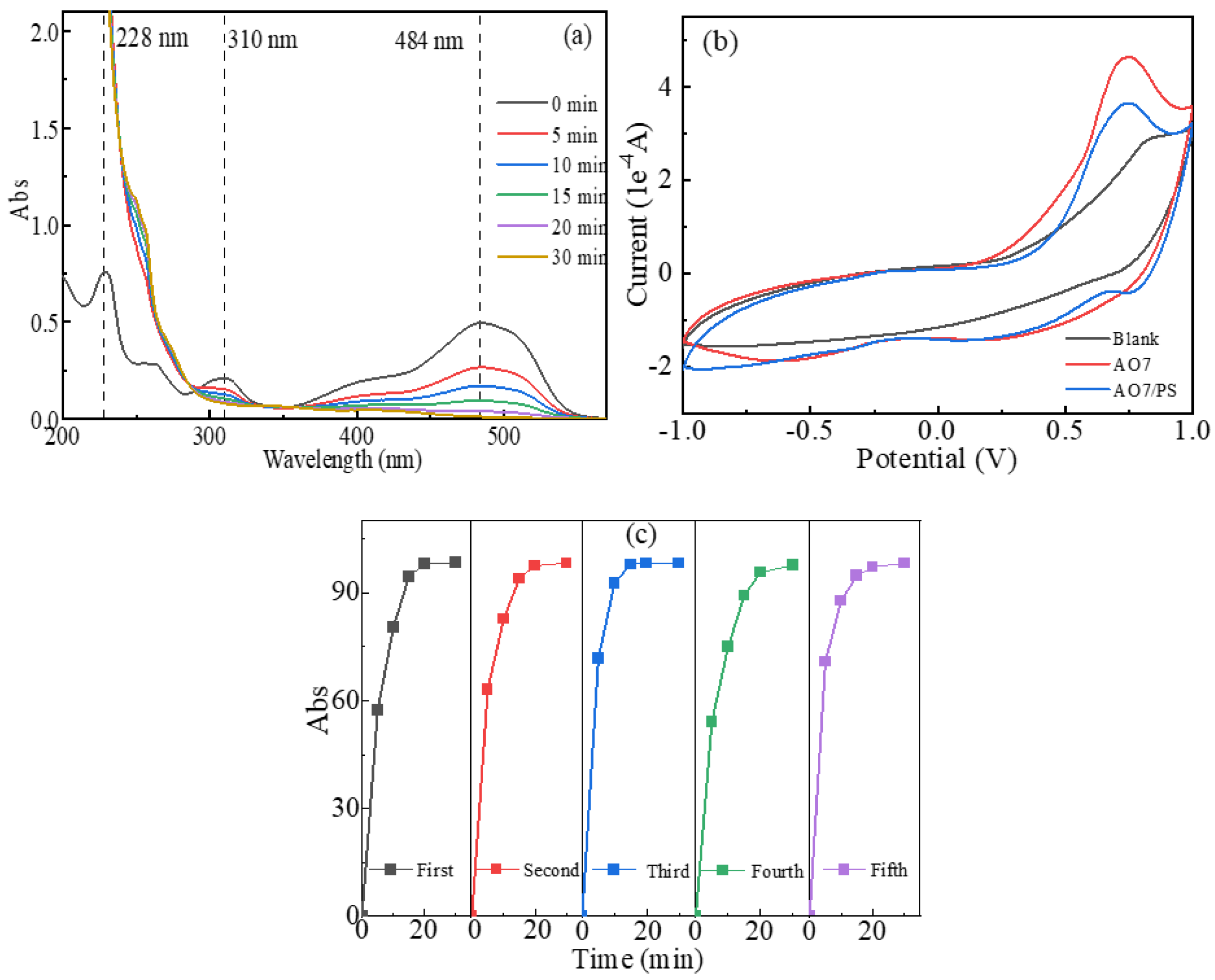
2.4. ROS Responsible for AO7 Degradation
2.5. Mechanisms of AO7 Degradation
3. Material and Methods
3.1. Reagents and Materials
3.2. Synthesis of MnxOy Materials
3.3. Degradation of AO7 by MnxOy Materials
3.4. Analytical Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, Y.; Wei, Z.; Duan, X.; Long, M.; Spinney, R.; Dionysiou, D.D.; Xiao, R.; Alvarez, P.J.J. Merits and Limitations of Radical vs. Nonradical Pathways in Persulfate-Based Advanced Oxidation Processes. Environ. Sci. Technol. 2023, 57, 12153–12179. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Ye, W.; Xie, M.; Seo, D.H.; Luo, J.; Wan, Y.; Van der Bruggen, B. Environmental impacts and remediation of dye-containing wastewater. Nat. Rev. Earth. Env. 2023, 4, 785–803. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2020, 717, 137222. [Google Scholar] [CrossRef]
- Ewuzie, U.; Saliu, O.D.; Dulta, K.; Ogunniyi, S.; Bajehg, A.O.; Iwuozori, K.O.; Ighaloj, J.O. A review on treatment technologies for printing and dyeing wastewater (PDW). J. Water. Process. Eng. 2022, 50, 103273. [Google Scholar] [CrossRef]
- Lin, J.; Lin, F.; Chen, X.; Ye, W.; Li, X.; Zeng, H.; Van der Bruggen, B. Sustainable Management of Textile Wastewater: A Hybrid Tight Ultrafiltration/Bipolar-Membrane Electrodialysis Process for Resource Recovery and Zero Liquid Discharge. Ind. Eng. Chem. Res. 2019, 58, 11003–11012. [Google Scholar] [CrossRef]
- Ye, W.; Ye, K.; Lin, F.; Liu, H.; Jiang, M.; Wang, J.; Liu, R.; Lin, J. Enhanced fractionation of dye/salt mixtures by tight ultrafiltration membranes via fast bio-inspired co-deposition for sustainable textile wastewater management. Chem. Eng. J. 2020, 379, 122321. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanoparticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Chen, X.; Su, X.; Cavaco-Paulo, A.; Wang, H.; Su, J. A backbone support structure and capillary effect-induced high-flux MOF-based mixed-matrix membrane for selective dye/salt separation. Sep. Purif. Technol. 2024, 344, 127221. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Lu, M.; Liang, L.; Zhang, H.; Wei, J. Biosynthesis based membrane filtration coupled with iron nanoparticles reduction process in removal of dyes. Chem. Eng. J. 2020, 387, 124202. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical development and prospects of photocatalysts for pollutant removal in water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Ammar, H.B.; Ben Brahim, M.; Abdelhedi, R.; Samet, Y. Green electrochemical process for metronidazole degradation at BDD anode in aqueous solutions via direct and indirect oxidation. Sep. Purif. Technol. 2016, 157, 9–16. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Guo, Z.; Zhang, W.; Li, H.; Huang, W. Recent developments and advances in boron-doped diamond electrodes for electrochemical oxidation of organic pollutants. Sep. Purif. Technol. 2019, 212, 802–821. [Google Scholar] [CrossRef]
- Zhang, M.-h.; Dong, H.; Zhao, L.; Wang, D.-x.; Meng, D. A review on Fenton process for organic wastewater treatment based on optimization perspective. Sci. Total Environ. 2019, 670, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; Zhu, G.; He, H. Strategies for enhancing the heterogeneous Fenton catalytic reactivity: A review. Appl. Catal. B-Environ. 2019, 255, 122599. [Google Scholar] [CrossRef]
- Wei, H.; Zhao, J.; Shang, B.; Zhai, J. Carbamazepine degradation through peroxymonosulfate activation by a-MnO2 with the different exposed facets: Enhanced radical oxidation by facet-dependent surface effect. J. Environ. Chem. Eng. 2022, 10, 108532. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y. A comprehensive review on persulfate activation treatment of wastewater. Sci. Total Environ. 2022, 831, 154906. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, X.; Duan, X.; Sarmah, A.K.; Zhao, X. Remediation of environmentally persistent organic pollutants (POPs) by persulfates oxidation system (PS): A review. Sci. Total Environ. 2023, 863, 160818. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D.; Gonzalez, M.A. Cobalt-mediated activation of peroxymonosulfate and sulfate radical attack on phenolic compounds. Implications of chloride ions. Environ. Sci. Technol. 2006, 40, 1000–1007. [Google Scholar] [CrossRef]
- Zhu, Y.; Sun, Z.; Deng, Y.; Liu, F.; Ruan, W.; Xie, L. Mn2O3/Mn3O4-Cu1.5Mn1.5O4 spinel as an efficient Fenton-like catalyst activating persulfate for the degradation of bisphenol A: Superoxide radicals dominate the reaction. Sci. Total Environ. 2022, 839, 156075. [Google Scholar] [CrossRef]
- Maitra, U.; Naidu, B.S.; Govindaraj, A.; Rao, C.N.R. Importance of trivalency and the eg1 configuration in the photocatalytic oxidation of water by Mn and Co oxides. Proc. Natl. Acad. Sci. USA 2013, 110, 11704–11707. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhou, J.; Liu, D.; Liu, S. Co isomorphic substitution for Cu-based metal organic framework based on electronic structure modulation boosts Fenton-like process. Sep. Purif. Technol. 2023, 306, 122526. [Google Scholar] [CrossRef]
- Zhang, R.; Wan, Y.; Peng, J.; Yao, G.; Zhang, Y.; Lai, B. Efficient degradation of atrazine by LaCoO3Al2O3 catalyzed peroxymonosulfate: Performance, degradation intermediates and mechanism. Chem. Eng. J. 2019, 372, 796–808. [Google Scholar] [CrossRef]
- Xu, H.; Yan, N.; Qu, Z.; Liu, W.; Mei, J.; Huang, W.; Zhao, S. Gaseous Heterogeneous Catalytic Reactions over Mn-Based Oxides for Environmental Applications: A Critical Review. Environ. Sci. Technol. 2017, 51, 8879–8892. [Google Scholar] [CrossRef]
- Li, Q.; Huang, X.; Su, G.; Zheng, M.; Huang, C.; Wang, M.; Ma, C.; Wei, D. The Regular/Persistent Free Radicals and Associated Reaction Mechanism for the Degradation of 1,2,4-Trichlorobenzene over Different MnO2 Polymorphs. Environ. Sci. Technol. 2018, 52, 13351–13360. [Google Scholar] [CrossRef]
- Meng, Y.; Song, W.; Huang, H.; Ren, Z.; Chen, S.-Y.; Suib, S.L. Structure–Property Relationship of Bifunctional MnO2 Nanostructures: Highly Efficient, Ultra-Stable Electrochemical Water Oxidation and Oxygen Reduction Reaction Catalysts Identified in Alkaline Media. J. Am. Chem. Soc. 2014, 136, 11452–11464. [Google Scholar] [CrossRef]
- Robinson, D.M.; Go, Y.B.; Mui, M.; Gardner, G.; Zhang, Z.; Mastrogiovanni, D.; Garfunkel, E.; Li, J.; Greenblatt, M.; Dismukes, G.C. Photochemical Water Oxidation by Crystalline Polymorphs of Manganese Oxides: Structural Requirements for Catalysis. J. Am. Chem. Soc. 2013, 135, 3494–3501. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhang, Q.; Chen, B.-Y.; Hong, J. Oxidation of bisphenol A by persulfate via Fe3O4-α-MnO2 nanoflower-like catalyst: Mechanism and efficiency. Chem. Eng. J. 2019, 357, 337–347. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Zheng, T.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Promoted catalytic transformation of polycyclic aromatic hydrocarbons by MnO2 polymorphs: Synergistic effects of Mn3+ and oxygen vacancies. Appl. Catal. B-Environ. 2020, 272, 119030. [Google Scholar] [CrossRef]
- Yan, J.; Li, Z.; Ma, L.; Han, P.; Zhao, W.; Ye, S. Oxidative degradation of thiocyanate via activation of persulfate by CuO/α-MnO2 composite: Effect of calcination temperature. J. Water. Process. Eng. 2024, 65, 105845. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Z.; Jia, H.; Li, C. Insights into the transformation of dissolved organic matter and carbon preservation on a MnO2 surface: Effect of molecular weight of dissolved organic matter. Sci. Total Environ. 2024, 945, 174022. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.A.; Alhumaimess, M.S.; Alshammari, K.; Al-Shammari, S.A.; Barnawi, A.A.; El-Aassar, M.R. Hydrothermal tailoring of MnFe2O4 nanospinel integrated with biodegradable polymers for improved CO oxidation efficiency. Colloid. Surface. A. 2024, 703, 135221. [Google Scholar] [CrossRef]
- Murtaza, G.; Khan, M.S.; Tahir, K.; Khan, A.U.; Zaki, M.E.A.; Almarhoon, Z.M.; Alanazi, A.A.; Al-Shehri, H.S.; Al-Saeedi, S.I.; Hassan, H.M.A. Photocatalytic applications of A5-mPmH-Zr/CuTiO3 nanocomposite synthesized by simple hydrothermal method. J. Environ. Chem. Eng. 2024, 12, 113345. [Google Scholar] [CrossRef]
- Sajid, M.; Batool, I.; Ullah Khan, A.; Tahir, K.; Alabbad, E.A.; Fahad Alabbosh, K.; Al-Shehri, H.S.; Hassan, H.M.A.; Al-Saeedi, S.I.; Zaki, M.E.A. Synthesis of Adenosine 5-monophosphate monohydrate functionalized mesoporous Ag/CuO/MCM-41 nanostructures for enormous mineralization of insistent organic contaminants and photoinhibition of bacteria. J. Mol. Li. 2023, 391, 123379. [Google Scholar] [CrossRef]
- Wan, J.; Zhou, L.; Deng, H.; Zhan, F.; Zhang, R. Oxidative degradation of sulfamethoxazole by different MnO2 nanocrystals in aqueous solution. J. Mol. Catal. A-Chem. 2015, 407, 67–74. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Li, A.; Zhou, J.; Zhang, Y.; Wang, T.; Jia, H.; Zhu, L. Enhancing Cu-EDTA decomplexation in a discharge plasma system coupled with nanospace confined iron oxide: Insights into electron transfer and high-valent iron species. Appl. Catal. B-Environ. 2024, 345, 123717. [Google Scholar] [CrossRef]
- Chen, Y.; Bai, X.; Ji, Y.; Chen, D. Enhanced activation of peroxymonosulfate using ternary MOFs-derived MnCoFeO for sulfamethoxazole degradation: Role of oxygen vacancies. J. Hazard. Mater. 2023, 441, 129912. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Z.; Cao, Y.; Chen, Y.; Du, X.; Yang, Y.; Wang, Z. Two-dimensional ultrathin perforated Co3O4 nanosheets enhanced PMS-Activated selective oxidation of organic micropollutants in environmental remediation. Chem. Eng. J. 2022, 427, 131953. [Google Scholar] [CrossRef]
- Wang, Z.; Jia, H.; Liu, Z.; Peng, Z.; Dai, Y.; Zhang, C.; Guo, X.; Wang, T.; Zhu, L. Greatly enhanced oxidative activity of δ-MnO2 to degrade organic pollutants driven by dominantly exposed facets. J. Hazard. Mater. 2021, 413, 125285. [Google Scholar] [CrossRef]
- Zhu, C.; Li, C.; Zheng, M.; Delaunay, J.-J. Plasma-Induced Oxygen Vacancies in Ultrathin Hematite Nanoflakes Promoting Photoelectrochemical Water Oxidation. ACS. Appl. Mater. Interfaces 2015, 7, 22355–22363. [Google Scholar] [CrossRef]
- Devi, R.; Kumar, V.; Kumar, S.; Bulla, M.; Sharma, S.; Sharma, A. Electrochemical analysis of MnO2 (α, β, and γ)-based electrode for high-performance supercapacitor application. Appl. Sci. 2023, 13, 7907. [Google Scholar] [CrossRef]
- Fu, J.; Luo, X. A first-principles investigation of α, β, and γ-MnO2 as potential cathode materials in Al-ion batteries. RSE Adv. 2020, 10, 39895–39900. [Google Scholar] [CrossRef]
- Wang, T.; Xu, K.-M.; Yan, K.-X.; Wu, L.-G.; Chen, K.-P.; Wu, J.-C.; Chen, H.-L. Comparative study of the performance of controlled release materials containing mesoporous MnOx in catalytic persulfate activation for the remediation of tetracycline contaminated groundwater. Sci. Total Environ. 2022, 846, 157217. [Google Scholar] [CrossRef]
- Li, H.; Li, T.; He, S.; Zhou, J.; Wang, T.; Zhu, L. Efficient degradation of antibiotics by non-thermal discharge plasma: Highlight the impacts of molecular structures and degradation pathways. Chem. Eng. J. 2020, 395, 125091. [Google Scholar] [CrossRef]
- Pi, L.; Yang, N.; Han, W.; Xiao, W.; Wang, D.; Xiong, Y.; Zhou, M.; Hou, H.; Mao, X. Heterogeneous activation of peroxymonocarbonate by Co-Mn oxides for the efficient degradation of chlorophenols in the presence of a naturally occurring level of bicarbonate. Chem. Eng. J. 2018, 334, 1297–1308. [Google Scholar] [CrossRef]
- Li, D.; Zhang, S.; Li, S.; Tang, J.; Hua, T.; Li, F. Mechanism of the application of single-atom catalyst-activated PMS/PDS to the degradation of organic pollutants in water environment: A review. J. Clean. Prod. 2023, 397, 136468. [Google Scholar] [CrossRef]
- Golshan, M.; Kakavandi, B.; Ahmadi, M.; Azizi, M. Photocatalytic activation of peroxymonosulfate by TiO2 anchored on cupper ferrite (TiO2@CuFe2O4) into 2,4-D degradation: Process feasibility, mechanism and pathway. J. Hazard. Mater. 2018, 359, 325–337. [Google Scholar] [CrossRef]
- Liang, F.; Liu, Z.; Jiang, X.; Li, J.; Xiao, K.; Xu, W.; Chen, X.; Liang, J.; Lin, Z.; Li, M.; et al. NaOH-modified biochar supported Fe/Mn bimetallic composites as efficient peroxymonosulfate activator for enhance tetracycline removal. Chem. Eng. J. 2023, 454, 139949. [Google Scholar] [CrossRef]
- Jin, X.; Huang, Y.; He, S.; Chen, G.; Liu, X.; He, C.; Du, C.; Chen, Q. Preparation of Co-Fe based Prussian blue analogs loaded nickel foams for Fenton-like degradation of tetracycline. Appl. Catal. A-Gen. 2023, 650, 118985. [Google Scholar] [CrossRef]
- Yuan, R.; Ramjaun, S.N.; Wang, Z.; Liu, J. Effects of chloride ion on degradation of Acid Orange 7 by sulfate radical-based advanced oxidation process: Implications for formation of chlorinated aromatic compounds. J. Hazard. Mater. 2011, 196, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, S.; Liao, W.; Wang, T. Enhanced sulfamethoxazole degradation using an efficient Co-doped MnO2 activator for peroxymonosulfate activation. Desalin. Water. Treat. 2024, 317, 100140. [Google Scholar] [CrossRef]
- Luo, F.; Yang, D.; Chen, Z.; Megharaj, M.; Naidu, R. One-step green synthesis of bimetallic Fe/Pd nanoparticles used to degrade Orange II. J. Hazard. Mater. 2016, 303, 145–153. [Google Scholar] [CrossRef]
- Lau, Y.-Y.; Wong, Y.-S.; Teng, T.-T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Coagulation-flocculation of azo dye Acid Orange 7 with green refined laterite soil. Chem. Eng. J. 2014, 246, 383–390. [Google Scholar] [CrossRef]
- Gong, C.; Chen, F.; Yang, Q.; Luo, K.; Yao, F.; Wang, S.; Wang, X.; Wu, J.; Li, X.; Wang, D.; et al. Heterogeneous activation of peroxymonosulfate by Fe-Co layered doubled hydroxide for efficient catalytic degradation of Rhoadmine B. Chem. Eng. J. 2017, 321, 222–232. [Google Scholar] [CrossRef]
- Zhao, C.; Meng, L.; Chu, H.; Wang, J.-F.; Wang, T.; Ma, Y.; Wang, C.-C. Ultrafast degradation of emerging organic pollutants via activation of peroxymonosulfate over Fe3C/Fe@N-C-x: Singlet oxygen evolution and electron-transfer mechanisms. Appl. Catal. B-Environ. 2023, 321, 122034. [Google Scholar] [CrossRef]
- Styanova, M.; Slavova, I.; Christoskova, S.; Ivanova, V. Catalytic performance of supported nanosized cobalt and iron-cobalt mixed oxides on MgO in oxidative degradation of Acid Orange 7 azo dye with peroxymonosulfate. Appl. Catal. A Gen. 2014, 476, 121–132. [Google Scholar] [CrossRef]
- Cai, C.; Liu, Y.; Xu, R.; Zhou, J.; Zhang, J.; Chen, Y.; Liu, L.Y.; Zhang, L.X.; Kang, S.; Xie, X. Bicarbonate enhanced heterogeneous activation of peroxymonosulfate by copper ferrite nanoparticles for the efficient degradation of refractory organic contaminants in water. Chemosphere 2023, 312, 137285. [Google Scholar] [CrossRef]
- Liu, X.; Xu, P.; Fu, Q.; Li, R.; He, C.; Yao, W.; Wang, L.; Xie, S.; Xie, Z.; Ma, J.; et al. Strong degradation of orange II by activation of peroxymonosulfate using combination of ferrous ion and zero-valent copper. Sep. Purif. Technol. 2022, 278, 119509. [Google Scholar] [CrossRef]
- Zuo, S.; Zhao, L.; Zou, X.; Wu, Y.; Wang, L.; Luo, L.; He, Y.; Zhang, Y. Efficient activation of peroxymonosulfate by CoMg2Mn-LDO for the degradation of orange II: The important synergy of Mg. Chemosphere 2023, 340, 139923. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Wang, T.; Zhu, L. Highly efficient photocatalytic degradation toward perfluorooctanoic acid by bromine doped BiOI with high exposure of (001) facet. Appl. Catal. B-Environ. 2020, 268, 118442. [Google Scholar] [CrossRef]
- Liang, R.; Shen, L.; Jing, F.; Qin, N.; Wu, L. Preparation of MIL-53(Fe)-Reduced Graphene Oxide Nanocomposites by a Simple Self-Assembly Strategy for Increasing Interfacial Contact: Efficient Visible-Light Photocatalysts. ACS. Appl. Mater. Interfaces 2015, 7, 9507–9515. [Google Scholar] [CrossRef]
- He, S.; Li, T.; Zhang, L.; Zhang, X.; Liu, Z.; Zhang, Y.; Wang, J.; Jia, H.; Wang, T.; Zhu, L. Highly effective photocatalytic decomplexation of Cu-EDTA by MIL-53(Fe): Highlight the important roles of Fe. Chem. Eng. J. 2021, 424, 130515. [Google Scholar] [CrossRef]

| System | Concentration | Condition | Degradation Rate | References |
|---|---|---|---|---|
| Co/MgO/PMS | 50 mg/L | Catalyst = 150 mg/L PMS: AO7 = 10:1, pH = 4.0 | >99% 10 min | [56] |
| CuFe2O4+HCO3/ PMS | 100 mg/L | PMS = 0.98 mmol/L HCO3 = 2 mmol/L pH = 5.9 ± 0.1 | 95 % 30 min | [57] |
| Fe(II)/PMS | 25 μM | Catalyst = 30 μM PMS = 1.5 mM pH = 3.15 | 39% 6 min | [58] |
| ZVC/PMS | 25 μM | Catalyst = 0.3 g/L PMS = 1.5 mM pH = 3.15 | 45% 10 min | |
| Fe(II)/ZVC/PMS | 25 μM | MS = 1.5 mM pH = 3.15 | 96% 10 min | |
| CoMg2Mn-LDO | 100 mg/L | Catalyst = 90 mg PMS = 100 mg pH = 3.0 | 97% 15 min | [59] |
| α-MnO2 | 50 mg | Catalyst = 50 mg PMS 0.05 mol/L pH = 3.0 | 98.3% 15 min | This study |
| Reagents | Molecular Formula | Grade | Manufacturer |
|---|---|---|---|
| Ammonium persulfate | (NH4)2S2O8 | AR | Shanghai Aladdin, Co., Ltd., Shanghai, China |
| Ammonium sulfate | (NH4)2SO4 | AR | |
| Manganese sulfate | MnSO4·H2O | AR | |
| Orange II | C16H11N2NaO4S | AR | |
| Sodium persulfate | Na2S2O8 | AR | |
| Methyl alcohol | CH3OH | AR | |
| Tertiary butanol | C4H10O | AR | |
| Furfuryl alcohol | C5H6O2 | AR | |
| Sodium nitrate | NaNO3 | AR | |
| Humic acid | C64O26H55N4 | AR | |
| Furfuryl alcohol | C5H6O2 | AR | |
| p-benzoquinone | C6H4O2 | AR | Shanghai Maclin Biochemical Technology, Co., Ltd., Shanghai, China |
| Sodium chloride | NaCl | AR | |
| Anhydrous sodium sulfate | Na2SO4 | AR | Sinopharm Group Chemical Reagent, Co., Ltd., Shanghai, China |
| Sodium carbonate anhydrous | Na2CO3 | AR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Guo, H.; Li, H.; Wang, T. Performance of Dye-Containing Wastewater Treatment Using MnxOy-Catalyzed Persulfate Oxidation. Catalysts 2024, 14, 758. https://doi.org/10.3390/catal14110758
Li Y, Guo H, Li H, Wang T. Performance of Dye-Containing Wastewater Treatment Using MnxOy-Catalyzed Persulfate Oxidation. Catalysts. 2024; 14(11):758. https://doi.org/10.3390/catal14110758
Chicago/Turabian StyleLi, Yujuan, He Guo, Hu Li, and Tiecheng Wang. 2024. "Performance of Dye-Containing Wastewater Treatment Using MnxOy-Catalyzed Persulfate Oxidation" Catalysts 14, no. 11: 758. https://doi.org/10.3390/catal14110758
APA StyleLi, Y., Guo, H., Li, H., & Wang, T. (2024). Performance of Dye-Containing Wastewater Treatment Using MnxOy-Catalyzed Persulfate Oxidation. Catalysts, 14(11), 758. https://doi.org/10.3390/catal14110758







