Abstract
A stable and efficient porous nickel phosphate (p-NiPO/Ti) electrocatalyst on titanium sheets was developed via electrochemical deposition and low-temperature phosphatization. For obtaining the optimal performance of the p-NiPO/Ti electrocatalyst, the optimized experimental parameters of deposition and phosphatization were determined by parallel experiments. After the preparation, XPS and XRD were used to validate the chemical and amorphous structure, with SEM and TEM simultaneously validating a distinct nanosheet/nanocluster crosslinked microstructure. In particular, with phosphatization conditions maintained at 300 °C for 10 min, the p-NiPO/Ti produced demonstrated excellent charge transfer and catalytic characteristics in 1.0 M KOH. The electrocatalytic results revealed that the optimal p-NiPO/Ti with excellent catalytic performance and excellent stability (~24 h) needs lower HER overpotentials (128 mV at 10 mA cm−2 and 242 mV at 100 mA cm−2) as inputs. This research provides a promising strategy with which to use transition metal materials as catalysts in alkaline electrocatalytic hydrogen production.
1. Introduction
Hydrogen energy, with its advantages of high energy density, cleanliness, and non-pollution, is regarded as an important source for future energy development [1,2,3]. The electrocatalytic hydrogen generation process, as an efficient and environmentally friendly energy conversion method, can convert electrical energy generated from renewable energy sources (e.g., solar energy, wind energy, etc.) into hydrogen, which provides important support for a sustainable energy system [4,5,6]. The most common catalysts for water electrolysis are the noble-metal-based (Pt) catalysts used in industry applications, with advantages of lower overpotentials and higher exchange current densities, but whose high cost, low earth abundance, and poor durability limit their further utilization, to some extent, in HER [7,8,9]. Compared to the regular noble catalysts, transition metal materials possess unique d-orbital electronic structures, which furnish empty orbitals and lone-pair electrons during chemical reactions, thereby reducing the reaction activation energy [10,11]. Transition metal ions and ligands interact through ligand bonds, making the electronic state and charge arrangement re-distributed, influencing the structure and properties of active sites, and further enhancing the reaction rate [12,13]. Furthermore, transition metal catalysts can engage in the reaction process via various methods, such as redox reactions, acid–base reactions, and the formation and cleavage of ligand bonds, and all of the catalytic reaction mechanisms of transition metals exhibit remarkable performances [14,15]. At present, the prevalent transition metal electrocatalysts encompass transition metal hydroxides/oxides, nitrides, sulfides, phosphides, and MXene materials [16,17,18,19,20].
Among the transition metal catalysts, transition metal phosphides (TMPs) exhibit superior electrical conductivity, electrochemical activity, and stability. These significant advantages endow them with broader application prospects in the field of electrocatalysis [21,22,23]. In particular, nickel–phosphate materials featuring three-dimensional mesh structures possess unique Ni-O-P and P-O-P bonds, which offer abundant active sites and facilitate efficient pathways for electron transport and catalytic reactions [24,25].
For example, Ying et al. [26] synthesized nickel metaphosphate (Ni2P4O12) electrocatalysts for the first time by a one-step calcination method using n-octyl ammonium hypophosphite as a phosphorus source. The prepared Ni2P4O12 has a porous structure, which increases the exposed area of the catalyst, thus enhancing the electrocatalytic effect and durability for HER in alkaline electrolytes. Syed et al. [27] used the hydrothermal method to directly synthesize unique one-dimensional (1D) nickel phosphate (NiPO) nano pins on nickel foam (NF). The in situ synthesis of NiPO/NF catalysts achieved simple electrode preparation and measurement operation, with an extra advantage of increasing the contact between the active sites and the conductive substrate. Under alkaline conditions, the NiPO/NF electrode exhibited a low overpotential of 374 mV for the hydrogen-evolution reaction (HER) and 377 mV for the oxygen-evolution reaction (OER) at a current density of 250 mA cm−2, as a bifunctional catalyst. However, further enhancement of the electrocatalytic stability and hydrogen precipitation performance of nickel phosphate is still required before industrial application.
In this paper, we employ two strategies to enhance the electrocatalytic stability and hydrogen evolution performance of nickel phosphate: (1) utilizing Ti sheets, known for their excellent electrical conductivity, high stability, and corrosion resistance, as the substrate material; and (2) depositing a porous Ni(OH)2 (p-Ni(OH)2) layer on the surface of the titanium plates via electrochemical deposition, followed by converting the p-Ni(OH)2 into porous amorphous NiPO (p-NiPO) through a straightforward low-temperature phosphatization process. The catalytic activity of the p-NiPO/Ti was optimized by adjusting the conditions of electrodeposition and phosphatization. The experimental results demonstrated that the prepared p-NiPO/Ti under optimal low-temperature phosphatization conditions exhibited high electrocatalytic performance and excellent stability for HER in alkaline electrolytes.
2. Results and Discussions
2.1. Schematic Diagram
Scheme 1 depicts the schematic of the synthesis of the p-NiPO on the Ti plates in two steps. Firstly, the p-Ni(OH)2/Ti was synthesized using hydrogen bubbles as templates formed in situ by electrochemical deposition technology [28]. The preparation conditions of the materials were optimized by testing their hydrogen evolution performance in an alkaline solution. As shown in Figures S1 and S2, the optimal deposition potential of the p-Ni(OH)2/Ti nanomaterial was set at −1.0 V (vs Ag/AgCl), and the amount of deposition charge was −0.25 C. Subsequently, the p-NiPO/Ti was prepared via a short-duration and high-temperature phosphating process. By optimizing the phosphating conditions, the temperature was set at 300 °C, the duration was 10 min, and the phosphorus-to-nickel ratio was 20:1, based on the sample morphology and hydrogen evolution performance of the p-NiPO (as shown in Figures S3–S5). The subsequent analysis and characterization of the p-NiPO nanomaterials were conducted under these optimal conditions. Both the s-Ni(OH)2/Ti and the s-NiPO/Ti were prepared with the optimal condition of p-Ni(OH)2/Ti and p-NiPO/Ti.

Scheme 1.
Schematic Diagram of p-NiPO/Ti Preparation Process.
2.2. Structural Properties (SEM, TEM, XRD, XPS)
2.2.1. SEM and TEM
The morphology and microstructures were examined by scanning electron microscopy (SEM) and transmission electron microscopy (TEM). Figure 1a and Figure 1c show the SEM images of the s-Ni(OH)2/Ti and p-Ni(OH)2/Ti nanostructures, respectively. The p-Ni(OH)2/Ti nanoclusters of varying sizes are stacked together with numerous cracks, resulting in a rough surface. On the other hand, the s-Ni(OH)2/Ti exhibits a tightly packed surface, forming a dense film, as shown in Figure 1c. After low-temperature phosphorization, the SEM images of the s-NiPO/Ti and p-NiPO/Ti were synthesized, as shown in Figure 1b and Figure 1d, respectively. Figure 1b shows that the s-NiPO/Ti is composed of a dense nanofilm with tiny nano protrusions on the surface. The p-NiPO/Ti nanocluster shows a stacking structure in Figure 1d, with thick nanosheets with thicknesses of 30–50 nm growing at the cracks in the stacking and arranged vertically within the cracks. The morphology structure provides the catalytic electrode with a high surface area and diffusion of electrolytes/ions, enhancing the electrocatalytic activity. The TEM and HRTEM images of the p-NiPO are shown in Figure 1e,f, revealing the presence of abundant microporous structures of p-NiPO. These microstructures are beneficial for enhancing the contact between the catalyst and the electrolyte, exposing more active sites for catalytic reactions. An elemental distribution analysis, depicted in the elemental mapping image in Figure 1g, demonstrates the uniform distribution of Ni, O, and P elements in the sample, indicating the material’s homogeneity. In Figure 1h, the electron diffraction pattern displays no distinct electron diffraction rings, suggesting that the generated p-NiPO exhibits an amorphous structure, corroborating the X-ray diffraction (XRD) results (as shown in Figure S6).
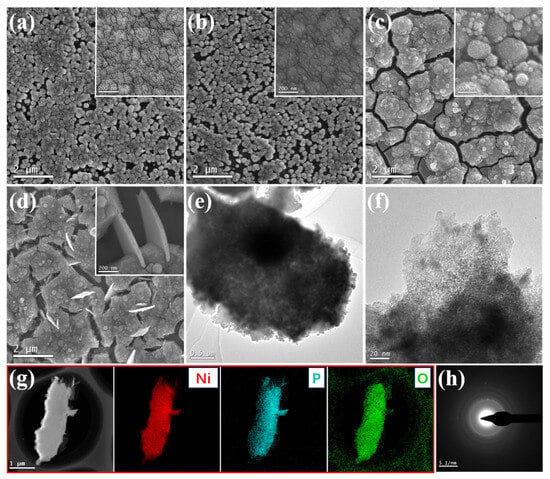
Figure 1.
The SEM images of (a) s-Ni(OH)2/Ti, (b) s-NiPO/Ti, (c) p-Ni(OH)2/Ti, and (d) p-NiPO/Ti; (e) TEM image of p-NiPO/Ti; (f) HRTEM image of p-NiPO; (g) EDS elemental mapping of p-NiPO; (h) SAED diffraction pattern of p-NiPO.
2.2.2. XPS
To further elucidate the electronic-state-associated catalytic activities of the main elements, an X-ray photoelectron spectroscopy (XPS) analysis was performed to assess the chemical composition and surface electronic states. Figure 2 shows the XPS spectrum of the p-Ni(OH)2/Ti and p-NiPO/Ti (Figure 2, the solid line represents the XPS fitting results). The wide-scan XPS spectrum confirms the presence of P elements in the p-NiPO/Ti compared with the p-Ni(OH)2/Ti. Regarding the XPS Ni 2p spectrum shown in Figure 2b, two main orbital peaks at 856.5 eV and 874.1 eV are observable in the p-NiPO/Ti, which corresponds to the spin–orbit splitting of the Ni2+ 2p3/2 and Ni2+ 2p1/2, respectively, attributed to the divalent nickel ions (Ni2+) [29,30]. Moreover, two broader peaks at 861.8 eV and 879.7 eV correspond to their satellite peaks [31]. At the same time, the p-Ni(OH)2/Ti also appears as the orbital peak of Ni2+ and its corresponding satellite peak. It is worth noting that the Ni 2p orbital peak of the p-NiPO/Ti has a significant positive shift relative to the p-Ni(OH)2/Ti, which is caused by the rearrangement of electrons on the Ni due to the formation of chemical bonds between the Ni2+ and the surrounding P elements. This electronic reallocation is beneficial for HER performance improvement. The P 2p spectrum of the p-NiPO is shown in Figure 2c, with one major peak observable at 133.5 eV, attributed to the P-O bond in the phosphate group. A small peak is shown at 130.7 eV, which corresponds to the Ni-P bond [32,33]. In Figure 2d, p-the Ni(OH)2/Ti is shown clearly -OH group in the O 1s spectrum at 531.4 eV [34]. The O 1s spectrum of the p-NiPO can be fitted with two peaks at 531.4 eV and 533.0 eV, which are assigned to the P-O-Ni bond and P=O bond, respectively [35,36]. These features in the XPS spectra demonstrate that we successfully synthesized the p-NiPO/Ti and p-Ni(OH)2/Ti catalysts.
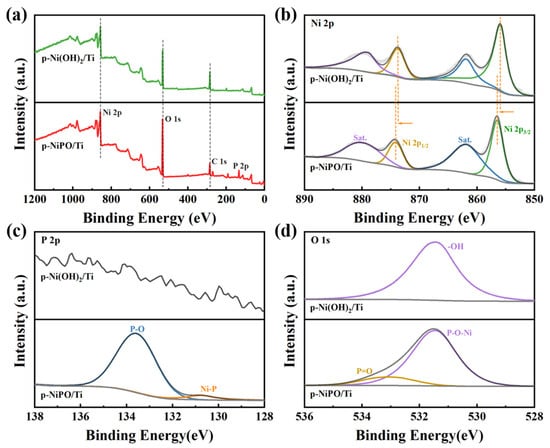
Figure 2.
XPS spectra of p-Ni(OH)2/Ti and p-NiPO/Ti: (a) Overall spectrum; (b) Ni 2p, (c) P 2p, and (d) O1s spectra, respectively.
2.3. The Electrocatalytic Testing for Hydrogen Generation
The electrochemical performances of the obtained samples were evaluated in a three-electrode configuration. Figure 3a shows the linear sweep voltammetry (LSV) curves for the HER of the s-NiPO/Ti, p-NiPO/Ti, s-Ni(OH)2/Ti, p-Ni(OH)2/Ti, and Ti sheet catalysts, with a scan rate of 5 mV/s in 1 M KOH. Among them, the Ti sheet shows the lowest HER performance. In the NiPO/Ti and Ni(OH)2/Ti catalysts, the porous materials show higher current density. The catalyst performance of the p-NiPO/Ti was superior to that of the others in this work. As shown in Figure 3b, the overpotentials of the p-NiPO/Ti at current densities of 10 mA cm−2 (η10) and 100 mA cm−2 (η100) are 128 mV and 242 mV, respectively, significantly lower than those of the s-Ni(OH)2/Ti (368 mV and 484 mV), p-Ni(OH)2/Ti (240 mV and 315 mV), and s-NiPO/Ti (236 mV and 356 mV), demonstrating a good HER catalytic performance. The Tafel slopes (Figure 3c) indicate that the p-NiPO/Ti presents the lowest value (79.2 mV dec−1) among s-Ni(OH)2/Ti (138.3 mV dec−1), p-Ni(OH)2/Ti (109.7 mV dec−1) and s-NiPO/Ti (116.7 mV dec−1). This result reveals that the p-NiPO/Ti catalyst has superior catalytic kinetics during the HER process. As shown in Figure 3d, it can be concluded that the charge transfer resistance (Rct) of the p-NiPO/Ti is 5.26 Ω, lower than those of the s-Ni(OH)2/Ti (88.04 Ω), p-Ni(OH)2/Ti (17.33 Ω), and s-NiPO/Ti (36.02 Ω). This indicates that in the HER process, the conductivity between the p-NiPO/Ti catalyst and the electrolyte is higher, leading to a faster charge transfer rate. This is consistent with the low overpotential and excellent Tafel slope exhibited by the p-NiPO/Ti.
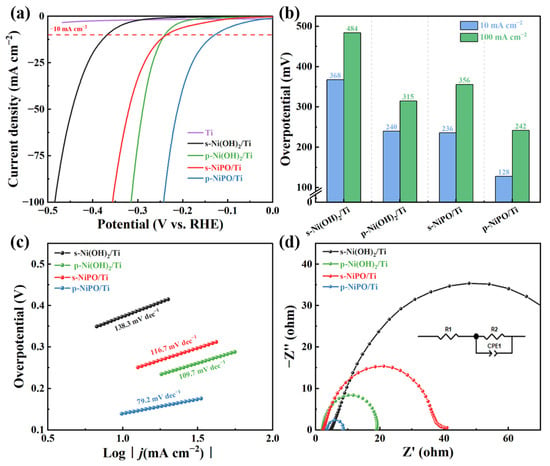
Figure 3.
Hydrogen evolution properties of s-Ni(OH)2/Ti, p-Ni(OH)2/Ti, s-NiPO/Ti, and p-NiPO/Ti: (a) LSV polarization curve; (b) comparison of overpotentials at 10 mA cm−2 and 100 mA cm−2; (c) Tafel slope; (d) electrochemical impedance spectroscopy (EIS) plots.
Furthermore, the HER values were tested for more than 24 h to assess the durability of the p-NiPO/Ti. As shown in Figure 4a, the p-NiPO/Ti still retained good HER (24 h at 10 mA cm−2) catalytic activity after the durability test. Figure 4b shows the LSV curve of the p-NiPO/Ti before and after the reaction, indicating its superior catalytic stability. Figures S8 and S9 present the SEM and XPS of the p-NiPO/Ti after the 24 h test. It shows good stability. The capacitance of the double layer (Cdl) is directly proportional to the electrochemical active surface area (ECSA) and can be used to compare the structural advantages and intrinsic activity of prepared electrocatalysts (more detailed information is shown in Figure S7) [37]. As shown in Figure 4c, the Cdl of the p-Ni(OH)2/Ti is 0.590 mF cm−2, which is 16.4 times that of the s-Ni(OH)2/Ti. After low-temperature phosphorization, the Cdl of the p-NiPO/Ti reaches 1.629 mF cm−2, which is 32.6 times that of the p-Ni(OH)2/Ti, as shown in Figure 4d. The maximum Cdl of the p-NiPO/Ti proves its maximum electrochemically active surface area, which is also the main reason for its superior performance over other catalysts. The test results indicate that low-temperature phosphorization can effectively increase the electrochemically active area of the catalyst, enhance the exposure of active sites in the electrolyte solution, and thereby improve catalytic performance.
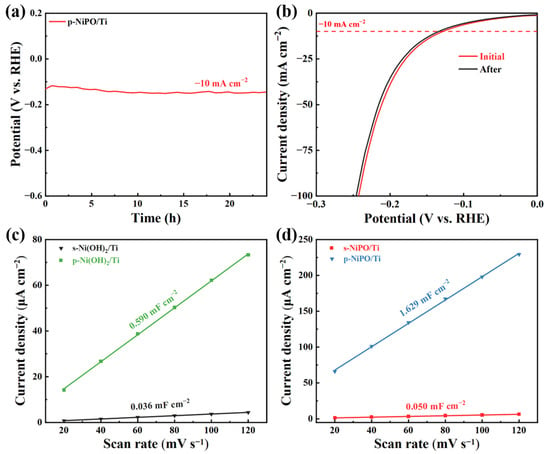
Figure 4.
(a) Stability test curve of p-NiPO; (b) LSV curves before and after stability testing. The capacitance properties of the double layer (Cdl) (c) s-Ni(OH)2/Ti, p-Ni(OH)2/Ti, (d) s-NiPO/Ti, and p-NiPO/Ti.
Table 1 summarizes the synthetic conditions (i.e., phosphorus source and heating treatments) and the corresponding HER performances of different Nickel–phosphorus catalysts reported in the literature. It is worth noting that the p-NiPO/Ti electrode material prepared in this work not only reduces the phosphating time and energy consumption during preparation, but shows excellent electrocatalytic hydrogen evolution performance as well, which could be potentially applied in industry in the future.

Table 1.
Comparison of HER Electrocatalytic Performance of p-NiPO/Ti in 1 M KOH with Materials Reported in the Literature.
3. Materials and Methods
NiPO/Ti electrodes were manufactured by electrodeposition and low-temperature phosphatization.
3.1. The Preparation of Porous/Smooth (p/s)-Ni(OH)2/Ti
The 0.5 cm × 0.5 cm Ti plate substrates were ultrasonically cleaned with 2 M HCl, acetone, and ethanol for 15 min, respectively. The substrates were then washed with deionized water and dried in a vacuum oven at 60 °C for 1 h. During the electrodeposition process, the following were used: an Ag/AgCl (3 M KCl) as the reference electrode, a graphite counter electrode, and the pre-processed Ti plate as the working electrode.
The electrolyte solution (0.1 M NiCl2·6H2O) was maintained at a temperature of 30 °C. The p-Ni(OH)2/Ti was synthesized by electrodeposition experiments using chronoamperometry (I-t) at a deposition potential of −1.0 V and a deposition charge of −0.25 C. The procedure for preparing s-Ni(OH)2/Ti was similar to that of the p-Ni(OH)2/Ti, except for using an electrolyte solution containing 0.1 M NiCl2·6H2O and 0.1 M H3BO3.
3.2. The Preparation of Porous/Smooth (p/s)-NiPO/Ti
The p/s-Ni(OH)2/Ti nanoparticles were further phosphorized using a tube furnace. The upstream side of the porcelain crucible was filled with NaH2PO2·H2O particles (12 mg), while the opposite side was filled with freshly manufactured p/s-Ni(OH)2/Ti. The p/s-Ni(OH)2/Ti was heated to 300 °C at a rate of 5 °C/min for 10 min under an Ar flow. After cooling to room temperature and washing with ethanol and deionized water, p/s-NiPO/Ti was synthesized. Supplementary Materials contain further information on the materials’ characterizations and electrocatalytic activity tests.
4. Conclusions
The p-NiPO/Ti electrocatalyst was synthesized by the electrochemical deposition and low-temperature phosphatization method on Ti plates. It was noted that the p-NiPO/Ti electrocatalyst prepared by this method has a unique nanosheet/nanocluster crosslinking structure. The optimized p-NiPO/Ti electrocatalyst shows superior charge transfer and catalytic performance in the alkaline water cracking process. The electrocatalytic results show that the optimized p-NiPO/Ti has a lower HER overpotential (128 mV at 10 mA cm−2 and 242 mV at 100 mA cm−2) and a higher stability within 24 h at 10 mA cm−2. In addition, the Ti sheet, as the substrate for the controllable growth of the catalytic material, helps to enhance electrode compatibility in industrial applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal14110757/s1. Figure S1: (a) LSV polarization curves of p-Ni(OH)2/Ti at different deposition potentials; (b) Comparison of hydrogen evolution overpotentials at different current densities; Figure S2: (a) LSV polarization curves of Ni(OH)2/Ti under different deposited charges; (b) Comparison of hydrogen evolution overpotentials at different current densities; Figure S3: (a) LSV polarization curves of p-NiPO/Ti at different phosphating temperatures; (b) Comparison of hydrogen evolution overpotentials at different current densities; Figure S4: (a) LSV polarization curves of p-NiPO/Ti at different phosphating times; (b) Comparison of hydrogen evolution overpotentials at different current densities; Figure S5: (a) LSV polarization curves of p-NiPO/Ti at different P/Ni ratios; (b) Comparison of hydrogen evolution overpotential at different current densities; Figure S6: The XRD images of p-Ni(OH)2/Ti and p-NiPO/Ti; Figure S7: CV curves at different scanning speeds: (a) s-Ni(OH)2/Ti, (b) p-Ni(OH)2/Ti, (c) s-NiPO/Ti, (d) p-NiPO/Ti. Figure S8: The SEM images of p-NiPO/Ti after 24-h test; Figure S9: XPS spectra of p-NiPO/Ti after the 24-h test: (a) Ni 2p; (b) P 2p and (c) O 1s.
Author Contributions
H.W. and L.L. designed the concept. T.H. and H.W. performed all the experiments. T.H. and H.W. analyzed the experiment results and revised the draft. Y.X. and L.Z. carried out most of the characterizations. L.P., H.W., L.L., and P.L. co-wrote the manuscript and Supplementary Materials. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Jiaxing Nanhu University research project (62314YL), the research start-up funds of Jiaxing Nanhu University (70500000/075), and the Jiaxing public welfare research project (2024AY10053).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, L.; Jia, C.; Bai, F.; Wang, W.; An, S.; Zhao, K.; Li, Z.; Li, J.; Sun, H. A comprehensive review of the promising clean energy carrier: Hydrogen production, transportation, storage, and utilization (HPTSU) technologies. Fuel 2024, 355, 129455. [Google Scholar] [CrossRef]
- Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M.; Al-Jiboory, A.K. Hydrogen energy future: Advancements in storage technologies and implications for sustainability. J. Energy Storage 2023, 72, 108404. [Google Scholar] [CrossRef]
- Xu, X.; Sun, H.; Jiang, S.P.; Shao, Z. Modulating metal–organic frameworks for catalyzing acidic oxygen evolution for proton exchange membrane water electrolysis. SusMat 2021, 1, 460–481. [Google Scholar] [CrossRef]
- Liu, X.; Han, Y.; Guo, Y.; Zhao, X.; Pan, D.; Li, K.; Wen, Z. Electrochemical Hydrogen Generation by Oxygen Evolution Reaction-Alternative Anodic Oxidation Reactions. Adv. Energy Sustain. Res. 2022, 3, 2200005. [Google Scholar] [CrossRef]
- Gong, Y.; Yao, J.; Wang, P.; Li, Z.; Zhou, H.; Xu, C. Perspective of hydrogen energy and recent progress in electrocatalytic water splitting. Chin. J. Chem. Eng. 2022, 43, 282–296. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, X.; Yu, B.; Zhang, W.; Zhu, X.; Liu, Z. Engineering of P vacancies and phosphate on Fe-doped Ni2P nanosheet arrays for enhanced oxygen evolution. J. Alloys Compd. 2022, 905, 164023. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Lee, E.B.; Jo, S.G.; Park, G.-R.; Lee, J.; Kim, C.-S.; Kim, S.J.; Lee, J.W. Facile and fast route of electrodepositing chloride ion-modified NiFePt layered double hydroxides for hydrogen evolution reaction. Appl. Surf. Sci. 2024, 662, 160112. [Google Scholar] [CrossRef]
- Sun, J.; Ren, M.; Yu, L.; Yang, Z.; Xie, L.; Tian, F.; Yu, Y.; Ren, Z.; Chen, S.; Zhou, H. Highly Efficient Hydrogen Evolution from a Mesoporous Hybrid of Nickel Phosphide Nanoparticles Anchored on Cobalt Phosphosulfide/Phosphide Nanosheet Arrays. Small 2019, 15, 1804272. [Google Scholar] [CrossRef]
- Bhavanari, M.; Lee, K.-R.; Tseng, C.-J.; Su, B.-J.; Chen, J.-M.; Chang, J.-K.; Bhattacharyya, A.J.; Su, C.-Y. New insights into interface charge-transfer mechanism of copper-iron layered double hydroxide cathodic electrocatalyst in alkaline electrolysis. J. Environ. Chem. Eng. 2022, 10, 107287. [Google Scholar] [CrossRef]
- Xiong, L.; Qiu, Y.; Peng, X.; Liu, Z.; Chu, P.K. Electronic structural engineering of transition metal-based electrocatalysts for the hydrogen evolution reaction. Nano Energy 2022, 104, 107882. [Google Scholar] [CrossRef]
- Jin, X.; Li, X.; Lei, H.; Guo, K.; Lv, B.; Guo, H.; Chen, D.; Zhang, W.; Cao, R. Comparing electrocatalytic hydrogen and oxygen evolution activities of first-row transition metal complexes with similar coordination environments. J. Energy Chem. 2021, 63, 659–666. [Google Scholar] [CrossRef]
- Sun, H.; Kim, H.; Song, S.; Jung, W. Copper foam-derived electrodes as efficient electrocatalysts for conventional and hybrid water electrolysis. Mater. Rep. Energy 2022, 2, 100092. [Google Scholar] [CrossRef]
- Zhang, Z.; Ye, K.; Du, H.; Li, X. In situ electrodeposition synthesis of CoP@NiFe LDH heterostructure as high-performance electrocatalyst for enhanced seawater electrolysis. Int. J. Hydrogen Energy 2024, 62, 722–731. [Google Scholar] [CrossRef]
- Chen, S.; Hu, J.; Zhou, H.Q.; Yu, F.; Wu, C.M.; Chung, L.H.; Yu, L.; He, J. Microenvironment Regulation of Metal-Organic Frameworks to Anchor Transition Metal Ions for the Electrocatalytic Hydrogen Evolution Reaction. Inorg Chem 2022, 61, 19475–19482. [Google Scholar] [CrossRef]
- Cao, F.; Li, M.; Hu, Y.; Wu, X.; Li, X.; Meng, X.; Zhang, P.; Li, S.; Qin, G. Kinetically accelerated oxygen evolution reaction in metallic (oxy)hydroxides enabled by Cr-dopant and heterostructure. Chem. Eng. J. 2023, 472, 144970. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, T.; Balogun, M.S.J.T.; Huang, Y.; Adekoya, D.; Zhang, S.; Tong, Y. Enhanced metallicity boosts hydrogen evolution capability of dual-bimetallic Ni–Fe nitride nanoparticles. Mater. Today Phys. 2020, 15, 100267. [Google Scholar] [CrossRef]
- Mondal, A.; Vomiero, A. 2D Transition Metal Dichalcogenides-Based Electrocatalysts for Hydrogen Evolution Reaction. Adv. Funct. Mater. 2022, 32, 2208994. [Google Scholar] [CrossRef]
- Gao, M.; Gao, P.; Lei, T.; Ouyang, C.; Wu, X.; Wu, A.; Du, Y. FeP/Ni2P nanosheet arrays as high-efficiency hydrogen evolution electrocatalysts. J. Mater. Chem. A 2022, 10, 15569–15579. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, B.; Peng, Q.; Yu, Y.; Zhou, J.; Sun, Z. MXenes modified by single transition metal atom for hydrogen evolution reaction catalysts. Appl. Surf. Sci. 2021, 562, 150151. [Google Scholar] [CrossRef]
- Anne Acedera, R.; Theresse Dumlao, A.; Donn Matienzo, D.J.; Divinagracia, M.; Anne Paraggua, J.; Abel Chuang, P.-Y.; Ocon, J. Templated synthesis of transition metal phosphide electrocatalysts for oxygen and hydrogen evolution reactions. J. Energy Chem. 2024, 89, 646–669. [Google Scholar] [CrossRef]
- Duan, D.; Feng, J.; Guo, D.; Gao, J.; Liu, S.; Wang, Y.; Zhou, X. MOF-derived cobalt manganese phosphide as highly efficient electrocatalysts for hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 12927–12936. [Google Scholar] [CrossRef]
- Luo, S.; Hei, P.; Wang, R.; Yin, J.; Hong, W.; Liu, S.; Bai, Z.; Jiao, T. Facile synthesis of cobalt phosphide nanoparticles as highly active electrocatalysts for hydrogen evolution reaction. Colloids Surf. A 2020, 600, 124925. [Google Scholar] [CrossRef]
- Liu, L.; Meng, F.; Wang, H.; Ma, S.; Lukyanov, D.; Zhu, W.; Li, Y.; Ren, P.; Kondratiev, V.; Yang, P.; et al. Insight into the electronic modulation on nickel-cobalt bimetallic phosphates towards high-efficiency electrocatalytic hydrogen evolution. J. Alloys Compd. 2024, 1002, 175259. [Google Scholar] [CrossRef]
- Wang, K.; Sun, X.; Huang, W.; Cao, Q.; Zhao, Y.; Ding, R.; Liu, E.; Gao, P.; Lin, W. Superhydrophilic nickel cyclotetraphosphate for the hydrogen evolution reaction in acidic solution. Dalton Trans. 2021, 50, 12435–12439. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, C.; Chen, T.; Zhao, X.; Li, Z.; Hao, J. A new phosphonium-based ionic liquid to synthesize nickel metaphosphate for hydrogen evolution reaction. Nanotechnology 2020, 31, 505402. [Google Scholar] [CrossRef]
- Mehdi, S.M.Z.; Ali, M.; Maqsood, M.F.; Abbas, N.; Lee, N. Synthesis of nickel phosphate nanowires as a bifunctional catalyst for water electrolysis in alkaline media. J. Alloys Compd. 2024, 988, 174250. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Liu, L.; Park, S. One-Pot Synthesis of Nanoporous Nickel Hydroxide Film as High-Performance Electrode for Asymmetric Supercapacitor. J. Electrochem. Soc. 2019, 166, D595–D602. [Google Scholar] [CrossRef]
- Bian, W.; Huang, Y.; Xu, X.; Ud Din, M.A.; Xie, G.; Wang, X. Iron Hydroxide-Modified Nickel Hydroxylphosphate Single-Wall Nanotubes as Efficient Electrocatalysts for Oxygen Evolution Reactions. ACS Appl. Mater. Interfaces 2018, 10, 9407–9414. [Google Scholar] [CrossRef]
- Lv, S.; Sun, Y.; Liu, D.; Song, C.; Wang, D. Construction of S-Scheme heterojunction Ni11(HPO3)8(OH)6/CdS photocatalysts with open framework surface for enhanced H2 evolution activity. J. Colloid Interface Sci. 2023, 634, 148–158. [Google Scholar] [CrossRef]
- Gupta, N.; Bhattacharya, P. Microwave-plasma induced one-step synthesis of Ni(PO3)2 nanosphere-loaded bio-waste derived N, P co-doped carbon for an asymmetric supercapacitor with prolonged life. J. Mater. Chem. C 2023, 11, 13503–13517. [Google Scholar] [CrossRef]
- Wang, J.; Huang, J.; Zhao, S.; Parkin, I.P.; Tian, Z.; Lai, F.; Liu, T.; He, G. Mo/Fe bimetallic pyrophosphates derived from Prussian blue analogues for rapid electrocatalytic oxygen evolution. Green Energy Environ. 2023, 8, 1450–1458. [Google Scholar] [CrossRef]
- Singh, T.I.; Maibam, A.; Cha, D.C.; Yoo, S.; Babarao, R.; Lee, S.U.; Lee, S. High-Alkaline Water-Splitting Activity of Mesoporous 3D Heterostructures: An Amorphous-Shell@Crystalline-Core Nano-Assembly of Co-Ni-Phosphate Ultrathin-Nanosheets and V- Doped Cobalt-Nitride Nanowires. Adv. Sci. 2022, 9, 2201311. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Y.; Shao, M.; Hou, J.; Chen, X.; Fan, L.; Kong, F.; Chen, M. Controlled synthesis of self-supportive hydrangea shaped Ni based hydroxide/phosphide for efficient overall water splitting and full pH hydrogen evolution. Fuel 2024, 367, 131551. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Guo, H.; Zhang, H.; Ren, J.; Song, R. Rational Regulation of Crystalline/Amorphous Microprisms-Nanochannels Based on Molecular Sieve (VSB-5) for Electrochemical Overall Water Splitting. Small 2022, 18, 2200832. [Google Scholar] [CrossRef]
- Li, S.-S.; Zhao, X.-H.; Wang, K.-X.; Chen, J.-S. Tailoring the growth route of lithium peroxide through the rational design of a sodium-doped nickel phosphate catalyst for lithium–oxygen batteries. Chem. Commun. 2023, 59, 11839–11842. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Ren, J.-T.; Wang, L.; Sun, M.-L.; Yang, H.-M.; Lv, X.-W.; Yuan, Z.-Y. Synergistically enhanced activity and stability of bifunctional nickel phosphide/sulfide heterointerface electrodes for direct alkaline seawater electrolysis. J. Energy Chem. 2022, 75, 66–73. [Google Scholar] [CrossRef]
- Surendran, S.; Sivanantham, A.; Shanmugam, S.; Sim, U.; Kalai Selvan, R. Ni2P2O7 microsheets as efficient Bi-functional electrocatalysts for water splitting application. Sustainable Energy Fuels 2019, 3, 2435–2446. [Google Scholar] [CrossRef]
- Chang, J.; Lv, Q.; Li, G.; Ge, J.; Liu, C.; Xing, W. Core-shell structured Ni12P5/Ni3(PO4)2 hollow spheres as difunctional and efficient electrocatalysts for overall water electrolysis. Appl. Catal. B 2017, 204, 486–496. [Google Scholar] [CrossRef]
- Babar, P.T.; Lokhande, A.C.; Jo, E.; Pawar, B.S.; Gang, M.G.; Pawar, S.M.; Kim, J.H. Facile electrosynthesis of Fe (Ni/Co) hydroxyphosphate as a bifunctional electrocatalyst for efficient water splitting. J. Ind. Eng. Chem. 2019, 70, 116–123. [Google Scholar] [CrossRef]
- Babar, P.T.; Lokhande, A.C.; Shim, H.J.; Gang, M.G.; Pawar, B.S.; Pawar, S.M.; Kim, J.H. SILAR deposited iron phosphate as a bifunctional electrocatalyst for efficient water splitting. J. Colloid Interface Sci. 2019, 534, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.; Zhou, X.-Y.; Zhao, Y.-X.; Liu, J.-L.; Chen, X.-Y. Optimization of Functional Blocks of Organic Ligands Regulating the Electrocatalytic Hydrogen Evolution Reaction of Co(II)- or Ni(II)-Based Organic Frameworks/Nickel Foam Composites. ACS Appl. Energy Mater. 2023, 6, 10682–10693. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, D. 3D nanoporous NiCoP as a highly efficient electrocatalyst for the hydrogen evolution reaction in alkaline electrolyte. New J. Chem. 2022, 46, 7490–7496. [Google Scholar] [CrossRef]
- Lv, X.; Tian, W.; Liu, Y.; Yuan, Z.-Y. Well-defined CoP/Ni2P nanohybrids encapsulated in a nitrogen-doped carbon matrix as advanced multifunctional electrocatalysts for efficient overall water splitting and zinc–air batteries. Mater. Chem. Front. 2019, 3, 2428–2436. [Google Scholar] [CrossRef]
- Qian, X.; Wu, J.; Yang, Y.; Zhang, W.; Zheng, H.; Xia, J.; Chen, M.; Chen, W. Ni-CoS2@MoS2 hollow nanorod array in-situ synthesized on Ti foil as Pt-free self-supporting electrode for efficient wide-pH hydrogen evolution. Appl. Surf. Sci. 2024, 655, 159629. [Google Scholar] [CrossRef]
- Barua, S.; Balčiūnaitė, A.; Vaičiūnienė, J.; Tamašauskaitė-Tamašiūnaitė, L.; Norkus, E. Bimetallic 3D Nickel-Manganese/Titanium Bifunctional Electrocatalysts for Efficient Hydrogen and Oxygen Evolution Reaction in Alkaline and Acidic Media. Coatings 2023, 13, 1102. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).