Evaluating the Photocatalytic Activity of Green Synthesized Iron Oxide Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
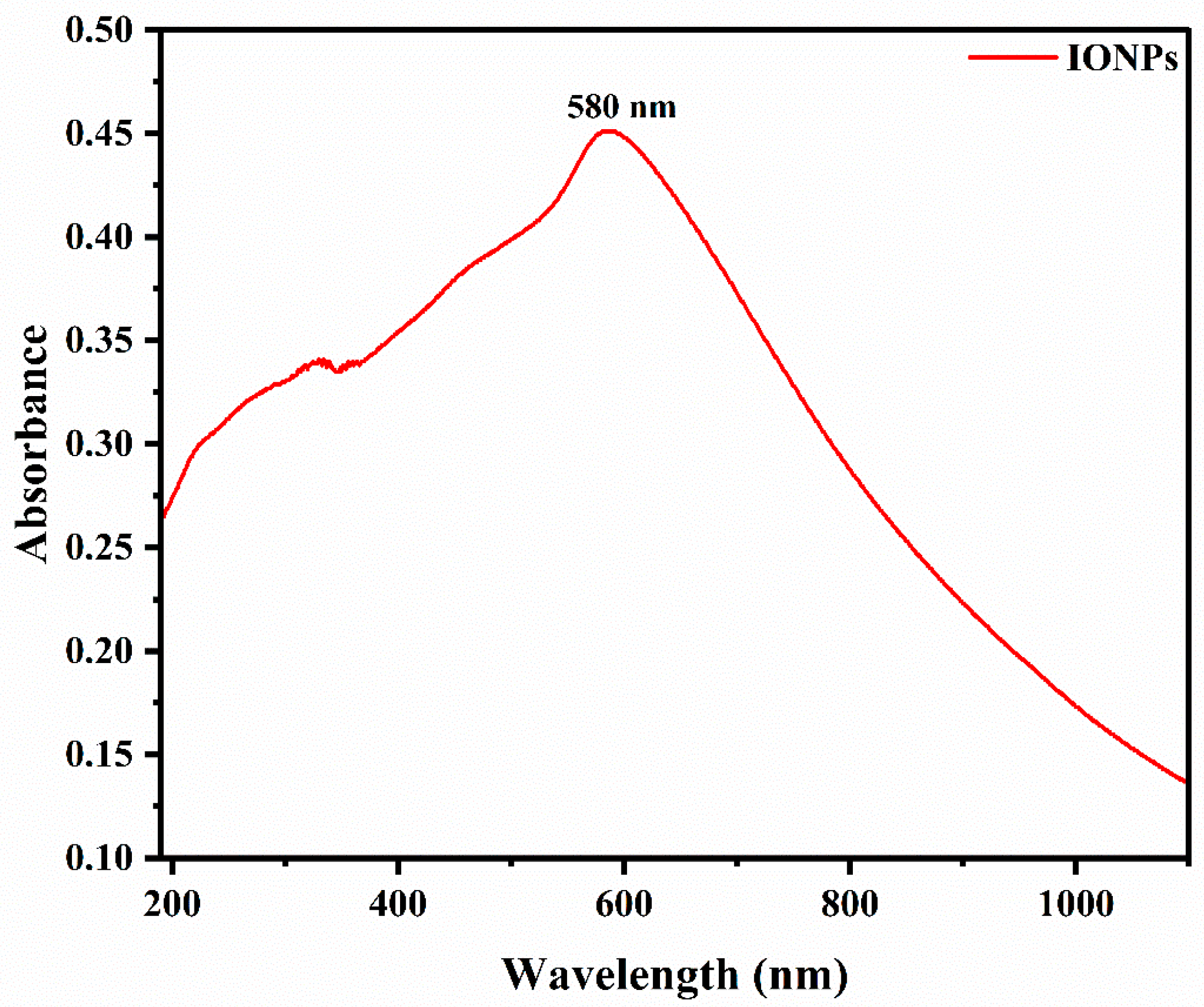
2.1. UV–Visible Spectroscopic Analysis
2.2. FTIR Analysis
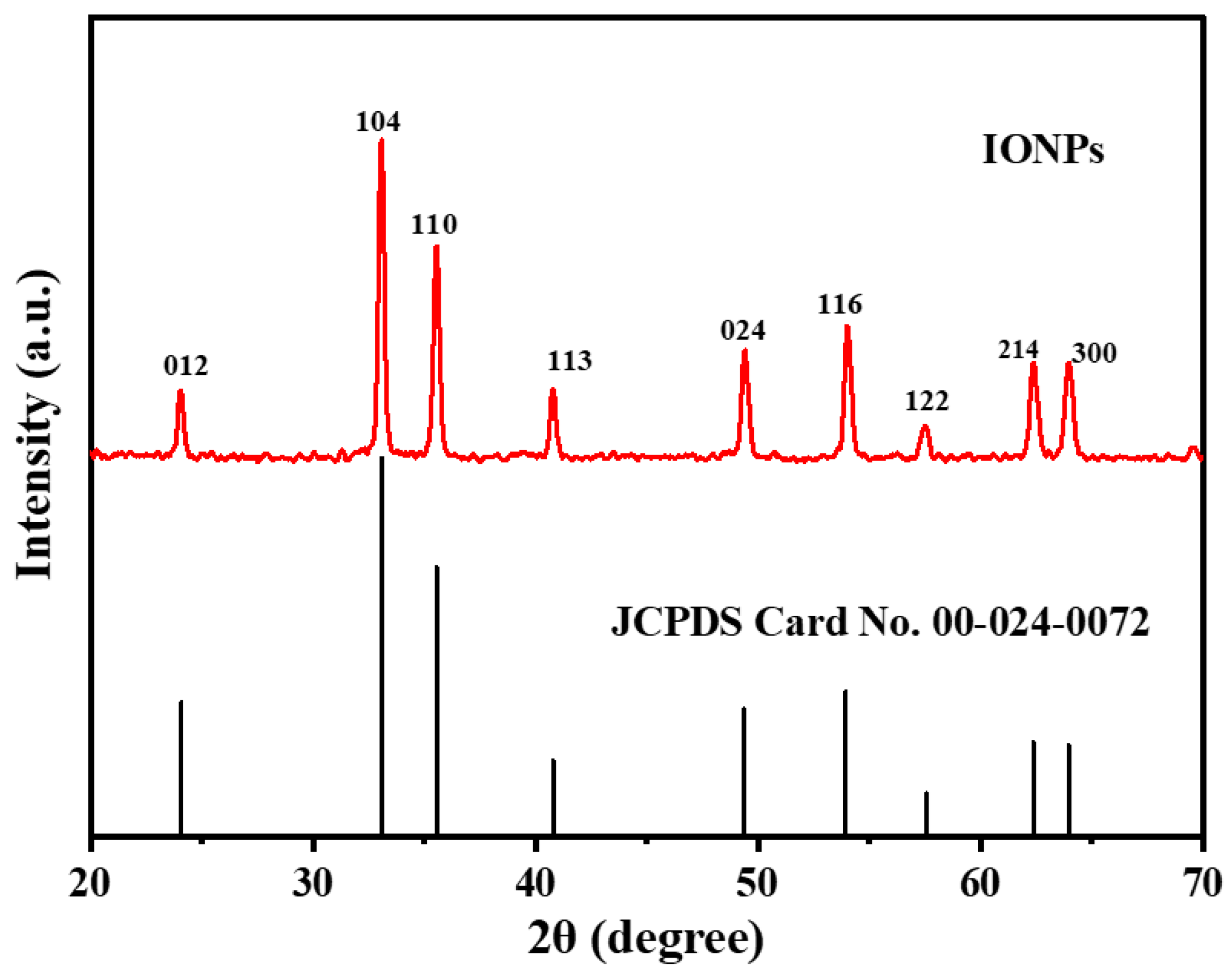
2.3. XRD Study
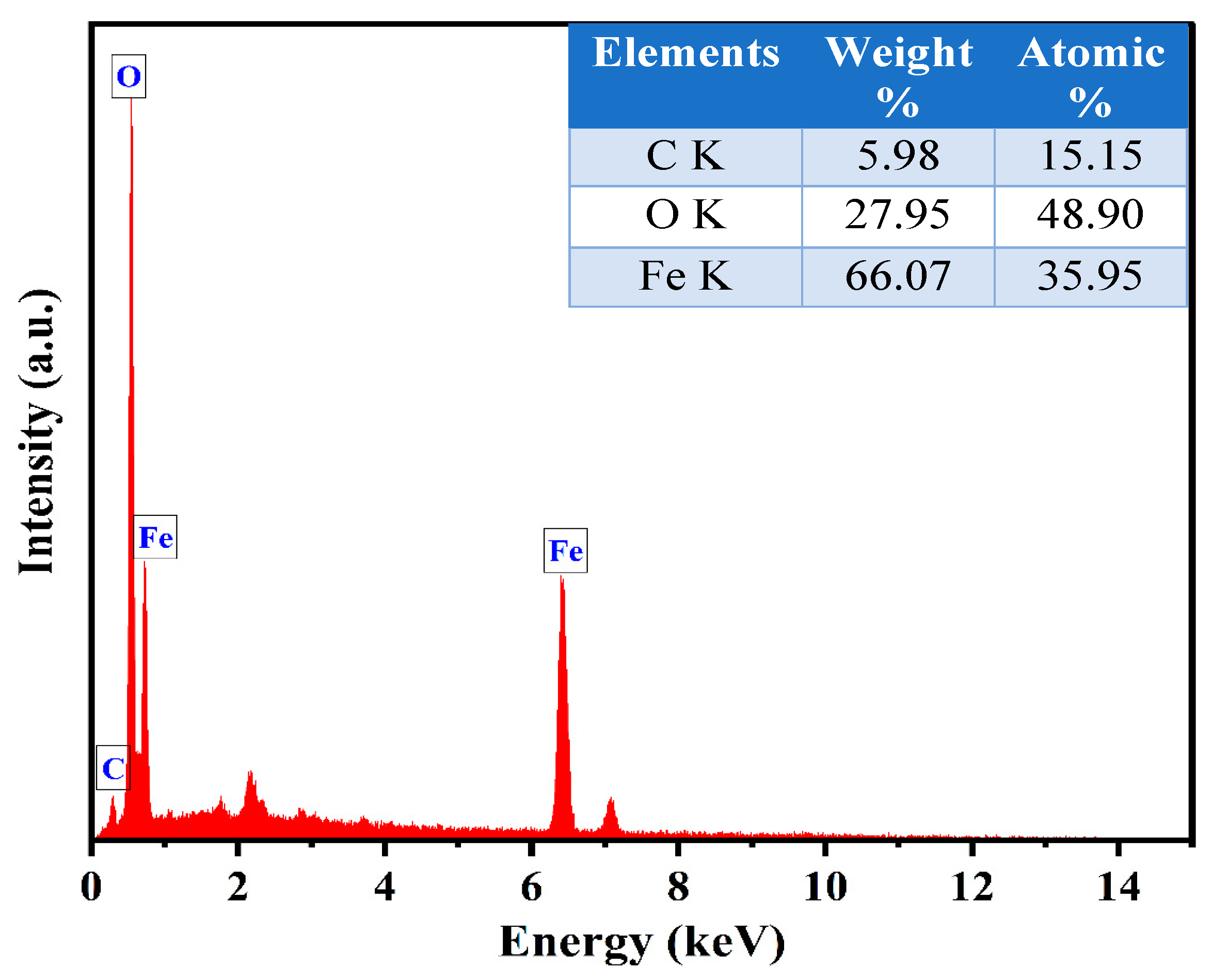
2.4. Morphological and Elemental Analysis
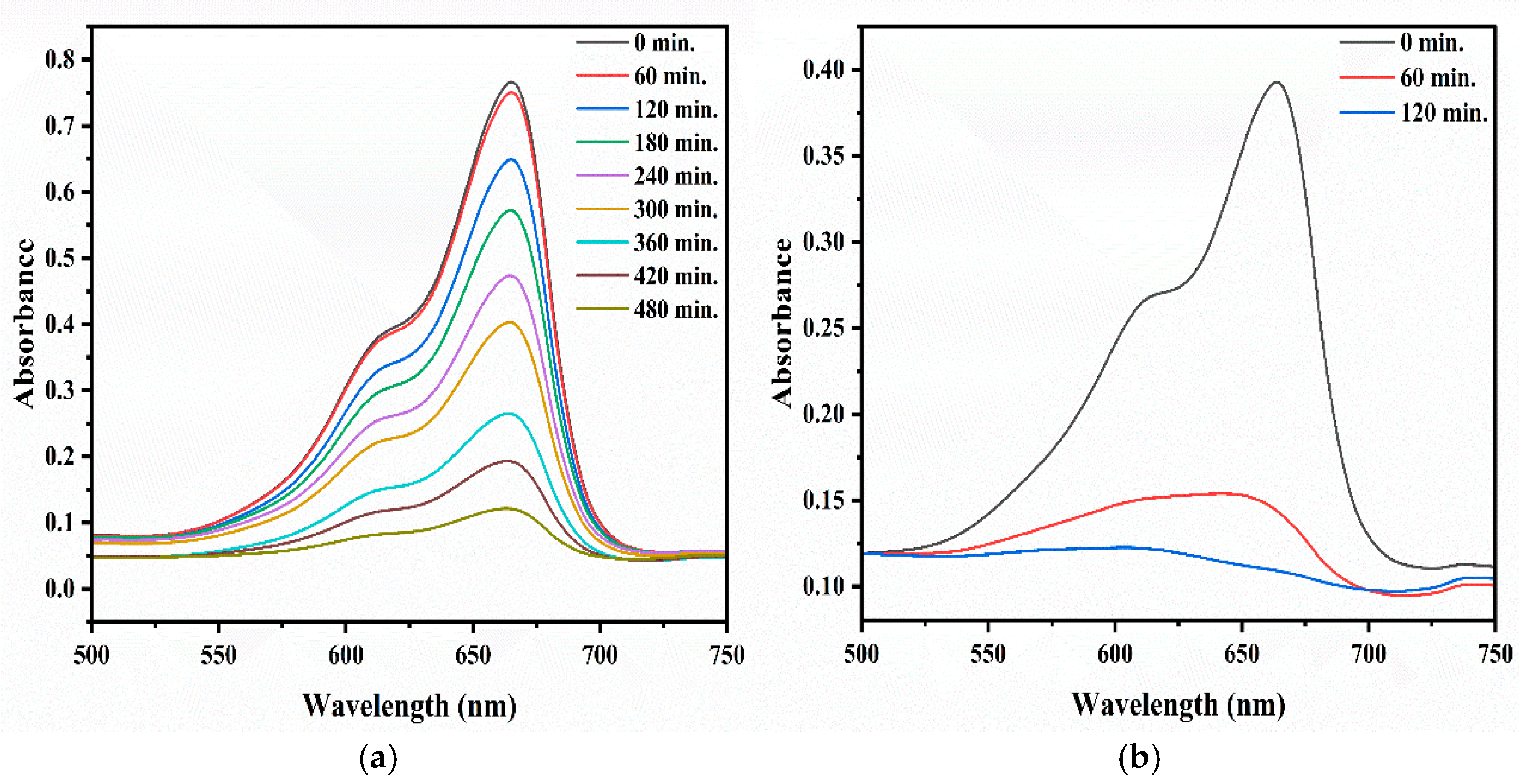
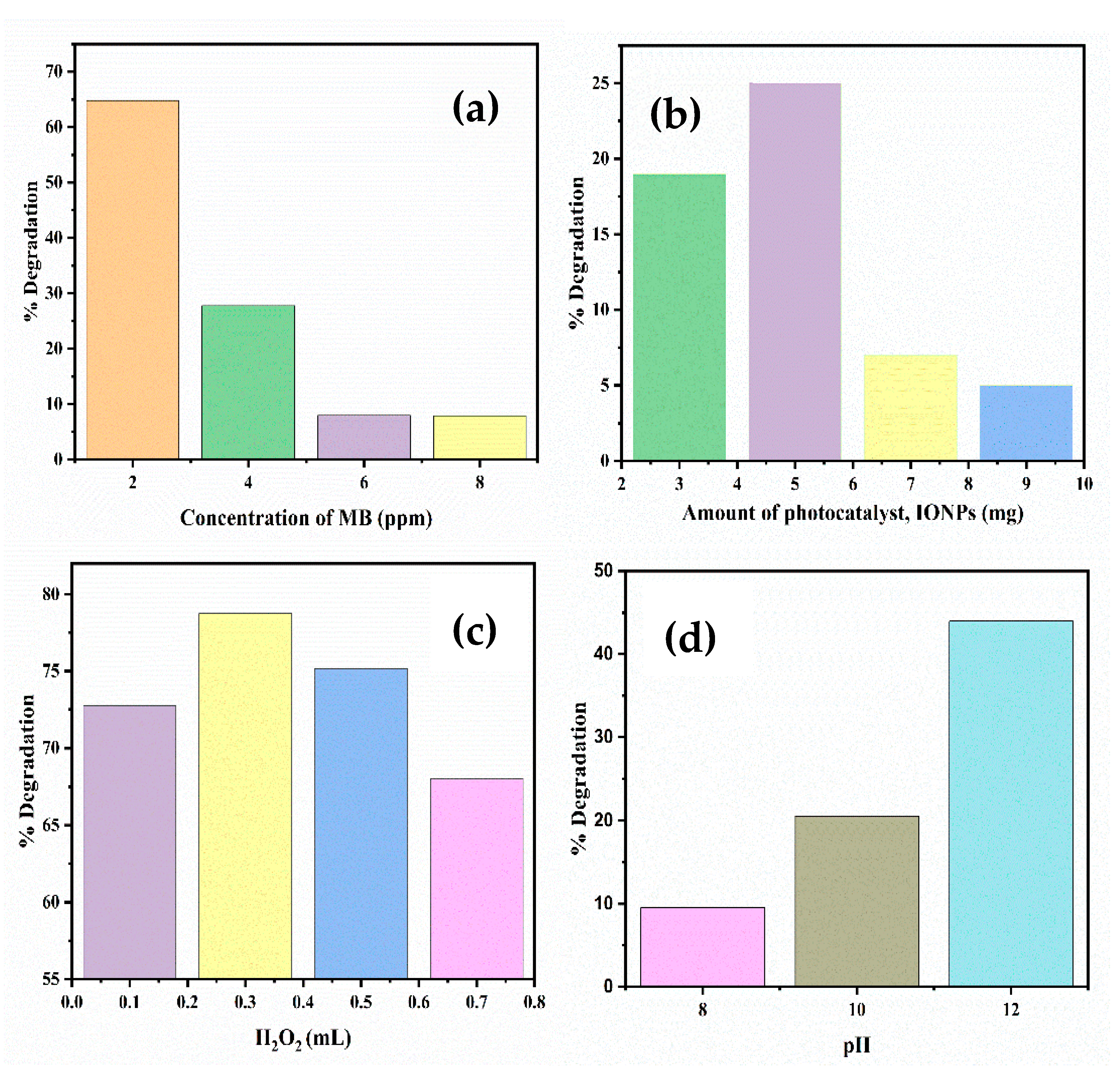
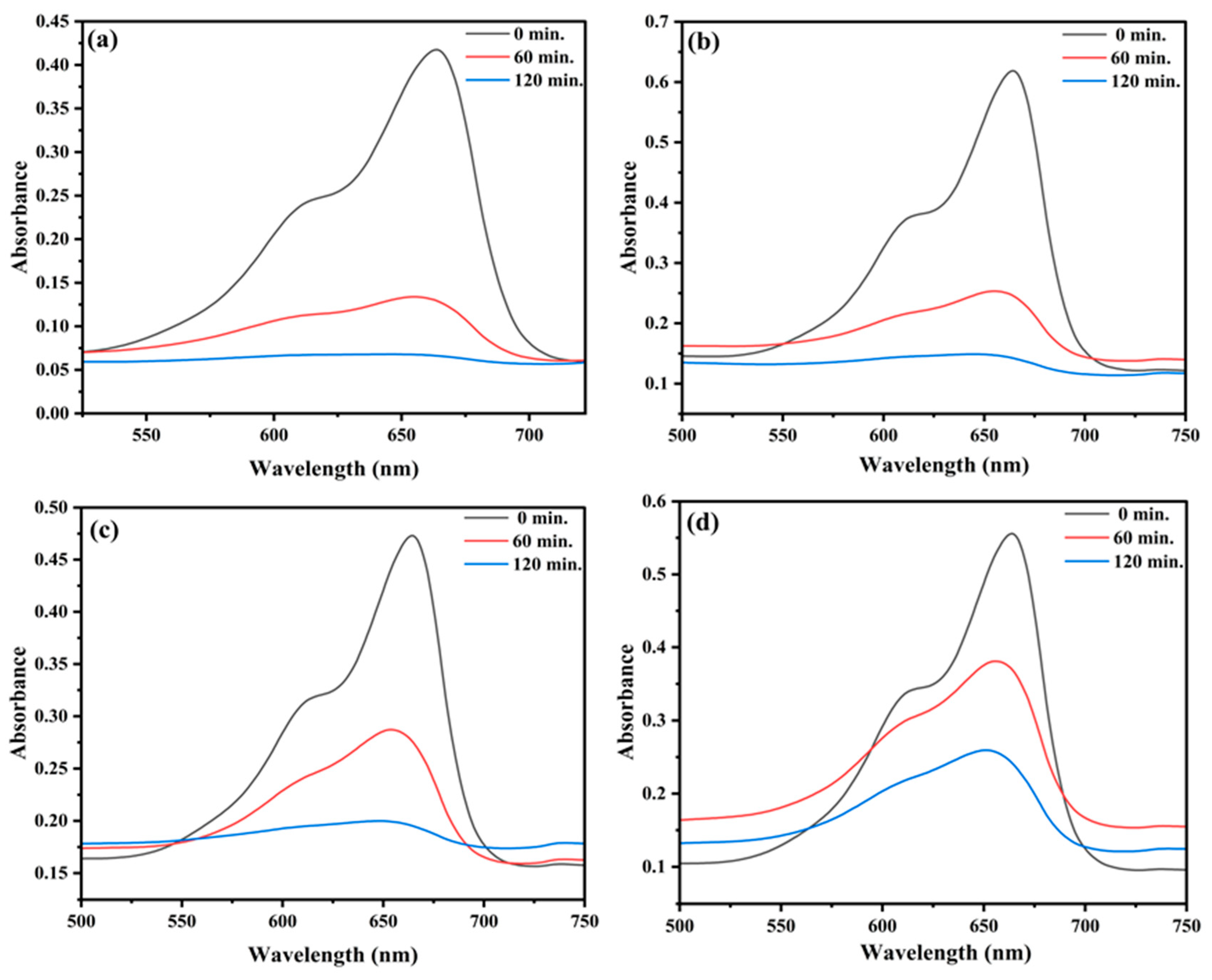
2.5. Photocatalytic Degradation of MB
2.5.1. Effect of Concentration of Dye
2.5.2. Effect of pH
2.5.3. Effect of Catalyst Dose
2.5.4. Effect of H2O2
2.5.5. Comparative Analysis of Photocatalytic Activity
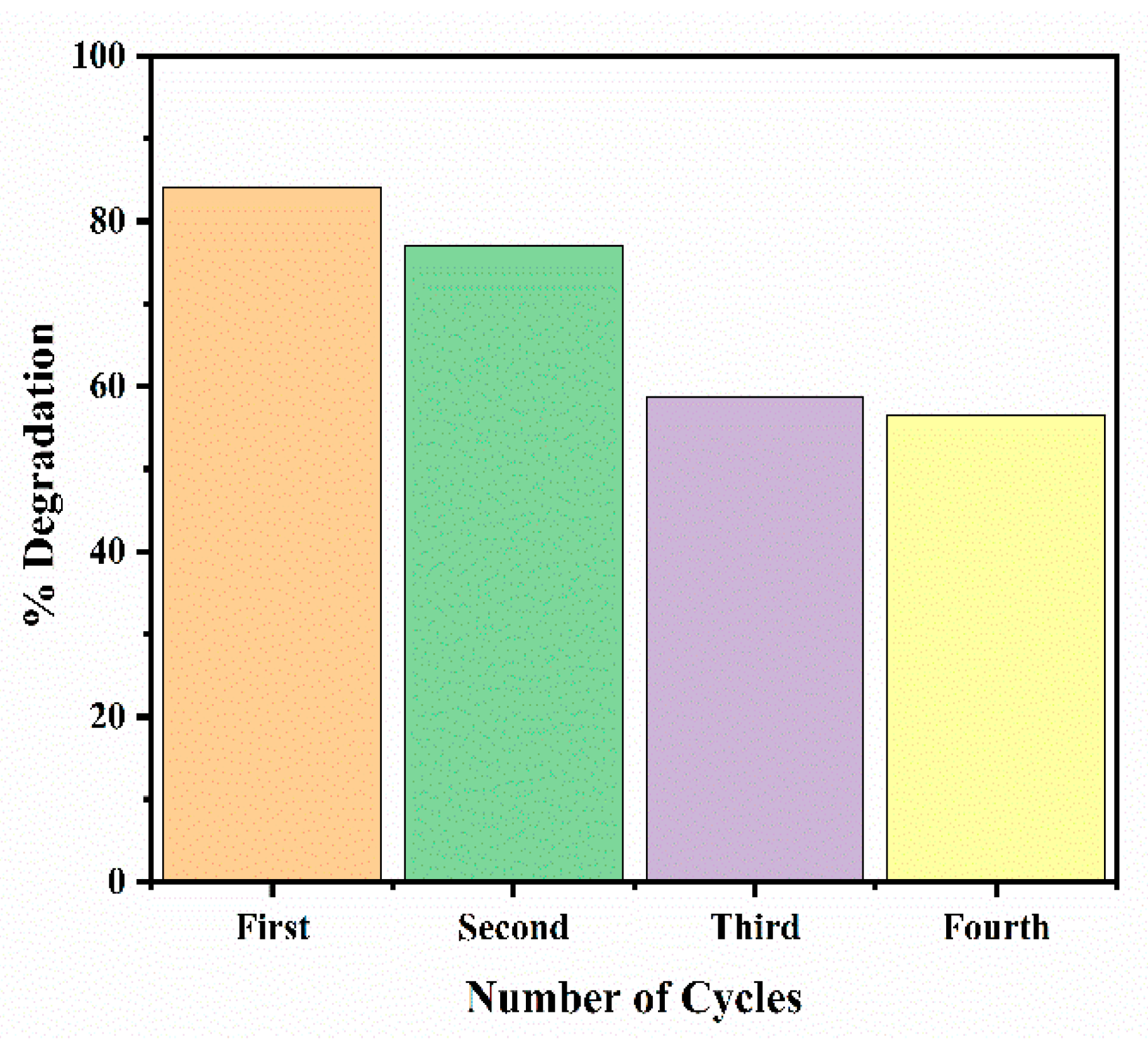
2.5.6. Regeneration and Reusability of Iron Oxide Nanoparticles
3. Materials and Methods
3.1. Chemicals Used
3.2. Preparation of Plant Extract
3.3. Synthesis of Iron Oxide Nanoparticles
3.4. Characterization of IONPs
- D = crystallite size (nm);
- K = Scherrer constant (0.9);
- λ = Wavelength of X-ray source (0.209013 nm);
- β = Full width at half maxima, FWHM (radians);
- θ = peak position (radians).
3.5. Photocatalytic Activity of Iron Oxide Nanoparticles
3.6. Reusability Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bilgiç, E.; Baba, A. Effect of urbanization on water resources: Challenges and prospects. In Ground Water in Arid and Semi-Arid Areas; Springer: Berlin/Heidelberg, Germany, 2023; pp. 81–108. [Google Scholar] [CrossRef]
- Ravulapalli, S.; Kunta, R. Effective removal of methylene blue, a hazardous dye from industrial effluents using active carbon of F. infectoria plant. Int. J. Environ. Sci. Technol. 2019, 16, 7837–7848. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Zekke, I.; Zhang, B.; Hendi, A.H.; Ahmad, A.; Ahmad, S.; Zada, N.; Ahmad, H.; Shah, L.A.; et al. Review on Methylene Blue: Its Properties, Uses, Toxicity and Photodegradation. Water 2022, 14, 242. [Google Scholar] [CrossRef]
- Fito, J.; Abrham, S.; Angassa, K. Adsorption of Methylene Blue from Textile Industrial Wastewater onto Activated Carbon of Parthenium hysterophorus. Int. J. Environ. Res. 2020, 14, 501–511. [Google Scholar] [CrossRef]
- Ajmal, A.; Majeed, I.; Malik, R.N.; Idriss, H.; Nadeem, M.A. Principles and mechanisms of photocatalytic dye degradation on TiO2based photocatalysts: A comparative overview. RSC Adv. 2014, 4, 37003–37026. [Google Scholar] [CrossRef]
- Khan, S.; Noor, T.; Iqbal, N.; Yaqoob, L. Photocatalytic Dye Degradation from Textile Wastewater: A Review. ACS Omega 2024, 9, 21751–21767. [Google Scholar] [CrossRef] [PubMed]
- Indhumathi, T.; Krishnamoorthy, N.; Valarmathy, R.; Saraswathi, K.; Dilwyn, S.; Prabhu, S. Green Synthesis of α-Fe2O3 Nanoparticles Mediated Musa Acuminata: A study of Their Applications as Photocatalytic Degradation and Antibacterial Agent. Nano Biomed. Eng. 2022, 14, 254–262. [Google Scholar] [CrossRef]
- Ayad, M.; Salahuddin, N.; Fayed, A.; Bastakoti, B.P.; Suzuki, N.; Yamauchi, Y. Chemical design of a smart chitosan–polypyrrole–magnetite nanocomposite toward efficient water treatment. Phys. Chem. Chem. Phys. 2014, 16, 21812–21819. [Google Scholar] [CrossRef]
- Chen, Y.H.; Lin, C.C. Effect of nano-hematite morphology on photocatalytic activity. Phys. Chem. Miner. 2014, 41, 727–736. [Google Scholar] [CrossRef]
- Fernandes, T.A.; Mendo, S.G.; Ferreira, L.P.; Neng, N.R.; Oliveira, M.C.; Gil, A.; Carvalho, M.D.; Monteiro, O.C.; Nogueira, J.M.F.; Calhorda, M.J. Photocatalytic degradation of acetaminophen and caffeine using magnetite–hematite combined nanoparticles: Kinetics and mechanisms. Environ. Sci. Pollut. Res. 2021, 28, 17228–17243. [Google Scholar] [CrossRef]
- Sakamoto, M.; Fujita, R.; Nishikawa, M.; Hirazawa, H.; Ueno, Y.; Yamamoto, M.; Takaoka, S. Hematite (α-Fe2O3) with Oxygen Defects: The Effect of Heating Rate for Photocatalytic Performance. Materials 2024, 17, 395. [Google Scholar] [CrossRef]
- Chauhan, S.; Upadhyay, L.S.B. Biosynthesis of iron oxide nanoparticles using plant derivatives of Lawsonia inermis (Henna) and its surface modification for biomedical application. Nanotechnol. Environ. Eng. 2019, 4, 8. [Google Scholar] [CrossRef]
- Malik, A.Q.; Mir, T.U.G.; Kumar, D.; Mir, I.A.; Rashid, A.; Ayoub, M.; Shukla, S. A review on the green synthesis of nanoparticles, their biological applications, and photocatalytic efficiency against environmental toxins. Environ. Sci. Pollut. Res. 2023, 30, 69796–69823. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Radha; Kumar, M.; Zhang, B.; Amarowicz, R.; Puri, S.; Pundir, A.; Rathour, S.; Kumari, N.; Chandran, D.; et al. Acacia catechu (L.f.) Willd.: A Review on Bioactive Compounds and Their Health Promoting Functionalities. Plants 2022, 11, 3091. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Lingha, R. A Recent Update on the Pharmacognostical as well as Pharmacological Profiles of the Acacia catechu Heartwood: A Mini Review. J. Ayurveda Integr. Med. 2021, 7, 188–192. [Google Scholar] [CrossRef]
- Da’na, E.; Taha, A.; Afkar, E. Green Synthesis of Iron Nanoparticles by Acacia nilotica Pods Extract and Its Catalytic, Adsorption, and Antibacterial Activities. Appl. Sci. 2018, 8, 1922. [Google Scholar] [CrossRef]
- Devatha, C.P.; Thalla, A.K.; Katte, S.Y. Green synthesis of iron nanoparticles using different leaf extracts for treatment of domestic waste water. J. Clean. Prod. 2016, 139, 1425–1435. [Google Scholar] [CrossRef]
- Badmapriya, D.; Asharani, I.V. Dye degradation studies catalysed by green synthesized Iron oxide nanoparticles. Int. J. ChemTech Res. 2016, 9, 409–416. [Google Scholar]
- Komal, K.; Kaur, H.; Kainth, M.; Meena, S.S.; Kang, T.S. Sustainable preparation of sunlight active α-Fe2O3 nanoparticles using iron containing ionic liquids for photocatalytic applications. RSC Adv. 2019, 9, 41803–41810. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, H.; Kang, T.S.; Singh, S. Sustainable preparation of Fe(OH)3 and α-Fe2O3 nanoparticles employing Acacia catechu extract for efficient removal of chromium (VI) from aqueous solution. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100593. [Google Scholar] [CrossRef]
- Lassoued, A.; Dkhil, B.; Gadri, A.; Ammar, S. Control of the shape and size of iron oxide (α-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys. 2017, 7, 3007–3015. [Google Scholar] [CrossRef]
- Hussain, A.; Yasar, M.; Ahmad, G.; Ijaz, M.; Aziz, A.; Nawaz, M.G.; Khan, F.A.; Iqbal, H.; Shakeel, W.; Momand, H.; et al. Synthesis, characterization, and applications of iron oxide nanoparticles. Int. J. Health Sci. 2023, 17, 3–10. [Google Scholar]
- Noor, R.; Yasmin, H.; Ilyas, N.; Nosheen, A.; Hassan, M.N.; Mumtaz, S.; Khan, N.; Ahmad, A.; Ahmad, P. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere 2021, 292, 133201. [Google Scholar] [CrossRef] [PubMed]
- Lohrasbi, S.; Kouhbanani, M.A.J.; Beheshtkhoo, N.; Ghasemi, Y.; Amani, A.M.; Taghizadeh, S. Green Synthesis of Iron Nanoparticles Using Plantago major Leaf Extract and Their Application as a Catalyst for the Decolorization of Azo Dye. BioNanoScience 2019, 9, 317–322. [Google Scholar] [CrossRef]
- Jain, R.; Mendiratta, S.; Kumar, L.; Srivastava, A. Green synthesis of iron nanoparticles using Artocarpus heterophyllus peel extract and their application as a heterogeneous Fenton-like catalyst for the degradation of Fuchsin Basic dye. Curr. Res. Green Sustain. Chem. 2021, 4, 100086. [Google Scholar] [CrossRef]
- Jamzad, M.; Bidkorpeh, M.K. Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity. J. Nanostruct. Chem. 2020, 10, 193–201. [Google Scholar] [CrossRef]
- Fardood, S.T.; Ramazani, A.; Golfar, Z.; Joo, S.W. Green Synthesis of α-Fe2O3 (hematite) Nanoparticles using Tragacanth Gel. J. Appl. Chem. Res. 2017, 11, 19–27. [Google Scholar]
- Jing, Z.; Wu, S. Synthesis and characterization of monodisperse hematite nanoparticles modified by surfactants via hydrothermal approach. Mater. Lett. 2004, 58, 3637–3640. [Google Scholar] [CrossRef]
- Joya, M.R.; Barón-Jaimez, J.; Barba-Ortega, J. Preparation and characterization of Fe2O3nanoparticles. J. Phys. Conf. Ser. 2013, 466, 012004. [Google Scholar] [CrossRef]
- Bastakoti, B.P.; Sukegawa, H.; Wu, K.C.-W.; Yamauchi, Y. Synthesis of porous iron oxide microspheres by a double hydrophilic block copolymer. RSC Adv. 2014, 4, 9986–9989. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Mapetla, S.; Makofane, A.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of iron oxide nanoparticles for the degradation of methylene blue dye, sulfisoxazole antibiotic and removal of bacteria from real water. Heliyon 2022, 8, e10536. [Google Scholar] [CrossRef]
- Balarabe, B.Y.; Oumarou, M.N.I.; Koroney, A.S.; Adjama, I.; Baraze, A.R.I. Photo-Oxidation of Organic Dye by Fe2O3 Nanoparticles: Catalyst, Electron Acceptor, and Polyurethane Membrane (PU-Fe2O3) Effects. J. Nanotechnol. 2023, 2023, 1292762. [Google Scholar] [CrossRef]
- Goudjil, M.B.; Dali, H.; Zighmi, S.; Mahcene, Z.; Bencheikh, S.E. Photocatalytic degradation of methylene blue dye with biosynthesized Hematite α-Fe2O3 nanoparticles under UV-Irradiation. Desalination Water Treat. 2024, 317, 100079. [Google Scholar] [CrossRef]
- Ali, M.A.; Maafa, I.M.; Qudsieh, I.Y. Photodegradation of Methylene Blue Using a UV/H2O2 Irradiation System. Water 2024, 16, 453. [Google Scholar] [CrossRef]
- Gondal, M.A.; Saleh, T.A.; Drmosh, Q.A. Synthesis of nickel oxide nanoparticles using pulsed laser ablation in liquids and their optical characterization. Appl. Surf. Sci. 2012, 258, 6982–6986. [Google Scholar] [CrossRef]
- Christy, A.J.; Umadevi, M. Novel combustion method to prepare octahedral NiO nanoparticles and its photocatalytic activity. Mater. Res. Bull. 2013, 48, 4248–4254. [Google Scholar] [CrossRef]
- Ali, M.H.H.; Goher, M.E.; Al-Afify, A.D.G.; El-Sayed, S.M. A facile method for synthesis rGO/Ag nanocomposite and its uses for enhancing photocatalytic degradation of Congo red dye. SN Appl. Sci. 2022, 4, 276. [Google Scholar] [CrossRef]
- Al-Hakkani, M.F.; Gouda, G.A.; Hassan, S.H.A.; Nagiub, A.M. Echinacea purpurea Mediated Hematite Nanoparticles (α-HNPs) Biofabrication, Characterization, Physicochemical Properties, and its In-vitro Biocompatibility Evaluation. Surf. Interfaces 2021, 24, 101113. [Google Scholar] [CrossRef]
- Pham, T.M.H.; Vu, M.T.; Cong, T.D.; Nguyen, N.S.; Doan, T.A.; Truong, T.T.; Nguyen, T.H. Green sonochemical process for preparation of polyethylene glycol–Fe3O4/ZnO magnetic nanocomposite using rambutan peel extract as photocatalyst, for removal of methylene blue in solution. Bull. Mater. Sci. 2022, 45, 13. [Google Scholar] [CrossRef]
- Qasim, S.; Zafar, A.; Saif, M.S.; Ali, Z.; Nazar, M.; Waqas, M.; Haq, A.U.; Tariq, T.; Hassan, S.G.; Iqbal, F.; et al. Green synthesis of iron oxide nanorods using Withania coagulans extract improved photocatalytic degradation and antimicrobial activity. J. Photochem. Photobiol. B Biol. 2020, 204, 111784. [Google Scholar] [CrossRef]
- Göktaş, S. Synergic Effects of pH, Reaction Temperature, and Various Light Sources on the Photodegradation of Methylene Blue Without Photocatalyst: A Relatively High Degradation Efficiency. Chem. Afr. 2024, 1–13. [Google Scholar] [CrossRef]
- Fox, M.A.; Dulay, M.T. Heterogeneous photocatalysis. Chem. Rev. 1993, 93, 341–357. [Google Scholar] [CrossRef]
- Torki, F.; Faghihian, H. Photocatalytic activity of NiS, NiO and coupled NiS–NiO for degradation of pharmaceutical pollutant cephalexin under visible light. RSC Adv. 2017, 7, 54651–54661. [Google Scholar] [CrossRef]
- Náfrádi, M.; Veréb, G.; Firak, D.S.; Alapi, T. Photocatalysis: Introduction, mechanism, and effective parameters. In Green Photocatalytic Semiconductors; Springer: Berlin/Heidelberg, Germany, 2022; pp. 3–31. [Google Scholar] [CrossRef]
- Saha, D.; Desipio, M.M.; Hoinkis, T.J.; Smeltz, E.J.; Thorpe, R.; Hensley, D.K.; Fischer-Drowos, S.G.; Chen, J. Influence of hydrogen peroxide in enhancing photocatalytic activity of carbon nitride under visible light: An insight into reaction intermediates. J. Environ. Chem. Eng. 2018, 6, 4927–4936. [Google Scholar] [CrossRef]
- Rather, M.Y.; Sundarapandian, S. Magnetic iron oxide nanorod synthesis by Wedelia urticifolia (Blume) DC. leaf extract for methylene blue dye degradation. Appl. Nanosci. 2020, 10, 2219–2227. [Google Scholar] [CrossRef]
- Madubuonu, N.; Aisida, S.O.; Ali, A.; Ahmad, I.; Zhao, T.-K.; Botha, S.; Maaza, M.; Ezema, F.I. Biosynthesis of iron oxide nanoparticles via a composite of Psidium guavaja-Moringa oleifera and their antibacterial and photocatalytic study. J. Photochem. Photobiol. B Biol. 2019, 199, 111601. [Google Scholar] [CrossRef]
- Vasantharaj, S.; Sathiyavimal, S.; Senthilkumar, P.; LewisOscar, F.; Pugazhendhi, A. Biosynthesis of iron oxide nanoparticles using leaf extract of Ruellia tuberosa: Antimicrobial properties and their applications in photocatalytic degradation. J. Photochem. Photobiol. B Biol. 2019, 192, 74–82. [Google Scholar] [CrossRef]
- Bhuiyan, M.S.H.; Miah, M.Y.; Paul, S.C.; Das Aka, T.; Saha, O.; Rahaman, M.M.; Sharif, M.J.I.; Habiba, O. Ashaduzzaman Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: Application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity. Heliyon 2020, 6, e04603. [Google Scholar] [CrossRef]
- Ren, L.; Huo, W.; Li, G.; Choi, W.; An, T. Photocatalytic mechanisms and photocatalyst deactivation during the degradation of 5-fluorouracil in water. Catal. Today 2022, 410, 45–55. [Google Scholar] [CrossRef]
- Enesca, A.; Isac, L. The Influence of Light Irradiation on the Photocatalytic Degradation of Organic Pollutants. Materials 2020, 13, 2494. [Google Scholar] [CrossRef]
- Mbachu, C.A.; Babayemi, A.K.; Egbosiuba, T.C.; Ike, J.I.; Ani, I.J.; Mustapha, S. Green synthesis of iron oxide nanoparticles by Taguchi design of experiment method for effective adsorption of methylene blue and methyl orange from textile wastewater. Results Eng. 2023, 19, 101198. [Google Scholar] [CrossRef]


| Plant Used | Pollutants | Light Source | Degradation Time (min.) | Degradation Efficiency (%) | References |
|---|---|---|---|---|---|
| Wedelia urticifolia | MB | Visible light | 360 | 98 | [46] |
| Psidium guavaja and Moringa oleifera | MB | Visible light | 60 | 20 | [47] |
| Mentha pulegium | MB | UV light | 120 | 78.68 | [33] |
| Ruellia tuberosa | Crystal violet | Visible light | 150 | 80 | [48] |
| Carica papaya | Remazol yellow | sunlight | 360 | 77 | [49] |
| Withania coagulans | Safranin | Visible light | 180 | 70 | [40] |
| Senegalia catechu | MB | sunlight | 120 | 80 | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khadka, D.; Gautam, P.; Dahal, R.; Ashie, M.D.; Paudyal, H.; Ghimire, K.N.; Pant, B.; Poudel, B.R.; Bastakoti, B.P.; Pokhrel, M.R. Evaluating the Photocatalytic Activity of Green Synthesized Iron Oxide Nanoparticles. Catalysts 2024, 14, 751. https://doi.org/10.3390/catal14110751
Khadka D, Gautam P, Dahal R, Ashie MD, Paudyal H, Ghimire KN, Pant B, Poudel BR, Bastakoti BP, Pokhrel MR. Evaluating the Photocatalytic Activity of Green Synthesized Iron Oxide Nanoparticles. Catalysts. 2024; 14(11):751. https://doi.org/10.3390/catal14110751
Chicago/Turabian StyleKhadka, Devendra, Prayas Gautam, Rabin Dahal, Moses D. Ashie, Hari Paudyal, Kedar Nath Ghimire, Bishweshwar Pant, Bhoj Raj Poudel, Bishnu Prasad Bastakoti, and Megh Raj Pokhrel. 2024. "Evaluating the Photocatalytic Activity of Green Synthesized Iron Oxide Nanoparticles" Catalysts 14, no. 11: 751. https://doi.org/10.3390/catal14110751
APA StyleKhadka, D., Gautam, P., Dahal, R., Ashie, M. D., Paudyal, H., Ghimire, K. N., Pant, B., Poudel, B. R., Bastakoti, B. P., & Pokhrel, M. R. (2024). Evaluating the Photocatalytic Activity of Green Synthesized Iron Oxide Nanoparticles. Catalysts, 14(11), 751. https://doi.org/10.3390/catal14110751










