The Effects of Support Specific Surface Area and Active Metal on the Performance of Biphenyl Selective Hydrogenation to Cyclohexylbenzene
Abstract
1. Introduction
2. Results and Discussion
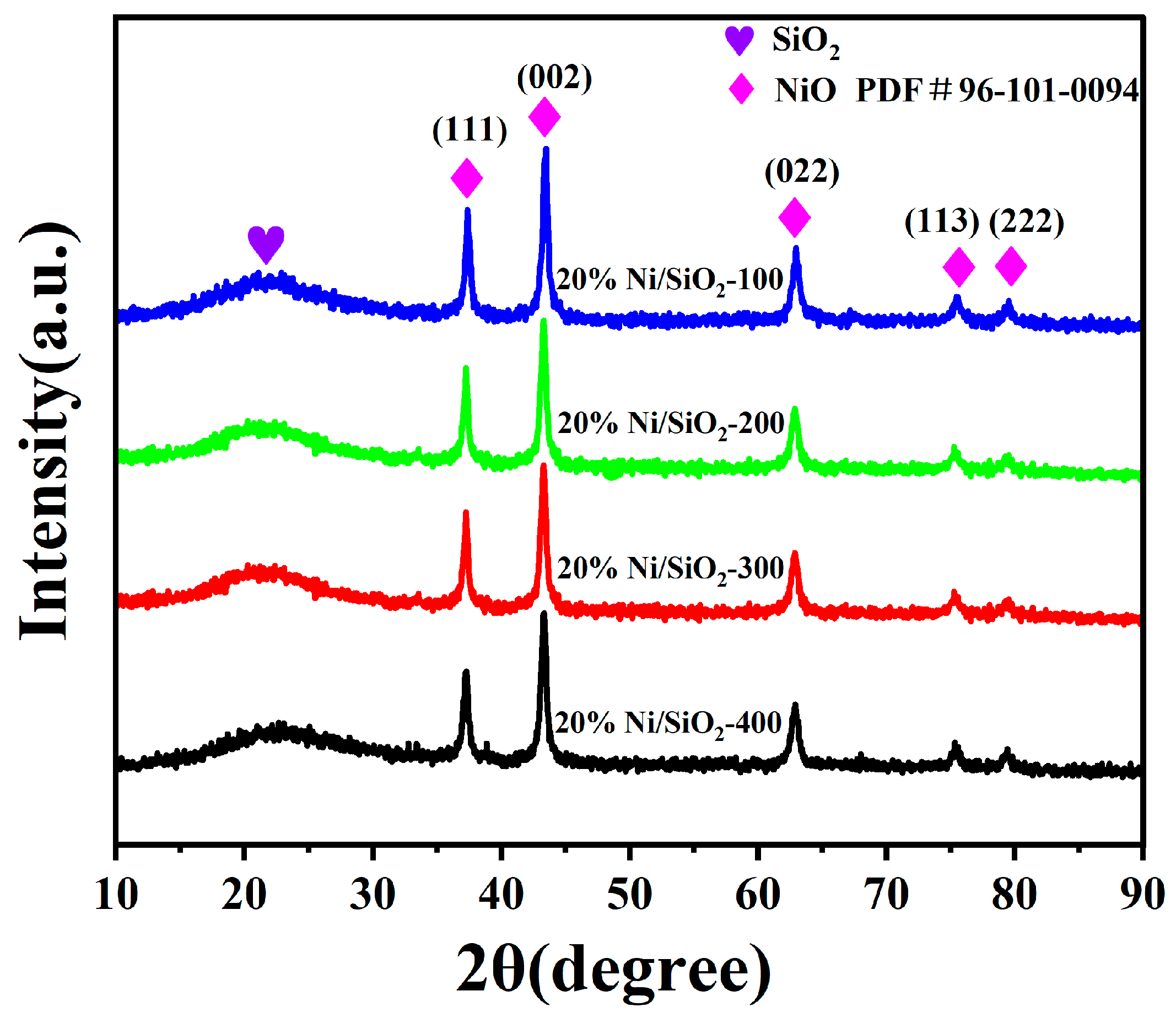
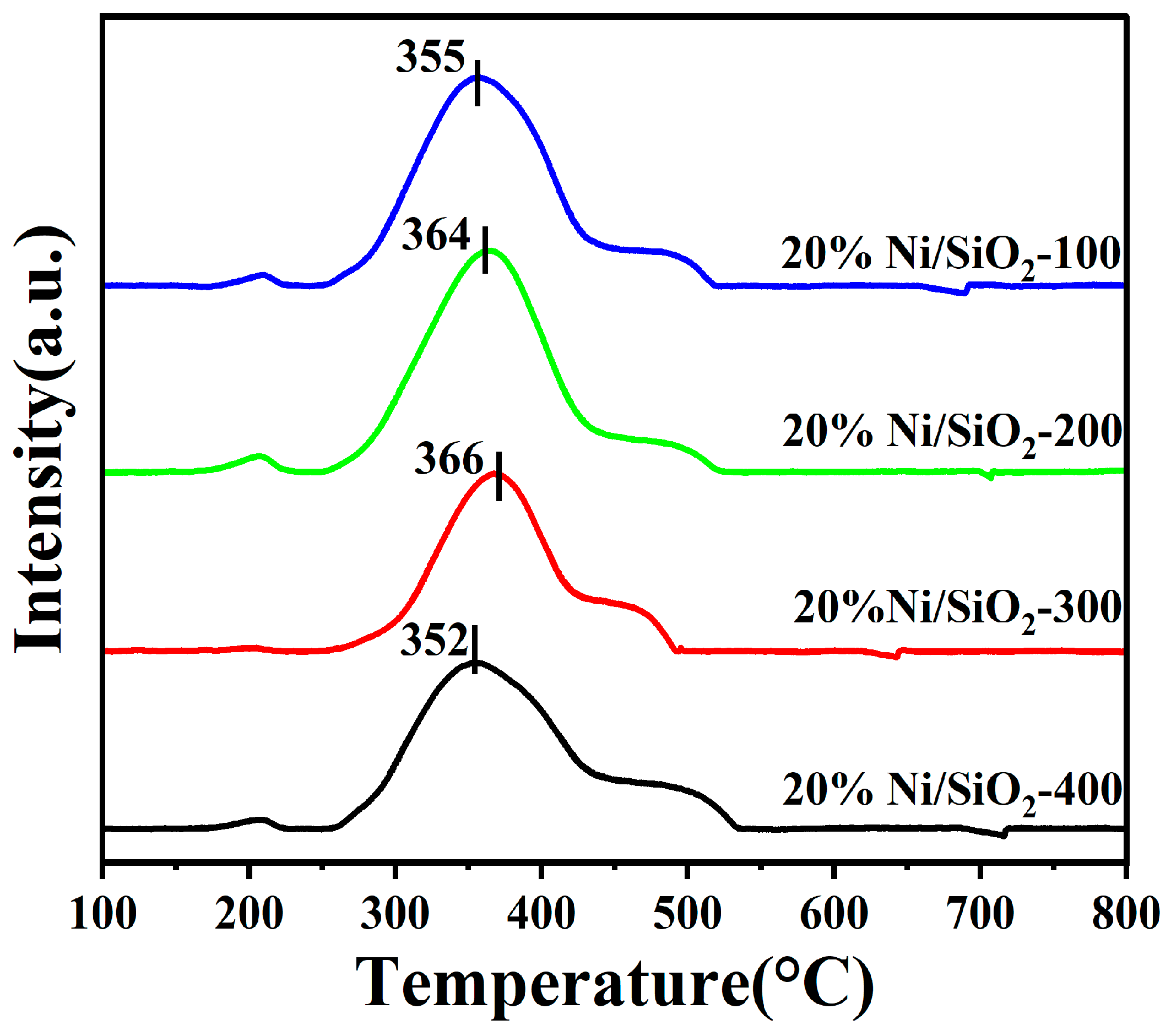
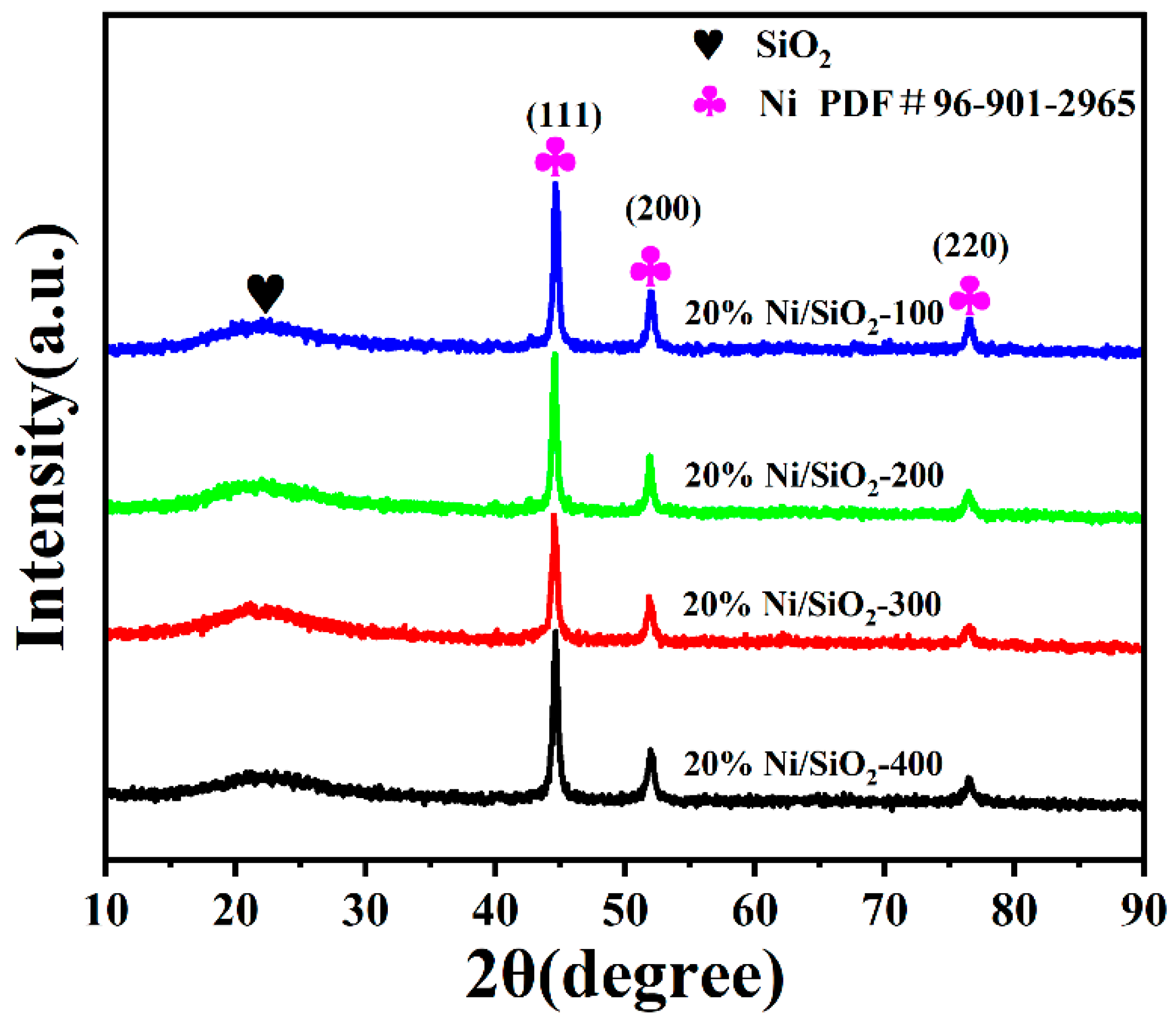
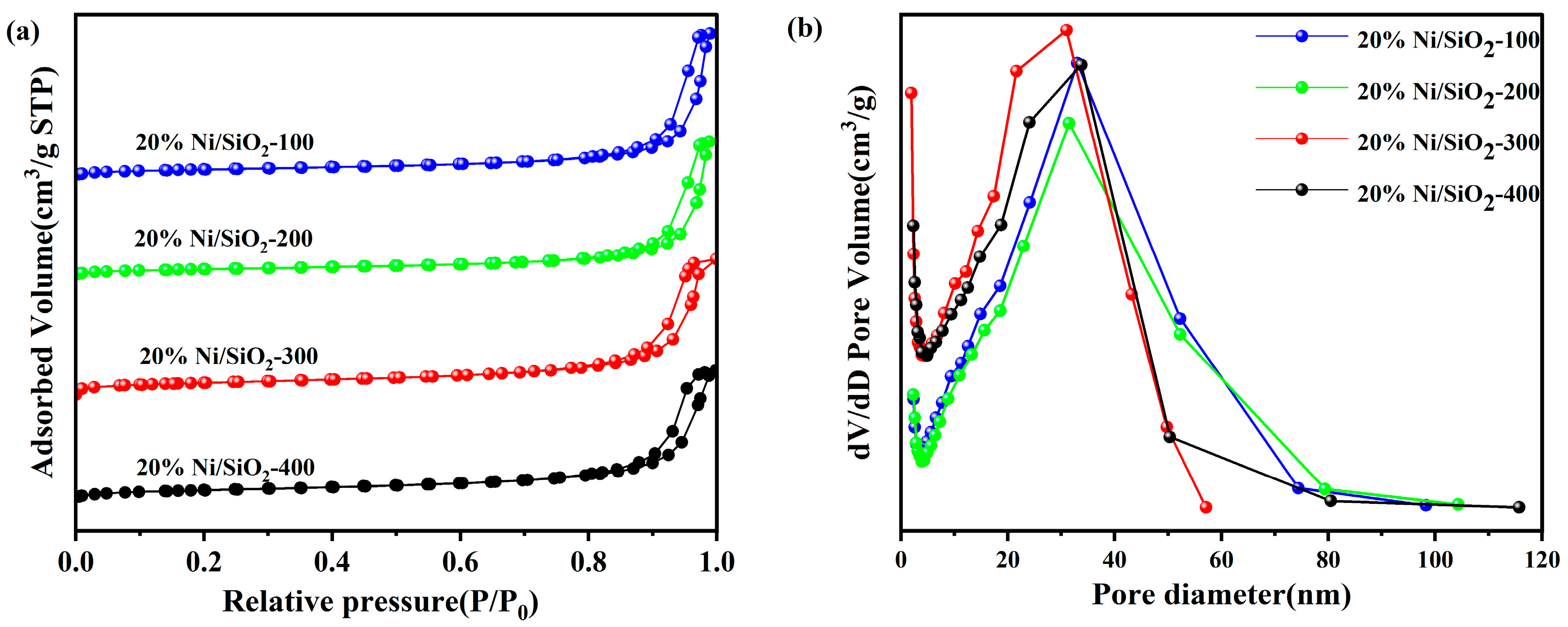
2.1. Characterization of Ni-Based Catalysts with Different SSAs
2.2. Characterization of 20%M/SiO2 (M = Fe, Cu, Co, and Ni) Catalysts
2.3. 20% Ni/SiO2 Catalysts with Different SSAs for BP Hydrogenation
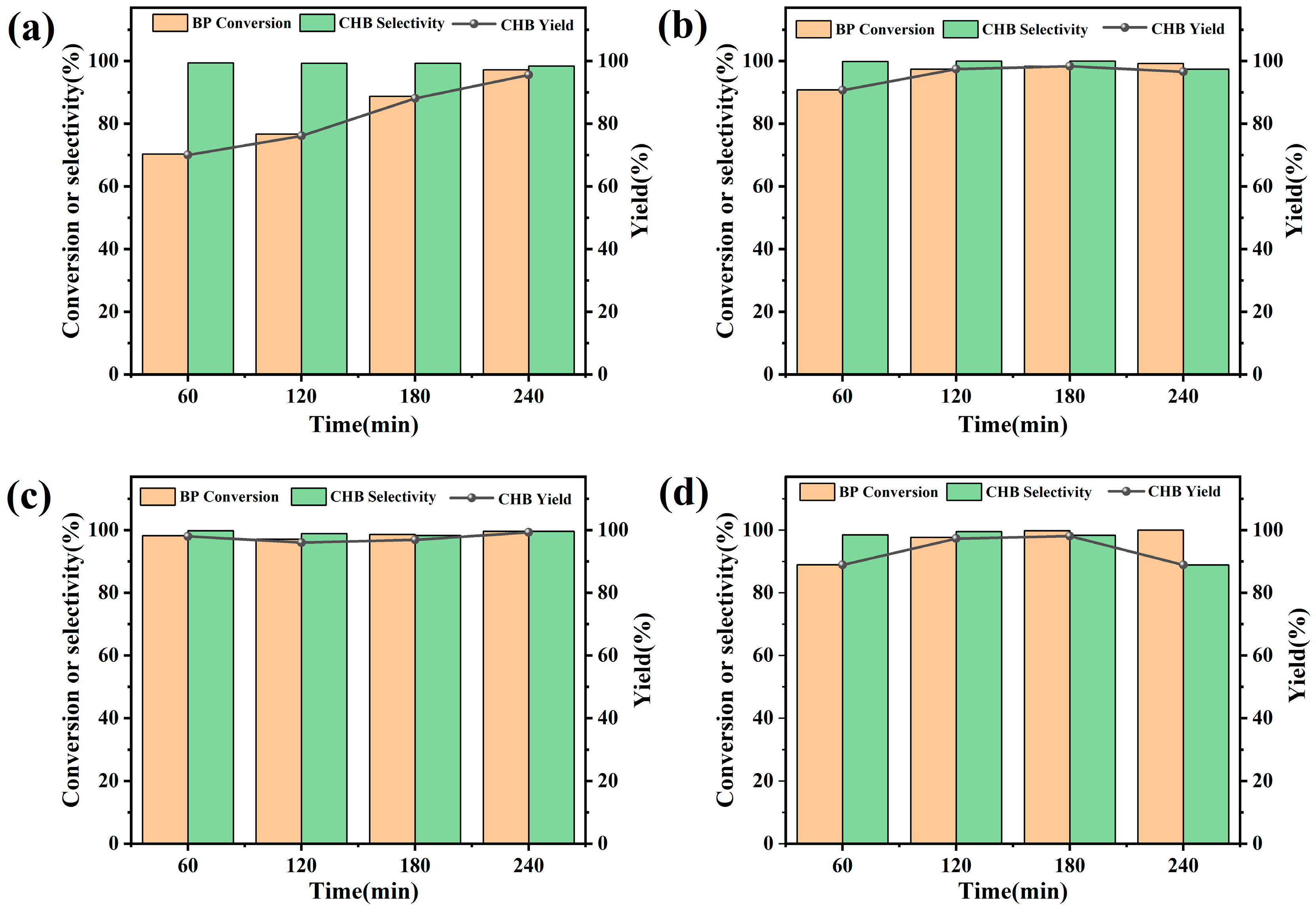
2.4. 20% M/SiO2 (M = Cu, Co, Fe) Catalysts for BP Hydrogenation
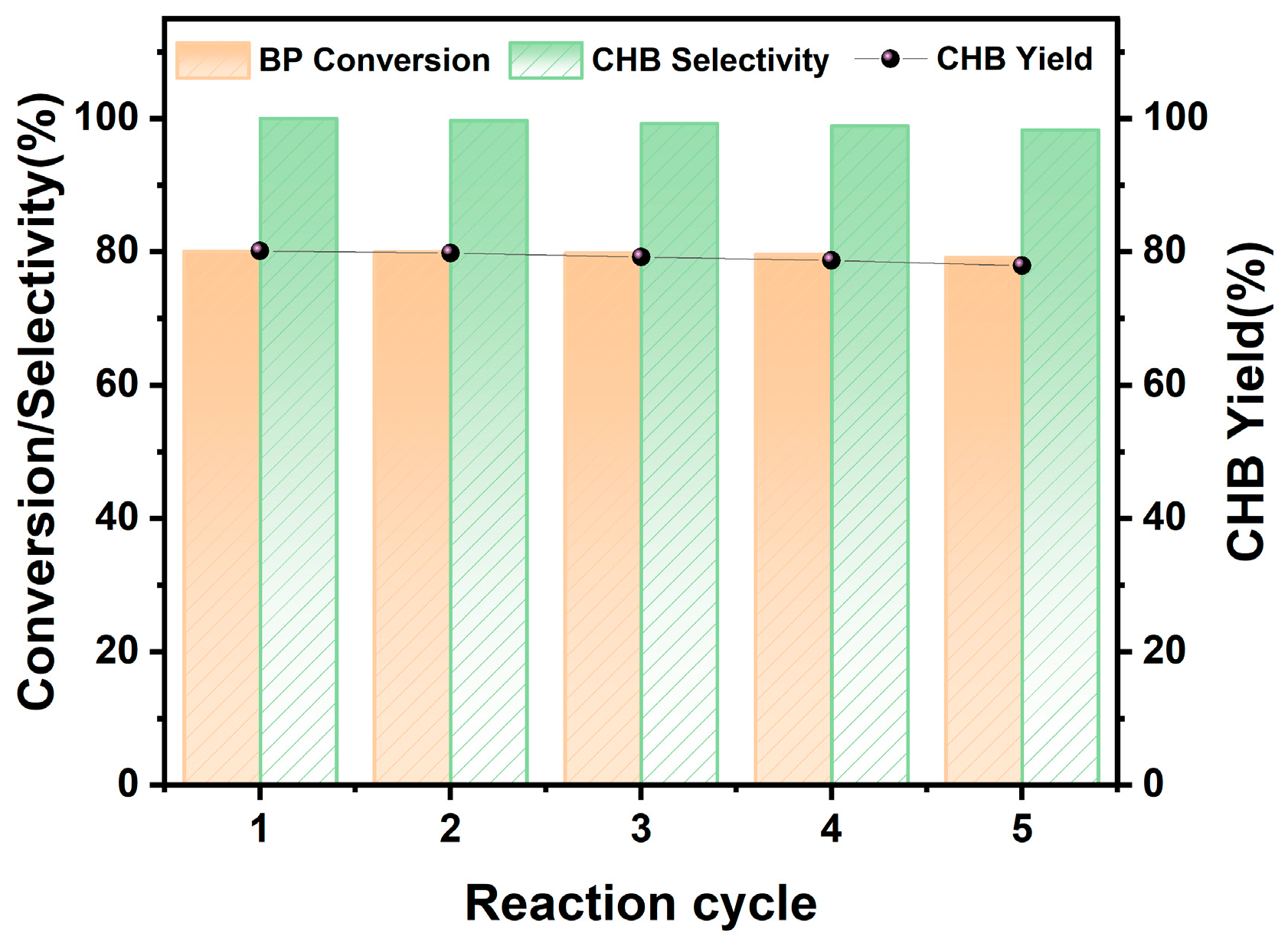
2.5. Cycle Stability of 20% Ni/SiO2-300
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Preparation of 20%Ni/SiO2 Catalysts
3.2.2. Preparation of 20%M/SiO2 Catalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharma, S.; Singh, P.; Bhardwaj, C.; Khandelwal, B.; Kumar, S. Investigations on combustion and emissions characteristics of aromatic fuel blends in a distributed combustor. Energy Fuels 2021, 35, 3150–3163. [Google Scholar] [CrossRef]
- Amirdivani, S.; Khorshidian, N.; Dana, M.G.; Mohammadi, R.; Mortazavian, A.M.; de Souza, S.L.Q.; Rocha, H.B.; Raices, R. Polycyclic aromatic hydrocarbons in milk and dairy products. Int. J. Dairy Technol. 2019, 72, 120–131. [Google Scholar] [CrossRef]
- Schifter, I.; Diaz, L.; Sanchez-Reyna, G.; Gonzalez-Macias, C.; Gonzalez, U.; Rodriguez, R. Influence of gasoline olefin and aromatic content on exhaust emissions of 15% ethanol blends. Fuel 2020, 265, 116950. [Google Scholar] [CrossRef]
- Stanislaus, A.; Marafi, A.; Rana, M.S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production. Catal. Today 2010, 153, 1–68. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Lee, W.-J.; Chen, C.-C.; Chen, C.-B. Saving energy and reducing emissions of both polycyclic aromatic hydrocarbons and particulate matter by adding bio-solution to emulsified diesel. Environ. Sci. Technol. 2006, 40, 5553–5559. [Google Scholar] [CrossRef]
- Song, Y.; Ding, X.; Li, F.; Zhang, D.; Zhao, X.; Wang, Y. A novel and green synthesis of methylcyclohexane diisocyanate: Reaction properties, deactivation and regeneration of Rh/γ-Al2O3 catalyst in benzene ring selective hydrogenation. Appl. Catal. A-Gen. 2023, 651, 119017. [Google Scholar] [CrossRef]
- Fahy, J.; Trimm, D.L.; Cookson, D.J. Four component catalysis for the hydroalkylation of benzene to cyclohexyl benzene. Appl. Catal. A-Gen. 2001, 211, 259–268. [Google Scholar] [CrossRef]
- Lu, L.; Rong, Z.; Du, W.; Ma, S.; Hu, S. Selective hydrogenation of single benzene ring in biphenyl catalyzed by skeletal Ni. ChemCatChem 2009, 1, 369–371. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, H.; Yang, G.; Liu, J.; Xu, J.; Wang, P.; Zhao, N.; Zhu, L.; Chen, B.H. Highly dispersed rhodium atoms supported on defect-rich Co(OH)2 for the chemoselective hydrogenation of nitroarenes. New J. Chem. 2022, 46, 1158–1167. [Google Scholar] [CrossRef]
- Hiyoshi, N.; Rode, C.V.; Sato, O.; Shirai, M. Biphenyl hydrogenation over supported transition metal catalysts under supercritical carbon dioxide solvent. Appl. Catal. A-Gen. 2005, 288, 43–47. [Google Scholar] [CrossRef]
- Olivas, A.; Gaxiola, E.; Cruz-Reyes, J.; Alvarez-Amparan, M.A.; Valdez, R. Transition-metal influence (Fe, Cu) on the MoWS catalyst for biphenyl hydrogenation. Fuel Process. Technol. 2020, 204, 106410. [Google Scholar] [CrossRef]
- Yang, Y.; You, Y.; Wu, J.; Feng, J.; Zhang, Y. Phosphotungstic acid encapsulated in USY zeolite as catalysts for the synthesis of cyclohexylbenzene. J. Iran Chem. Soc. 2021, 18, 573–580. [Google Scholar] [CrossRef]
- Xu, M.Q.; Xing, L.D.; Li, W.S.; Zuo, X.X.; Shu, D.; Li, G.L. Application of cyclohexyl benzene as electrolyte additive for overcharge protection of lithium ion battery. J. Power Sources 2008, 184, 427–431. [Google Scholar] [CrossRef]
- Liu, L.; Zhao, J.; Liu, J. Relationship analysis of lithium battery over charge performance and CHB. Chin. J. Power Sources 2017, 41, 205–207. [Google Scholar]
- Li, W.; Zhao, Z.; Li, J.; Liu, Y.; Gao, B.; Li, X.; Zhang, M.; Liu, Z. Effects of Ni-loading on the performance of Ni/SiO2 catalysts for the highly selective hydrogenation of biphenyl to cyclohexylbenzene. ChemistrySelect 2021, 6, 3897–3902. [Google Scholar] [CrossRef]
- Kalenchuk, A.N.; Koklin, A.E.; Bogdan, V.I.; Kustov, L.M. Hydrogenation of biphenyl and isomeric terphenyls over a Pt-containing catalyst. Russ. Chem. Bull. 2017, 66, 1208–1212. [Google Scholar] [CrossRef]
- Yang, J.; Ma, J.-J.; Zhang, D.-M.; Xue, T.; Guan, Y.-J. Effect of organic moieties (phenyl, naphthalene, and biphenyl) in Zr-MIL-140 on the hydrogenation activity of Pd nanoparticles. Chin. Chem. Lett. 2016, 27, 1679–1682. [Google Scholar] [CrossRef]
- Baigildin, I.G.; Karakhanov, E.A.; Maximov, A.L.; Vutolkina, A.V. Biphenyl hydrogenation with syngas for hydrogen purification and transportation: Performance of dispersed catalytic systems based on transition metal sulfides. Pet. Chem. 2021, 61, 1131–1137. [Google Scholar] [CrossRef]
- Philippov, A.A.; Chibiryaev, A.M.; Martyanov, O.N. Catalyzed transfer hydrogenation by 2-propanol for highly selective PAHs reduction. Catal. Today 2021, 379, 15–22. [Google Scholar] [CrossRef]
- Nunez, S.; Montesinos-Castellanos, A.; Zepeda, T.A.; Los Reyes, J.A. Performance and S resistance of novel supported PdPt catalysts on Al2O3-TiO2 material in hydrogenation of biphenyl. Mater. Res. Innov. 2008, 12, 55–59. [Google Scholar] [CrossRef]
- Kondrateva, V.Y.; Martynenko, E.A.; Pimerzin, A.A.; Verevkin, S.P. Effect of support on catalytic properties of platinum-containing catalysts in hydrogenation of a biphenyl-diphenylmethane eutectic mixture. Russ. J. Appl. Chem. 2022, 95, 126–134. [Google Scholar] [CrossRef]
- Yang, J.; Gao, Y.; Fan, J.; Wang, J.; Yang, T.; Bing, Z.; Zhang, M.; Liu, Z. Boosting the selective hydrogenation of biphenyl to cyclohexylbenzene over bimetallic Ni-Ru/SiO2 catalyst via enhancing strong metal-support interaction. Appl. Surf. Sci. 2024, 660, 160012. [Google Scholar] [CrossRef]
- Chernova, M.M.; Minayev, P.P.; Martynenko, Y.A.; Pimerzin, A.A.; Yeremina, Y.V.; Verevkin, S.P.; Pimerzin, A.A. An effect of a support nature and active phase morphology on catalytic properties of Ni-containing catalysts in hydrogenation of biphenyl. Russ. J. Appl. Chem. 2018, 91, 1701–1710. [Google Scholar] [CrossRef]
- Yang, F.; Liu, D.; Zhao, Y.; Wang, H.; Han, J.; Ge, Q.; Zhu, X. Size dependence of vapor phase hydrodeoxygenation of m-Cresol on Ni/SiO2 catalysts. ACS Catal. 2018, 8, 1672–1682. [Google Scholar] [CrossRef]
- Hongmanorom, P.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of the Pd/MCM-41 pore size on the catalytic activity and cis-trans selectivity for partial hydrogenation of canola biodiesel. Energy Fuels 2017, 31, 8202–8209. [Google Scholar] [CrossRef]
- Yang, Y.; Miao, C.; Wang, R.; Zhang, R.; Li, X.; Wang, J.; Wang, X.; Yao, J. Advances in morphology-controlled alumina and its supported Pd catalysts: Synthesis and applications. Chem. Soc. Rev. 2024, 53, 5014–5053. [Google Scholar] [CrossRef]
- Zhu, L.; Zheng, T.; Zheng, J.; Yu, C.; Zhang, N.; Zhou, Q.; Zhang, W.; Chen, B.H. Shape control of nickel crystals and catalytic hydrogenation performance of ruthenium-on-Ni crystals. Crystengcomm 2018, 20, 113–121. [Google Scholar] [CrossRef]
- Garcia-Perez, D.; Alvarez-Galvan, M.C.; Campos-Martin, J.M.; Fierro, J.L.G. Influence of the reduction temperature and the nature of the support on the performance of zirconia and alumina-supported Pt catalysts for n-dodecane hydroisomerization. Catalysts 2021, 11, 88. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, Y.; Tan, X.; Qi, S.; Smith, K.J.; Yi, C.; Yang, B. The effects of alumina morphology and Pt electron property on reversible hydrogenation and dehydrogenation of dibenzyltoluene as a liquid organic hydrogen carrier. Int. J. Hydrogen Energy 2022, 47, 4704–4715. [Google Scholar] [CrossRef]
- Castano, P.; Zepeda, T.A.; Pawelec, B.; Makkee, M.; Fierro, J.L.G. Enhancement of biphenyl hydrogenation over gold catalysts supported on Fe-, Ce- and Ti-modified mesoporous silica (HMS). J. Catal. 2009, 267, 30–39. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Ramesh, A.; KriShnan, P.S.; Mangesh, V.L.; Umasankar, S.; Narayanan, S.; Ragupathi, C.; Shanthi, K. Hydrogenation of dicyclopentadiene into endo-tetrahydrodicyclopentadie over supported different metal catalysts. Microporous Mesoporous Mat. 2019, 290, 109678. [Google Scholar] [CrossRef]
- O’Driscoll, Á.; Leahy, J.J.; Curtin, T. The influence of metal selection on catalyst activity for the liquid phase hydrogenation of furfural to furfuryl alcohol. Catal. Today 2017, 279, 194–201. [Google Scholar] [CrossRef]
- Wang, L.; Chen, T.; Zhang, J.; Jiao, Y.; Wang, J.; Zhu, Q.; Li, X. High catalytic activity and stability quasi homogeneous alkali metal promoted Ni/SiO2 aerogel catalysts for catalytic cracking of n-decane. Fuel 2020, 268, 117384. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, N.; Chen, J. Enhanced direct deoxygenation of anisole to benzene on SiO2-supported NiGa alloy and intermetallic compound. Appl. Catal. B-Environ. 2019, 250, 280–291. [Google Scholar] [CrossRef]
- Mahlaba, S.V.L.; Mahomed, A.S.; Friedrich, H.B. Regeneration of a 15% Ni/SiO2 phosphorus poisoned catalyst and subsequent effects of the support on recovery of the catalytic activity. Appl. Catal. A-Gen. 2018, 565, 163–169. [Google Scholar] [CrossRef]
- Li, B.; Zhang, B.; Guan, Q.; Chen, S.; Ning, P. Activity of Ni/CeO2 catalyst for gasification of phenol in supercritical water. Int. J. Hydrogen Energy 2018, 43, 19010–19018. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, J.; Chen, X.; Liang, C. Nickel molybdenum bimetallic nitrides as efficient catalysts for the hydrodeoxygenation of methyl palmitate. Eur. J. Inorg. Chem. 2023, 26, e202300073. [Google Scholar] [CrossRef]
- Ren, S.; Zhang, P.; Shui, H.; Lei, Z.; Wang, Z.; Kang, S. Promotion of Ni/SBA-15 catalyst for hydrogenation of naphthalene by pretreatment with ammonia/water vapour. Catal. Commun. 2010, 12, 132–136. [Google Scholar] [CrossRef]
- Zhou, S.; Kang, L.; Xu, Z.; Zhu, M. Catalytic performance and deactivation of Ni/MCM-41 catalyst in the hydrogenation of pure acetylene to ethylene. RSC Adv. 2020, 10, 1937–1945. [Google Scholar] [CrossRef]
- Ghuge, S.P.; Saroha, A.K. Catalytic ozonation of dye industry effluent using mesoporous bimetallic Ru-Cu/SBA-15 catalyst. Process Saf. Environ. Protect. 2018, 118, 125–132. [Google Scholar] [CrossRef]
- Yin, Y.; Ren, Y.; Lu, J.; Zhang, W.; Shan, C.; Hua, M.; Lv, L.; Pan, B. The nature and catalytic reactivity of UiO-66 supported Fe3O4 nanoparticles provide new insights into Fe-Zr dual active centers in Fenton-like reactions. Appl. Catal. B-Environ. 2021, 286, 119943. [Google Scholar] [CrossRef]
- Liu, M.; Shi, Y.; Bi, Y.; Xing, E.; Wu, Y.; Huang, S.; Yang, M. Influence of porosity on product distribution over Co/H-ZSM-22 catalysts in the upgrading of palmitic acid. Energy Technol. 2018, 6, 406–415. [Google Scholar] [CrossRef]
- Liu, L.; Lou, H.; Chen, M. Selective hydrogenation of furfural to tetrahydrofurfuryl alcohol over Ni/CNTs and bimetallic Cu-Ni/CNTs catalysts. Int. J. Hydrogen Energy 2016, 41, 14721–14731. [Google Scholar] [CrossRef]
- Armenta, M.A.; Maytorena, V.M.; Flores-Sanchez, L.A.; Quintana, J.M.; Valdez, R.; Olivas, A. Dimethyl ether production via methanol dehydration using Fe3O4and CuO over γ-χ-Al2O3 nanocatalysts. Fuel 2020, 280, 118545. [Google Scholar] [CrossRef]
- Chen, H.; Lin, W.; Zhang, Z.; Yang, Z.; Jie, K.; Fu, J.; Yang, S.-Z.; Dai, S. Facile benzene reduction promoted by a synergistically coupled Cu-Co-Ce ternary mixed oxide. Chem. Sci. 2020, 11, 5766–5771. [Google Scholar] [CrossRef]
- Nie, X.; Zhang, Z.; Wang, H.; Guo, X.; Song, C. Effect of surface structure and Pd doping of Fe catalysts on the selective hydrodeoxygenation of phenol. Catal. Today 2021, 371, 189–203. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, P.; Li, S.; Shi, H.; Jia, X.; Wang, Q.; Yang, F.; Song, Z.; Guo, C.; Hu, J.; et al. Bimetallic Ni-Co nanoparticles on SiO2 as robust catalyst for CO methanation: Effect of homogeneity of Ni-Co alloy. Appl. Catal. B-Environ. 2020, 278, 119307. [Google Scholar] [CrossRef]
- Audemar, M.; Ramdani, W.; Junhui, T.; Ifrim, A.; Ungureanu, A.; Jerome, F.; Royer, S.; Vigier, K.D.O. Selective hydrogenation of xylose to xylitol over Co/SiO2 catalysts. ChemCatChem 2020, 12, 1973–1978. [Google Scholar] [CrossRef]
- Huang, Z.; Barnett, K.J.; Chada, J.P.; Brentzel, Z.J.; Xu, Z.; Dumesic, J.A.; Huber, G.W. Hydrogenation of γ-butyrolactone to 1,4-butanediol over CuCo/TiO2 bimetallic catalysts. ACS Catal. 2017, 7, 8429–8440. [Google Scholar] [CrossRef]
- Huang, Z.; Chen, J.; Jia, Y.; Liu, H.; Xia, C.; Liu, H. Selective hydrogenolysis of xylitol to ethylene glycol and propylene glycol over copper catalysts. Appl. Catal. B-Environ. 2014, 147, 377–386. [Google Scholar] [CrossRef]
- Shao, Y.; Fan, M.; Sun, K.; Gao, G.; Li, C.; Li, D.; Jiang, Y.; Zhang, L.; Zhang, S.; Hu, X. The quantitative conversion of polyethylene terephthalate (PET) and Coca-Cola bottles to p-xylene over Co-based catalysts with tailored activities for deoxygenation and hydrogenation. Green Chem. 2023, 25, 10513–10529. [Google Scholar] [CrossRef]
- Yang, W.; Zhu, W.; Liu, H.; Niu, H.; Luo, J.; Liang, C. Regulating the coordination environment of Co@C catalysts for selective hydrogenation of adiponitrile to hexamethylenediamine. J. Catal. 2024, 430, 115312. [Google Scholar] [CrossRef]
- Bavykina, A.; Yarulina, I.; Al Abdulghani, A.J.; Gevers, L.; Hedhili, M.N.; Miao, X.; Galilea, A.R.; Pustovarenko, A.; Dikhtiarenko, A.; Cadiau, A.; et al. Turning a methanation Co catalyst into an In-Co methanol producer. ACS Catal. 2019, 9, 6910–6918. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Santos, V.P.; Nasalevich, M.A.; Warringa, Q.S.E.; Dugulan, A.I.; Chojecki, A.; Koeken, A.C.J.; Ruitenbeek, M.; Meima, G.; Islam, H.-U.; et al. Elucidating the nature of Fe species during pyrolysis of the Fe-BTC MOF into highly active and stable Fischer-Tropsch catalysts. ACS Catal. 2016, 6, 3236–3247. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yang, Y.; Zhang, B.; Wang, S.; Lin, J.; Wan, S.; Wang, Y. Selective hydrogenolysis of aryl ether bond over Ru-Fe bimetallic catalyst. Catal. Today 2021, 365, 199–205. [Google Scholar] [CrossRef]
- Su, W.; Yang, J.; Zhang, M.; Zhao, Z.; Han, J.; Yang, Y.; Yang, J.-H.; Liu, Z. Highly dispersed and ultra-small Ru nanoparticles deposited on silica support as highly active and stable catalyst for biphenyl hydrogenation. Mol. Catal. 2021, 508, 111577. [Google Scholar] [CrossRef]
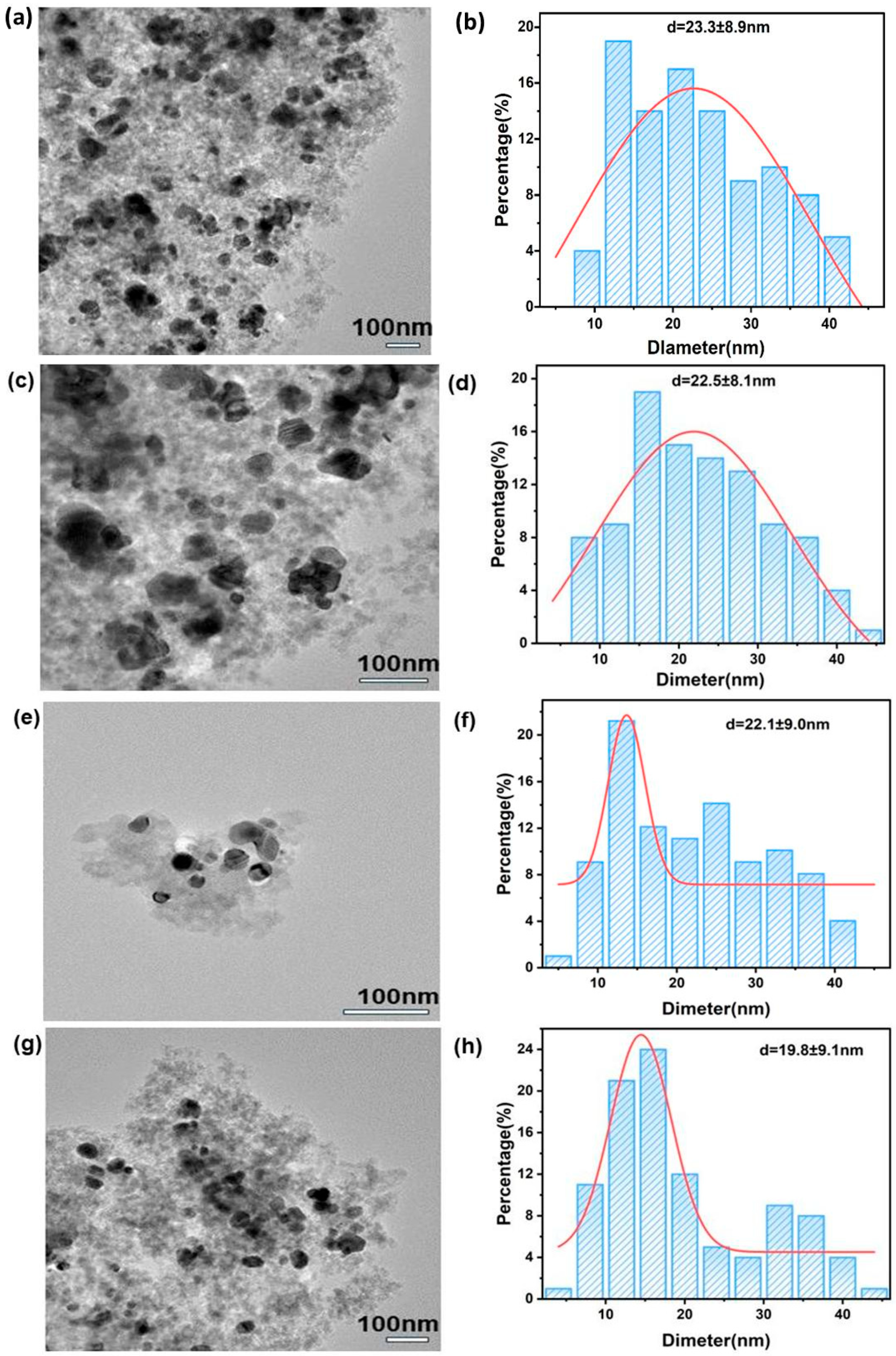

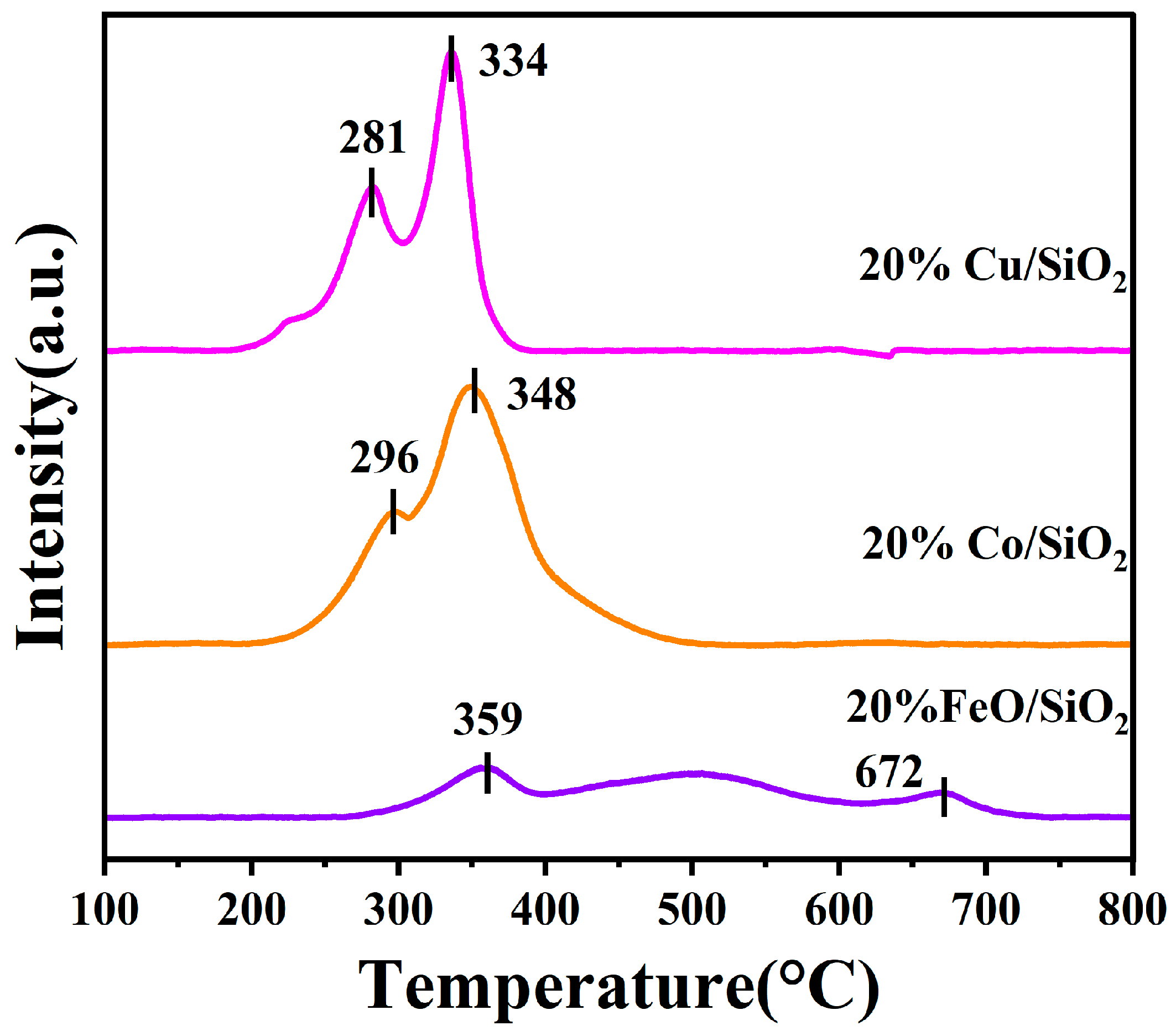



| Sample | SSA a (m2·g−1) | Pore Volume b (cm3g−1) | Average Pore Diameter b (nm) | Metal Particle Size c (nm) |
|---|---|---|---|---|
| 20% Ni/SiO2-100 | 152 | 1.1 | 25.9 | 23.5 |
| 20% Ni/SiO2-200 | 164 | 1.1 | 26.3 | 23.4 |
| 20% Ni/SiO2-300 | 207 | 1.0 | 17.3 | 22.0 |
| 20% Ni/SiO2-400 | 229 | 1.0 | 19.3 | 19.8 |
| Sample | SSA a (m2·g−1) | Pore Volume b (cm3g−1) | Average Pore Diameter b (nm) | Metal Particle Size c (nm) |
|---|---|---|---|---|
| 20%Co/SiO2 | 216 | 1.2 | 20.8 | 23.1 |
| 20%Cu/SiO2 | 204 | 1.2 | 21.7 | 19.2 |
| 20%Fe/SiO2 | 212 | 1.1 | 20.3 | 24.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, J.; Li, W.; Yang, J.; Yang, T.; Liu, Z.; Zhang, M. The Effects of Support Specific Surface Area and Active Metal on the Performance of Biphenyl Selective Hydrogenation to Cyclohexylbenzene. Catalysts 2024, 14, 727. https://doi.org/10.3390/catal14100727
Fan J, Li W, Yang J, Yang T, Liu Z, Zhang M. The Effects of Support Specific Surface Area and Active Metal on the Performance of Biphenyl Selective Hydrogenation to Cyclohexylbenzene. Catalysts. 2024; 14(10):727. https://doi.org/10.3390/catal14100727
Chicago/Turabian StyleFan, Jie, Wei Li, Jingyi Yang, Tao Yang, Zhongyi Liu, and Meng Zhang. 2024. "The Effects of Support Specific Surface Area and Active Metal on the Performance of Biphenyl Selective Hydrogenation to Cyclohexylbenzene" Catalysts 14, no. 10: 727. https://doi.org/10.3390/catal14100727
APA StyleFan, J., Li, W., Yang, J., Yang, T., Liu, Z., & Zhang, M. (2024). The Effects of Support Specific Surface Area and Active Metal on the Performance of Biphenyl Selective Hydrogenation to Cyclohexylbenzene. Catalysts, 14(10), 727. https://doi.org/10.3390/catal14100727







