Synthesis of Tetrahydrocarbazole-Tethered Triazoles as Compounds Targeting Telomerase in Human Breast Cancer Cells
Abstract
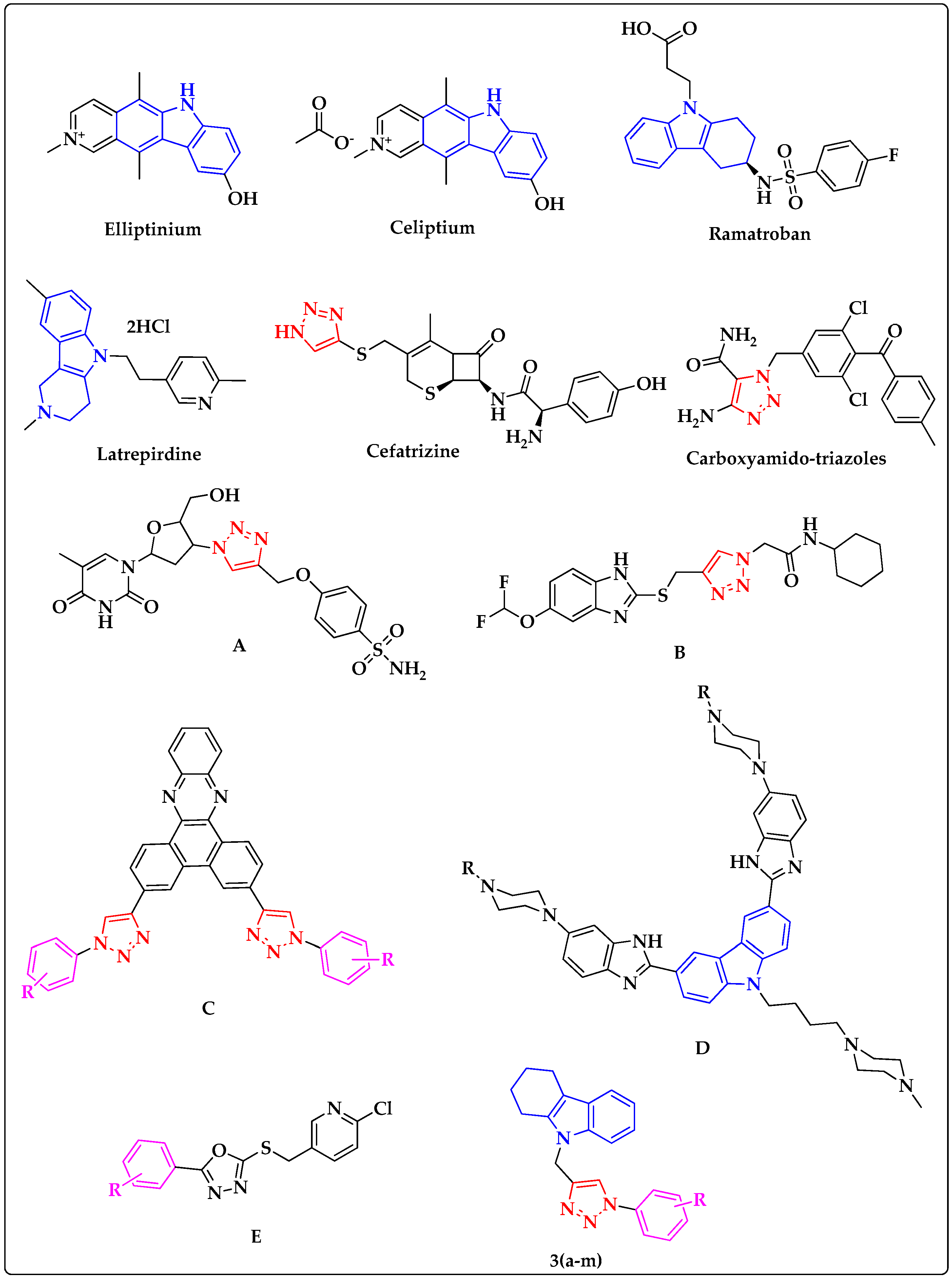
1. Introduction
2. Results and Discussion
2.1. Synthesis of Tetrahydrocarbazole
2.2. Synthesis of Tetrahydrocarbazole–Triazole Derivatives 5(a–m)
2.3. Effect of Newly Synthesized Triazoles on MCF-7 Breast Cancer Cells
2.4. Structural Analysis
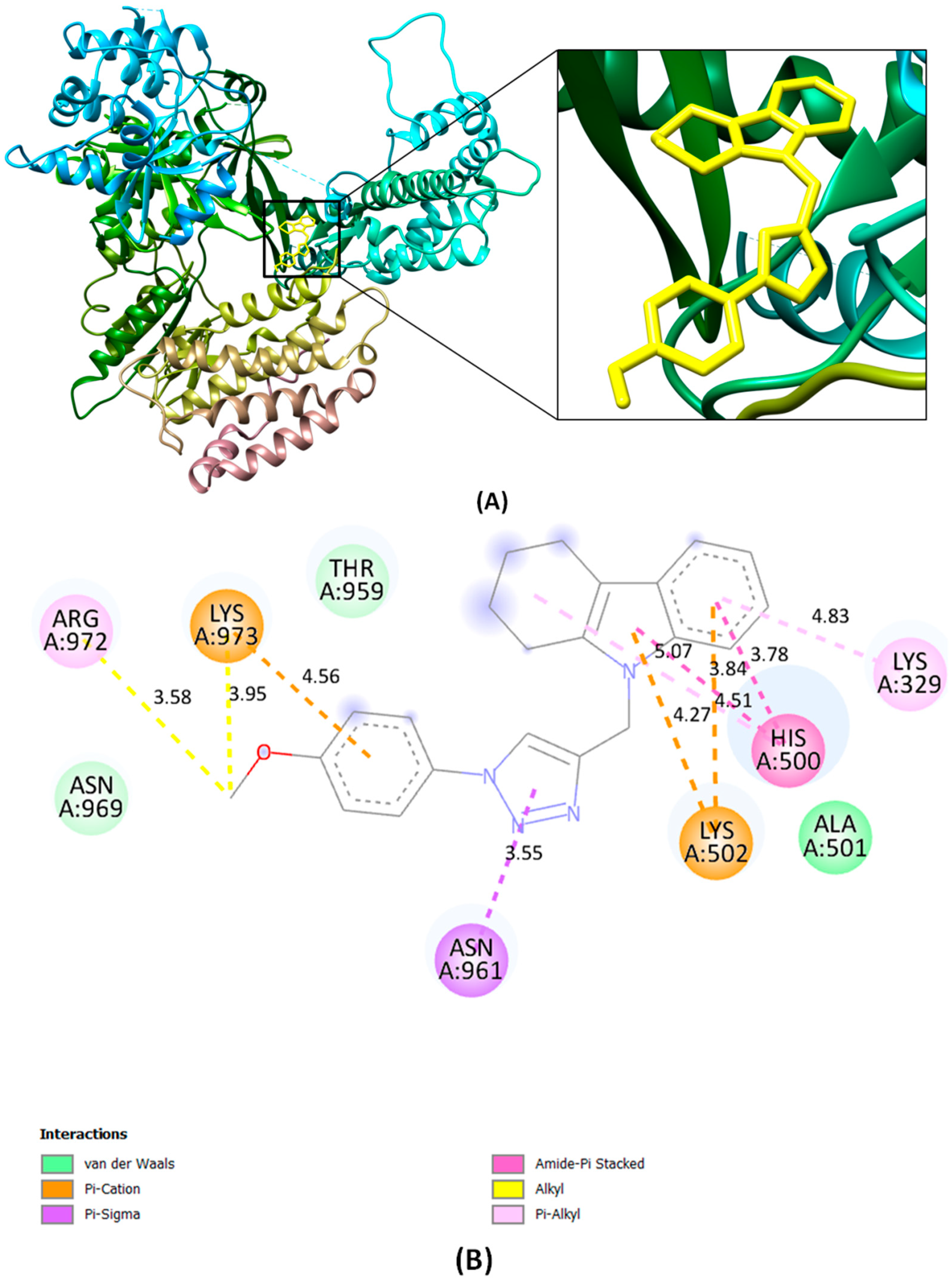
2.5. In Silico Analysis of Novel Compound 5g Targeting Telomerase Reverse Transcriptase
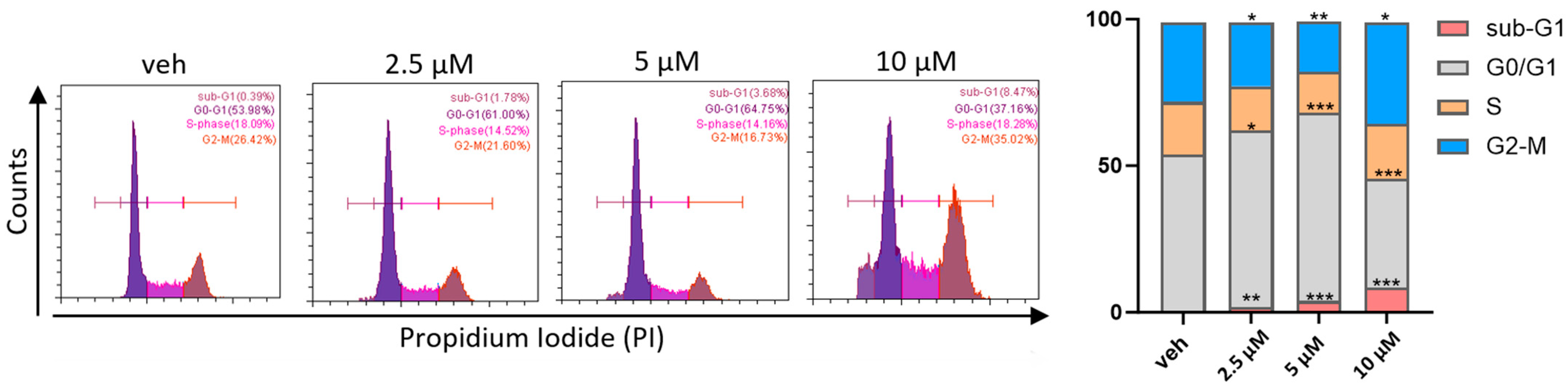
2.6. The Title Compound Arrested Cell Cycle at the Sub-G1 and G2-M Phases in MCF-7 Cells
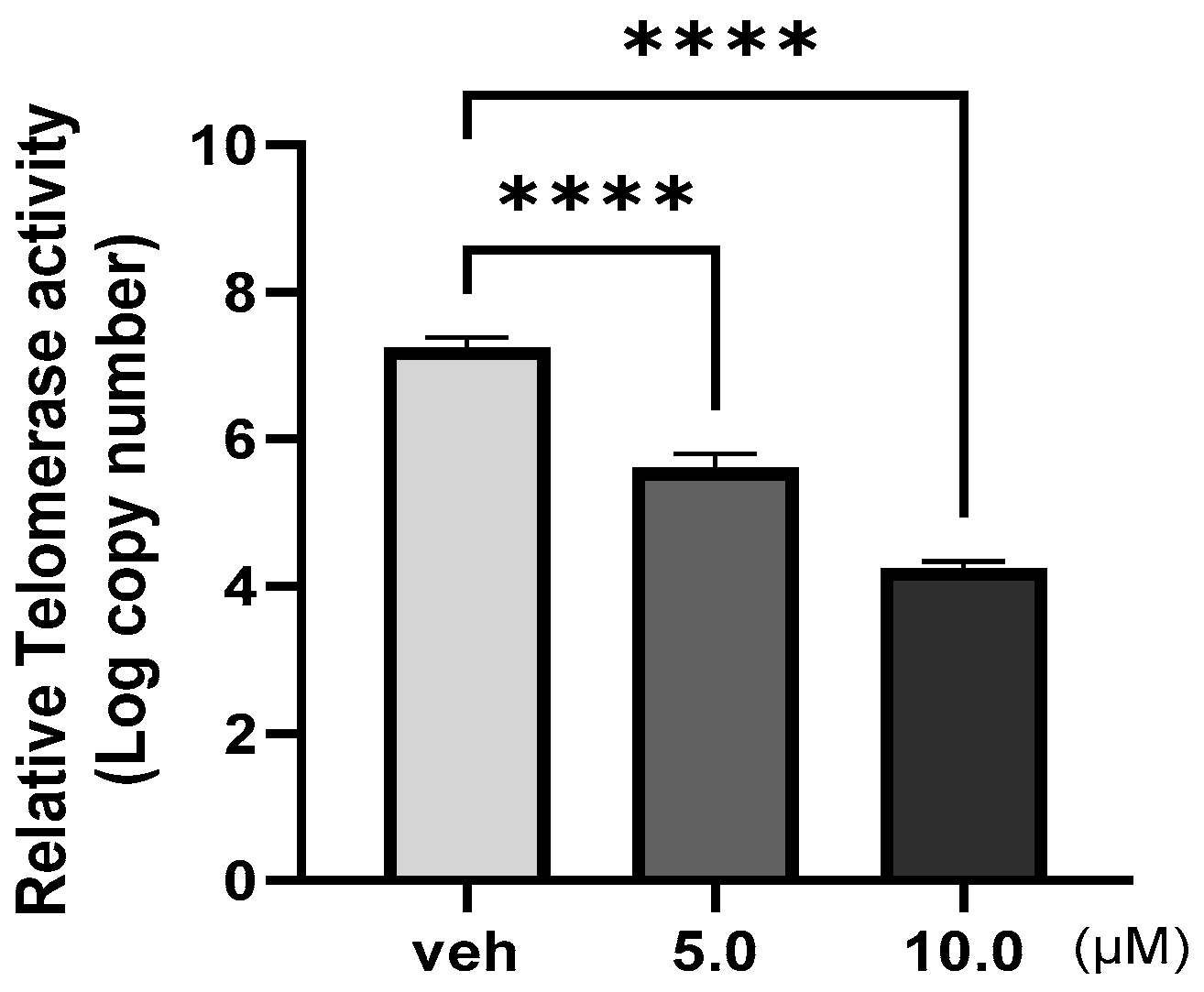
2.7. The Title Compound Inhibited Telomerase Enzymatic Activity in MCF–7 Cells
3. Materials and Methods
3.1. Synthesis of Tetrahydrocarbazole–Triazole Derivatives
3.2. 9-((1-(o-Tolyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5a)
3.3. 9-((1-(4-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5c)
3.4. 9-((1-(3,4-Dichlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5d)
3.5. 9-((1-(4-Bromophenyl)-1H-1,2,3-triazol-4-yl) methyl)-2,3,4,9-tetrahydro-1H-carbazole (5e)
3.6. 9-((1-(3-Chlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5f)
3.7. 9-((1-(4-Methoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5g)
3.8. 9-((1-(p-Tolyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5h)
3.9. 9-((1-(3-Bromophenyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5i)
3.10. 9-((1-Phenyl-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5j)
3.11. 9-((1-(5-(Furan-2-yl)-1H-pyrazol-3-yl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5k)
3.12. 9-((1-(4-Nitrophenyl)-1H-1,2,3-triazol-4-yl) methyl)-2,3,4,9-tetrahydro-1H-carbazole (5l)
3.13. 9-((1-(3-Nitro-4-methyl-phenyl)-1H-1,2,3-triazol-4-yl)methyl)-2,3,4,9-tetrahydro-1H-carbazole (5m)
3.14. Cell Lines and Culture Conditions
3.15. X-ray Data Collection and Refinement Methods
3.16. Molecular Docking Studies
3.17. Annexin V Cell Cycle Analysis Assay
3.18. Telomerase Enzymatic Activity
3.19. Data Analysis and Statistics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Robinson, N.J.; Schiemann, W.P. Telomerase in Cancer: Function, Regulation, and Clinical Translation. Cancers 2022, 14, 808. [Google Scholar] [CrossRef]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of Telomeres and Telomerase in Cancer, and Advances in Telomerase-Targeted Therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef]
- Shay, J.W.; Wright, W.E. Telomerase: A Target for Cancer Therapeutics. Cancer Cell 2002, 2, 257–265. [Google Scholar] [CrossRef]
- Cong, Y.-S.; Wright, W.E.; Shay, J.W. Human Telomerase and Its Regulation. Microbiol. Mol. Biol. Rev. 2002, 66, 407–425. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. TERT—Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Li, Y.; Tergaonkar, V. Noncanonical Functions of Telomerase: Implications in Telomerase-Targeted Cancer Therapies. Cancer Res. 2014, 74, 1639–1644. [Google Scholar] [CrossRef]
- Gaspar, T.B.; Sá, A.; Lopes, J.M.; Sobrinho-Simões, M.; Soares, P.; Vinagre, J. Telomere Maintenance Mechanisms in Cancer. Genes 2018, 9, 241. [Google Scholar] [CrossRef]
- Kheimar, A.; Trimpert, J.; Groenke, N.; Kaufer, B.B. Overexpression of Cellular Telomerase RNA Enhances Virus-Induced Cancer Formation. Oncogene 2018, 38, 1778–1786. [Google Scholar] [CrossRef]
- Bayat, M.; Tanny, R.E.; Wang, Y.; Herden, C.; Daniel, J.; Andersen, E.C.; Liebau, E.; Waschk, D.E.J. Effects of Telomerase Overexpression in the Model Organism Caenorhabditis Elegans. Gene 2020, 732, 144367. [Google Scholar] [CrossRef]
- Cunningham, A.P.; Love, W.K.; Zhang, R.W.; Andrews, L.G.; Tollefsbol, T.O. Telomerase Inhibition in Cancer Therapeutics: Molecular-Based Approaches. Curr. Med. Chem. 2006, 13, 2875–2888. [Google Scholar] [CrossRef]
- Torres-Montaner, A. The Telomere Complex and the Origin of the Cancer Stem Cell. Biomark. Res. 2021, 9, 81. [Google Scholar] [CrossRef]
- Betori, R.C.; Liu, Y.; Mishra, R.K.; Cohen, S.B.; Kron, S.J.; Scheidt, K.A. Targeted Covalent Inhibition of Telomerase. ACS Chem. Biol. 2020, 15, 706–717. [Google Scholar] [CrossRef]
- Melana, S.M.; Holland, J.F.; Pogo, B.G. Inhibition of cell growth and telomerase activity of breast cancer cells in vitro by 3′-azido-3′-deoxythymidine. Clin. Cancer Res. 1998, 4, 693. [Google Scholar]
- Gomez, D.E.; Armando, R.G.; Alonso, D.F. AZT as a Telomerase Inhibitor. Front. Oncol. 2012, 2, 113. [Google Scholar] [CrossRef]
- Pendino, F.; Flexor, M.; Delhommeau, F.; Buet, D.; Lanotte, M.; Ségal-Bendirdjian, E. Retinoids Down-Regulate Telomerase and Telomere Length in a Pathway Distinct from Leukemia Cell Differentiation. Proc. Natl. Acad. Sci. USA 2001, 98, 6662–6667. [Google Scholar] [CrossRef]
- Aldous, W.K.; Marean, A.J.; DeHart, M.J.; Matej, L.A.; Moore, K.H. Effects of Tamoxifen on Telomerase Activity in Breast Carcinoma Cell Lines. Cancer 1999, 85, 1523–1529. [Google Scholar] [CrossRef]
- Naasani, I.; Seimiya, H.; Tsuruo, T. Telomerase Inhibition, Telomere Shortening, and Senescence of Cancer Cells by Tea Catechins. Biochem. Biophys. Res. Commun. 1998, 249, 391–396. [Google Scholar] [CrossRef]
- Fu, W.; Begley, J.G.; Killen, M.W.; Mattson, M.P. Anti-Apoptotic Role of Telomerase in Pheochromocytoma Cells. J. Biol. Chem. 1999, 274, 7264–7271. [Google Scholar] [CrossRef]
- Gomez, D. Telomerase Downregulation Induced by the G-Quadruplex Ligand 12459 in A549 Cells Is Mediated by hTERT RNA Alternative Splicing. Nucleic Acids Res. 2004, 32, 371–379. [Google Scholar] [CrossRef]
- Malonne, H.; Atassi, G. DNA Topoisomerase Targeting Drugs: Mechanisms of Action and Perspectives. Anti-Cancer Drugs 1997, 8, 811–822. [Google Scholar] [CrossRef]
- Arteaga, C.L.; Kisner, D.L.; Goodman, A.; Von Hoff, D.D. Elliptinium, a DNA Intercalating Agent with Broad Antitumor Activity in a Human Tumor Cloning System. Eur. J. Cancer Clin. Oncol. 1987, 23, 1621–1626. [Google Scholar] [CrossRef]
- Issa, S.; Prandina, A.; Bedel, N.; Rongved, P.; Yous, S.; Le Borgne, M.; Bouaziz, Z. Carbazole Scaffolds in Cancer Therapy: A Review from 2012 to 2018. J. Enzym. Inhib. Med. Chem. 2019, 34, 1321–1346. [Google Scholar] [CrossRef]
- Basappa, B.; Jung, Y.Y.; Ravish, A.; Xi, Z.; Swamynayaka, A.; Madegowda, M.; Pandey, V.; Lobie, P.E.; Sethi, G.; Ahn, K.S. Methyl-Thiol-Bridged Oxadiazole and Triazole Heterocycles as Inhibitors of NF-κB in Chronic Myelogenous Leukemia Cells. Biomedicines 2023, 11, 1662. [Google Scholar] [CrossRef]
- Vishwanath, D.; Xi, Z.; Ravish, A.; Mohan, A.; Basappa, S.; Krishnamurthy, N.P.; Gaonkar, S.L.; Pandey, V.; Lobie, P.E.; Basappa, B. Electrochemical Synthesis of New Isoxazoles and Triazoles Tethered with Thiouracil Base as Inhibitors of Histone Deacetylases in Human Breast Cancer Cells. Molecules 2023, 28, 5254. [Google Scholar] [CrossRef]
- Penthala, N.R.; Madhukuri, L.; Thakkar, S.; Madadi, N.R.; Lamture, G.; Eoff, R.L.; Crooks, P.A. Synthesis and Anti-Cancer Screening of Novel Heterocyclic-(2H)-1,2,3-Triazoles as Potential Anti-Cancer Agents. MedChemComm 2015, 6, 1535–1543. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, S.-J.; Liu, Y. 1,2,3-Triazole-Containing Hybrids as Potential Anticancer Agents: Current Developments, Action Mechanisms and Structure-Activity Relationships. Eur. J. Med. Chem. 2019, 183, 111700. [Google Scholar] [CrossRef]
- Yakkala, P.A.; Panda, S.R.; Shafi, S.; Naidu, V.G.M.; Yar, M.S.; Ubanako, P.N.; Adeyemi, S.A.; Kumar, P.; Choonara, Y.E.; Radchenko, E.V.; et al. Synthesis and Cytotoxic Activity of 1,2,4-Triazolo-Linked Bis-Indolyl Conjugates as Dual Inhibitors of Tankyrase and PI3K. Molecules 2022, 27, 7642. [Google Scholar] [CrossRef]
- Praveena Devi, C.B.; Vijay, K.; Hari Babu, B.; Adil, S.F.; Mujahid Alam, M.; Vijjulatha, M.; Ansari, M.B. CuSO4/Sodium Ascorbate Catalysed Synthesis of Benzosuberone and 1,2,3-Triazole Conjugates: Design, Synthesis and in Vitro Anti-Proliferative Activity. J. Saudi Chem. Soc. 2019, 23, 980–991. [Google Scholar] [CrossRef]
- Ashram, M.; Habashneh, A.Y.; Bardaweel, S.; Taha, M.O. A Click Synthesis, Molecular Docking and Biological Evaluation of 1,2,3-Triazoles-Benzoxazepine Hybrid as Potential Anticancer Agents. Med. Chem. Res. 2022, 32, 271–287. [Google Scholar] [CrossRef]
- Berrino, E.; Angeli, A.; Zhdanov, D.D.; Kiryukhina, A.P.; Milaneschi, A.; De Luca, A.; Bozdag, M.; Carradori, S.; Selleri, S.; Bartolucci, G.; et al. Azidothymidine “Clicked” into 1,2,3-Triazoles: First Report on Carbonic Anhydrase–Telomerase Dual-Hybrid Inhibitors. J. Med. Chem. 2020, 63, 7392–7409. [Google Scholar] [CrossRef]
- Singu, P.S.; Chilakamarthi, U.; Mahadik, N.S.; Keerti, B.; Valipenta, N.; Mokale, S.N.; Nagesh, N.; Kumbhare, R.M. Benzimidazole-1,2,3-Triazole Hybrid Molecules: Synthesis and Study of Their Interaction with G-Quadruplex DNA. RSC Med. Chem. 2021, 12, 416–429. [Google Scholar] [CrossRef]
- Pal, S.; Fatma, K.; Ravichandiran, V.; Dash, J. Triazolyl Dibenzo[a,c]Phenazines Stabilize Telomeric G-quadruplex and Inhibit Telomerase. Asian J. Org. Chem. 2021, 10, 2921–2926. [Google Scholar] [CrossRef]
- Maji, B.; Kumar, K.; Kaulage, M.; Muniyappa, K.; Bhattacharya, S. Design and Synthesis of New Benzimidazole–Carbazole Conjugates for the Stabilization of Human Telomeric DNA, Telomerase Inhibition, and Their Selective Action on Cancer Cells. J. Med. Chem. 2014, 57, 6973–6988. [Google Scholar] [CrossRef]
- Zheng, Q.-Z.; Zhang, X.-M.; Xu, Y.; Cheng, K.; Jiao, Q.-C.; Zhu, H.-L. Synthesis, Biological Evaluation, and Molecular Docking Studies of 2-Chloropyridine Derivatives Possessing 1,3,4-Oxadiazole Moiety as Potential Antitumor Agents. Bioorg. Med. Chem. 2010, 18, 7836–7841. [Google Scholar] [CrossRef]
- Schotten, C.; Nicholls, T.P.; Bourne, R.A.; Kapur, N.; Nguyen, B.N.; Willans, C.E. Making Electrochemistry Easily Accessible to the Synthetic Chemist. Green Chem. 2020, 22, 3358–3375. [Google Scholar] [CrossRef]
- Zhu, C.; Ang, N.W.J.; Meyer, T.H.; Qiu, Y.; Ackermann, L. Organic Electrochemistry: Molecular Syntheses with Potential. ACS Cent. Sci. 2021, 7, 415–431. [Google Scholar] [CrossRef]
- Rogers, C.U.; Corson, B.B. One-Step Synthesis of 1,2,3,4-Tetrahydrocarbazole and 1,2-Benzo-3,4-Dihydrocarbazole. J. Am. Chem. Soc. 1947, 69, 2910–2911. [Google Scholar] [CrossRef]
- Chaudhari, T.Y.; Tandon, V. Recent Approaches to the Synthesis of Tetrahydrocarbazoles. Org. Biomol. Chem. 2021, 19, 1926–1939. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Gammon, D.; van Steen, E. Synthesis of 1,2,3,4-tetrahydrocarbazole over zeolite catalysts. Catal. Lett. 1999, 61, 93–97. [Google Scholar] [CrossRef]
- Kumar, N.; Kumar, V.; Chowdhary, Y. A Review on Synthesis Methods of Tricyclic 1,2,3,4-Tetrahydrocarbazoles. World J. Adv. Res. Rev. 2022, 13, 160–171. [Google Scholar] [CrossRef]
- Scott, T.L.; Burke, N.; Carrero-Martínez, G.; Söderberg, B.C.G. Synthesis of 1,2,3,4-Tetrahydrocarbazoles and Related Tricyclic Indoles. Tetrahedron 2007, 63, 1183–1190. [Google Scholar] [CrossRef]
- Kehl, A.; Schupp, N.; Breising, V.M.; Schollmeyer, D.; Waldvogel, S.R. Electrochemical Synthesis of Carbazoles by Dehydrogenative Coupling Reaction. Chem.-Eur. J. 2020, 26, 15847–15851. [Google Scholar] [CrossRef]
- He, J.; Liu, A.; Yu, Y.; Wang, C.; Mei, H.; Han, J. Electrochemical Annulation of Indole-Tethered Alkynes Enabling Synthesis of Exocyclic Alkenyl Tetrahydrocarbazoles. J. Org. Chem. 2023, 88, 6962–6972. [Google Scholar] [CrossRef]
- Wirtanen, T.; Rodrigo, E.; Waldvogel, S.R. Selective and Scalable Electrosynthesis of 2H-2-(Aryl)-benzo[d]-1,2,3-triazoles and Their N-Oxides by Using Leaded Bronze Cathodes. Chem.-Eur. J. 2020, 26, 5592–5597. [Google Scholar] [CrossRef]
- Krishnan, M.; Kathiresan, M.; Praveen, C. Electrochemically Generated Copper(I)-Catalyzed Click Chemistry: Triazole Synthesis and Insights into Their Photophysical Properties. Eur. J. Org. Chem. 2023, 26, e202201405. [Google Scholar] [CrossRef]
- Carre-Rangel, L.; Espinoza, K.; Oropeza-Guzmán, M.; Rivero, I. Synthesis of Triazoles by Electro-Assisted Click Reaction Using a Copper Foil Electrode. J. Braz. Chem. Soc. 2021, 32, 1373–1380. [Google Scholar] [CrossRef]
- Ravish, A.; Siddappa, T.P.; Xi, Z.; Vishwanath, D.; Mohan, A.; Basappa, S.; Krishnamurthy, N.P.; Lobie, P.E.; Pandey, V.; Basappa, B. Electrochemical Synthesis of Versatile Pyrimidine and Oxadiazoles Tethered Triazoles as Inhibitors of VEGFR-2 in Human Breast Cancer Cells. Catalysts 2023, 13, 1353. [Google Scholar] [CrossRef]
- Ramachandran, C.; Fonseca, H.B.; Jhabvala, P.; Escalon, E.A.; Melnick, S.J. Curcumin Inhibits Telomerase Activity through Human Telomerase Reverse Transcritpase in MCF-7 Breast Cancer Cell Line. Cancer Lett. 2002, 184, 1–6. [Google Scholar] [CrossRef]
- Chakraborty, S.; Ghosh, U.; Bhattacharyya, N.P.; Bhattacharya, R.K.; Roy, M. Inhibition of Telomerase Activity and Induction of Apoptosis by Curcumin in K-562 Cells. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2006, 596, 81–90. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Telomerase Inhibitors from Natural Products and Their Anticancer Potential. Int. J. Mol. Sci. 2017, 19, 13. [Google Scholar] [CrossRef]
- Basappa, B.; Chumadathil Pookunoth, B.; Shinduvalli Kempasiddegowda, M.; Kanchugarakoppal Subbegowda, R.; Lobie, P.E.; Pandey, V. Novel Biphenyl Amines Inhibit Oestrogen Receptor (ER)-α in ER-Positive Mammary Carcinoma Cells. Molecules 2021, 26, 783. [Google Scholar] [CrossRef]
- SAINT-Plus, B. and SADABS, version 2004/1; Bruker AXS Inc.: Madison, WI, USA, 2004.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON, an integrated tool for the analysis of the results of a single crystal structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, c34. [Google Scholar]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0– New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Huey, R.; Morris, G.M.; Olson, A.J.; Goodsell, D.S. A Semiempirical Free Energy Force Field with Charge-based Desolvation. J. Comput. Chem. 2007, 28, 1145–1152. [Google Scholar] [CrossRef]
- BIOVIA Dassault Systèmes. Discovery Studio Visualizer, version 21.1.0.20298; Dassault Systèmes: San Diego, CA, USA, 2020. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization System for Exploratory Research and Analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.; Chen, X.; Huang, P.; Ma, L.; Ding, H.; Basappa, B.; Zhu, T.; Lobie, P.E.; Pandey, V. Combined Inhibition of BADSer99 Phosphorylation and PARP Ablates Models of Recurrent Ovarian Carcinoma. Commun. Med. 2022, 2, 82. [Google Scholar] [CrossRef]
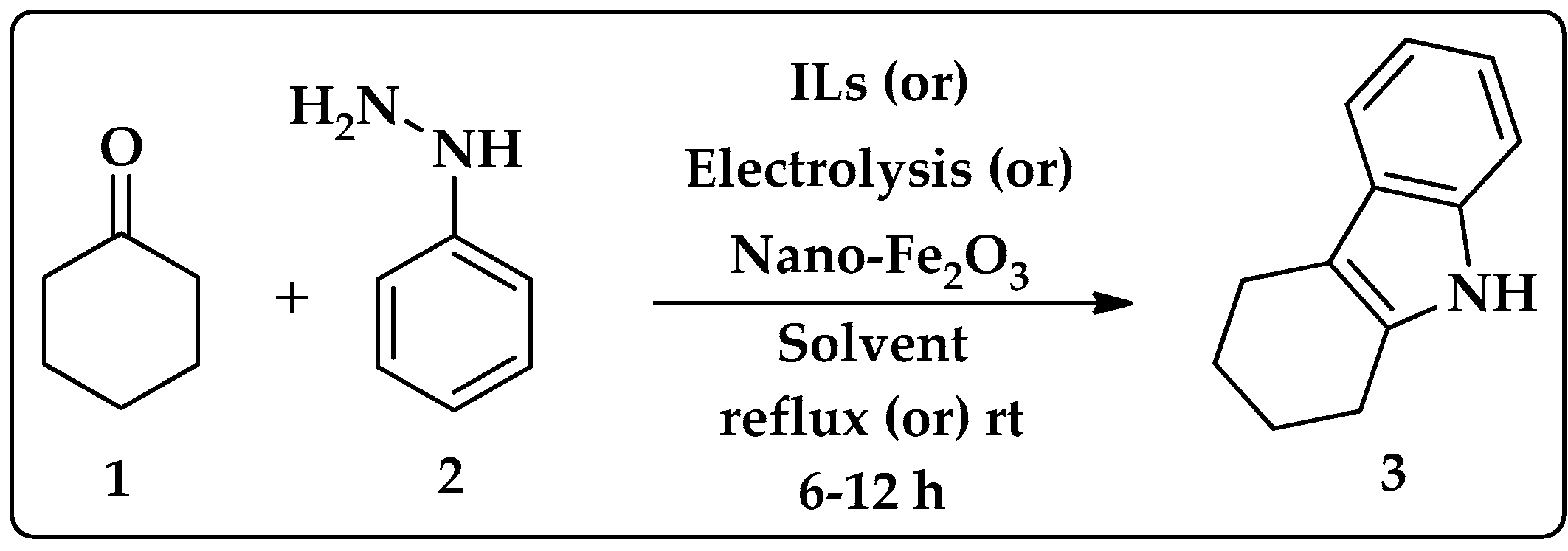

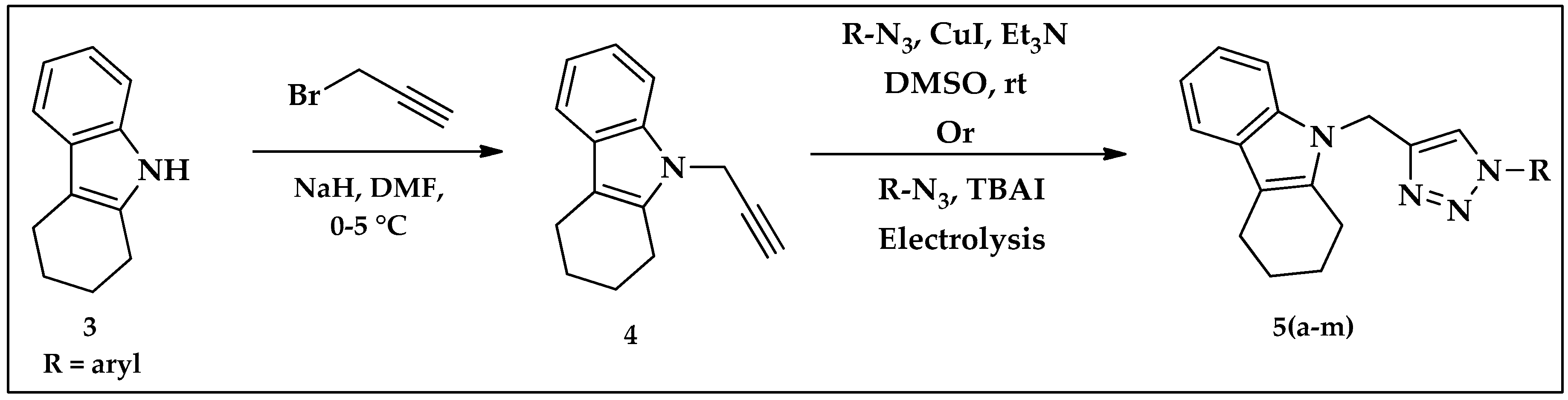

| Entry | Solvent | Catalyst | Reaction Condition | Time | Yield (%) |
|---|---|---|---|---|---|
| 1. | [BMIM]BF4 | Reflux | 12 | 75 | |
| 2. | [EMIM]BF4 | Reflux | 12 | 70 | |
| 3. | Electrochemical method | rt | 8 | 80 | |
| 4. | DMSO | 0.1 eq nano-Fe2O3 | rt | 12 | 50 |
| 5. | DMSO | 0.3 eq nano-Fe2O3 | rt | 12 | 65 |
| 6. | DMSO | 0.5 eq nano-Fe2O3 | rt | 12 | 75 |
| 7. | DMSO | 0.5 eq nano-Fe2O3 | Reflux | 8 | 60 |
| 8. | DMF | 0.5 eq nano-Fe2O3 | rt | 12 | 70 |
| 9. | DMF | 0.5 eq nano-Fe2O3 | Reflux | 8 | 55 |
| 10. | ACN | 0.5 eq nano-Fe2O3 | rt | nr | nr |
| 11. | Dioxane | 0.5 eq nano-Fe2O3 | rt | 12 | 55 |
| 12. | Acetone | 0.5 eq nano-Fe2O3 | rt | nr | nr |
| 13. | THF | 0.5 eq nano-Fe2O3 | rt | nr | nr |
| 14. | EtOH | 0.5 eq nano-Fe2O3 | rt | 10 | 70 |
| 15. | MeOH | 0.5 eq nano-Fe2O3 | rt | 11 | 65 |
| 16. | H2O | 0.5 eq nano-Fe2O3 | rt | 6 | 85 |
| 17. | EtOH:H2O | 0.5 eq nano-Fe2O3 | rt | 7 | 80 |
| Entry | R–N3 | Product | IC50 (µM) |
|---|---|---|---|
| 5a | 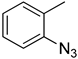 | 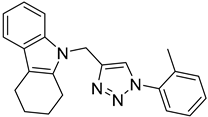 | >100 |
| 5b |  |  | 21.38 |
| 5c |  | 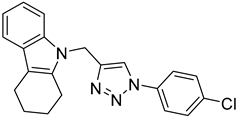 | >100 |
| 5d | 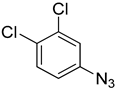 | 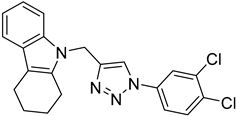 | >100 |
| 5e |  |  | >100 |
| 5f |  | 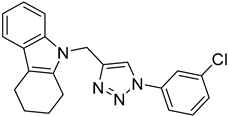 | >100 |
| 5g | 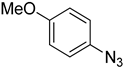 | 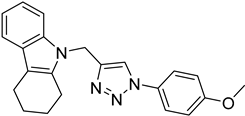 | 15.14 |
| 5h |  |  | >100 |
| 5i | 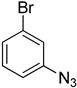 | 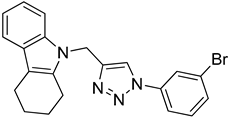 | >100 |
| 5j | 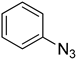 |  | 27.19 |
| 5k | 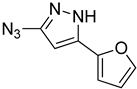 | 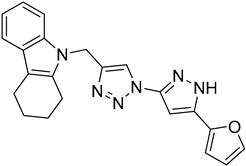 | 34.8 |
| 5l |  | 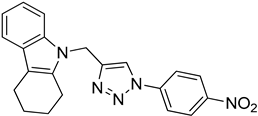 | 64.33 |
| 5m |  |  | 79.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uppar, P.M.; Ravish, A.; Xi, Z.; Kumar Harish, K.; Kumar, A.M.; Poonacha, L.K.; Swaroop, T.R.; Somu, C.; Gaonkar, S.L.; Madegowda, M.; et al. Synthesis of Tetrahydrocarbazole-Tethered Triazoles as Compounds Targeting Telomerase in Human Breast Cancer Cells. Catalysts 2024, 14, 726. https://doi.org/10.3390/catal14100726
Uppar PM, Ravish A, Xi Z, Kumar Harish K, Kumar AM, Poonacha LK, Swaroop TR, Somu C, Gaonkar SL, Madegowda M, et al. Synthesis of Tetrahydrocarbazole-Tethered Triazoles as Compounds Targeting Telomerase in Human Breast Cancer Cells. Catalysts. 2024; 14(10):726. https://doi.org/10.3390/catal14100726
Chicago/Turabian StyleUppar, Pradeep M., Akshay Ravish, Zhang Xi, Keshav Kumar Harish, Arun M. Kumar, Lisha K. Poonacha, Toreshettahally R. Swaroop, Chaithanya Somu, Santosh L. Gaonkar, Mahendra Madegowda, and et al. 2024. "Synthesis of Tetrahydrocarbazole-Tethered Triazoles as Compounds Targeting Telomerase in Human Breast Cancer Cells" Catalysts 14, no. 10: 726. https://doi.org/10.3390/catal14100726
APA StyleUppar, P. M., Ravish, A., Xi, Z., Kumar Harish, K., Kumar, A. M., Poonacha, L. K., Swaroop, T. R., Somu, C., Gaonkar, S. L., Madegowda, M., Lobie, P. E., Pandey, V., & Basappa, B. (2024). Synthesis of Tetrahydrocarbazole-Tethered Triazoles as Compounds Targeting Telomerase in Human Breast Cancer Cells. Catalysts, 14(10), 726. https://doi.org/10.3390/catal14100726







