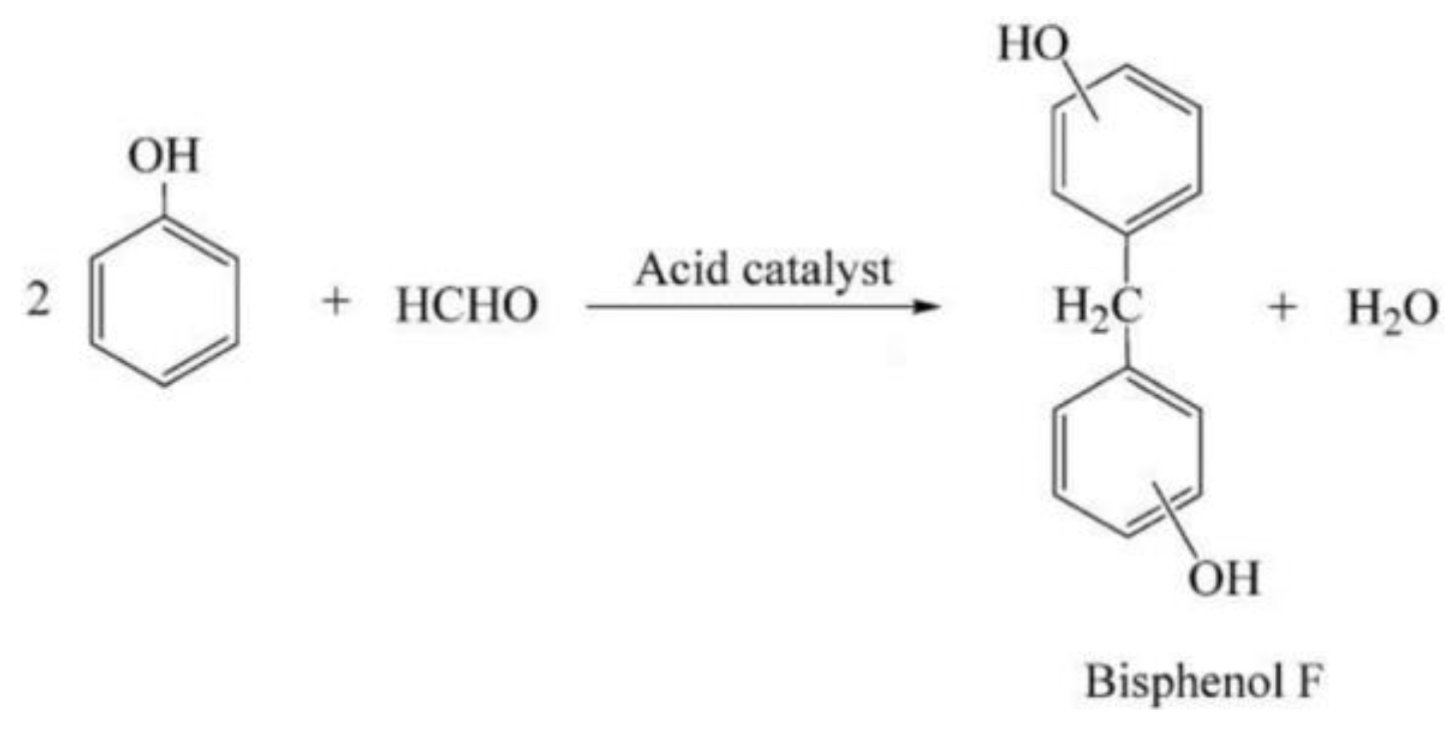
Bisphenol F Synthesis from Formaldehyde and Phenol over Zeolite Y Extrudate Catalysts in a Catalyst Basket Reactor and a Fixed-Bed Reactor
Abstract
1. Introduction
2. Results and Discussion
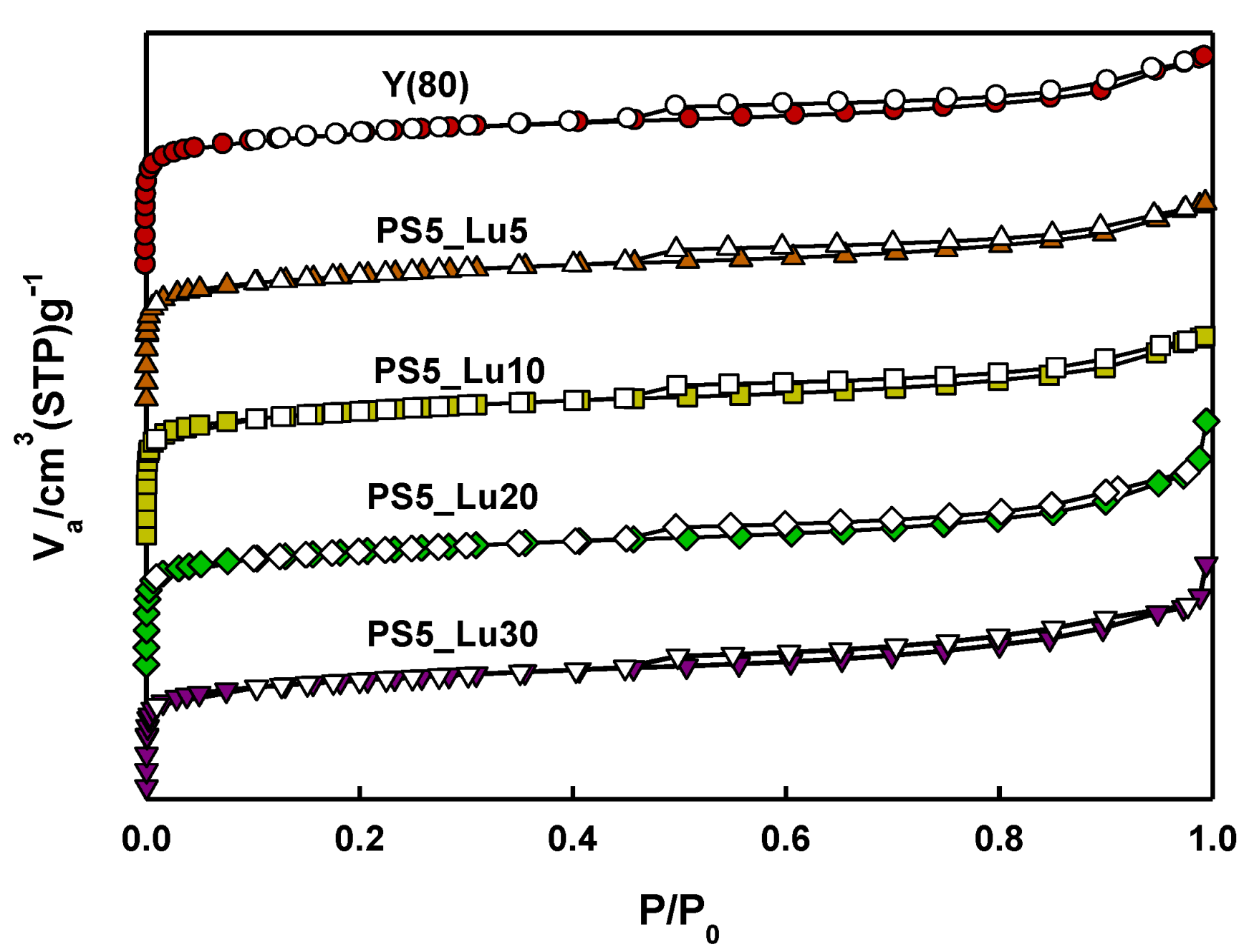
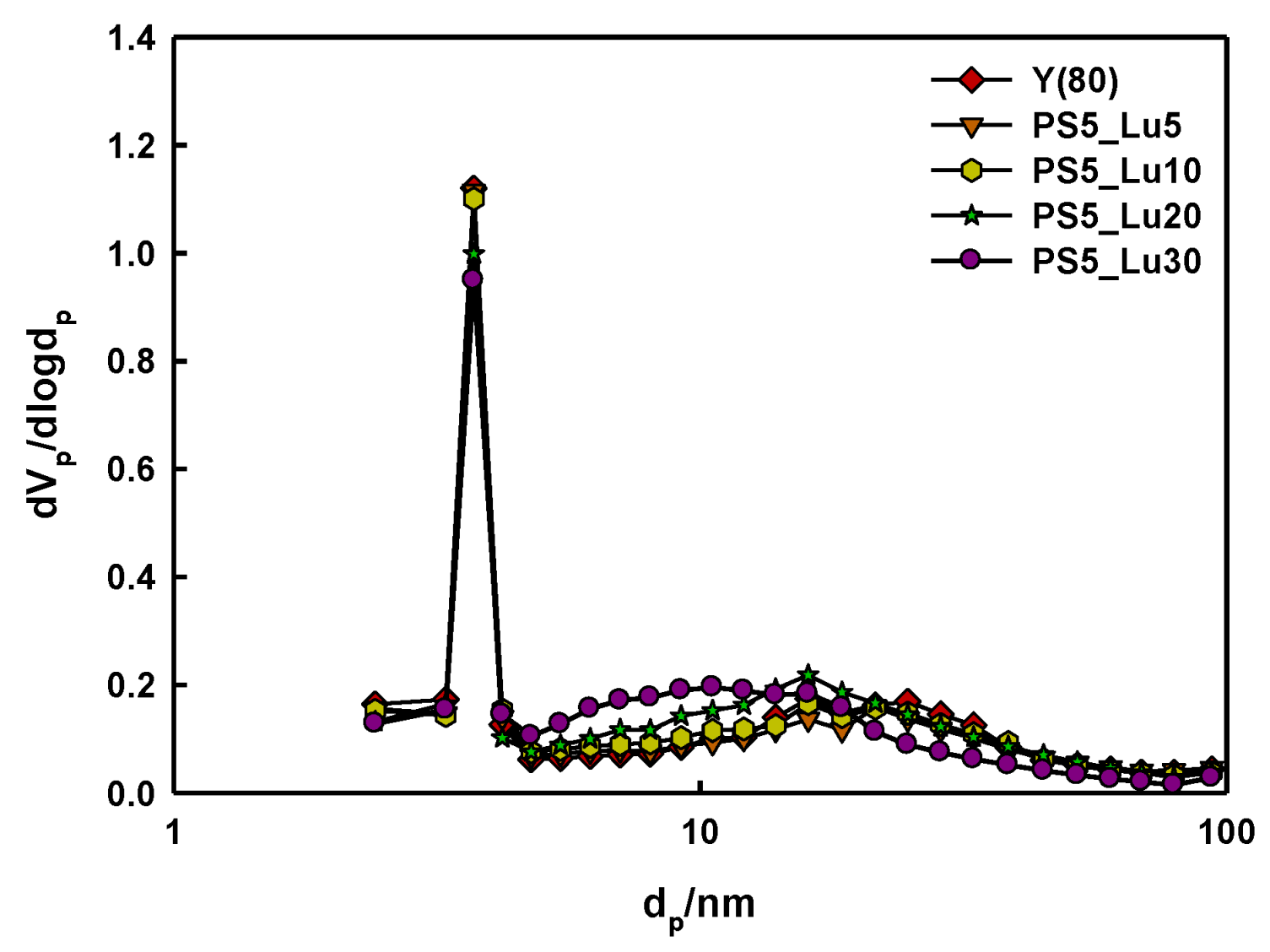
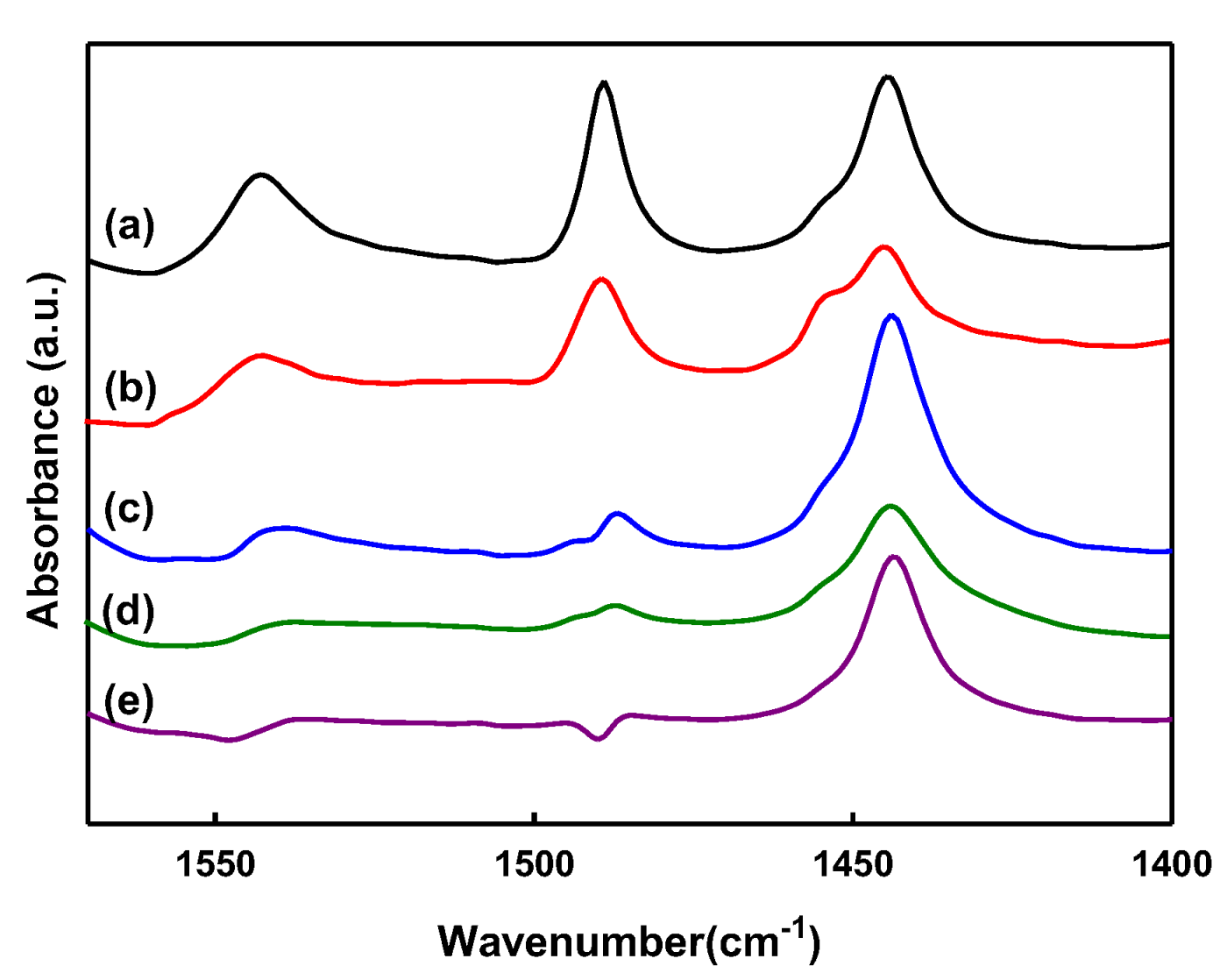
2.1. Characteristics of Catalyst Extrudates
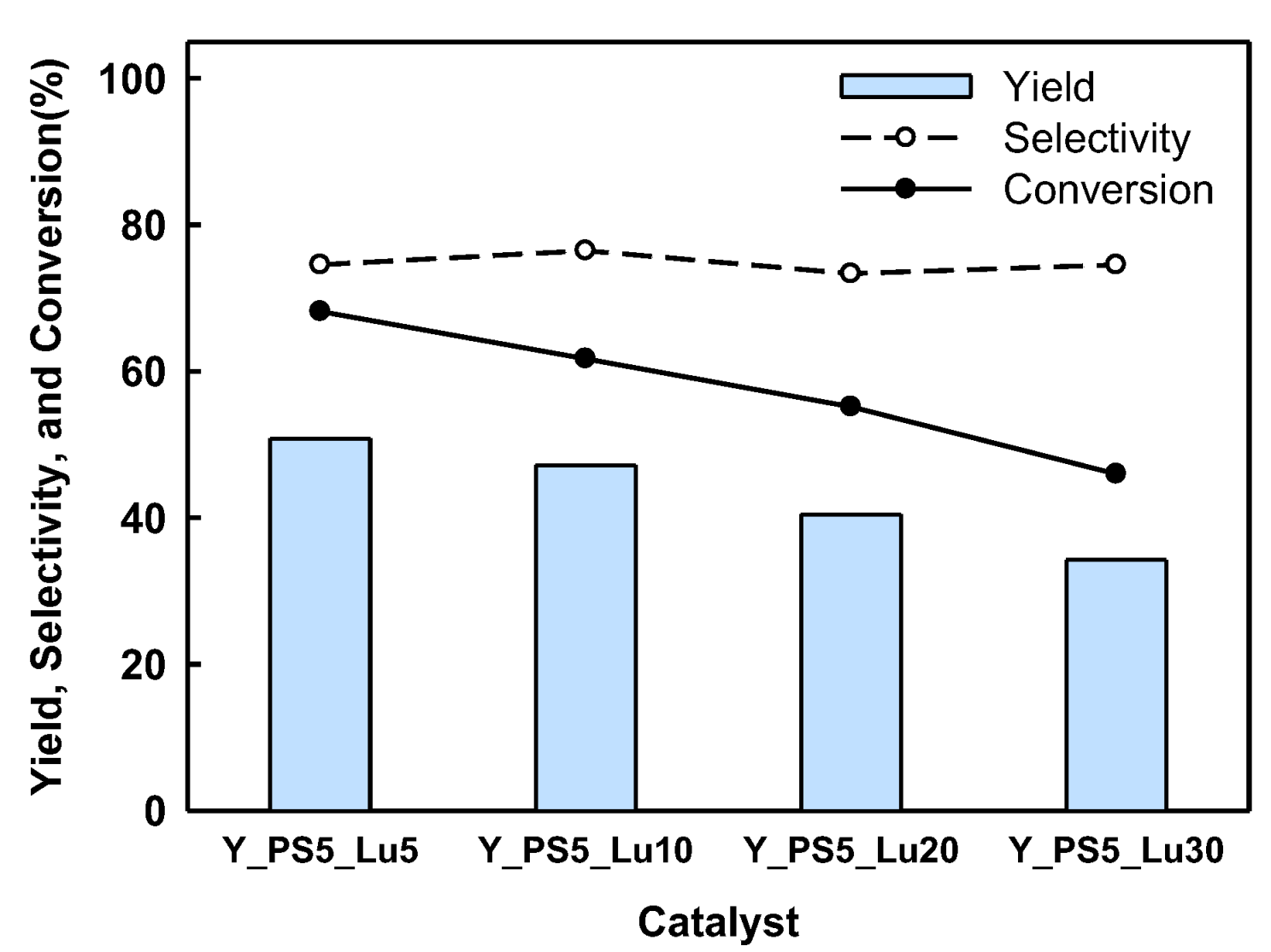
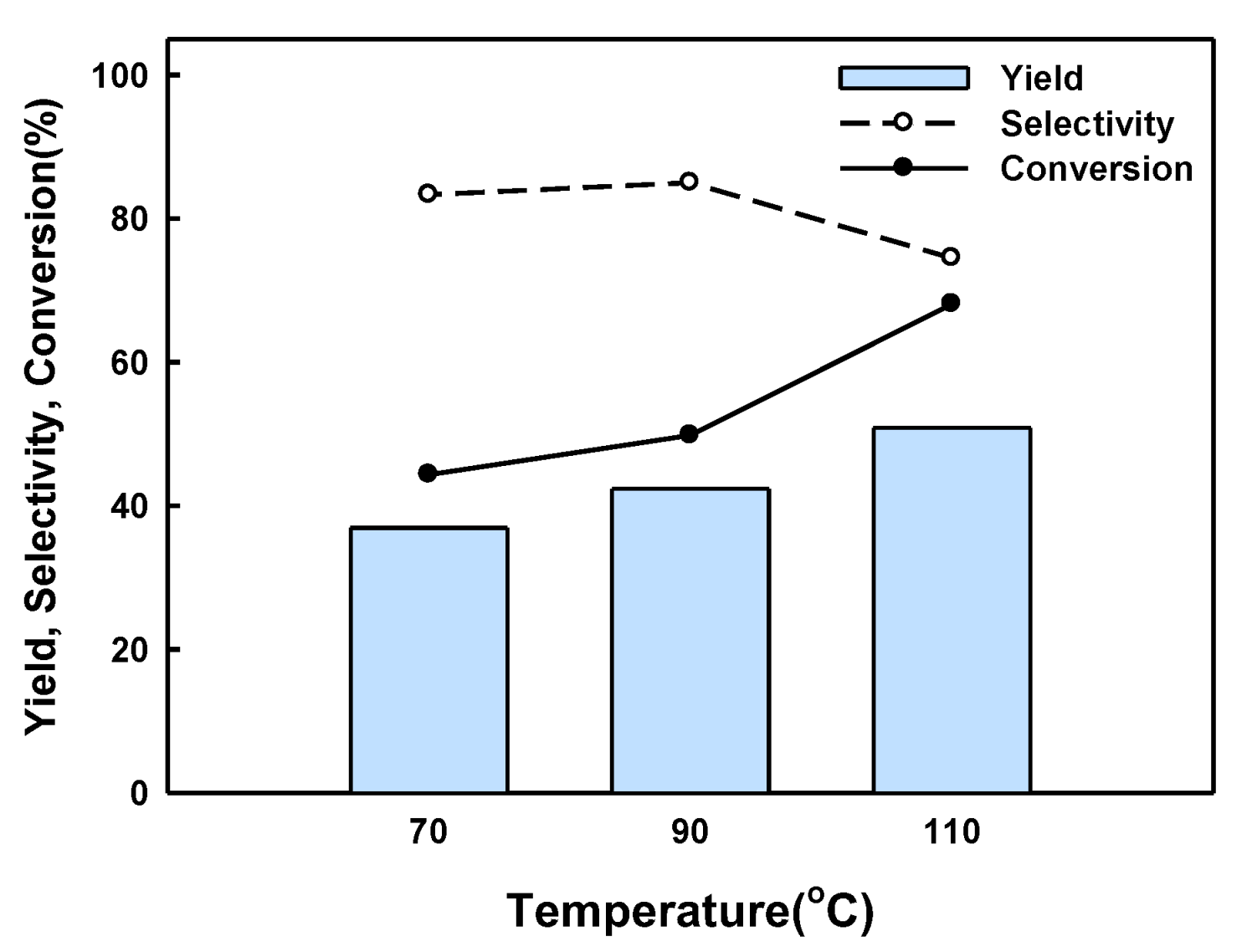
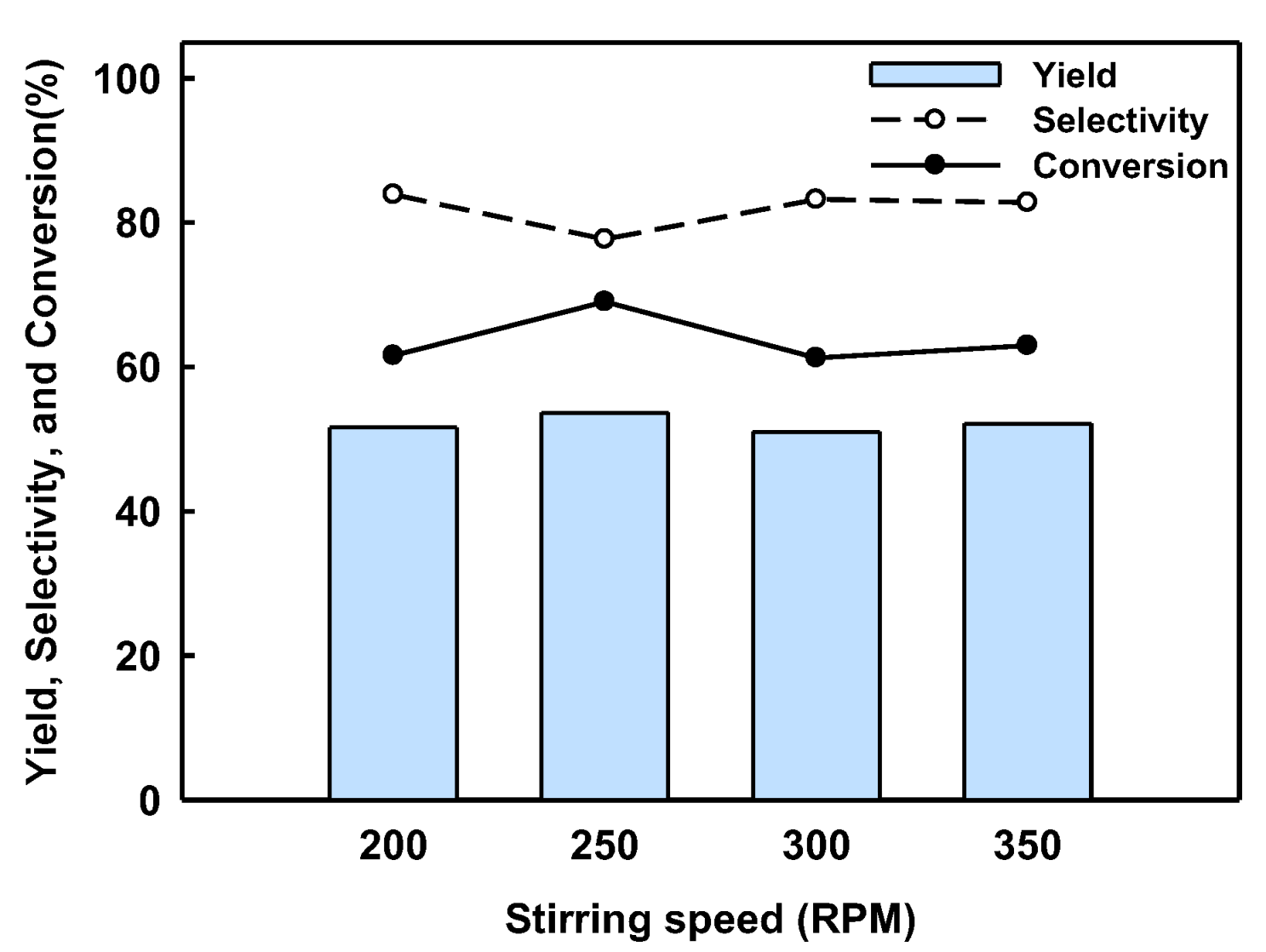
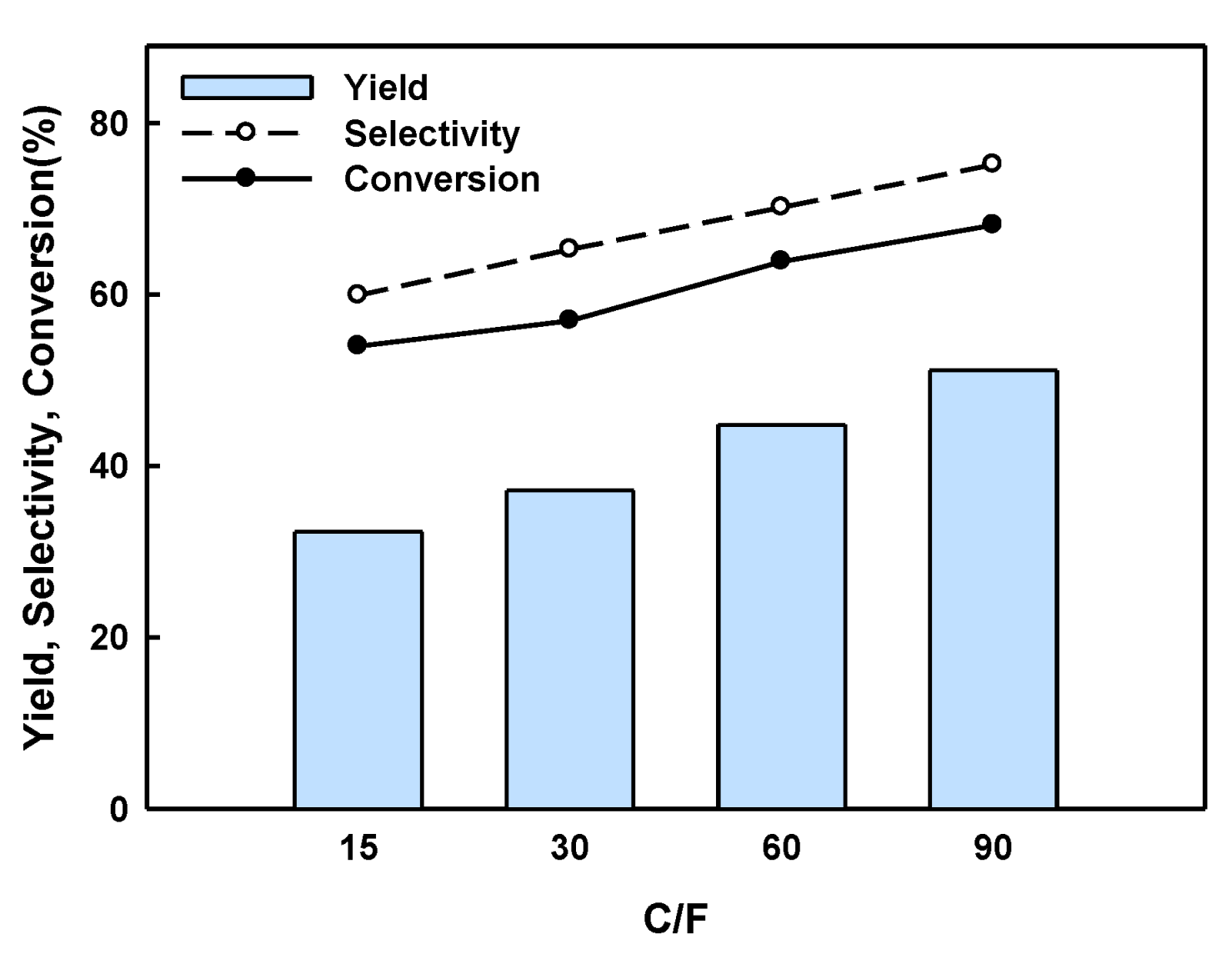
2.2. BPF Synthesis in a Batch Reactor Equipped with Catalyst Basket
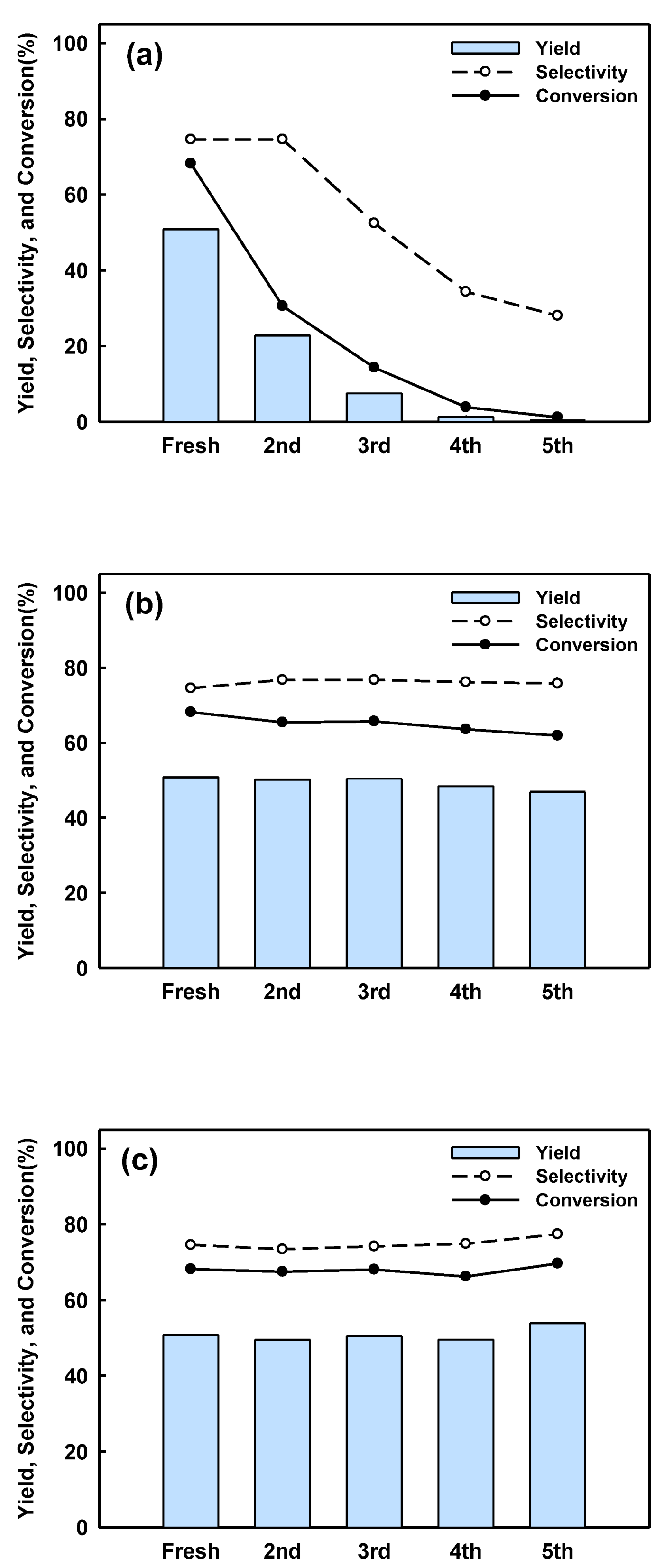
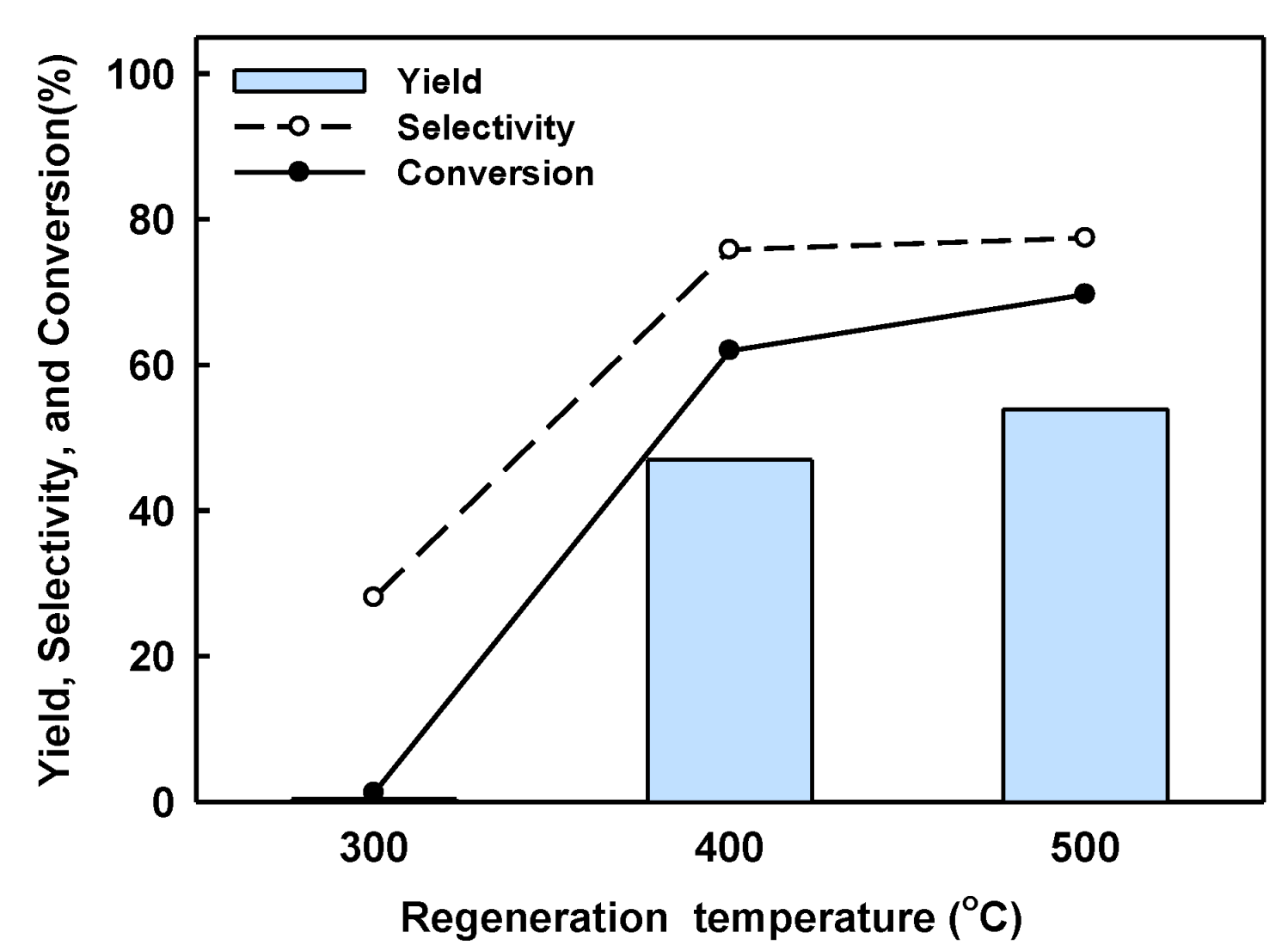
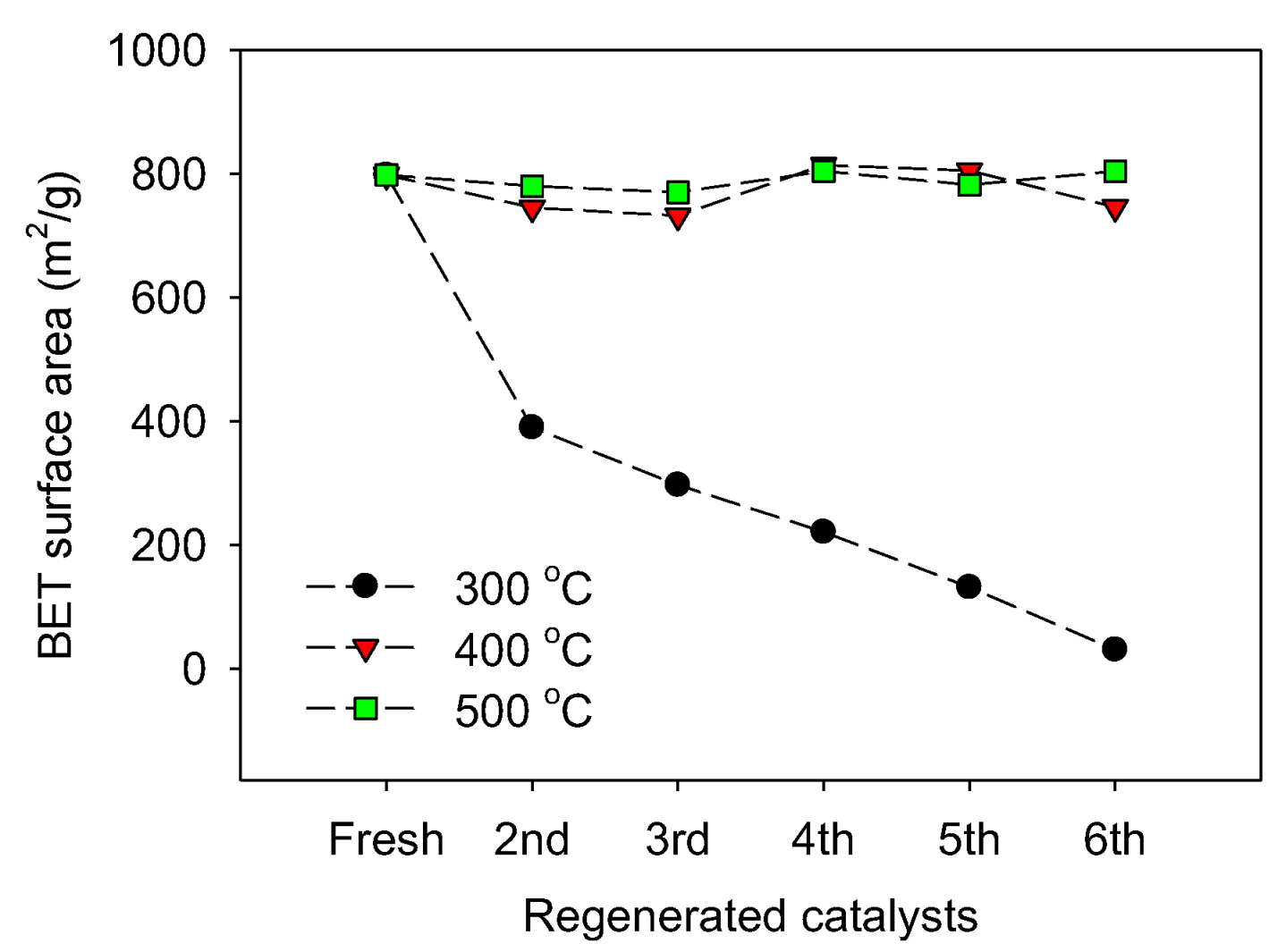
2.3. Catalyst Regeneration and Reuse in Batch Reactor
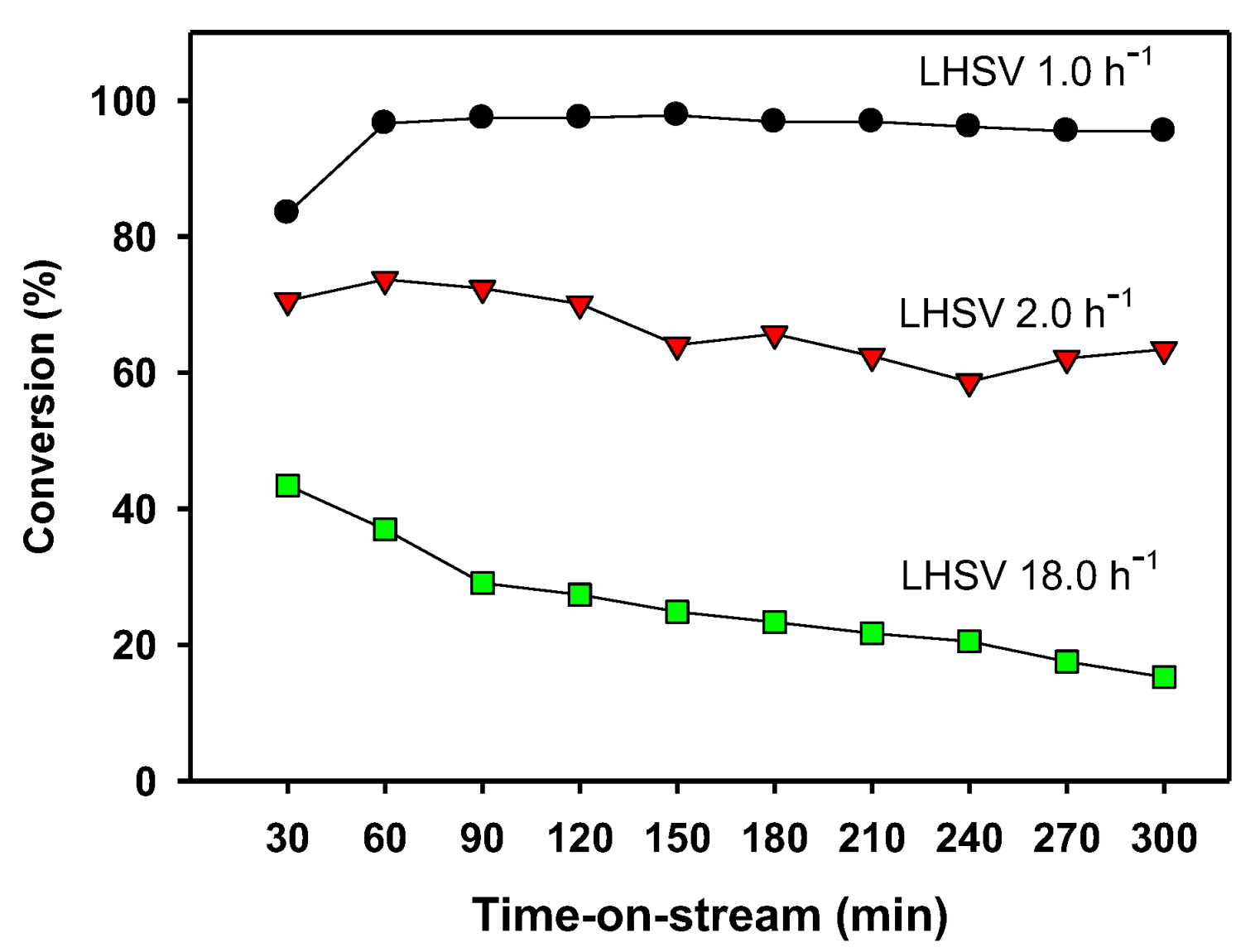
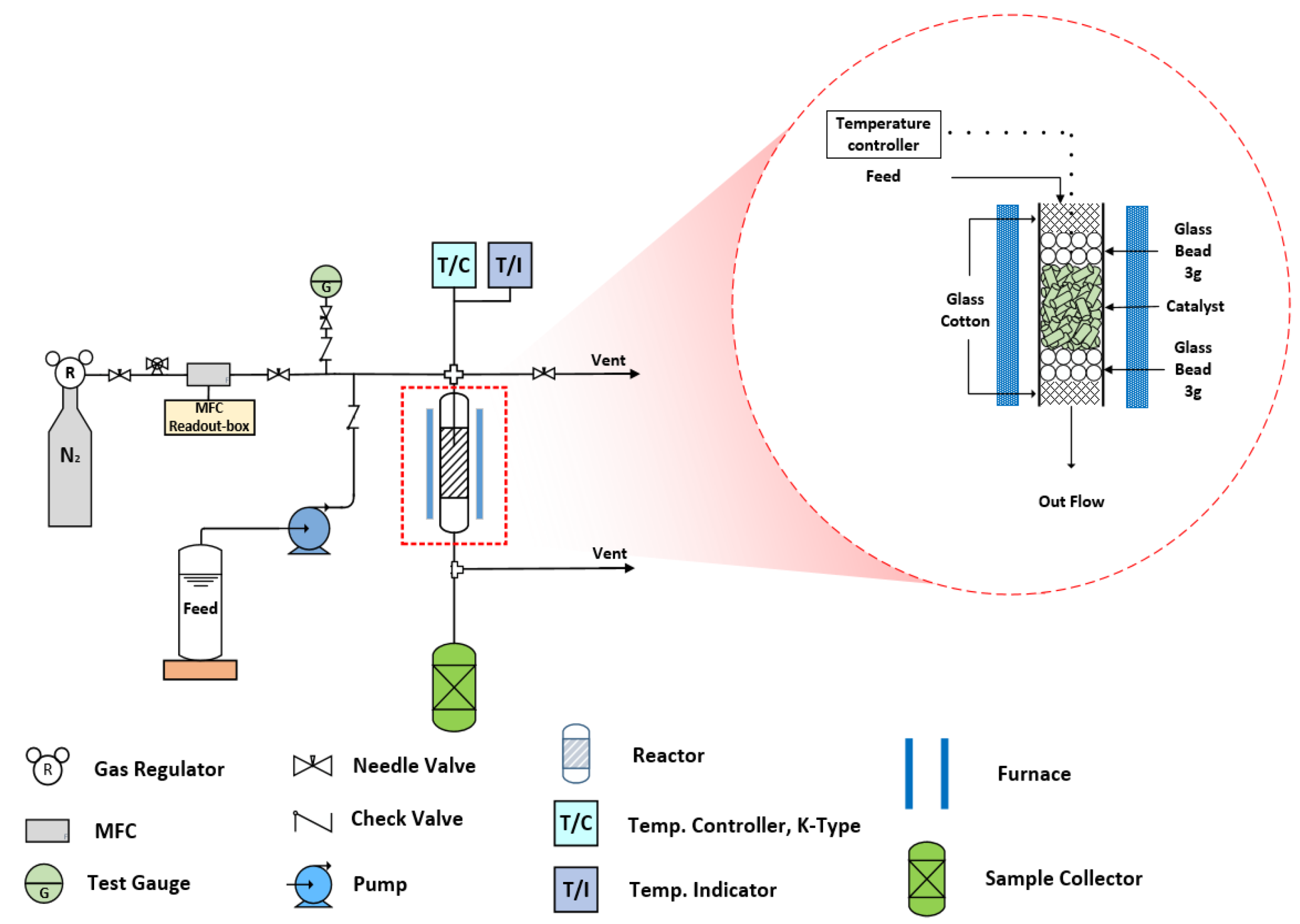
2.4. Continuous BPF Synthesis in a Fixed-Bed Reactor
3. Experimental Details
3.1. Preparation of Catalyst Extrudates
3.2. Characterization of Catalyst Extrudates
3.3. Synthesis of Bisphenol F in a Catalyst Basket Reactor and a Fixed-Bed Reactor
3.4. Synthesis of Bisphenol F in a Fixed-Bed Reactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, R.; Niu, X.; Xia, X.; Zeng, Z.; Zhang, G.; Lu, Y. Green synthesis of bisphenol F over 12-phosphotungstic acid supported on acid-activated palygorskite. RSC Adv. 2015, 5, 62394–62401. [Google Scholar] [CrossRef]
- Jana, S.K.; Okamoto, T.; Kugita, T.; Namba, S. Selective synthesis of bisphenol F catalyzed by microporous H-beta zeolite. Appl. Catal. A Gen. 2005, 288, 80–85. [Google Scholar] [CrossRef]
- Wang, H.; Li, M.; Chen, H.; Fu, Y.; Shen, J. Selective Synthesis of Bisphenol F from Phenol and PODE2 over an Acidic Resin–Carbon Composite Material. Ind. Eng. Chem. Res. 2019, 58, 9223–9230. [Google Scholar] [CrossRef]
- Wei, Y.; Li, Y.; Tan, Y.; Wu, Z.; Pan, L.; Liu, Y. Short-channeled mesoporous Zr–Al-SBA-15 as a highly efficient catalyst for hydroxyalkylation of phenol with formaldehyde to bisphenol F. Chem. Eng. J. 2016, 298, 271–280. [Google Scholar] [CrossRef]
- Xia, X.; Xu, Y.; Chen, Y.; Liu, Y.; Lu, Y. The distinct catalytic behaviours of calcium silicate hydrate for the high selectivity of 2, 2′-isomer in reaction of phenol with formaldehyde. Catal. Commun. 2019, 118, 15–18. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Liu, R.; Xia, X.; Wang, G.; Zhang, Y. Hydroxyalkylation of phenol to bisphenol F over heteropolyacid catalysts: The effect of catalyst acid strength on isomer distribution and kinetics. J. Colloid Interface Sci. 2016, 481, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.K.; Kugita, T.; Namba, S. Aluminum-grafted MCM-41 molecular sieve: An active catalyst for bisphenol F synthesis process. Appl. Catal. A Gen. 2004, 266, 245–250. [Google Scholar] [CrossRef]
- Li, H.; Cao, X.; He, H.; Liu, J.; Xiang, W.; She, J.; Yin, D. Catalytic condensation of phenol with formaldehyde into BPF on recyclable anchoring sulfuric acid. In Proceedings of the 2nd International Conference on Applied Chemistry and Industrial Catalysis (ACIC 2020), Dalian, China, 16–19 October 2020; Volume 213, p. 01003. [Google Scholar]
- Liu, R.; Xia, X.; Niu, X.; Zhang, G.; Lu, Y.; Jiang, R.; He, S. 12-Phosphotungstic acid immobilized on activated-bentonite as an efficient heterogeneous catalyst for the hydroxyalkylation of phenol. Appl. Clay Sci. 2015, 105, 71–77. [Google Scholar] [CrossRef]
- Jana, S.K.; Kugita, T.; Namba, S. Bisphenol F synthesis over mesoporous aluminosilicate MCM-41 molecular sieves. Catal. Lett. 2003, 90, 143–147. [Google Scholar] [CrossRef]
- Garade, A.C.; Kshirsagar, V.S.; Rode, C.V. Selective hydroxyalkylation of phenol to bisphenol F over dodecatungstophosphoric acid (DTP) impregnated on fumed silica. Appl. Catal. A Gen. 2009, 354, 176–182. [Google Scholar] [CrossRef]
- Vajglova, Z.; Kumar, N.; Mäki-Arvela, P.; Eränen, K.; Peurla, M.; Hupa, L.; Murzin, D.Y. Effect of Binders on the Physicochemical and Catalytic Properties of Extrudate-Shaped Beta Zeolite Catalysts for Cyclization of Citronellal. Org. Process Res. Dev. 2019, 23, 2456–2463. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Lee, H.-K.; Ihm, S.-K. Influence of Catalyst Binders on the Acidity and Catalytic Performance of HZSM-5 Zeolites for Methanol-to-Propylene (MTP) Process: Single and Binary Binder System. Top. Catal. 2010, 53, 247–253. [Google Scholar] [CrossRef]
- Bingre, R.; Louis, B.; Nguyen, P. An Overview on Zeolite Shaping Technology and Solutions to Overcome Diffusion Limitations. Catalysts 2018, 8, 163. [Google Scholar] [CrossRef]
- Almeida, A.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Grande, C. Extrusion and Characterization of High Si/Al Ratio ZSM-5 Using Silica Binder. Energies 2020, 13, 1201. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Huang, C.; Li, A.; Chao, Z.-S. Heterogeneous catalytic synthesis of quinoline compounds from aniline and C1–C4 alcohols over zeolite-based catalysts. RSC Adv. 2017, 7, 48275–48285. [Google Scholar] [CrossRef]
- Lakiss, L.; Gilson, J.-P.; Valtchev, V.; Mintova, S.; Vicente, A.; Vimont, A.; Bedard, R.; Abdo, S.; Bricker, J. Zeolites in a good shape: Catalyst forming by extrusion modifies their performances. Microporous Mesoporous Mater. 2020, 299, 110114. [Google Scholar] [CrossRef]
- Jung, E.; Jeon, S.; Kim, C.-U.; Jeong, S.-Y.; Park, Y.-K.; Jeon, J.-K. Hydro-upgrading of n-octadecane over Pt–Mg/HY catalysts. Catal. Today 2016, 265, 124–130. [Google Scholar] [CrossRef]
- Jung, E.; Choi, H.; Jeon, J.-K. Effect of Si/Al2 Ratio on 2-butanol Dehydration over HY Zeolite Catalysts. Korean Chem. Eng. Res. 2015, 53, 116–120. [Google Scholar] [CrossRef][Green Version]
- Seo, G.; Kim, G.J. Catalyst: Basic Concept, Structure, Function; Cheongmungak: Paju, Republic of Korea, 2014; pp. 144–146. [Google Scholar]



| Catalysts | Catalyst Shape | Binder | SiO2/Al2O3 | Compressive Strength (N/mm2) | |
|---|---|---|---|---|---|
| PS (wt%) a | Lu (wt%) b | ||||
| Y(80) | powder | - | - | 80.0 | - |
| Y_PS5_Lu5 | extrudates | 5 | 5 | 41.8 | 0.31 |
| Y_PS5_Lu10 | extrudates | 5 | 10 | 43.7 | 0.32 |
| Y_PS5_Lu20 | extrudates | 5 | 20 | 46.5 | 0.46 |
| Y_PS5_Lu30 | extrudates | 5 | 30 | 49.4 | 0.57 |
| Catalysts | SBET a (m2/g) | SLangmuir b (m2/g) | Average dp c (nm) | Vp d (cm3/g) |
|---|---|---|---|---|
| Y(80) | 853 | 819 | 2.5 | 0.3 |
| Y_PS5_Lu5 | 798 | 757 | 2.5 | 0.2 |
| Y_PS5_Lu10 | 800 | 776 | 2.6 | 0.2 |
| Y_PS5_Lu20 | 746 | 713 | 3.1 | 0.4 |
| Y_PS5_Lu30 | 715 | 684 | 3.0 | 0.3 |
| Catalysts | Weak Acid Sites (mmol/g) | Strong Acid Sites (mmol/g) | Total Acid (mmol/g) | Weak Acid Sites/Strong Acid Sites |
|---|---|---|---|---|
| Y(80) | 7.6 | 26.5 | 34.1 | 0.29 |
| Y_PS5_Lu5 | 16.3 | 25.4 | 41.7 | 0.64 |
| Y_PS5_Lu10 | 14.3 | 22.2 | 36.5 | 0.64 |
| Y_PS5_Lu20 | 12.1 | 18.3 | 30.4 | 0.66 |
| Y_PS5_Lu30 | 10.7 | 14.9 | 25.6 | 0.72 |
| Catalysts | Brønsted (μmol/g) | Lewis (μmol/g) | Brønsted/Lewis |
|---|---|---|---|
| Y(80) | 115 | 142 | 0.81 |
| Y_PS5_Lu5 | 55 | 116 | 0.48 |
| Y_PS5_Lu10 | 41 | 219 | 0.18 |
| Y_PS5_Lu20 | 29 | 122 | 0.23 |
| Y_PS5_Lu30 | 21 | 137 | 0.15 |
|
Liquid Hourly Space Velocity (h−1) |
Time-on-Stream (min) |
Formaldehyde Conversion (%) |
Selectivity to BPF (%) |
Yield of BPF (%) |
|---|---|---|---|---|
| 1.0 | 60 | 97.4 | 75.8 | 73.8 |
| 300 | 95.5 | 72.3 | 69.0 | |
| 2.0 | 60 | 73.7 | 74.8 | 55.1 |
| 300 | 63.4 | 76.6 | 48.6 | |
| 18.0 | 60 | 37.0 | 73.7 | 27.3 |
| 300 | 15.3 | 58.2 | 8.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.; Hwang, S.; Won, S.; Kim, Y.; Hong, S.; Lee, J.; Lee, S.; Jeon, J.-K. Bisphenol F Synthesis from Formaldehyde and Phenol over Zeolite Y Extrudate Catalysts in a Catalyst Basket Reactor and a Fixed-Bed Reactor. Catalysts 2024, 14, 656. https://doi.org/10.3390/catal14100656
Park Y, Hwang S, Won S, Kim Y, Hong S, Lee J, Lee S, Jeon J-K. Bisphenol F Synthesis from Formaldehyde and Phenol over Zeolite Y Extrudate Catalysts in a Catalyst Basket Reactor and a Fixed-Bed Reactor. Catalysts. 2024; 14(10):656. https://doi.org/10.3390/catal14100656
Chicago/Turabian StylePark, Yeongseo, Seoyeon Hwang, Seyeon Won, Yehee Kim, Sooyeon Hong, Jungyeop Lee, Simon Lee, and Jong-Ki Jeon. 2024. "Bisphenol F Synthesis from Formaldehyde and Phenol over Zeolite Y Extrudate Catalysts in a Catalyst Basket Reactor and a Fixed-Bed Reactor" Catalysts 14, no. 10: 656. https://doi.org/10.3390/catal14100656
APA StylePark, Y., Hwang, S., Won, S., Kim, Y., Hong, S., Lee, J., Lee, S., & Jeon, J.-K. (2024). Bisphenol F Synthesis from Formaldehyde and Phenol over Zeolite Y Extrudate Catalysts in a Catalyst Basket Reactor and a Fixed-Bed Reactor. Catalysts, 14(10), 656. https://doi.org/10.3390/catal14100656







