Synergistic Effect of NiAl-Layered Double Hydroxide and Cu-MOF for the Enhanced Photocatalytic Degradation of Methyl Orange and Antibacterial Properties
Abstract
1. Introduction
2. Results and Discussion
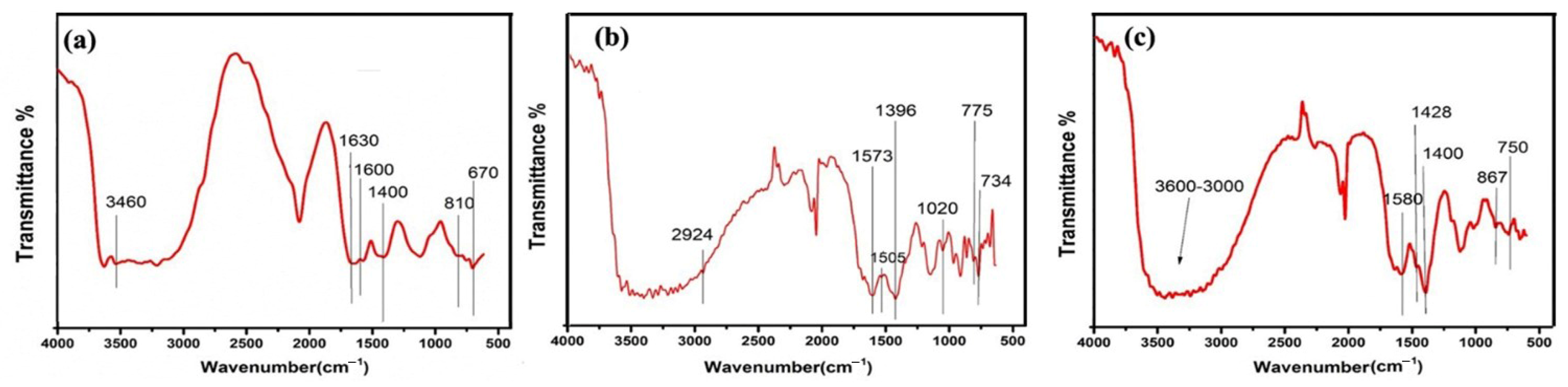
2.1. FTIR Analysis
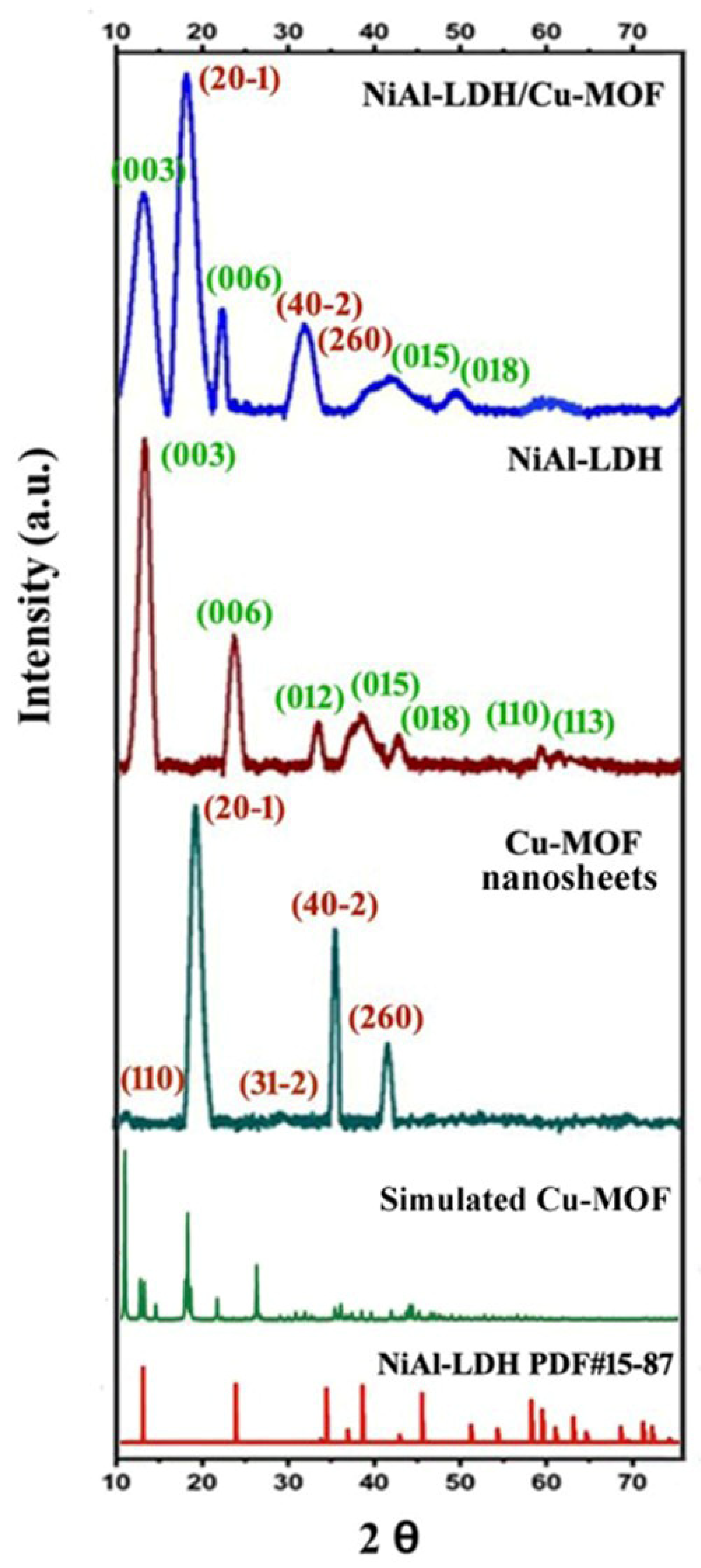
2.2. PXRD Analysis
2.3. Morphology Analysis
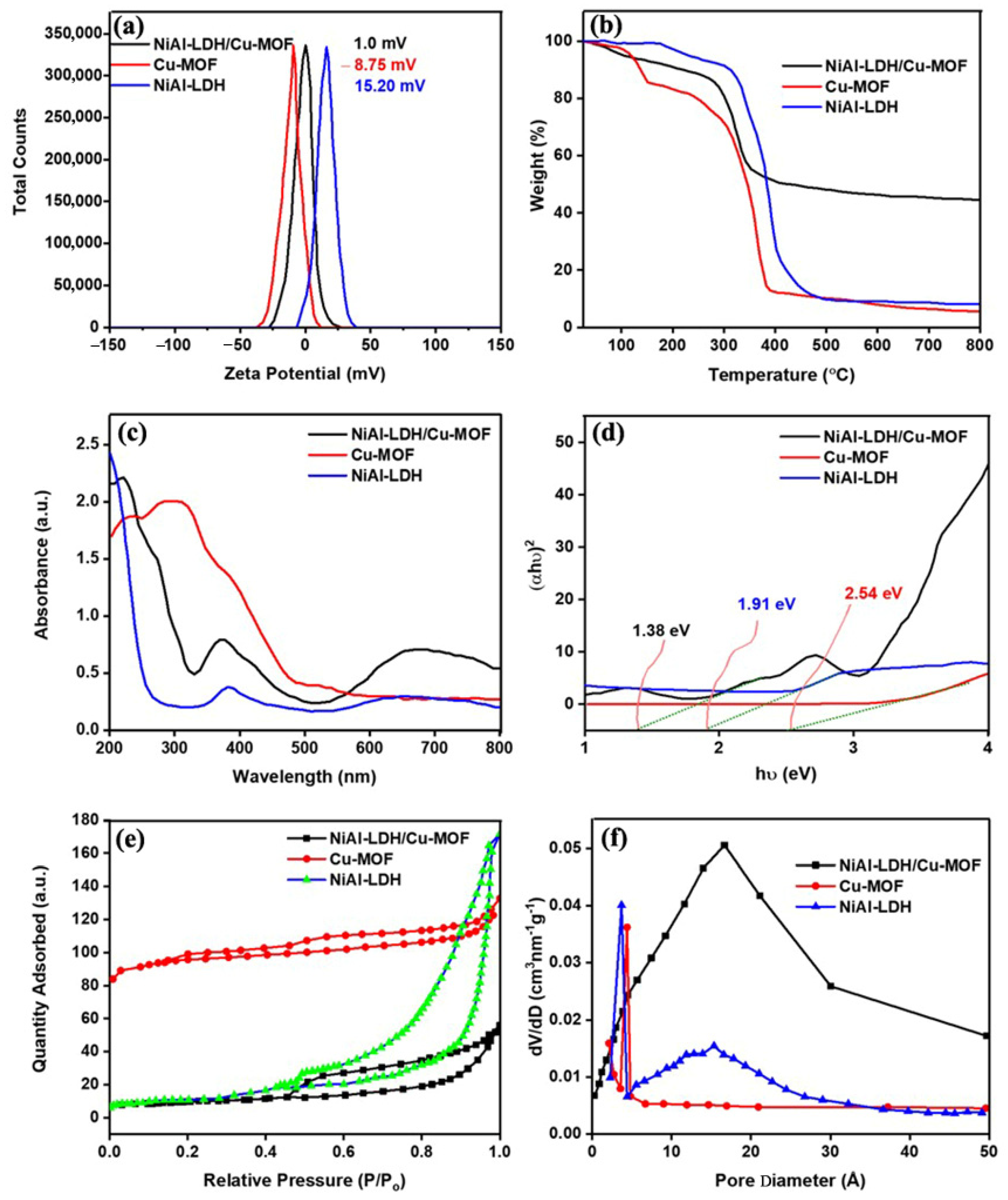
2.4. Zeta Potential
2.5. Thermogravimetric Analysis
2.6. UV–Vis DRS Analysis
2.7. N2 Adsorption–Desorption Isotherm
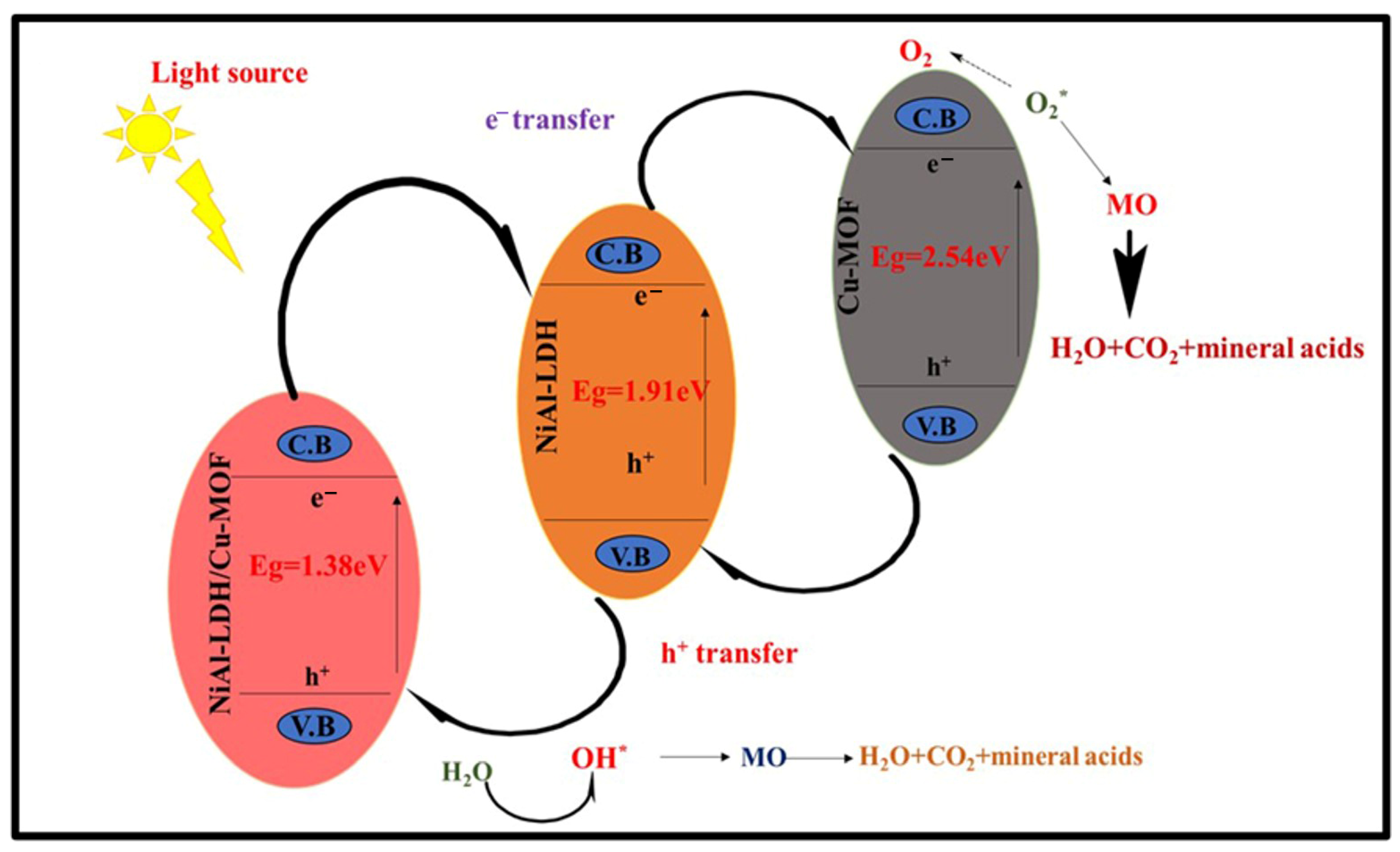
2.8. Reaction Mechanism of NiAl-LDH/Cu-MOF
2.9. Mechanism of Deterioration of MO Dye
2.10. Factors Affecting Degradation Efficiency of MO
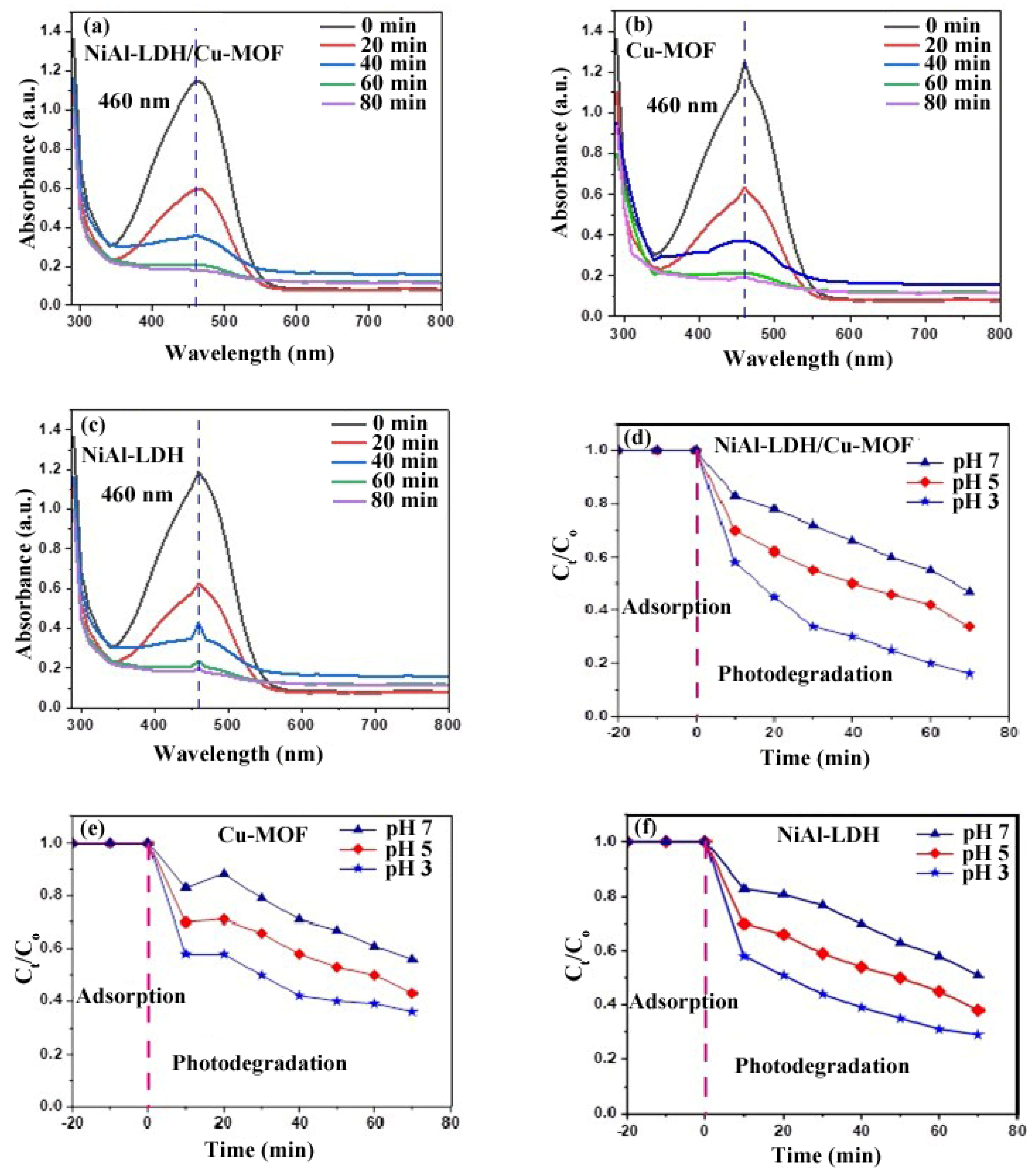
2.10.1. Effect of Contact Time
2.10.2. pH Effect
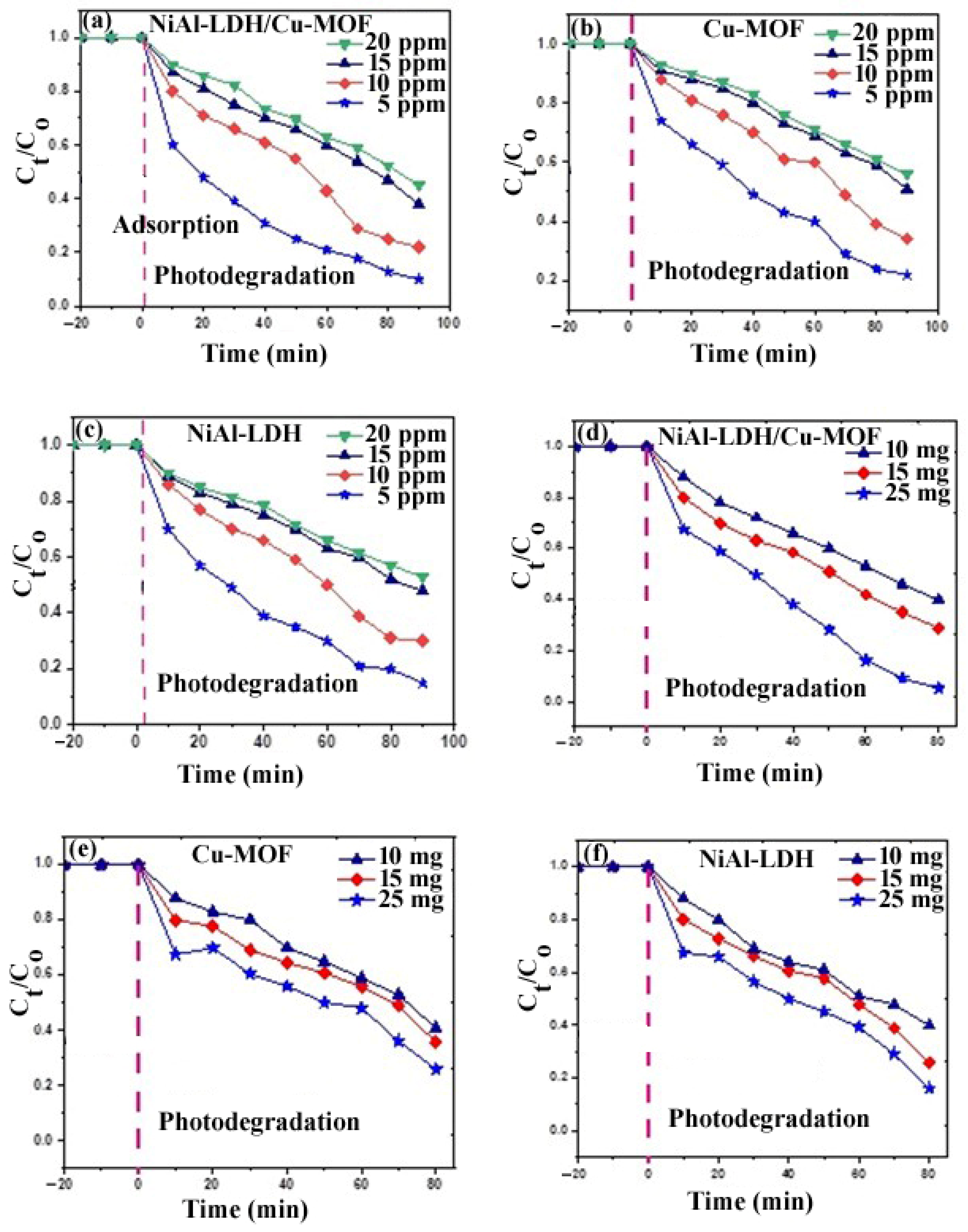
2.10.3. Initial Dye Concentration Effect
2.10.4. Effect of Photocatalyst Amount
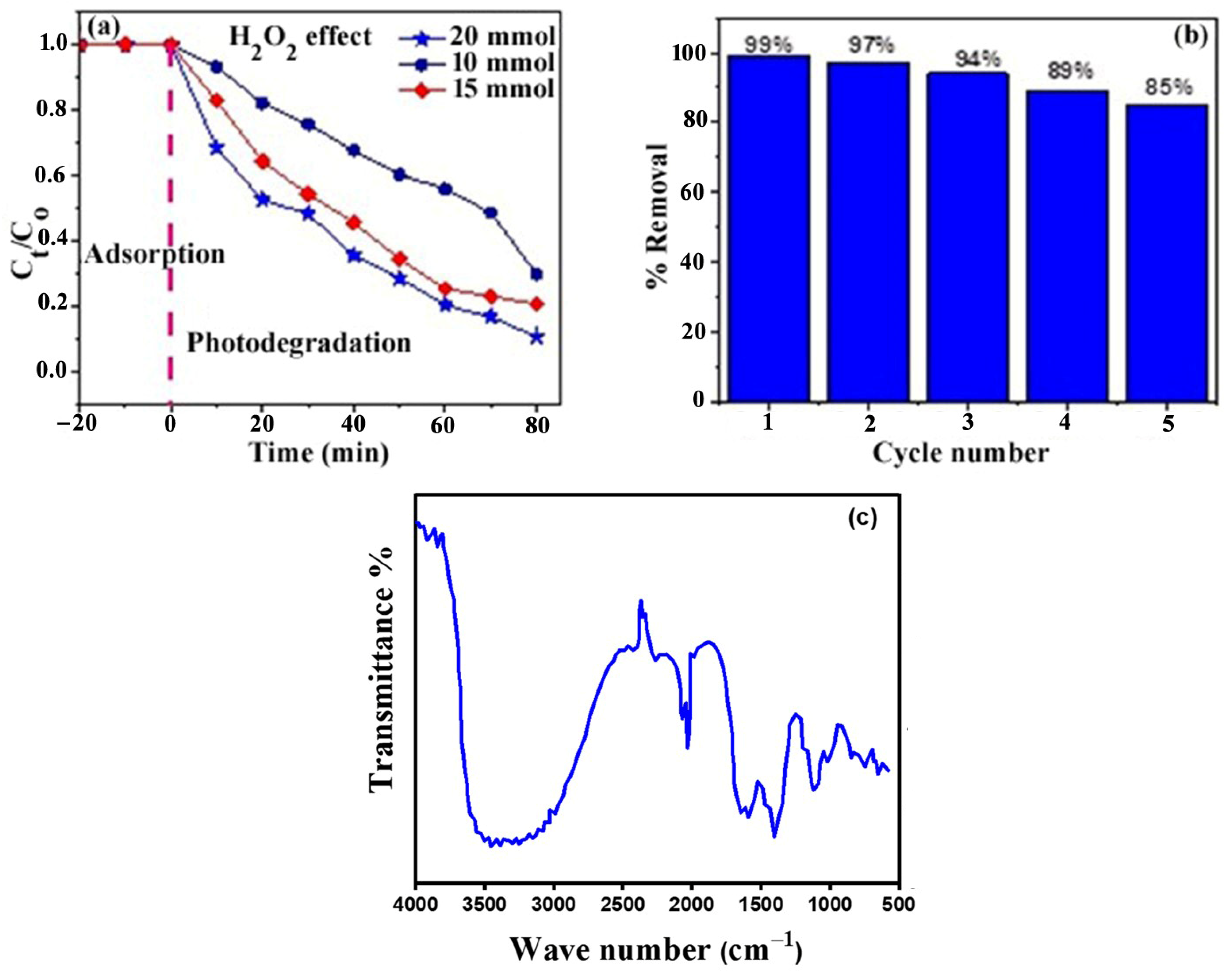
2.10.5. Hydrogen Peroxide (H2O2) Concentration Effect
2.10.6. Recyclability of the Photocatalyst
Photodegradation Kinetics
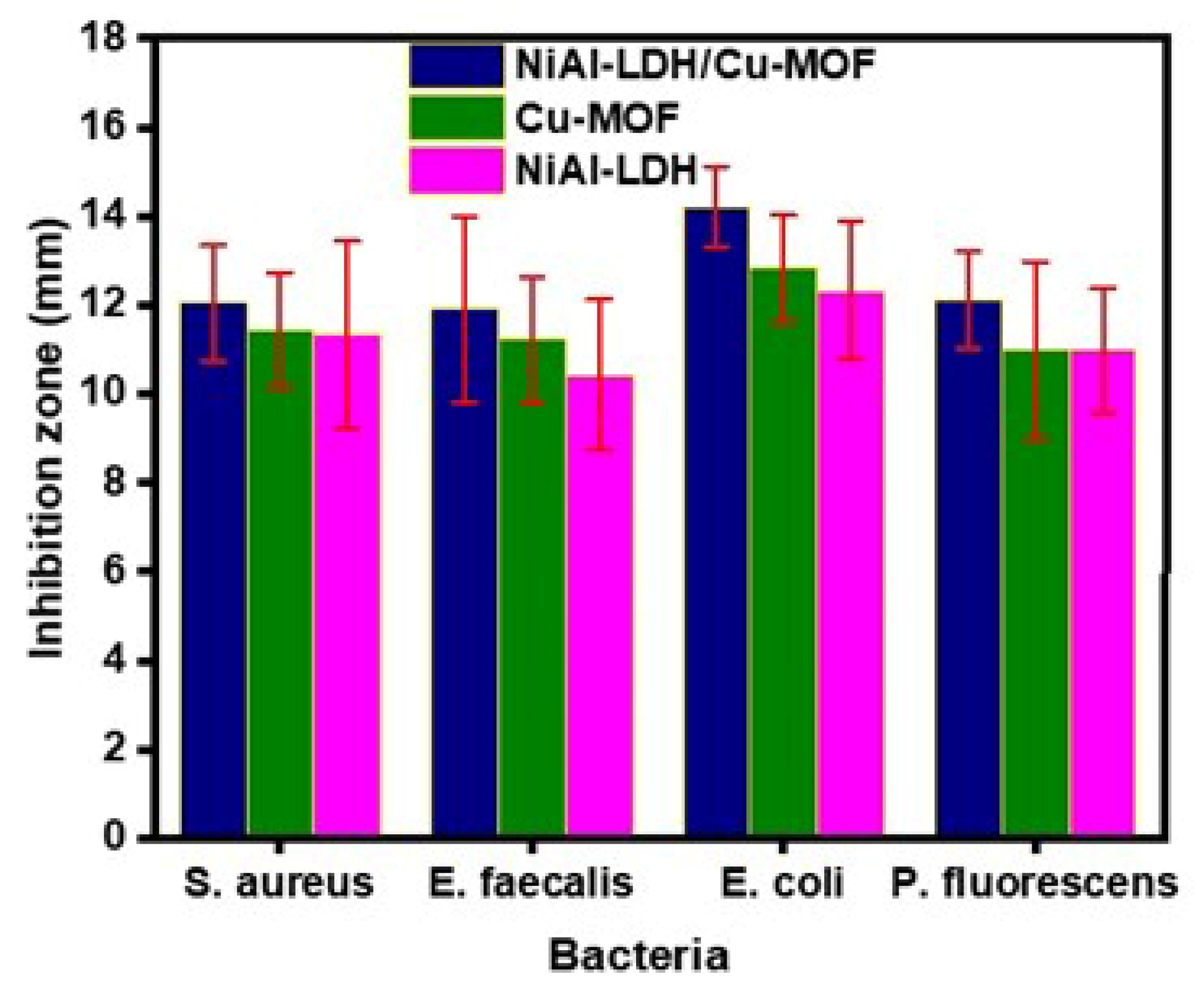
Antibacterial Activity
3. Experimental Section
3.1. Materials and Synthesis Methods
3.2. Preparation of NiAl-LDH
3.3. Synthesis of Cu-MOF
3.4. Synthesis of NiAl-LDH/Cu-MOF Composite
3.5. Photocatalytic Degradation Experiment
3.6. Reusability of the Photocatalyst
3.6.1. Antibacterial Study
3.6.2. Minimal Inhibitory Concentration (MIC)
3.6.3. Minimum Bactericidal Concentration (MBC)
3.6.4. Disk Diffusion Method for Testing Antibacterial Sensibility
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvi, M.; Al-Ghamdi, A.; ShaheerAkhtar, M. Synthesis of ZnO nanostructures via low temperature solution process for photocatalytic degradation of rhodamine B dye. Mater. Lett. 2017, 204, 12–15. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Yan, R.; Chen, X. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr (VI) reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Classification of dye and pigments. In Dyes and Pigments; Springer: Berlin/Heidelberg, Germany, 2016; pp. 31–45. [Google Scholar]
- Jana, S.; Konar, S.; Mitra, B.C.; Mondal, A.; Mukhopadhyay, S. Fabrication of a new heterostructure Au/Pt/SnO2: An excellent catalyst for fast reduction of para-nitrophenol and visible light assisted photodegradation of dyes. Mater. Res. Bull. 2021, 141, 111351. [Google Scholar] [CrossRef]
- Alghamdi, Y.G.; Krishnakumar, B.; Malik, M.A.; Alhayyani, S. Design and preparation of biomass-derived activated carbon loaded TiO2 photocatalyst for photocatalytic degradation of reactive red 120 and ofloxacin. Polymers 2022, 14, 880. [Google Scholar] [CrossRef]
- Darvishi Cheshmeh Soltani, R.; Rezaee, A.; Safari, M.; Khataee, A.; Karimi, B. Photocatalytic degradation of formaldehyde in aqueous solution using ZnO nanoparticles immobilized on glass plates. Desalin. Water Treat. 2015, 53, 1613–1620. [Google Scholar] [CrossRef]
- Alsulaim, G.M. New BaTi0.96Cu0.02X0.02O3 (X = V, Nb) Photocatalysts for Dyes Effluent Remediation: Broad Visible Light Response. Catalysts 2023, 13, 1365. [Google Scholar] [CrossRef]
- Pahalagedara, M.N.; Samaraweera, M.; Dharmarathna, S.; Kuo, C.-H.; Pahalagedara, L.R.; Gascón, J.A.; Suib, S.L. Removal of azo dyes: Intercalation into sonochemically synthesized NiAl layered double hydroxide. J. Phys. Chem. C 2014, 118, 17801–17809. [Google Scholar] [CrossRef]
- Islam, D.; Borah, D.; Acharya, H. Controlled synthesis of monodisperse silver nanoparticles supported layered double hydroxide catalyst. RSC Adv. 2015, 5, 13239–13245. [Google Scholar] [CrossRef]
- Cheng, Z.-L.; Liu, Y.-Y.; Cao, B.-C. Two-dimensional layered structure-templated synthesis of graphene nanosheets using CoAl-LDH under low carbonization temperature. Mater. Lett. 2016, 175, 215–218. [Google Scholar] [CrossRef]
- Gao, Z.; Liang, J.; Yao, J.; Meng, Q.; He, G.; Chen, H. Synthesis of Ce-doped NiAl LDH/RGO composite as an efficient photocatalyst for photocatalytic degradation of ciprofloxacin. J. Environ. Chem. Eng. 2021, 9, 105405. [Google Scholar] [CrossRef]
- Haque, E.; Lee, J.E.; Jang, I.T.; Hwang, Y.K.; Chang, J.-S.; Jegal, J.; Jhung, S.H. Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J. Hazard. Mater. 2010, 181, 535–542. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef]
- Li, S.; Dong, K.; Cai, M.; Li, X.; Chen, X. A plasmonic S-scheme Au/MIL-101 (Fe)/BiOBr photocatalyst for efficient synchronous decontamination of Cr (VI) and norfloxacin antibiotic. EScience 2024, 4, 100208. [Google Scholar] [CrossRef]
- Salama, R.S.; El-Hakam, S.; Samra, S.; El-Dafrawy, S.; Ahmed, A. Adsorption, equilibrium and kinetic studies on the removal of methyl orange dye from aqueous solution by using of copper metal organic framework (Cu-BDC). Int. J. Mod. Chem 2018, 10, 195–207. [Google Scholar]
- Elizalde-Velázquez, A.; Gómez-Oliván, L.M.; Galar-Martínez, M.; Islas-Flores, H.; Dublán-García, O.; SanJuan-Reyes, N. Amoxicillin in the aquatic environment, its fate and environmental risk. InTech 2016, 1, 247–267. [Google Scholar]
- Bhuyan, A.; Ahmaruzzaman, M. Ultrasonic-assisted synthesis of highly efficient and robust metal oxide QDs immobilized-MOF-5/Ni-Co-LDH photocatalyst for sunlight-mediated degradation of multiple toxic dyes. J. Alloys Compd. 2024, 972, 172781. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, A.; Liu, Z.; Yao, S.; Zhou, R.; Fu, Y.; Zhou, Q. A study on the role of high-energy holes and reactive oxygen species in photocatalytic degradation using oxygen-doped/biochar-modified 2D carbon nitride. J. Water Process Eng. 2024, 65, 105808. [Google Scholar] [CrossRef]
- Mohamadpour, F.; Amani, A.M. Photocatalytic systems: Reactions, mechanism, and applications. RSC Adv. 2024, 14, 20609–20645. [Google Scholar] [CrossRef]
- Ubaldi, F.; Valeriani, F.; Volpini, V.; Lofrano, G.; Romano Spica, V. Antimicrobial Activity of Photocatalytic Coatings on Surfaces: A Systematic Review and Meta-Analysis. Coatings 2024, 14, 92. [Google Scholar] [CrossRef]
- Du, T.; Zhang, H.; Ruan, J.; Jiang, H.; Chen, H.-Y.; Wang, X. Adjusting the linear range of Au-MOF fluorescent probes for real-time analyzing intracellular GSH in living cells. ACS Appl. Mater. Interfaces 2018, 10, 12417–12423. [Google Scholar] [CrossRef]
- Carson, C.G.; Hardcastle, K.; Schwartz, J.; Liu, X.; Hoffmann, C.; Gerhardt, R.A.; Tannenbaum, R. Synthesis and structure characterization of copper terephthalate metal–organic frameworks. Eur. J. Inorg. Chem. 2009, 2009, 2338–2343. [Google Scholar] [CrossRef]
- Wang, R.; Li, Q.; Duan, N.; Zhang, T.; Lu, H. Preparation of biomorphic Ni–Al LDHs using cotton from discarded T–shirt as a template and the adsorption capability for Congo red. Res. Chem. Intermed. 2015, 41, 7899–7914. [Google Scholar] [CrossRef]
- Lesbani, A.; Normah, N.; Palapa, N.R.; Taher, T.; Andreas, R.; Mohadi, R. Ni/Al Layered Double Hydroxide Intercalated with Keggin Ion [α-SiW12O40]4-for Iron (II) Removal in Aqueous Solution (Similarity). Molekul 2020, 15, 149–157. [Google Scholar] [CrossRef]
- Li, L.; San Hui, K.; Hui, K.N.; Xia, Q.; Fu, J.; Cho, Y.-R. Facile synthesis of NiAl layered double hydroxide nanoplates for high-performance asymmetric supercapacitor. J. Alloys Compd. 2017, 721, 803–812. [Google Scholar] [CrossRef]
- Zheng, W.; Sun, S.; Xu, Y.; Yu, R.; Li, H. Sulfidation of hierarchical NiAl−LDH/Ni−MOF composite for high-performance supercapacitor. ChemElectroChem 2019, 6, 3375–3382. [Google Scholar] [CrossRef]
- Yan, X.; Jin, Z. Interface engineering: NiAl-LDH in-situ derived NiP2 quantum dots and Cu3P nanoparticles ingeniously constructed pn heterojunction for photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 420, 127682. [Google Scholar] [CrossRef]
- Verónica, M.; Graciela, B.; Norma, A.; Miguel, L. Ethanol steam reforming using Ni(II)-Al(III) layered double hydroxide as catalyst precursor: Kinetic study. Chem. Eng. J. 2008, 138, 602–607. [Google Scholar] [CrossRef]
- Perez-Bernal, M.E.; Ruano-Casero, R.J.; Benito, F.; Rives, V. Nickel–aluminum layered double hydroxides prepared via inverse micelles formation. J. Solid State Chem. 2009, 182, 1593–1601. [Google Scholar] [CrossRef]
- Mas, V.; Dieuzeide, M.L.; Jobbágy, M.; Baronetti, G.; Amadeo, N.; Laborde, M. Ni(II)-Al(III) layered double hydroxide as catalyst precursor for ethanol steam reforming: Activation treatments and kinetic studies. Catal. Today 2008, 133, 319–323. [Google Scholar] [CrossRef]
- Yang, J.-F.; Zhou, Z.-T. Use of spray technique to prepare Ni/Al-layered double hydroxides. J. Alloys Compd. 2009, 473, 458–461. [Google Scholar] [CrossRef]
- Wang, H.; Zheng, C.; Li, F. Biopolymer-induced microstructure-controlled fabrication of Ni–Al layered double hydroxide films. Chem. Eng. J. 2010, 158, 633–640. [Google Scholar] [CrossRef]
- Popova, L.; Pancheva, T.; Uzunova, A. Salicylic acid: Properties, biosynthesis and physiological role. Bulg. J. Plant Physiol 1997, 23, 85–93. [Google Scholar]
- Abdolmohammad-Zadeh, H.; Kohansal, S.; Sadeghi, G. Nickel–aluminum layered double hydroxide as a nanosorbent for selective solid-phase extraction and spectrofluorometric determination of salicylic acid in pharmaceutical and biological samples. Talanta 2011, 84, 368–373. [Google Scholar] [CrossRef]
- Rodenas, T.; Luz, I.; Prieto, G.; Seoane, B.; Miro, H.; Corma, A.; Kapteijn, F.; Llabrés i Xamena, F.X.; Gascon, J. Metal–organic framework nanosheets in polymer composite materials for gas separation. Nat. Mater. 2015, 14, 48–55. [Google Scholar] [CrossRef]
- Zhan, G.; Fan, L.; Zhao, F.; Huang, Z.; Chen, B.; Yang, X.; Zhou, S.F. Fabrication of ultrathin 2D Cu-BDC nanosheets and the derived integrated MOF nanocomposites. Adv. Funct. Mater. 2019, 29, 1806720. [Google Scholar] [CrossRef]
- Liu, P.-F.; Tao, K.; Li, G.-C.; Wu, M.-K.; Zhu, S.-R.; Yi, F.-Y.; Zhao, W.-N.; Han, L. In situ growth of ZIF-8 nanocrystals on layered double hydroxide nanosheets for enhanced CO2 capture. Dalton Trans. 2016, 45, 12632–12635. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Pan, J.H.; Steinbach, F.; Caro, J.R. In situ synthesis of MOF membranes on ZnAl-CO3 LDH buffer layer-modified substrates. J. Am. Chem. Soc. 2014, 136, 14353–14356. [Google Scholar] [CrossRef]
- Rani, R.; Deep, A.; Mizaikoff, B.; Singh, S.J.V. Enhanced hydrothermal stability of Cu MOF by post synthetic modification with amino acids. Vacuum 2019, 164, 449–457. [Google Scholar] [CrossRef]
- Abdelmoaty, A.S.; El-Beih, A.A.; Hanna, A.A. Synthesis, characterization and antimicrobial activity of copper-metal organic framework (Cu-MOF) and its modification by melamine. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1778–1785. [Google Scholar] [CrossRef]
- dos Santos, R.M.M.; Gonçalves, R.G.L.; Constantino, V.R.L.; Santilli, C.V.; Borges, P.D.; Tronto, J.; Pinto, F.G. Adsorption of Acid Yellow 42 dye on calcined layered double hydroxide: Effect of time, concentration, pH and temperature. Appl. Clay Sci. 2017, 140, 132–139. [Google Scholar] [CrossRef]
- Constantino, V.R.; Pinnavaia, T.J. Basic properties of Mg2+ 1-xAl3+ x layered double hydroxides intercalated by carbonate, hydroxide, chloride, and sulfate anions. Inorg. Chem. 1995, 34, 883–892. [Google Scholar] [CrossRef]
- Guo, H.; Wang, Y.; Li, C.; Zhou, K. Construction of sandwich-structured CoAl-layered double hydroxide@zeolitic imidazolate framework-67 (CoAl-LDH@ZIF-67) hybrids: Towards enhancing the fire safety of epoxy resins. RSC Adv. 2018, 8, 36114–36122. [Google Scholar] [CrossRef]
- Taghavi Fardood, S.; Moradnia, F.; Heidarzadeh, S.; Naghipour, A. Green synthesis, characterization, photocatalytic and antibacterial activities of copper oxide nanoparticles of copper oxide nanoparticles. Nanochem. Res. 2023, 8, 134–140. [Google Scholar]
- Jubu, P.R.; Obaseki, O.; Ajayi, D.; Danladi, E.; Chahrour, K.M.; Muhammad, A.; Landi, S., Jr.; Igbawua, T.; Chahul, H.; Yam, F. Considerations about the determination of optical bandgap from diffuse reflectance spectroscopy using the Tauc plot. J. Opt. 2024, 1–11. [Google Scholar] [CrossRef]
- Jubu, P.R.; Danladi, E.; Ndeze, U.; Adedokun, O.; Landi, S., Jr.; Haider, A.; Adepoju, A.; Yusof, Y.; Obaseki, O.; Yam, F. Comment about the use of unconventional Tauc plots for bandgap energy determination of semiconductors using UV–Vis spectroscopy. Results Opt. 2024, 14, 100606. [Google Scholar] [CrossRef]
- Rajput, A.; Rahman, M.A.; Rahman, M.H.; Kuila, A. Visible light photocatalytic degradation of organic pollutants in industrial wastewater by engineered TiO2 nanoparticles. Biomass Convers. Biorefinery 2024, 14, 17301–17311. [Google Scholar] [CrossRef]
- Kong, C.; Min, S.; Lu, G. Dye-sensitized NiSx catalyst decorated on graphene for highly efficient reduction of water to hydrogen under visible light irradiation. ACS Catal. 2014, 4, 2763–2769. [Google Scholar] [CrossRef]
- Maseeh, I.; Anwar, F.; Aroob, S.; Javed, T.; Bibi, I.; Almasoudi, A.; Raheel, A.; Javid, M.A.; Carabineiro, S.A.; Taj, M.B. Multifunctional MgAl LDH/Zn-MOF S-scheme heterojunction: Efficient hydrogen production, methyl red removal, and CO2 adsorption. Mater. Adv. 2024, 5, 5080–5095. [Google Scholar] [CrossRef]
- Li, X.; Yu, J.; Low, J.; Fang, Y.; Xiao, J.; Chen, X. Engineering heterogeneous semiconductors for solar water splitting. J. Mater. Chem. A 2015, 3, 2485–2534. [Google Scholar] [CrossRef]
- Derikvandi, H.; Vosough, M.; Nezamzadeh-Ejhieh, A. A novel double Ag@AgCl/Cu@Cu2O plasmonic nanostructure: Experimental design and LC-Mass detection of tetracycline degradation intermediates. Int. J. Hydrogen Energy 2021, 46, 2049–2064. [Google Scholar] [CrossRef]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J. Colloid Interface Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Xu, Y.; He, Q.; Yin, R.; Sun, P.; Dong, X. Phenanthroline bridging graphitic carbon nitride framework and Fe (II) ions to promote transfer of photogenerated electrons for selective photocatalytic reduction of Nitrophenols. J. Colloid Interface Sci. 2022, 608, 2088–2099. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Li, J.; Ng, D.H.; Yang, P.; Song, P.; Zuo, M. Synthesis of novel hierarchically porous Fe3O4@MgAl–LDH magnetic microspheres and its superb adsorption properties of dye from water. J. Ind. Eng. Chem. 2017, 46, 315–323. [Google Scholar] [CrossRef]
- González, M.; Pavlovic, I.; Rojas-Delgado, R.; Barriga, C. Removal of Cu2+, Pb2+ and Cd2+ by layered double hydroxide–humate hybrid. Sorbate and sorbent comparative studies. Chem. Eng. J. 2014, 254, 605–611. [Google Scholar] [CrossRef]
- Baran, W.; Adamek, E.; Makowski, A. The influence of selected parameters on the photocatalytic degradation of azo-dyes in the presence of TiO2 aqueous suspension. Chem. Eng. J. 2008, 145, 242–248. [Google Scholar] [CrossRef]
- Shah, R.K. Efficient photocatalytic degradation of methyl orange dye using facilely synthesized α-Fe2O3 nanoparticles. Arab. J. Chem. 2023, 16, 104444. [Google Scholar] [CrossRef]
- Nyachhyon, A.R.; Neupane, G. Photocatalytical degradation of Methyl Orange using laboratory prepared Copper Oxide photocatalyst. Sci. World 2024, 17, 106–113. [Google Scholar] [CrossRef]
- Ni, Z.-M.; Xia, S.-J.; Wang, L.-G.; Xing, F.-F.; Pan, G.-X. Treatment of methyl orange by calcined layered double hydroxides in aqueous solution: Adsorption property and kinetic studies. J. Colloid Interface Sci. 2007, 316, 284–291. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, K.; Gao, Z.; Gao, H.; Xie, Z.; Du, X.; Huang, H. Reversing the dye adsorption and separation performance of metal–organic frameworks via introduction of− SO3H groups. Ind. Eng. Chem. Res. 2017, 56, 4496–4501. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Meng, L. Adsorption of methyl orange from aqueous solution on hydrothermal synthesized Mg–Al layered double hydroxide. J. Chem. Eng. Data 2011, 56, 4217–4225. [Google Scholar] [CrossRef]
- Asfaram, A.; Ghaedi, M.; Hajati, S.; Rezaeinejad, M.; Goudarzi, A.; Purkait, M.K. Rapid removal of Auramine-O and Methylene blue by ZnS: Cu nanoparticles loaded on activated carbon: A response surface methodology approach. J. Taiwan Inst. Chem. Eng. 2015, 53, 80–91. [Google Scholar] [CrossRef]
- Bulut, Y.; Aydın, H. A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination 2006, 194, 259–267. [Google Scholar] [CrossRef]
- Han, R.; Zhang, J.; Han, P.; Wang, Y.; Zhao, Z.; Tang, M. Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem. Eng. J. 2009, 145, 496–504. [Google Scholar] [CrossRef]
- Rashed, S.H.; Abd-Elhamid, A.; Abdalkarim, S.Y.H.; El-Sayed, R.H.; El-Bardan, A.A.; Soliman, H.M.; Nayl, A. Preparation and characterization of layered-double hydroxides decorated on graphene oxide for dye removal from aqueous solution. J. Mater. Res. Technol. 2022, 17, 2782–2795. [Google Scholar] [CrossRef]
- González, A.S.; Martínez, S.S. Study of the sonophotocatalytic degradation of basic blue 9 industrial textile dye over slurry titanium dioxide and influencing factors. Ultrason. Sonochem. 2008, 15, 1038–1042. [Google Scholar] [CrossRef]
- Saquib, M.; Tariq, M.A.; Haque, M.; Muneer, M.J. Photocatalytic degradation of disperse blue 1 using UV/TiO2/H2O2 process. J. Environ. Manag. 2008, 88, 300–306. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Balakrishnan, A.; Chinthala, M. Comprehensive review on advanced reusability of g-C3N4 based photocatalysts for the removal of organic pollutants. Chemosphere 2022, 297, 134190. [Google Scholar] [CrossRef]
- Bisaria, K.; Sinha, S.; Singh, R.; Iqbal, H.M. Recent advances in structural modifications of photo-catalysts for organic pollutants degradation–a comprehensive review. Chemosphere 2021, 284, 131263. [Google Scholar] [CrossRef]
- Crepaldi, E.L.; Tronto, J.; Cardoso, L.P.; Valim, J.B. Sorption of terephthalate anions by calcined and uncalcined hydrotalcite-like compounds. Colloids Surf. A Physicochem. Eng. Asp. 2002, 211, 103–114. [Google Scholar] [CrossRef]
- Zhu, M.-X.; Li, Y.-P.; Xie, M.; Xin, H.-Z. Sorption of an anionic dye by uncalcined and calcined layered double hydroxides: A case study. J. Hazard. Mater. 2005, 120, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Hadjltaief, H.B.; Zina, M.B.; Galvez, M.E.; Da Costa, P. Photocatalytic degradation of methyl green dye in aqueous solution over natural clay-supported ZnO–TiO2 catalysts. J. Photochem. Photobiol. A Chem. 2016, 315, 25–33. [Google Scholar] [CrossRef]
- Dhatwalia, J.; Kumari, A.; Chauhan, A.; Mansi, K.; Thakur, S.; Saini, R.V.; Guleria, I.; Lal, S.; Kumar, A.; Batoo, K.M.; et al. Rubus ellipticus Sm. Fruit extract mediated zinc oxide nanoparticles: A green approach for dye degradation and biomedical applications. Materials 2022, 15, 3470. [Google Scholar] [CrossRef]
- Mani, S.K.; Saroja, M.; Venkatachalam, M.; Rajamanickam, T.J. Antimicrobial activity and photocatalytic degradation properties of zinc sulfide nanoparticles synthesized by using plant extracts. J. Nanostruct. 2018, 8, 107–118. [Google Scholar]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Monash, P.; Pugazhenthi, G. Utilization of calcined Ni-Al layered double hydroxide (LDH) as an Adsorbent for removal of methyl orange dye from aqueous solution. Environ. Prog. Sustain. Energy 2014, 33, 154–159. [Google Scholar] [CrossRef]
- Javed, T.; Thumma, A.; Uddin, A.N.; Akhter, R.; Babar Taj, M.; Zafar, S.; Mahmood Baig, M.; Shoaib Ahmad Shah, S.; Wasim, M.; Amin Abid, M. Batch adsorption study of Congo Red dye using unmodified Azadirachta indica leaves: Isotherms and kinetics. Water Pract. Technol. 2024, 19, 546–566. [Google Scholar] [CrossRef]
- Urooj, H.; Javed, T.; Taj, M.B.; Nouman Haider, M. Adsorption of crystal violet dye from wastewater on Phyllanthus emblica fruit (PEF) powder: Kinetic and thermodynamic. Int. J. Environ. Anal. Chem. 2023, 1–26. [Google Scholar] [CrossRef]
- Ikram, M.; Rashid, M.; Haider, A.; Naz, S.; Haider, J.; Raza, A.; Ansar, M.; Uddin, M.K.; Ali, N.M.; Ahmed, S.S. A review of photocatalytic characterization, and environmental cleaning, of metal oxide nanostructured materials. Sustain. Mater. Technol. 2021, 30, e00343. [Google Scholar] [CrossRef]
- Periyasamy, A.P. Recent advances in the remediation of textile-dye-containing wastewater: Prioritizing human health and sustainable wastewater treatment. Sustainability 2024, 16, 495. [Google Scholar] [CrossRef]
- Attar, R.M.; Alkhamis, K.M.; Alsharief, H.H.; Alaysuy, O.; Alrashdi, K.S.; Mattar, H.; Alkhatib, F.; El-Metwaly, N.M. Remarkable photodegradation breakdown cost, antimicrobial activity, photocatalytic efficiency, and recycling of SnO2 quantum dots throughout industrial hazardous pollutants treatment. Ceram. Int. 2024, 50, 36194–36209. [Google Scholar] [CrossRef]
- Kamal, A.-B.; Hassane, A.M.; An, C.; Deng, Q.; Hu, N.; Abolibda, T.Z.; Altaleb, H.A.; Gomha, S.M.; Selim, M.M.; Shenashen, M.A. Developing a cost-effective and eco-friendly adsorbent/photocatalyst using biomass and urban waste for crystal violet removal and antimicrobial applications. Biomass Convers. Biorefinery 2024, 1–19. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E. Cu and CuO nanoparticles immobilized by silica thin films as antibacterial materials and photocatalysts. Surf. Coat. Technol. 2010, 205, 219–223. [Google Scholar] [CrossRef]
- Jannesari, M.; Akhavan, O.; Madaah Hosseini, H.R.; Bakhshi, B. Graphene/CuO2 nanoshuttles with controllable release of oxygen nanobubbles promoting interruption of bacterial respiration. ACS Appl. Mater. Interfaces 2020, 12, 35813–35825. [Google Scholar] [CrossRef]


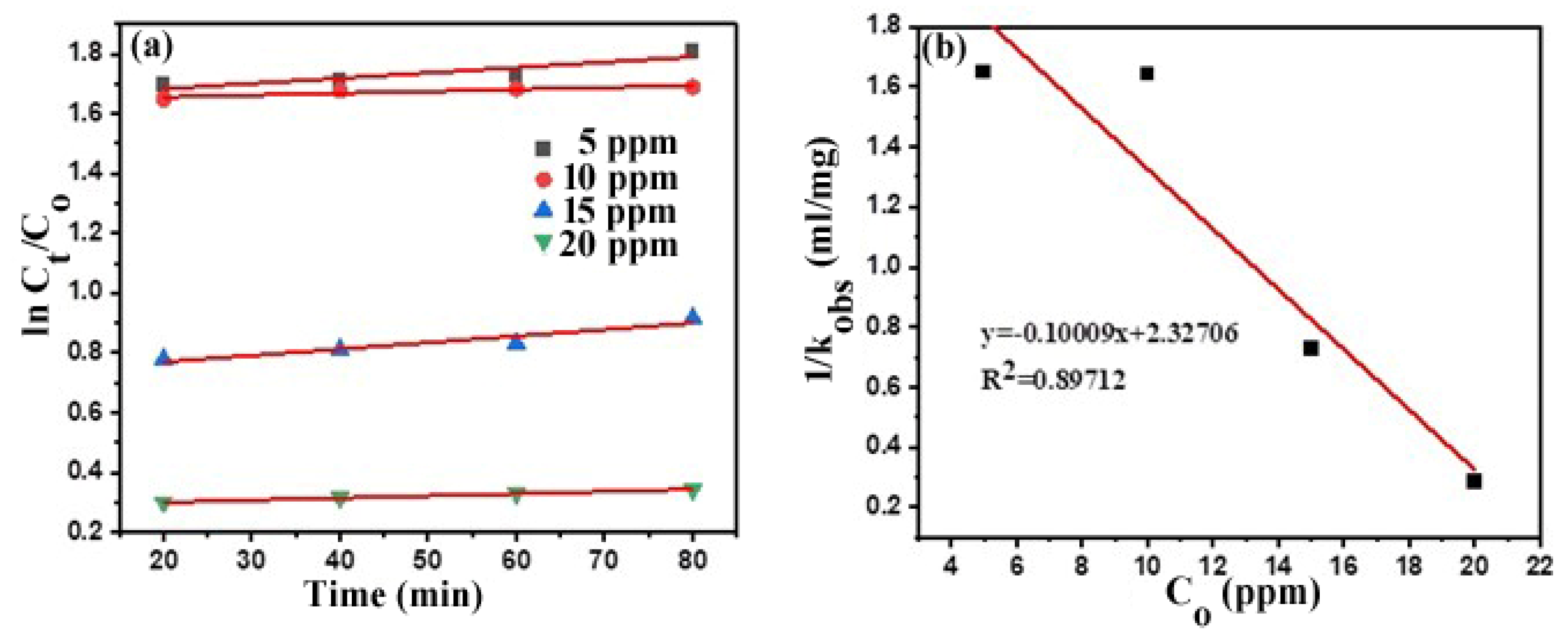
| Initial Concentration (ppm) | R2 | 1/kobs | Equation of a Straight Line |
|---|---|---|---|
| 5 | 0.79 | 1.648 | y = −1.0 × 10−3 x + 1.648 |
| 10 | 0.73 | 1.643 | y = 6.5 × 10−4 x + 1.643 |
| 15 | 0.86 | 0.726 | y = 2.0 × 10−3 x + 0.726 |
| 20 | 0.99 | 0.285 | y = 7.2 × 10−4 x + 0.285 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, I.; Aroob, S.; Anwar, F.; Taj, M.B.; Baamer, D.F.; Almasoudi, A.; Ali, O.M.; Aldahiri, R.H.; Alsulami, F.M.H.; Khan, M.I.; et al. Synergistic Effect of NiAl-Layered Double Hydroxide and Cu-MOF for the Enhanced Photocatalytic Degradation of Methyl Orange and Antibacterial Properties. Catalysts 2024, 14, 719. https://doi.org/10.3390/catal14100719
Batool I, Aroob S, Anwar F, Taj MB, Baamer DF, Almasoudi A, Ali OM, Aldahiri RH, Alsulami FMH, Khan MI, et al. Synergistic Effect of NiAl-Layered Double Hydroxide and Cu-MOF for the Enhanced Photocatalytic Degradation of Methyl Orange and Antibacterial Properties. Catalysts. 2024; 14(10):719. https://doi.org/10.3390/catal14100719
Chicago/Turabian StyleBatool, Iqra, Sadia Aroob, Farheen Anwar, Muhammad Babar Taj, Doaa F. Baamer, Afaf Almasoudi, Omar Makram Ali, Reema H. Aldahiri, Fatimah Mohammad H. Alsulami, Muhammad Imran Khan, and et al. 2024. "Synergistic Effect of NiAl-Layered Double Hydroxide and Cu-MOF for the Enhanced Photocatalytic Degradation of Methyl Orange and Antibacterial Properties" Catalysts 14, no. 10: 719. https://doi.org/10.3390/catal14100719
APA StyleBatool, I., Aroob, S., Anwar, F., Taj, M. B., Baamer, D. F., Almasoudi, A., Ali, O. M., Aldahiri, R. H., Alsulami, F. M. H., Khan, M. I., Nawaz, A., Maseeh, I., Nazir, M. K., Carabineiro, S. A. C., Shanableh, A., & Fernandez-Garcia, J. (2024). Synergistic Effect of NiAl-Layered Double Hydroxide and Cu-MOF for the Enhanced Photocatalytic Degradation of Methyl Orange and Antibacterial Properties. Catalysts, 14(10), 719. https://doi.org/10.3390/catal14100719








