Kinetic Modelling of Aromaticity and Colour Changes during the Degradation of Sulfamethoxazole Using Photo-Fenton Technology
Abstract
1. Introduction
2. Results and Discussion
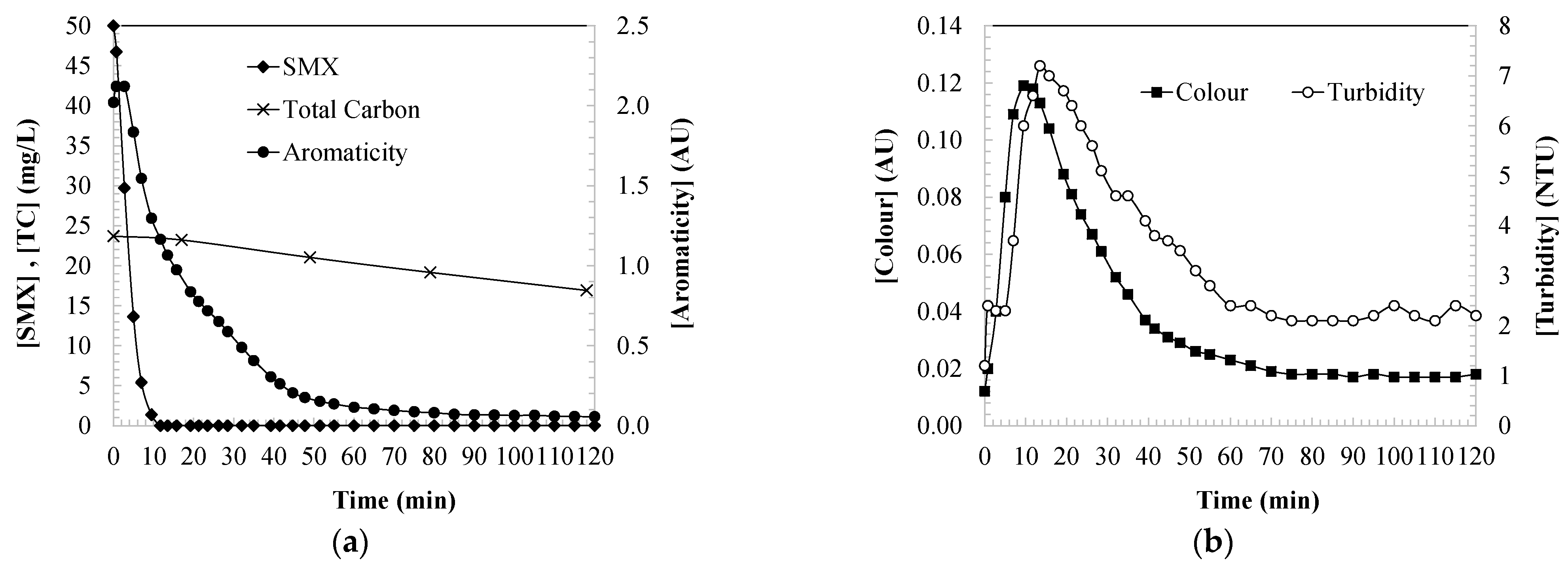
2.1. Parameters Indicating the Water Quality during the SMX Oxidation
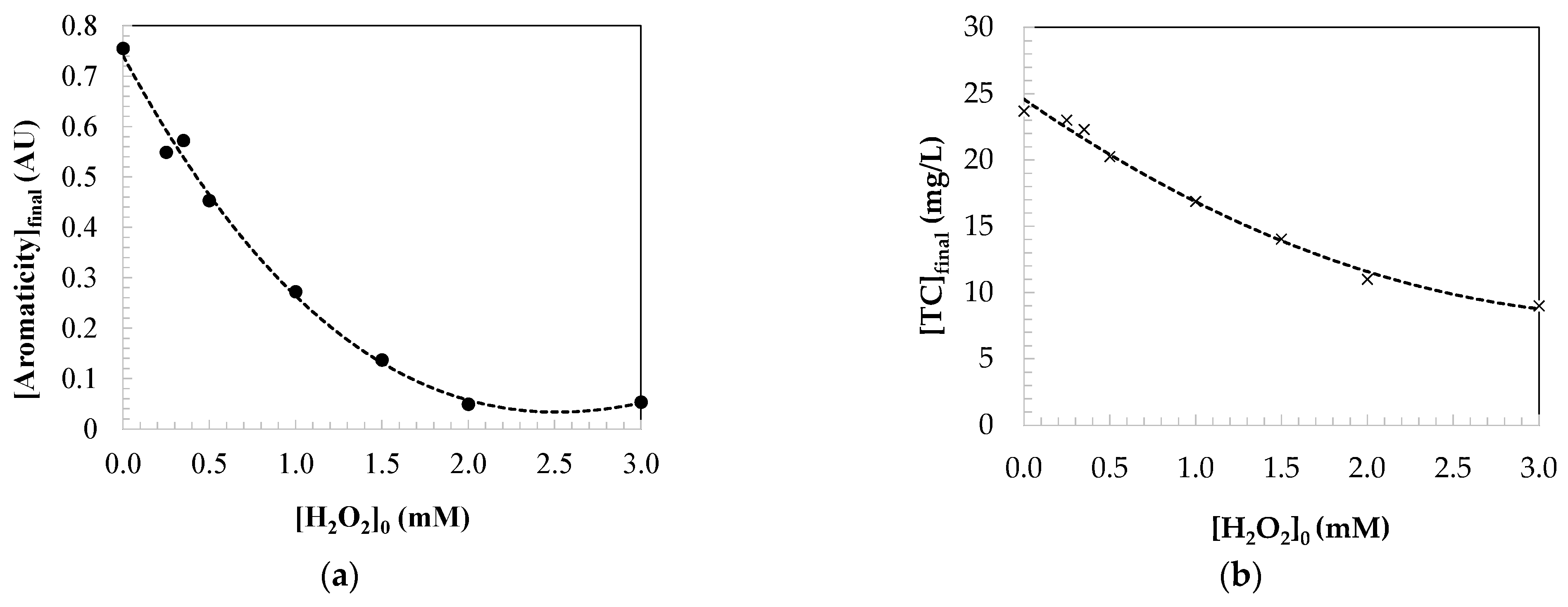
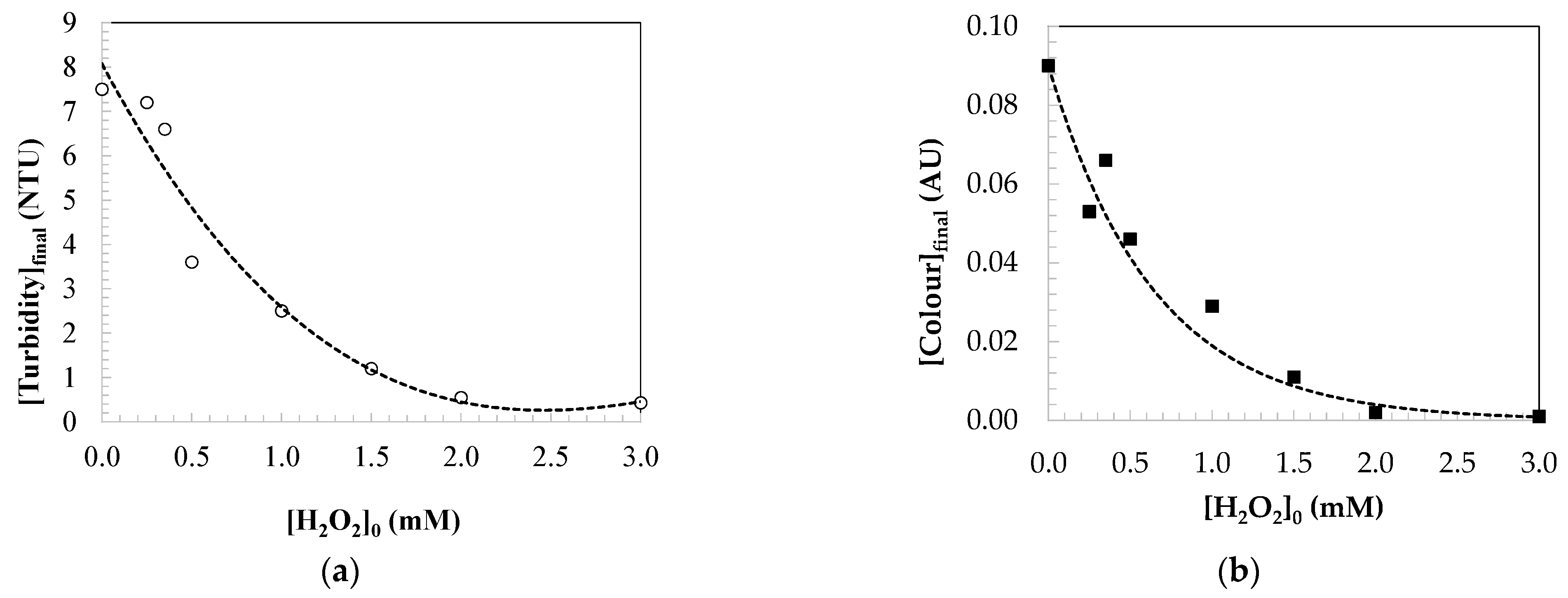
2.2. Kinetic Modelling of the Parameters Indicating the Water Quality
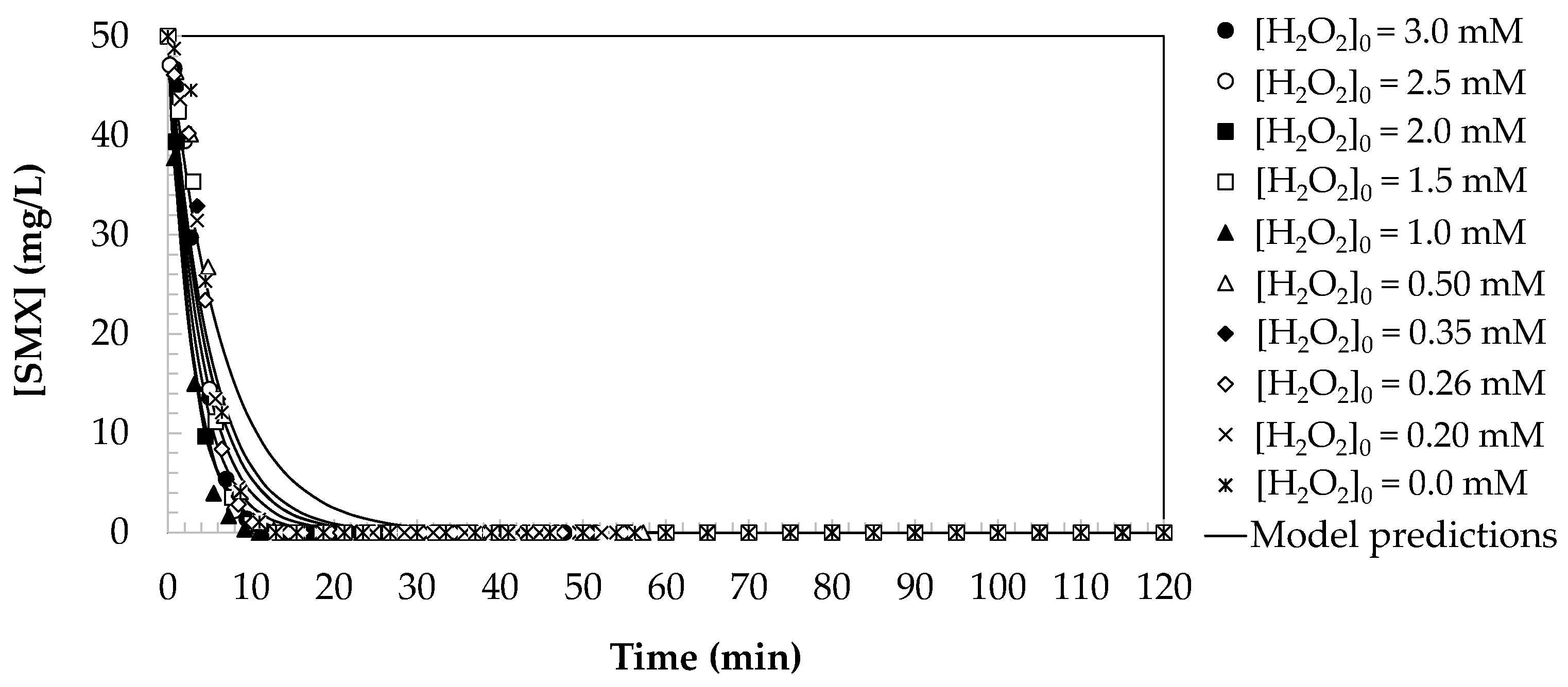
2.2.1. Kinetic Modelling of Pseudo-First Order for SMX Oxidation
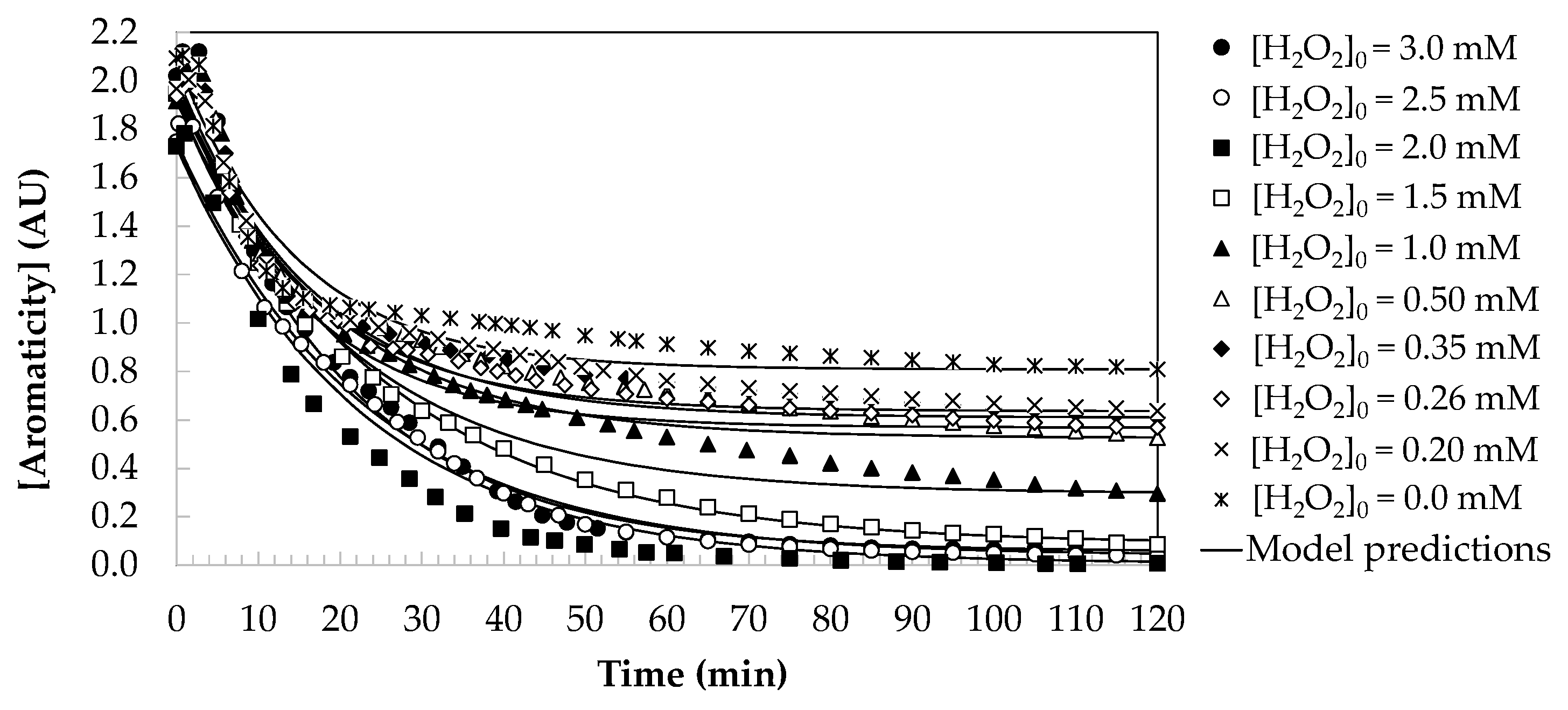
2.2.2. Kinetic Modelling for Aromaticity Loss
2.2.3. Kinetic Modelling for Colour Changes
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lauzurique, Y.; Miralles-Cuevas, S.; Godoy, M.; Sepúlveda, P.; Bollo, S.; Cabrera-Reina, A.; Huiliñir, C.; Malato, S.; Oller, I.; Salazar-González, R. Elimination of sulfamethoxazole by anodic oxidation using mixed metal oxide anodes. J. Water Process Eng. 2023, 54, 103922. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Buschmann, A.H.; Dölz, H.J. Aquaculture as yet another environmental gateway to the development and globalisation of antimicrobial resistance. Lancet Infect. Dis. 2016, 16, 127–133. [Google Scholar] [CrossRef]
- Isidori, M.; Lavorgna, M.; Nardelli, A.; Pascarella, L.; Parrella, A. Toxic and genotoxic evaluation of six antibiotics on non-target organisms. Sci. Total Environ. 2005, 346, 87–98. [Google Scholar] [CrossRef]
- Kohantorabi, M.; Moussavi, G.; Oulego, P.; Giannakis, S. Radical-based degradation of sulfamethoxazole via UVA/PMS-assisted photocatalysis, driven by magnetically separable Fe3O4@CeO2@BiOI nanospheres. Sep. Purif. Technol. 2021, 267, 118665. [Google Scholar] [CrossRef]
- Huang, Z.; Dai, X.; Huang, Z.; Wang, T.; Cui, L.; Ye, J.; Wu, P. Simultaneous and efficient photocatalytic reduction of Cr(VI) and oxidation of trace sulfamethoxazole under LED light by rGO@Cu2O/BiVO4 p-n heterojunction composite. Chemosphere 2019, 221, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Guo, X.; Yang, C.; Ouyang, Z. Synthesis of g-C3N4 by different precursors under burning explosion effect and its photocatalytic degradation for tylosin. Appl. Catal. B Environ. 2018, 230, 65–76. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Gahrouei, A.E.; Vakili, S.; Zandifar, A.; Pourebrahimi, S. From wastewater to clean water: Recent advances on the removal of metronidazole, ciprofloxacin, and sulfamethoxazole antibiotics from water through adsorption and advanced oxidation processes (AOPs). Environ. Res. 2024, 252, 119029. [Google Scholar] [CrossRef]
- Zyoud, S.H. The state of current research on COVID-19 and antibiotic use: Global implications for antimicrobial resistance. J. Health Popul. Nutr. 2023, 42, 42. [Google Scholar] [CrossRef]
- Oharisi, O.L.; Ncube, S.; Nyoni, H.; Madikizela, M.L.; Olowoyo, O.J.; Maseko, B.R. Occurrence and Prevalence of Antibiotics in Wastewater Treatment Plants and Effluent Receiving Rivers in South Africa Using UHPLC-MS Determination. J. Environ. Manag. 2023, 345, 118621. [Google Scholar] [CrossRef]
- Mertah, O.; Gómez-Avilés, A.; Kherbeche, A.; Belver, C.; Bedia, J. Peroxymonosulfate enhanced photodegradation of sulfamethoxazole with TiO2@CuCo2O4 catalysts under simulated solar light. J. Environ. Chem. Eng. 2022, 10, 108438. [Google Scholar] [CrossRef]
- de Matos, M.H.; Afonso, P.; Borges, K.C.M.; de Melo, L.; de Fátima, R.; Daldin, M.; Vilella, F.; Maribondo, R.; Godinho, M. Enhanced degradation of the antibiotic sulfamethoxazole by heterogeneous photocatalysis using Ce0,8 Gd0,2 O2-d/TiO2 particles. J. Alloys Compd. 2019, 808, 151711. [Google Scholar] [CrossRef]
- Bao, Y.; Lim, T.T.; Goei, R.; Zhong, Z.; Wang, R.; Hu, X. One-step construction of heterostructured metal-organics@Bi2O3 with improved photoinduced charge transfer and enhanced activity in photocatalytic degradation of sulfamethoxazole under solar light irradiation. Chemosphere 2018, 205, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Yazdanbakhsh, A.R.; Eslami, A.; Mohamadreza Massoudinejad, M.; Avazpour, M. Enhanced degradation of sulfamethoxazole antibiotic from aqueous solution using Mn-WO3/LED photocatalytic process: Kinetic, mechanism, degradation pathway and toxicity reduction. Chem. Eng. J. 2020, 380, 122497. [Google Scholar] [CrossRef]
- Murillo-Sierra, J.C.; Ruiz-Ruiz, E.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Machuca-Martínez, F.; Hernández-Ramírez, A. Sulfamethoxazole mineralization by solar photo electro-Fenton process in a pilot plant. Catalysis Today. 2018, 313, 175–181. [Google Scholar] [CrossRef]
- Keerthanan, S.; Jayasinghe, C.; Bolan, N.; Rinklebe, J.; Vithanage, M. Retention of sulfamethoxazole by cinnamon wood biochar and its efficacy of reducing bioavailability and plant uptake in soil. Chemosphere 2022, 297, 134073. [Google Scholar] [CrossRef]
- Dirany, A.; Sirés, I.; Oturan, N.; Oturan, M.A. Electrochemical abatement of the antibiotic sulfamethoxazole from water. Chemosphere 2010, 81, 594–602. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, H.; Pan, Z.; Liu, Y.; Yao, G.; Guo, Y.; Lai, B. Degradation of sulfamethoxazole by cobalt-nickel powder composite catalyst coupled with peroxymonosulfate: Performance, degradation pathways and mechanistic consideration. J. Hazard. Mater. 2020, 400, 123322. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Yerushalmi, L.; Chen, Z.; Haghighat, F.; Guo, J. Enhanced photocatalytic degradation of sulfamethoxazole by zinc oxide photocatalyst in the presence of fluoride ions: Optimization of parameters and toxicological evaluation. Water Res. 2018, 132, 241–251. [Google Scholar] [CrossRef]
- Gong, H.; Chu, W. Permanganate with a double-edge role in photodegradation of sulfamethoxazole: Kinetic, reaction mechanism and toxicity. Chemosphere 2018, 191, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Poza-Nogueiras, V.; Gomis-Berenguer, A.; Pazos, M.; Sanroman, A.; Ania, C.O. Exploring the use of carbon materials as cathodes in electrochemical advanced oxidation processes for the degradation of antibiotics. J. Environ. Chem. Eng. 2022, 10, 107506. [Google Scholar] [CrossRef]
- Wei, R.; Ge, F.; Huang, S.; Chen, M.; Wang, R. Occurrence of veterinary antibiotics in animal wastewater and surface water around farms in Jiangsu Province, China. Chemosphere 2011, 82, 1408–1414. [Google Scholar] [CrossRef] [PubMed]
- Martini, J.; Orge, C.A.; Faria, J.L.; Pereira, M.F.R.; Soares, O.S.G.P. Catalytic Advanced Oxidation Processes for Sulfamethoxazole Degradation. Appl. Sci. 2019, 9, 2652. [Google Scholar] [CrossRef]
- González, O.; Sans, C.; Esplugas, S. Sulfamethoxazole abatement by photo-Fenton Toxicity, inhibition and biodegradability assessment of intermediates. J. Hazard. Mater. 2007, 146, 459–464. [Google Scholar] [CrossRef]
- Wu, C.H.; Wu, J.T.; Lin, Y.H. Mineralization of sulfamethizole in photo-Fenton and photo-Fenton-like systems. Water Sci. Technol. 2016, 73, 746–750. [Google Scholar] [CrossRef]
- Villegas-Guzman, P.; Oppenheimer-Barrot, S.; Silva-Agredo, J.; Torres-Palma, R.A. Comparative Evaluation of Photo-Chemical AOPs for Ciprofoxacin Degradation: Elimination in Natural Waters and Analysis of pH Effect, Primary Degradation By-Products, and the Relationship with the Antibiotic Activity. Water Air. Soil. Pollut. 2017, 228, 209. [Google Scholar] [CrossRef]
- Sun, S.; Yao, H.; Li, X.; Deng, S.; Zhao, S.; Zhang, W. Enhanced Degradation of Sulfamethoxazole (SMX) in Toilet Wastewater by Photo-Fenton Reactive Membrane Filtration. Nanomaterials 2020, 10, 180. [Google Scholar] [CrossRef]
- Ameta, R.; Chohadia, A.K.; Jain, A.; Punjabi, P.B. Chapter 3—Fenton and Photo-Fenton Processes. In Advanced Oxidation Processes for Waste Water Treatment; Ameta, S.C., Ameta, R., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 49–87. [Google Scholar] [CrossRef]
- Soriano-Molina, P.; De la Obra, I.; Miralles-Cuevas, S.; Gualda-Alonso, E.; Casas López, J.L.; Sánchez Pérez, J.A. Assessment of Different Iron Sources for Continuous Flow Solar Photo-Fenton at Neutral pH for Sulfamethoxazole Removal in Actual MWWTP Effluents. J. Water Process Eng. 2021, 42, 102109. [Google Scholar] [CrossRef]
- Martínez-Costa, J.I.; Rivera-Utrilla, J.; Leyva-Ramos, R.; Sánchez-Polo, M.; Velo-Gala, I.; Mota, A.J. Individual and Simultaneous Degradation of the Antibiotics Sulfamethoxazole and Trimethoprim in Aqueous Solutions by Fenton, Fenton-like and Photo-Fenton Processes Using Solar and UV Radiations. J. Photochem. Photobiol. A Chem. 2018, 360, 95–108. [Google Scholar] [CrossRef]
- Elmolla, E.S. Effect of Photo-Fenton Operating Conditions on the Performance of Photo-Fenton-SBR Process for Recalcitrant Wastewater Treatment. J. Appl. Sci. 2010, 10, 3236–3242. [Google Scholar] [CrossRef]
- Tian, L.; Wang, L.; Wei, S.; Zhang, L.; Dong, D.; Guo, Z. Enhanced degradation of enoxacin using ferrihydrite-catalyzed heterogeneous photo-Fenton process. Environ. Res. 2024, 251, 118650. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Fan, Y.; Liu, K.; Kong, D.; Lu, J. Thermo activated persulfate oxidation of antibiotic sulfamethoxazole and structurally related compounds. Water Res. 2015, 87, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moradi, M.; Moussavi, G. Investigation of chemical-less UVC/VUV process for advanced oxidation of sulfamethoxazole in aqueous solutions: Evaluation of operational variables and degradation mechanism. Sep. Purif. Technol. 2018, 190, 90–99. [Google Scholar] [CrossRef]
- Trovó, A.G.; Nogueira, R.F.P.; Agüera, A.; Fernandez-Alba, A.R.; Sirtori, C.; Malato, S. Degradation of sulfamethoxazole in water by solar photo-Fenton. Chemical and toxicological evaluation. Water Res. 2009, 43, 3922–3931. [Google Scholar] [CrossRef]
- Villota, N.; Echevarria, B.; Duoandicoechea, U.; Lombrana, J.I.; De Luis, A.M. Kinetic Study of the Water Quality Parameters during the Oxidation of Diclofenac by UV Photocatalytic Variants. Catalysts 2024, 14, 580. [Google Scholar] [CrossRef]
- Villota, N.; Cruz-Alcalde, A.; Ferreiro, C.; Lombrana, J.I.; Esplugas, S. Changes in solution turbidity and color during paracetamol removal in laboratory and pilot-scale semicontinuous ozonation reactors. Sci. Total. Environ. 2023, 854, 158682. [Google Scholar] [CrossRef]
- Villota, N.; Jankelevitch, S.; Lomas, J.M. Kinetic modelling of colour and turbidity formation in aqueous solutions of sulphamethoxazole degraded by UV/H2O2. Environ. Technol. 2024, 45, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Villota, N.; Ferreiro, C.; Qulatein, H.A.; Lomas, J.M.; Camarero, L.M.; Lombrana, J.I. Colour Changes during the Carbamazepine Oxidation by Photo-Fenton. Catalysts 2021, 11, 386. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Umukoro, E.H.; Tshwenya, L.; Moutloali, R.; Babalola, J.O.; Arotiba, O.A. Photoelectrocatalytic Water Treatment Systems: Degradation, Kinetics and Intermediate Products Studies of Sulfamethoxazole on a TiO2–Exfoliated Graphite Electrode. RSC Adv. 2017, 7, 40571–40583. [Google Scholar] [CrossRef]
- Ren, M.; Sun, S.; Wu, Y.; Shi, Y.; Wang, Z.; Cao, H.; Xie, Y. The structure-activity relationship of aromatic compounds in advanced oxidation processes: A review. Chemosphere 2022, 296, 134071. [Google Scholar] [CrossRef] [PubMed]

| [H2O2]0 (mM) | kSMX (1/min) | karom (1/min) | kcolour,form (1/min) | kcolour,deg (1/min) | αcolour (-) |
|---|---|---|---|---|---|
| 0.00 | 0.15 | 7.0 × 10−2 | 1.9 × 10−4 | 2.9 × 10−2 | 2.00 |
| 0.20 | 0.20 | 6.3 × 10−2 | 3.7 × 10−4 | 3.0 × 10−2 | 1.83 |
| 0.25 | 0.22 | 6.5 × 10−2 | 3.4 × 10−4 | 3.0 × 10−2 | 1.80 |
| 0.35 | 0.22 | 6.0 × 10−2 | 3.6 × 10−4 | 3.5 × 10−2 | 1.77 |
| 0.50 | 0.25 | 5.5 × 10−2 | 4.8 × 10−4 | 3.5 × 10−2 | 1.62 |
| 1.00 | 0.35 | 4.7 × 10−2 | 8.0 × 10−4 | 4.0 × 10−2 | 1.25 |
| 1.50 | 0.35 | 4.0 × 10−2 | 9.9 × 10−4 | 4.6 × 10−2 | 1.11 |
| 2.00 | 0.36 | 4.5 × 10−2 | 1.0 × 10−3 | 5.5 × 10−2 | 1.00 |
| 2.50 | 0.35 | 4.4 × 10−2 | 9.6 × 10−4 | 5.8 × 10−2 | 1.00 |
| 3.00 | 0.29 | 5.0 × 10−2 | 9.0 × 10−4 | 5.8 × 10−2 | 1.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villota, N.; Duoandicoechea, U.; Lombraña, J.I.; De Luis, A.M. Kinetic Modelling of Aromaticity and Colour Changes during the Degradation of Sulfamethoxazole Using Photo-Fenton Technology. Catalysts 2024, 14, 718. https://doi.org/10.3390/catal14100718
Villota N, Duoandicoechea U, Lombraña JI, De Luis AM. Kinetic Modelling of Aromaticity and Colour Changes during the Degradation of Sulfamethoxazole Using Photo-Fenton Technology. Catalysts. 2024; 14(10):718. https://doi.org/10.3390/catal14100718
Chicago/Turabian StyleVillota, Natalia, Unai Duoandicoechea, Jose Ignacio Lombraña, and Ana María De Luis. 2024. "Kinetic Modelling of Aromaticity and Colour Changes during the Degradation of Sulfamethoxazole Using Photo-Fenton Technology" Catalysts 14, no. 10: 718. https://doi.org/10.3390/catal14100718
APA StyleVillota, N., Duoandicoechea, U., Lombraña, J. I., & De Luis, A. M. (2024). Kinetic Modelling of Aromaticity and Colour Changes during the Degradation of Sulfamethoxazole Using Photo-Fenton Technology. Catalysts, 14(10), 718. https://doi.org/10.3390/catal14100718







