Ultradurable Pt-Based Catalysts for Oxygen Reduction Electrocatalysis
Abstract
1. Introduction
2. Operation Mechanism of ORR Electrocatalysis
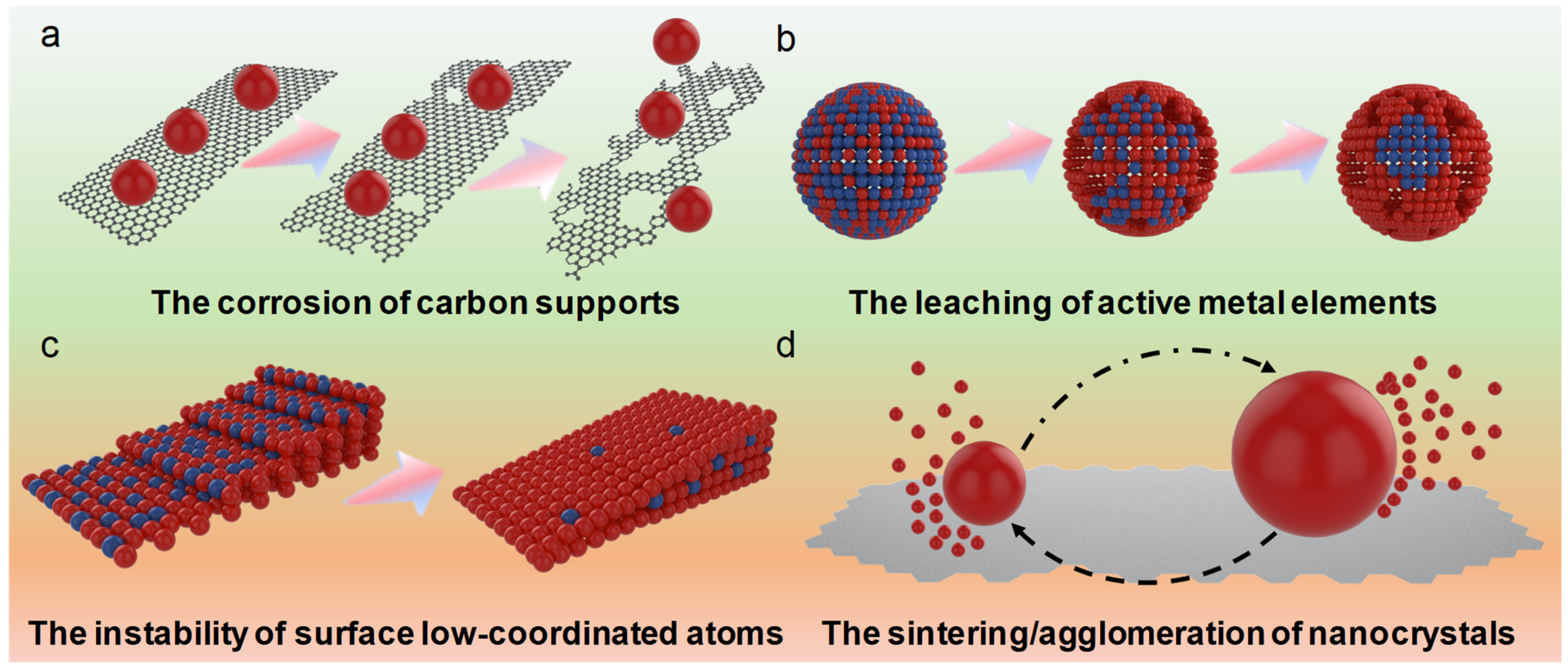
3. Limitation of Traditional Pt-Based ORR Catalysts
4. Main Catalyst Systems for Ultradurable ORR Electrocatalysis
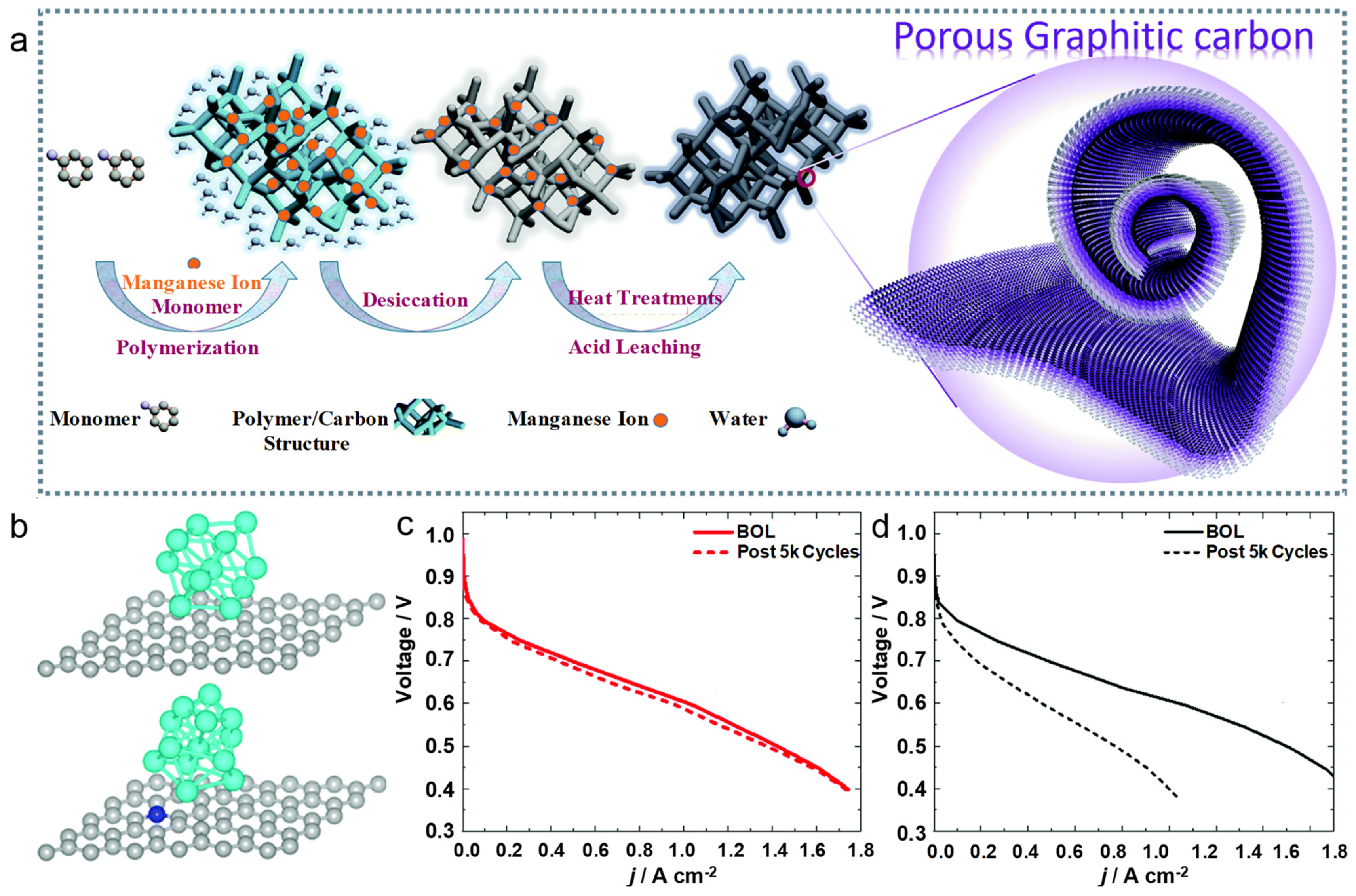
4.1. The Optimization of Supports
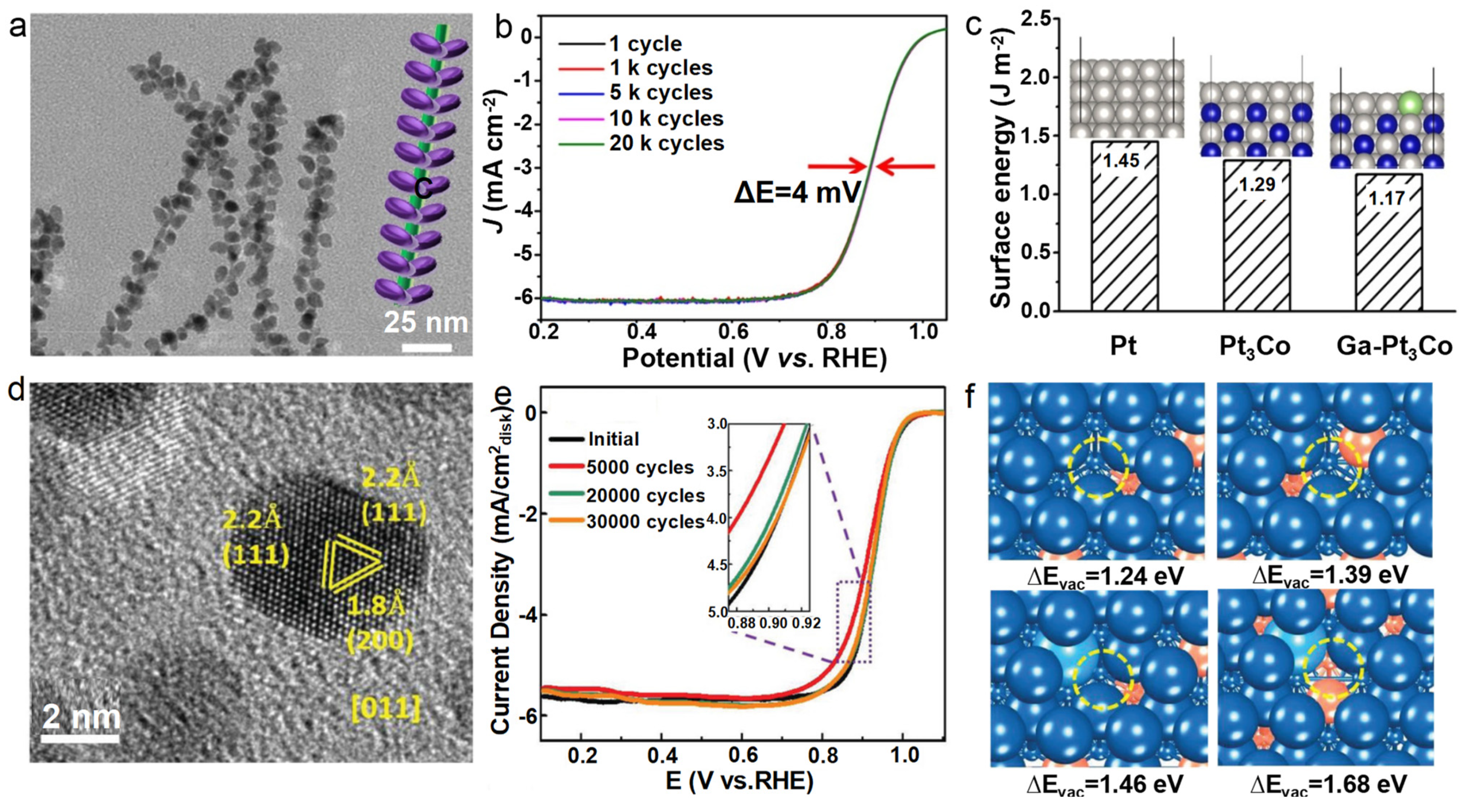
4.2. Heteroatom-Doped Alloys
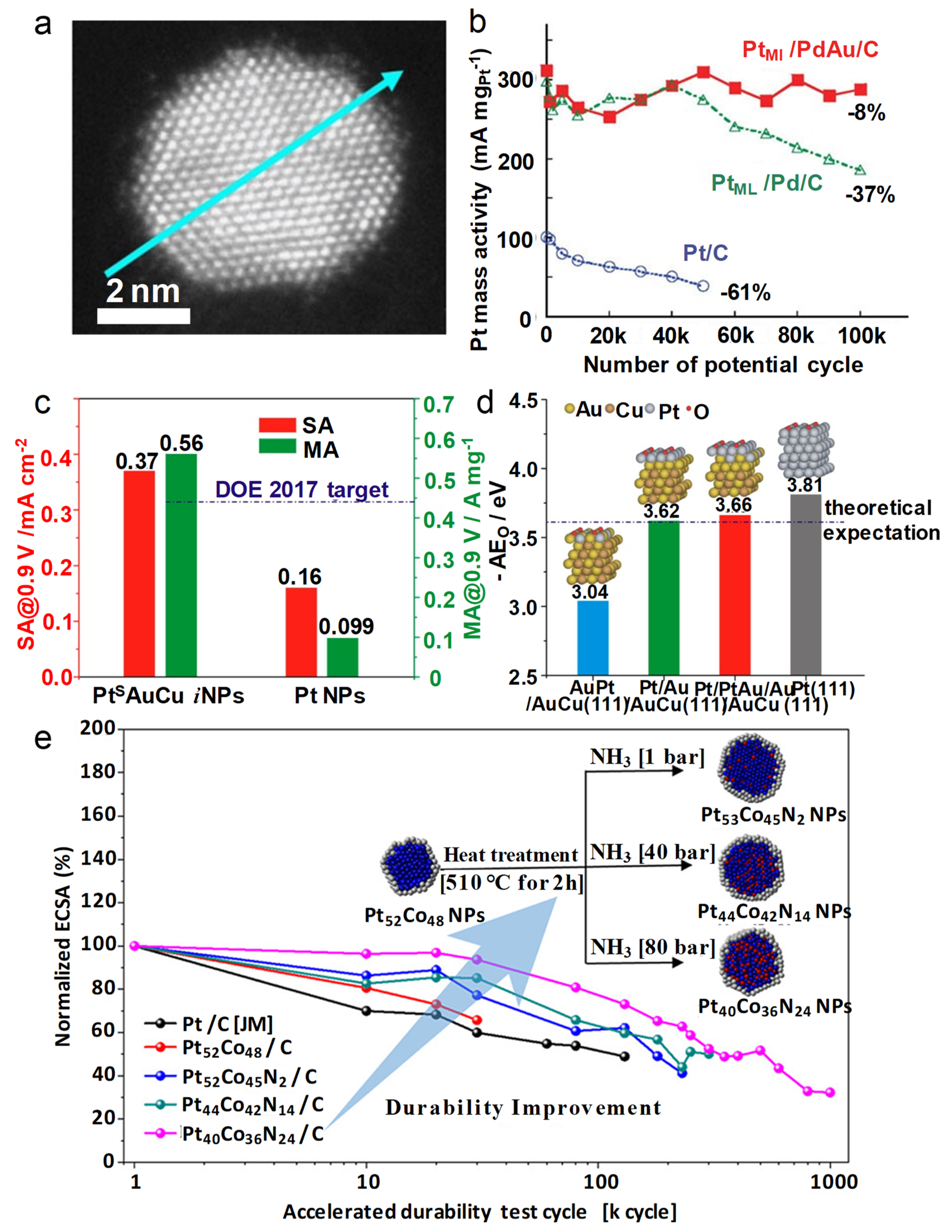
4.3. Core/Shell Structures
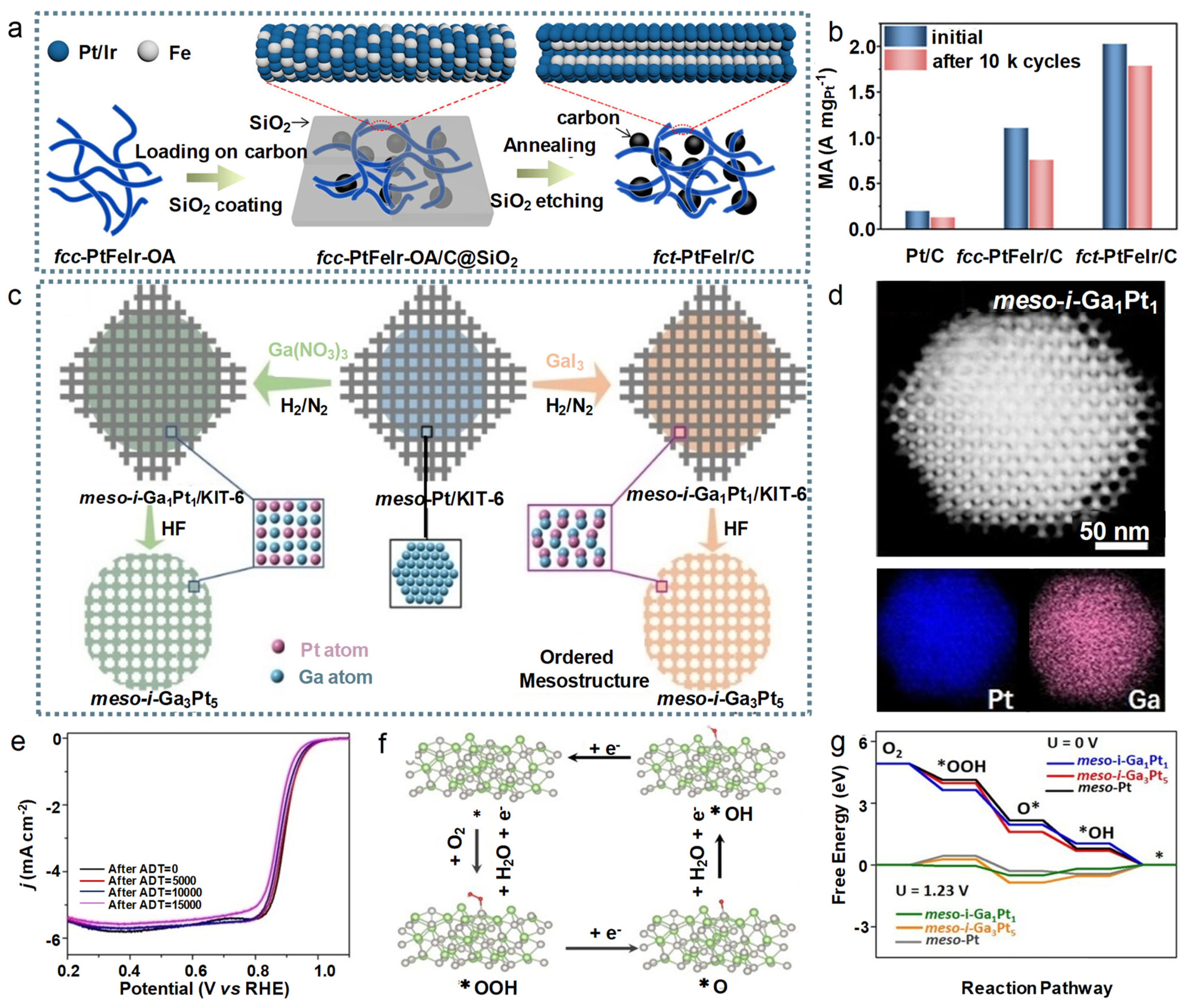
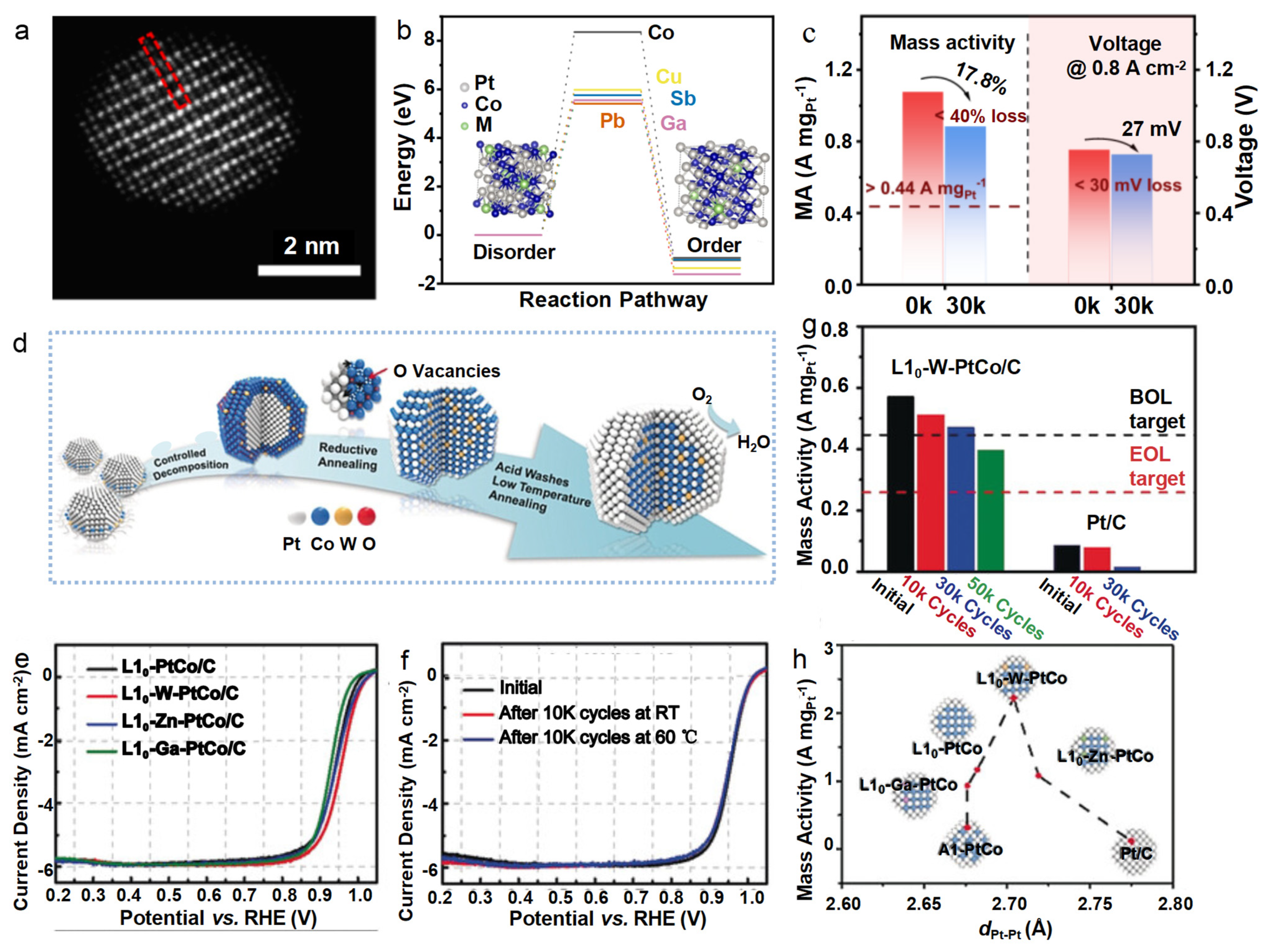
4.4. Intermetallics

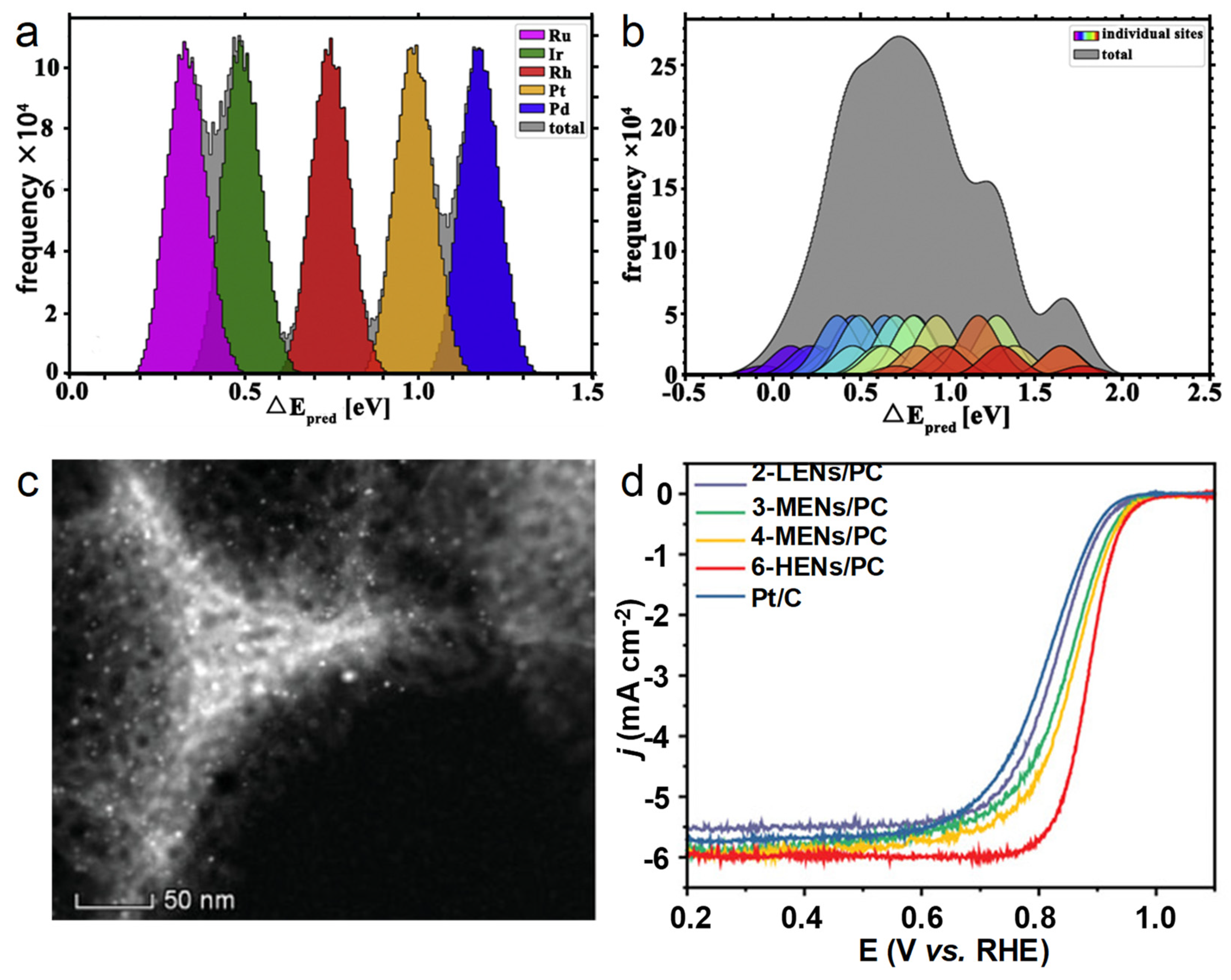
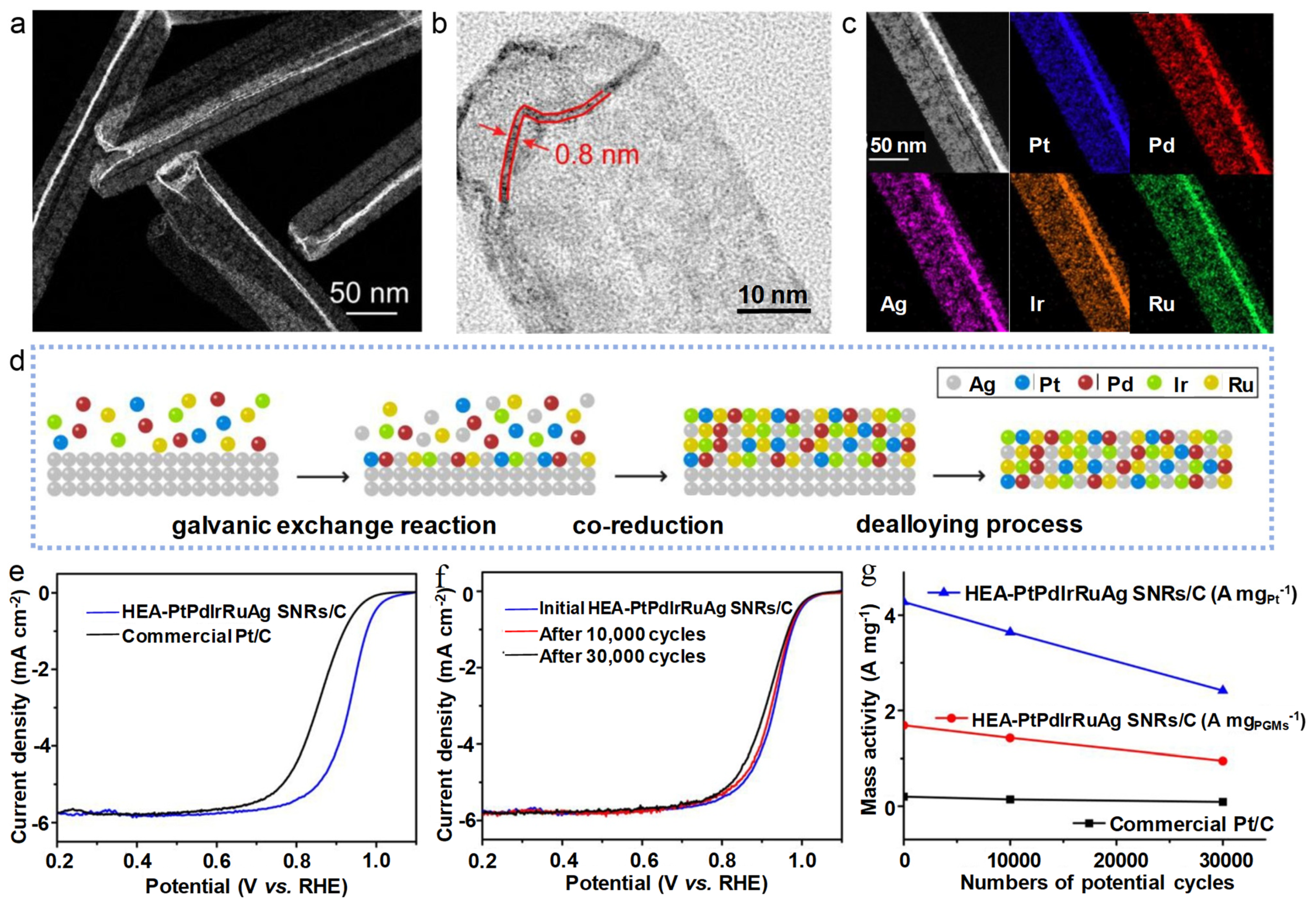
4.5. High-Entropy Alloys
4.6. Others
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lewis, N.S. Research Opportunities to Advance Solar Energy Utilization. Science 2016, 351, aad1920. [Google Scholar] [CrossRef] [PubMed]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the Next Generation of Proton-Exchange Membrane Fuel Cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Tan, H.; Rui, X.; Yu, Y. Vanadium-Based Materials: Next Generation Electrodes Powering the Battery Revolution? Acc. Chem. Res. 2020, 53, 1660–1671. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, J.; Guo, X.; Li, J.; Huang, Y.; Geng, S.; Yu, Y.; Liu, Y.; Yang, W. Structural Engineering of Fe-Doped Ni2P Nanosheets Arrays for Enhancing Bifunctional Electrocatalysis towards Overall Water Splitting. Appl. Surf. Sci. 2021, 536, 147909. [Google Scholar] [CrossRef]
- Gu, Y.; Xi, B.; Wei, R.; Fu, Q.; Qain, Y.; Xiong, S. Sponge Assembled by Graphene Nanocages with Double Active Sites to Accelerate Alkaline HER Kinetics. Nano Lett. 2020, 20, 8375–8383. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Z.; Zhang, W.; Guo, S. Electronic-Structure Tuning of Water-Splitting Nanocatalysts. Trends Chem. 2019, 1, 259–271. [Google Scholar] [CrossRef]
- Li, L.; Tian, F.; Qiu, L.; Wu, F.; Yang, W.; Yu, Y. Recent Progress on Ruthenium-Based Electrocatalysts towards the Hydrogen Evolution Reaction. Catalysts 2023, 13, 1497. [Google Scholar] [CrossRef]
- Shao, M.; Chang, Q.; Dodelet, J.-P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New Roads and Challenges for Fuel Cells in Heavy-Duty Transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Kim, J.; Hong, Y.; Lee, K.; Kim, J.Y. Highly Stable Pt-Based Ternary Systems for Oxygen Reduction Reaction in Acidic Electrolytes. Adv. Energy Mater. 2020, 10, 2002049. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, S.; Shao, P.; Zhu, Y.; Mu, Z.; Sheng, D.; Zhang, T.; Jiang, X.; Shao, R.; Ren, Z.; et al. Covalent Organic Framework–Based Porous Ionomers for High-Performance Fuel Cells. Science 2022, 378, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.-Y.; Ma, T.; Hu, Q.; Yang, H.-P.; He, C.-X. Ternary PtRuTe Alloy Nanofibers as an Efficient and Durable Electrocatalyst for Hydrogen Oxidation Reaction in Alkaline Media. Sci. China Mater. 2022, 65, 3462–3469. [Google Scholar] [CrossRef]
- Su, L.; Jin, Y.; Fan, X.; Liu, Z.; Luo, W. pH-Dependent Binding Energy-Induced Inflection-Point Behaviors for pH-Universal Hydrogen Oxidation Reaction. Sci. China Chem. 2023, 66, 3262–3268. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, L.; Li, X.; Liu, Y.; Wang, Y.; Yao, Q.; Xie, J.; Xue, Q.; Yan, Z.; Yuan, X.; et al. Atomic-Precision Pt6 Nanoclusters for Enhanced Hydrogen Electro-Oxidation. Nat. Commun. 2022, 13, 1596. [Google Scholar] [CrossRef]
- Yuan, S.; Zhang, J.; Hu, L.; Li, J.; Li, S.; Gao, Y.; Zhang, Q.; Gu, L.; Yang, W.; Feng, X.; et al. Decarboxylation-Induced Defects in MOF-Derived Single Cobalt Atom@Carbon Electrocatalysts for Efficient Oxygen Reduction. Angew. Chem. Int. Ed. 2021, 60, 21685–21690. [Google Scholar] [CrossRef]
- Li, M.; Tian, F.; Lin, T.; Tao, L.; Guo, X.; Chao, Y.; Guo, Z.; Zhang, Q.; Gu, L.; Yang, W.; et al. High-Index Faceted PdPtCu Ultrathin Nanorings Enable Highly Active and Stable Oxygen Reduction Electrocatalysis. Small Methods 2021, 5, 2100154. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, C.; Liu, Z.; Huang, J.; Wu, M.; Liu, H.; Li, Y.; Huang, Y. Pt-Based Nanocrystal for Electrocatalytic Oxygen Reduction. Adv. Mater. 2019, 31, 1808115. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, J.; He, F.; Chen, Y.; Zhu, J.; Wang, D.; Mu, S.; Yang, H.Y. Defect and Doping Co-Engineered Non-Metal Nanocarbon ORR Electrocatalyst. Nano-Micro Lett. 2021, 13, 65. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in Applying Highly Active Pt-Based Nanostructured Catalysts for Oxygen Reduction Reactions to Fuel Cell Vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Nie, Y.; Li, L.; Wei, Z. Recent Advancements in Pt and Pt-Free Catalysts for Oxygen Reduction Reaction. Chem. Soc. Rev. 2015, 44, 2168–2201. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Yang, J.; Lei, Y.; Wang, C.; Wang, J.; Tang, Y.; Mao, Z. Recent Advances in Pt-Based Electrocatalysts for PEMFCs. RSC Adv. 2021, 11, 13316–13328. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.-Y.; Park, J.E.; Kim, S.; Kim, O.-H.; Hwang, W.; Her, M.; Kang, S.Y.; Park, S.; Kwon, O.J.; Park, H.S.; et al. Differences in the Electrochemical Performance of Pt-Based Catalysts Used for Polymer Electrolyte Membrane Fuel Cells in Liquid Half- and Full-Cells. Chem. Rev. 2021, 121, 15075–15140. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Sun, T.; Li, J.-R.; Li, Q.-Z.; Hou, C.-C.; Sun, Q. The Cathode Catalysts of Hydrogen Fuel Cell: From Laboratory toward Practical Application. Nano Res. 2023, 16, 4365–4380. [Google Scholar] [CrossRef]
- Luo, M.; Koper, M.T.M. A Kinetic Descriptor for the Electrolyte Effect on the Oxygen Reduction Kinetics on Pt (111). Nat. Catal. 2022, 5, 615–623. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Fu, H. Research Progress of Fe-N-C Catalysts for the Electrocatalytic Oxygen Reduction Reaction. Sci. China Mater. 2022, 65, 1701–1722. [Google Scholar] [CrossRef]
- Wu, X.; Xie, W.; Liu, X.; Liu, X.; Zhao, Q. Two-Dimensional Fe-N-C Nanosheets for Efficient Oxygen Reduction Reaction. Catalysts 2022, 12, 1276. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Lv, Z.; Liu, R.; Li, L.; Wang, J.; Yang, W.; Jiang, X.; Feng, X.; Wang, B. Tuning the Spin State of the Iron Center by Bridge-Bonded Fe-O-Ti Ligands for Enhanced Oxygen Reduction. Angew. Chem. Int. Ed. 2022, 61, e202117617. [Google Scholar] [CrossRef]
- Gu, Y.; Xi, B.J.; Zhang, H.; Ma, Y.C.; Xiong, S.L. Activation of Main-Group Antimony Atomic Sites for Oxygen Reduction Catalysis. Angew. Chem. Int. Ed. 2022, 61, e202202200. [Google Scholar] [CrossRef]
- Ma, Z.; Cano, Z.P.; Yu, A.; Chen, Z.; Jiang, G.; Fu, X.; Yang, L.; Wu, T.; Bai, Z.; Lu, J. Enhancing Oxygen Reduction Activity of Pt-Based Electrocatalysts: From Theoretical Mechanisms to Practical Methods. Angew. Chem. Int. Ed. 2020, 59, 18334–18348. [Google Scholar] [CrossRef]
- Sapkota, P.; Lim, S.; Aguey-Zinsou, K.-F. Superior Performance of an Iron-Platinum/Vulcan Carbon Fuel Cell Catalyst. Catalysts 2022, 12, 1369. [Google Scholar] [CrossRef]
- Hou, B.; Luo, X.; Zheng, Z.; Tang, R.; Zhang, Q.; Gholizadeh, M.; Wang, C.; Tan, Z. An Efficient Electrocatalyst (PtCo/C) for the Oxygen Reduction Reaction. Catalysts 2022, 12, 794. [Google Scholar] [CrossRef]
- Huang, L.; Su, Y.-Q.; Qi, R.; Dang, D.; Qin, Y.; Xi, S.; Zaman, S.; You, B.; Ding, S.; Xia, B.Y. Boosting Oxygen Reduction via Integrated Construction and Synergistic Catalysis of Porous Platinum Alloy and Defective Graphitic Carbon. Angew. Chem. Int. Ed. 2021, 60, 25530–25537. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Gao, Y.; Ma, T.; Bai, M.; He, C.; Ren, X.; Luo, X.; Wu, C.; Li, S.; Cheng, C. Metal Alloys-Structured Electrocatalysts: Metal-Metal Interactions, Coordination Microenvironments, and Structural Property-Reactivity Relationships. Adv. Mater. 2023, 35, 2301836. [Google Scholar] [CrossRef]
- Kim, D.-G.; Sohn, Y.; Jang, I.; Yoo, S.J.; Kim, P. Formation Mechanism of Carbon-Supported Hollow PtNi Nanoparticles via One-Step Preparations for Use in the Oxygen Reduction Reaction. Catalysts 2022, 12, 513. [Google Scholar] [CrossRef]
- Li, C.; Huang, B.; Luo, M.; Qin, Y.; Sun, Y.; Li, Y.; Yang, Y.; Wu, D.; Li, M.; Guo, S. An Efficient Ultrathin PtFeNi Nanowire/Ionic Liquid Conjugate Electrocatalyst. Appl. Catal. B 2019, 256, 117828. [Google Scholar] [CrossRef]
- Yoshida, T.; Kojima, K. Toyota MIRAI Fuel Cell Vehicle and Progress toward a Future Hydrogen Society. Electrochem. Soc. Interface 2015, 24, 45. [Google Scholar] [CrossRef]
- Borup, R.L.; Kusoglu, A.; Neyerlin, K.C.; Mukundan, R.; Ahluwalia, R.K.; Cullen, D.A.; More, K.L.; Weber, A.Z.; Myers, D.J. Recent Developments in Catalyst-Related PEM Fuel Cell Durability. Curr. Opin. Electrochem. 2020, 21, 192–200. [Google Scholar] [CrossRef]
- Gao, L.; Li, X.; Yao, Z.; Bai, H.; Lu, Y.; Ma, C.; Lu, S.; Peng, Z.; Yang, J.; Pan, A.; et al. Unconventional p-d Hybridization Interaction in PtGa Ultrathin Nanowires Boosts Oxygen Reduction Electrocatalysis. J. Am. Chem. Soc. 2019, 141, 18083–18090. [Google Scholar] [CrossRef]
- Li, K.; Li, X.; Huang, H.; Luo, L.; Li, X.; Yan, X.; Ma, C.; Si, R.; Yang, J.; Zeng, J. One-Nanometer-Thick PtNiRh Trimetallic Nanowires with Enhanced Oxygen Reduction Electrocatalysis in Acid Media: Integrating Multiple Advantages into One Catalyst. J. Am. Chem. Soc. 2018, 140, 16159–16167. [Google Scholar] [CrossRef]
- Wei, M.; Huang, L.; Li, L.; Ai, F.; Su, J.; Wang, J. Coordinatively Unsaturated PtCo Flowers Assembled with Ultrathin Nanosheets for Enhanced Oxygen Reduction. ACS Catal. 2022, 12, 6478–6485. [Google Scholar] [CrossRef]
- Zhu, X.; Huang, L.; Wei, M.; Tsiakaras, P.; Shen, P.K. Highly Stable Pt-Co Nanodendrite in Nanoframe with Pt Skin Structured Catalyst for Oxygen Reduction Electrocatalysis. Appl. Catal. B 2021, 281, 119460. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, X.; Su, Y.-Q.; Wang, L.; Wang, H.; Dang, D.; Chi, B.; Liu, H.; Hensen, E.J.M.; Lou, X.W.; et al. Engineering Bunched Pt-Ni Alloy Nanocages for Efficient Oxygen Reduction in Practical Fuel Cells. Science 2019, 366, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Petersen, A.S.; Wan, H.; Hübner, R.; Zhang, J.; Wang, J.; Qi, H.; Ye, Y.; Liang, C.; Yang, J.; et al. Scalable and Controllable Synthesis of Pt-Ni Bunched-Nanocages Aerogels as Efficient Electrocatalysts for Oxygen Reduction Reaction. Adv. Energy Mater. 2023, 13, 2204257. [Google Scholar] [CrossRef]
- Luo, M.; Qin, Y.; Li, M.; Sun, Y.; Li, C.; Li, Y.; Yang, Y.; Lv, F.; Wu, D.; Zhou, P.; et al. Interface Modulation of Twinned PtFe Nanoplates Branched 3D Architecture for Oxygen Reduction Catalysis. Sci. Bull. 2020, 65, 97–104. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, R.; Yin, K.; Zhang, S.; Chung-Yen Jung, J.; Zhao, Y.; Li, M.; Xia, Z.; Zhang, J. Integration of Multiple Advantages into One Catalyst: Non-CO Pathway of Methanol Oxidation Electrocatalysis on Surface Ir-Modulated PtFeIr Jagged Nanowires. J. Colloid Interface Sci. 2023, 640, 348–358. [Google Scholar] [CrossRef]
- Shi, Y.; Lyu, Z.; Zhao, M.; Chen, R.; Nguyen, Q.N.; Xia, Y. Noble-Metal Nanocrystals with Controlled Shapes for Catalytic and Electrocatalytic Applications. Chem. Rev. 2021, 121, 649–735. [Google Scholar] [CrossRef]
- Zhang, S.; Ling, F.; Wang, L.; Xu, R.; Ma, M.; Cheng, X.; Bai, R.; Shao, Y.; Huang, H.; Li, D.; et al. An Open-Ended Ni3S2-Co9S8 Heterostructures Nanocage Anode with Enhanced Reaction Kinetics for Superior Potassium-Ion Batteries. Adv. Mater. 2022, 34, 2201420. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z.; Luo, M.; He, L.; Tao, L.; Yang, W.; Yu, Y.; Guo, S. Structural Regulation of Pd-Based Nanoalloys for Advanced Electrocatalysis. Small Sci. 2021, 1, 2100061. [Google Scholar] [CrossRef]
- Cheng, H.; Gui, R.; Liu, S.; Xie, Y.; Wu, C. Local Structure Engineering for Active Sites in Fuel Cell Electrocatalysts. Sci. China Chem. 2020, 63, 1543–1556. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, W.; Guo, K.; Liu, X.; Liu, J.; Liang, X.; Wang, X.; Gao, D.; Gan, L.; Zhu, Y.; et al. Fine-Tuning Intrinsic Strain in Penta-Twinned Pt-Cu-Mn Nanoframes Boosts Oxygen Reduction Catalysis. Adv. Funct. Mater. 2020, 30, 1910107. [Google Scholar] [CrossRef]
- Guo, N.; Xue, H.; Bao, A.; Wang, Z.; Sun, J.; Song, T.; Ge, X.; Zhang, W.; Huang, K.; He, F.; et al. Achieving Superior Electrocatalytic Performance by Surface Copper Vacancy Defects during Electrochemical Etching Process. Angew. Chem. Int. Ed. 2020, 59, 13778–13784. [Google Scholar] [CrossRef] [PubMed]
- Takimoto, D.; Toma, S.; Suda, Y.; Shirokura, T.; Tokura, Y.; Fukuda, K.; Matsumoto, M.; Imai, H.; Sugimoto, W. Platinum Nanosheets Synthesized via Topotactic Reduction of Single-Layer Platinum Oxide Nanosheets for Electrocatalysis. Nat. Commun. 2023, 14, 19. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Yang, K.; Li, M.; Xu, D.; Ma, Z. Engineering the Spin Configuration of Electrocatalysts for Electrochemical Renewable Conversions. Mater. Chem. Front. 2024. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; Gao, L.; Zeng, G.; Li, M.; Huang, H. Stabilizing Pt-Based Electrocatalysts for Oxygen Reduction Reaction: Fundamental Understanding and Design Strategies. Adv. Mater. 2021, 33, 2006494. [Google Scholar] [CrossRef] [PubMed]
- Zaman, S.; Chen, S. A Perspective on Inaccurate Measurements in Oxygen Reduction and Carbon Dioxide Reduction Reactions. J. Catal. 2023, 421, 221–227. [Google Scholar] [CrossRef]
- Fan, J.; Chen, M.; Zhao, Z.; Zhang, Z.; Ye, S.; Xu, S.; Wang, H.; Li, H. Bridging the Gap between Highly Active Oxygen Reduction Reaction Catalysts and Effective Catalyst Layers for Proton Exchange Membrane Fuel Cells. Nat. Energy 2021, 6, 475–486. [Google Scholar] [CrossRef]
- Zaman, S.; Tian, X.; Xia, B.Y. Bridging Oxygen Reduction Performance Gaps in Half and Full Cells: Challenges and Perspectives. Mater. Chem. Front. 2023, 7, 4605–4612. [Google Scholar] [CrossRef]
- Lei, W.; Li, M.; He, L.; Meng, X.; Mu, Z.; Yu, Y.; Ross, F.M.; Yang, W. A General Strategy for Bimetallic Pt-Based Nano-Branched Structures as Highly Active and Stable Oxygen Reduction and Methanol Oxidation Bifunctional Catalysts. Nano Res. 2020, 13, 638–645. [Google Scholar] [CrossRef]
- Stamenkovic, V.R.; Fowler, B.; Mun, B.S.; Wang, G.; Ross, P.N.; Lucas, C.A.; Marković, N.M. Improved Oxygen Reduction Activity on Pt3Ni(111) via Increased Surface Site Availability. Science 2007, 315, 493–497. [Google Scholar] [CrossRef]
- Tao, L.; Xia, Z.; Zhang, Q.; Sun, Y.; Li, M.; Yin, K.; Gu, L.; Guo, S. Spiny Pd/PtFe Core/Shell Nanotubes with Rich High-Index Facets for Efficient Electrocatalysis. Sci. Bull. 2021, 66, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Bando, Y.; Sun, Z.; Wu, K.C.W.; Rowan, A.E.; Na, J.; Guan, B.Y.; Yamauchi, Y. In Search of Excellence: Convex versus Concave Noble Metal Nanostructures for Electrocatalytic Applications. Adv. Mater. 2021, 33, 2004554. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Lu, B.-A.; Xue, P.; Tian, N.; Zhou, Z.-Y.; Lin, X.; Lin, W.-F.; Sun, S.-G. High-Index-Facet- and High-Surface-Energy Nanocrystals of Metals and Metal Oxides as Highly Efficient Catalysts. Joule 2020, 4, 2562–2598. [Google Scholar] [CrossRef]
- Ioroi, T.; Siroma, Z.; Yamazaki, S.-I.; Yasuda, K. Electrocatalysts for PEM Fuel Cells. Adv. Energy Mater. 2019, 9, 1801284. [Google Scholar] [CrossRef]
- Alekseenko, A.; Pavlets, A.; Moguchikh, E.; Tolstunov, M.; Gribov, E.; Belenov, S.; Guterman, V. Platinum-Containing Nanoparticles on N-Doped Carbon Supports as an Advanced Electrocatalyst for the Oxygen Reduction Reaction. Catalysts 2022, 12, 414. [Google Scholar] [CrossRef]
- Castanheira, L.; Dubau, L.; Mermoux, M.; Berthomé, G.; Caqué, N.; Rossinot, E.; Chatenet, M.; Maillard, F. Carbon Corrosion in Proton-Exchange Membrane Fuel Cells: From Model Experiments to Real-Life Operation in Membrane Electrode Assemblies. ACS Catal. 2014, 4, 2258–2267. [Google Scholar] [CrossRef]
- Zhu, S.; Yang, L.; Bai, J.; Chu, Y.; Liu, J.; Jin, Z.; Liu, C.; Ge, J.; Xing, W. Ultra-Stable Pt5La Intermetallic Compound towards Highly Efficient Oxygen Reduction Reaction. Nano Res. 2023, 16, 2035–2040. [Google Scholar] [CrossRef]
- Liu, B.; Feng, R.; Busch, M.; Wang, S.; Wu, H.; Liu, P.; Gu, J.; Bahadoran, A.; Matsumura, D.; Tsuji, T.; et al. Synergistic Hybrid Electrocatalysts of Platinum Alloy and Single-Atom Platinum for an Efficient and Durable Oxygen Reduction Reaction. ACS Nano 2022, 16, 14121–14133. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Q.; Xu, G.-L.; Qin, X.; Hwang, I.; Sun, C.-J.; Liu, M.; Hua, W.; Wu, H.-W.; Zhu, S.; et al. Atomically Dispersed Pt and Fe Sites and Pt-Fe Nanoparticles for Durable Proton Exchange Membrane Fuel Cells. Nat. Catal. 2022, 5, 503–512. [Google Scholar] [CrossRef]
- Cheng, Q.; Yang, S.; Fu, C.; Zou, L.; Zou, Z.; Jiang, Z.; Zhang, J.; Yang, H. High-Loaded Sub-6 nm Pt1Co1 Intermetallic Compounds with Highly Efficient Performance Expression in PEMFCs. Energy Environ. Sci. 2022, 15, 278–286. [Google Scholar] [CrossRef]
- Liang, J.; Li, N.; Zhao, Z.; Ma, L.; Wang, X.; Li, S.; Liu, X.; Wang, T.; Du, Y.; Lu, G.; et al. Tungsten-Doped L10-PtCo Ultrasmall Nanoparticles as a High-Performance Fuel Cell Cathode. Angew. Chem. Int. Ed. 2019, 58, 15471–15477. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Mahmood, A.; Liang, Z.; Zou, R.; Guo, S. Earth-Abundant Nanomaterials for Oxygen Reduction. Angew. Chem. Int. Ed. 2016, 55, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Yang, M.; Ke, C.; Wei, G.; Priest, C.; Qiao, Z.; Wu, G.; Zhang, J. Platinum-Group-Metal Catalysts for Proton Exchange Membrane Fuel Cells: From Catalyst Design to Electrode Structure Optimization. EnergyChem 2020, 2, 100023. [Google Scholar] [CrossRef]
- Guo, S.; Zhang, S.; Sun, S. Tuning Nanoparticle Catalysis for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2013, 52, 8526–8544. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Qu, Y.; Yuan, T.; Wang, W.; Wu, Y.; Li, Y. Review of Metal Catalysts for Oxygen Reduction Reaction: From Nanoscale Engineering to Atomic Design. Chem 2019, 5, 1486–1511. [Google Scholar] [CrossRef]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Stamenkovic, V.; Mun, B.S.; Mayrhofer, K.J.J.; Ross, P.N.N.; Markovic, N.M.; Rossmeisl, J.; Greeley, J.; Nørskov, J.K. Changing the Activity of Electrocatalysts for Oxygen Reduction by Tuning the Surface Electronic Structure. Angew. Chem. Int. Ed. 2006, 45, 2897–2901. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-López, H.M.; Valdés-Madrigal, M.A.; Rojas-Chávez, H.; Cruz-Martínez, H.; Padilla-Islas, M.A.; Tellez-Cruz, M.M.; Solorza-Feria, O. A Trimetallic Pt2NiCo/C Electrocatalyst with Enhanced Activity and Durability for Oxygen Reduction Reaction. Catalysts 2020, 10, 170. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Z.; Peng, B.; Duan, X.; Huang, Y. Beyond Extended Surfaces: Understanding the Oxygen Reduction Reaction on Nanocatalysts. J. Am. Chem. Soc. 2020, 142, 17812–17827. [Google Scholar] [CrossRef]
- Sun, Y.; Tian, J.; Mu, Z.; Tian, B.; Zhou, Q.; Liu, C.; Liu, S.; Wu, Q.; Ding, M. Unravelling the Critical Role of Surface Nafion Adsorption in Pt-Catalyzed Oxygen Reduction Reaction by in situ Electrical Transport Spectroscopy. Sci. China Chem. 2022, 65, 2290–2298. [Google Scholar] [CrossRef]
- Maillard, F.; Silva, W.O.; Castanheira, L.; Dubau, L.; Lima, F.H.B. Carbon Corrosion in Proton-Exchange Membrane Fuel Cells: Spectrometric Evidence for Pt-Catalysed Decarboxylation at Anode-Relevant Potentials. ChemPhysChem 2019, 20, 3106–3111. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-Herrera, J.C.; Tellez-Cruz, M.M.; Solorza-Feria, O.; Medina, D.I. Effect of Different Carbon Supports on the Activity of PtNi Bimetallic Catalysts toward the Oxygen Reduction. Catalysts 2022, 12, 477. [Google Scholar] [CrossRef]
- Li, C.; Clament Sagaya Selvam, N.; Fang, J. Shape-Controlled Synthesis of Platinum-Based Nanocrystals and Their Electrocatalytic Applications in Fuel Cells. Nano Micro Lett. 2023, 15, 83. [Google Scholar] [CrossRef]
- Nakaya, Y.; Furukawa, S. Catalysis of Alloys: Classification, Principles, and Design for a Variety of Materials and Reactions. Chem. Rev. 2023, 123, 5859–5947. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Yang, X.; Zhao, F.; Li, Y.; Zhang, D.; Gan, L.-Y.; Yao, K.X.; Yuan, Q. Low-Coordinated Surface Sites Make Truncated Pd Tetrahedrons as Robust ORR Electrocatalysts Outperforming Pt for DMFC Devices. Nano Res. 2022, 15, 7951–7958. [Google Scholar] [CrossRef]
- Liu, B.; Yang, H.; Hu, P.; Wang, G.-S.; Guo, Y.; Zhao, H. Dimension Engineering in Noble-Metal-Based Nanocatalysts. Catalysts 2024, 14, 9. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Virkar, A.V. Electrochemical Ostwald Ripening of Pt and Ag Catalysts Supported on Carbon. J. Power Sources 2013, 234, 82–90. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, T.; Goddard, W.A., III. Atomistic Explanation of the Dramatically Improved Oxygen Reduction Reaction of Jagged Platinum Nanowires, 50 Times Better than Pt. J. Am. Chem. Soc. 2020, 142, 8625–8632. [Google Scholar] [CrossRef]
- Lin, G.; Ju, Q.; Jin, Y.; Qi, X.; Liu, W.; Huang, F.; Wang, J. Suppressing Dissolution of Pt-Based Electrocatalysts Through the Electronic Metal-Support Interaction. Adv. Energy Mater. 2021, 11, 2101050. [Google Scholar] [CrossRef]
- Bu, L.; Ning, F.; Zhou, J.; Zhan, C.; Sun, M.; Li, L.; Zhu, Y.; Hu, Z.; Shao, Q.; Zhou, X.; et al. Three-Dimensional Porous Platinum-Tellurium-Rhodium Surface/Interface Achieve Remarkable Practical Fuel Cell Catalysis. Energy Environ. Sci. 2022, 15, 3877–3890. [Google Scholar] [CrossRef]
- Liu, J.; Liu, S.; Yan, F.; Wen, Z.; Chen, W.; Liu, X.; Liu, Q.; Shang, J.; Yu, R.; Su, D.; et al. Ultrathin Nanotube Structure for Mass-Efficient and Durable Oxygen Reduction Reaction Catalysts in PEM Fuel Cells. J. Am. Chem. Soc. 2022, 144, 19106–19114. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-W.; Xu, C.; Sheng, Z.-T.; Yan, H.-K.; Tong, L.; Liu, J.; Zeng, W.-J.; Zuo, L.-J.; Yin, P.; Zuo, M.; et al. Small Molecule-Assisted Synthesis of Carbon Supported Platinum Intermetallic Fuel Cell Catalysts. Nat. Commun. 2022, 13, 6521. [Google Scholar] [CrossRef]
- Göhl, D.; Garg, A.; Paciok, P.; Mayrhofer, K.J.J.; Heggen, M.; Shao-Horn, Y.; Dunin-Borkowski, R.E.; Román-Leshkov, Y.; Ledendecker, M. Engineering Stable Electrocatalysts by Synergistic Stabilization between Carbide Cores and Pt Shells. Nat. Mater. 2020, 19, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Y.; Yang, J.; Meng, L.; Ma, C.; Peng, L.; Chen, D.; Chen, Q. Polyaniline-Derived Carbon Nanofibers with a High Graphitization Degree Loading Ordered PtNi Intermetallic Nanoparticles for Oxygen Reduction Reaction. Ind. Chem. Mater. 2023, 1, 458–464. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Z.; Zhang, A.; Yan, X.; Xue, W.; Peng, B.; Xin, H.L.; Pan, X.; Duan, X.; Huang, Y. Graphene-Nanopocket-Encaged PtCo Nanocatalysts for Highly Durable Fuel Cell Operation under Demanding Ultralow-Pt-Loading Conditions. Nat. Nanotech. 2022, 17, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zaman, S.; Tian, X.; Wang, Z.; Fang, W.; Xia, B.Y. Advanced Platinum-Based Oxygen Reduction Electrocatalysts for Fuel Cells. Acc. Chem. Res. 2021, 54, 311–322. [Google Scholar] [CrossRef]
- Yin, P.; Yan, Q.Q.; Liang, H.W. Strong Metal-Support Interactions through Sulfur-Anchoring of Metal Catalysts on Carbon Supports. Angew. Chem. Int. Ed. 2023, 62, e202302819. [Google Scholar] [CrossRef]
- Qiao, Z.; Hwang, S.; Li, X.; Wang, C.; Samarakoon, W.; Karakalos, S.; Li, D.; Chen, M.; He, Y.; Wang, M.; et al. 3D Porous Graphitic Nanocarbon for Enhancing the Performance and Durability of Pt Catalysts: A Balance between Graphitization and Hierarchical Porosity. Energy Environ. Sci. 2019, 12, 2830–2841. [Google Scholar] [CrossRef]
- Huang, L.; Wei, M.; Qi, R.; Dong, C.-L.; Dang, D.; Yang, C.-C.; Xia, C.; Chen, C.; Zaman, S.; Li, F.-M.; et al. An Integrated Platinum-Nanocarbon Electrocatalyst for Efficient Oxygen Reduction. Nat. Commun. 2022, 13, 6703. [Google Scholar] [CrossRef]
- Zeng, L.; Zhao, Z.; Huang, Q.; Zhou, C.; Chen, W.; Wang, K.; Li, M.; Lin, F.; Luo, H.; Gu, Y.; et al. Single-Atom Cr-N4 Sites with High Oxophilicity Interfaced with Pt Atomic Clusters for Practical Alkaline Hydrogen Evolution Catalysis. J. Am. Chem. Soc. 2023, 145, 21432–21441. [Google Scholar] [CrossRef]
- Lai, J.; Chen, S.; Liu, X.; Yan, X.; Qin, Z.; Xie, L.; Lin, Z.; Cai, Z.; Zhao, Y.; Wang, H.-L.; et al. Synergy between Intermetallic Pt Alloy and Porous Co-N4 Carbon Nanofibers Enables Durable Fuel Cells with Low Mass Transport Resistance. ACS Catal. 2023, 13, 11996–12006. [Google Scholar] [CrossRef]
- Xiao, F.; Wang, Y.; Xu, G.-L.; Yang, F.; Zhu, S.; Sun, C.-J.; Cui, Y.; Xu, Z.; Zhao, Q.; Jang, J.; et al. Fe-N-C Boosts the Stability of Supported Platinum Nanoparticles for Fuel Cells. J. Am. Chem. Soc. 2022, 144, 20372–20384. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.-Y.; Ganesan, P.; Park, S.; Popov, B.N. Development of a Titanium Dioxide-Supported Platinum Catalyst with Ultrahigh Stability for Polymer Electrolyte Membrane Fuel Cell Applications. J. Am. Chem. Soc. 2009, 131, 13898–13899. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhu, J.; Zheng, Y.; Xiao, M.; Gao, R.; Zhang, Z.; Wen, G.; Dou, H.; Deng, Y.-P.; Yu, A.; et al. Materials Engineering toward Durable Electrocatalysts for Proton Exchange Membrane Fuel Cells. Adv. Energy Mater. 2022, 12, 2102665. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Cheng, C.; Yang, Z. Recent Progress of MXenes as the Support of Catalysts for the CO Oxidation and Oxygen Reduction Reaction. Chin. Chem. Lett. 2020, 31, 931–936. [Google Scholar] [CrossRef]
- Xu, C.; Fan, C.; Zhang, X.; Chen, H.; Liu, X.; Fu, Z.; Wang, R.; Hong, T.; Cheng, J. MXene (Ti3C2Tx) and Carbon Nanotube Hybrid-Supported Platinum Catalysts for the High-Performance Oxygen Reduction Reaction in PEMFC. ACS Appl. Mater. Interfaces 2020, 12, 19539–19546. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Huang, Y.; Chen, Z.; Hu, S.; Zhu, J.; Tsiakaras, P.; Kang Shen, P. Novel PtNi Nanoflowers Regulated by a Third Element (Rh, Ru, Pd) as Efficient Multifunctional Electrocatalysts for ORR, MOR and HER. Chem. Eng. J. 2023, 454, 140131. [Google Scholar] [CrossRef]
- Liu, H.; Jia, R.; Qin, C.; Yang, Q.; Tang, Z.; Li, M.; Ma, Z. Anti-CO Poisoning FePtRh Nanoflowers with Rh-Rich Core and Fe-Rich Shell Boost Methanol Oxidation Electrocatalysis. Adv. Funct. Mater. 2023, 33, 2210626. [Google Scholar] [CrossRef]
- Duan, X.; Cao, F.; Ding, R.; Li, X.; Li, Q.; Aisha, R.; Zhang, S.; Hua, K.; Rui, Z.; Wu, Y.; et al. Cobalt-Doping Stabilized Active and Durable Sub-2 nm Pt Nanoclusters for Low-Pt-Loading PEMFC Cathode. Adv. Energy Mater. 2022, 12, 2103144. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Zhang, W.; Luo, M.; Tao, L.; Sun, Y.; Xia, Z.; Chao, Y.; Yin, K.; Zhang, Q.; et al. Sub-Monolayer YOx/MoOx on Ultrathin Pt Nanowires Boosts Alcohol Oxidation Electrocatalysis. Adv. Mater. 2021, 33, 2103762. [Google Scholar] [CrossRef]
- Jin, C.; Wang, Q.; Liu, J. Pb Induced Dislocation Defects of PtCo Systems: Strain-Triggered Oxygen Reduction Reaction for PEMFC. Nano Res. 2023. [Google Scholar] [CrossRef]
- Huang, Y.; Li, M.; Yang, W.; Yu, Y.; Hao, S. Ce-Doped Ordered Mesoporous Cobalt Ferrite Phosphides as Robust Catalysts for Water Oxidation. Chem. Eur. J. 2020, 26, 13305–13310. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, M.; He, L.; Geng, S.; Tian, F.; Song, Y.; Yang, W.; Yu, Y. Industrially Promising NiCoP Nanorod Arrays Tailored with Trace W and Mo Atoms for Boosting Large-Current-Density Overall Water Splitting. Nanoscale 2021, 13, 14179–14185. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Xia, Z.; Yang, Y.; Luo, M.; Huang, Y.; Sun, Y.; Chao, Y.; Yang, W.; Yang, W.; et al. Lavender-Like Ga-Doped Pt3Co Nanowires for Highly Stable and Active Electrocatalysis. ACS Catal. 2020, 10, 3018–3026. [Google Scholar] [CrossRef]
- Zhang, S.; Saji, S.E.; Yin, Z.; Zhang, H.; Du, Y.; Yan, C.-H. Rare-Earth Incorporated Alloy Catalysts: Synthesis, Properties, and Applications. Adv. Mater. 2021, 33, 2005988. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, L.-F.; Li, G.; Lu, B.-A.; Zhou, Z.-Y.; Tian, N.; Sun, S.-G. Improved Stability of Octahedral PtCu by Rh Doping for the Oxygen Reduction Reaction. ChemElectroChem 2021, 8, 2425–2430. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, Z.; Cao, L.; Chen, Y.; Zhu, E.; Lin, Z.; Li, M.; Yan, A.; Zettl, A.; Wang, Y.M.; et al. High-Performance Transition Metal-Doped Pt3Ni Octahedra for Oxygen Reduction Reaction. Science 2015, 348, 1230–1234. [Google Scholar] [CrossRef]
- Huang, H.; Li, K.; Chen, Z.; Luo, L.; Gu, Y.; Zhang, D.; Ma, C.; Si, R.; Yang, J.; Peng, Z.; et al. Achieving Remarkable Activity and Durability toward Oxygen Reduction Reaction Based on Ultrathin Rh-Doped Pt Nanowires. J. Am. Chem. Soc. 2017, 139, 8152–8159. [Google Scholar] [CrossRef]
- Beermann, V.; Gocyla, M.; Willinger, E.; Rudi, S.; Heggen, M.; Dunin-Borkowski, R.E.; Willinger, M.-G.; Strasser, P. Rh-Doped Pt-Ni Octahedral Nanoparticles: Understanding the Correlation Between Elemental Distribution, Oxygen Reduction Reaction, and Shape Stability. Nano Lett. 2016, 16, 1719–1725. [Google Scholar] [CrossRef]
- Lim, J.; Shin, H.; Kim, M.; Lee, H.; Lee, K.-S.; Kwon, Y.; Song, D.; Oh, S.; Kim, H.; Cho, E. Ga-Doped Pt-Ni Octahedral Nanoparticles as a Highly Active and Durable Electrocatalyst for Oxygen Reduction Reaction. Nano Lett. 2018, 18, 2450–2458. [Google Scholar] [CrossRef]
- Tu, W.; Chen, K.; Zhu, L.; Zai, H.; Bin, E.; Ke, X.; Chen, C.; Sui, M.; Chen, Q.; Li, Y. Tungsten-Doping-Induced Surface Reconstruction of Porous Ternary Pt-Based Alloy Electrocatalyst for Oxygen Reduction. Adv. Funct. Mater. 2019, 29, 1807070. [Google Scholar] [CrossRef]
- Wang, H.; Gao, J.; Chen, C.; Zhao, W.; Zhang, Z.; Li, D.; Chen, Y.; Wang, C.; Zhu, C.; Ke, X.; et al. PtNi-W/C with Atomically Dispersed Tungsten Sites Toward Boosted ORR in Proton Exchange Membrane Fuel Cell Devices. Nano-Micro Lett. 2023, 15, 143. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Luo, W.; Chen, C.; Chen, K.; Zhu, E.; Zhao, Z.; Wang, Z.; Hu, T.; Zai, H.; Ke, X.; et al. Tungsten as “Adhesive” in Pt2CuW0.25 Ternary Alloy for Highly Durable Oxygen Reduction Electrocatalysis. Adv. Funct. Mater. 2020, 30, 1908230. [Google Scholar] [CrossRef]
- Zhu, S.; Sun, M.; Mei, B.; Yang, L.; Chu, Y.; Shi, Z.; Bai, J.; Wang, X.; Jiang, Z.; Liu, C.; et al. Intrinsic Spin Shielding Effect in Platinum-Rare-Earth Alloy Boosts Oxygen Reduction Activity. Natl. Sci. Rev. 2023, 10, nwad162. [Google Scholar] [CrossRef]
- Liu, Y.; Sheng, S.; Wu, M.; Wang, S.; Wang, Y.; Yang, H.; Chen, J.; Hao, X.; Zhi, C.; Wang, Y.; et al. Controllable Synthesis of PtIrCu Ternary Alloy Ultrathin Nanowires for Enhanced Ethanol Electrooxidation. ACS Appl. Mater. Interfaces 2023, 15, 3934–3940. [Google Scholar] [CrossRef]
- Cao, J.; Cao, H.; Wang, F.; Zhu, H. Zigzag PtCo Nanowires Modified in situ with Au Atoms as Efficient and Durable Electrocatalyst for Oxygen Reduction Reaction. J. Power Sources 2021, 489, 229425. [Google Scholar] [CrossRef]
- Greeley, J.; Stephens, I.E.L.; Bondarenko, A.S.; Johansson, T.P.; Hansen, H.A.; Jaramillo, T.F.; Rossmeisl, J.; Chorkendorff, I.; Nørskov, J.K. Alloys of Platinum and Early Transition Metals as Oxygen Reduction Electrocatalysts. Nat. Chem. 2009, 1, 552–556. [Google Scholar] [CrossRef]
- Gao, L.; Sun, T.; Tan, X.; Liu, M.; Xue, F.; Wang, B.; Zhang, J.; Lu, Y.-F.; Ma, C.; Tian, H.; et al. Trace Doping of Early Transition Metal Enabled Efficient and Durable Oxygen Reduction Catalysis on Pt-Based Ultrathin Nanowires. Appl. Catal. B 2022, 303, 120918. [Google Scholar] [CrossRef]
- Chao, T.; Luo, X.; Zhu, M.; Hu, Y.; Zhang, Y.; Qu, Y.; Peng, H.; Shen, X.; Zheng, X.; Zhang, L.; et al. The Promoting Effect of Interstitial Hydrogen on the Oxygen Reduction Performance of PtPd Alloy Nanotubes for Fuel Cells. Nano Res. 2023, 16, 2366–2372. [Google Scholar] [CrossRef]
- Li, Z.; Niu, W.; Yang, Z.; Zaman, N.; Samarakoon, W.; Wang, M.; Kara, A.; Lucero, M.; Vyas, M.V.; Cao, H.; et al. Stabilizing Atomic Pt with Trapped Interstitial F in Alloyed PtCo Nanosheets for High-Performance Zinc-Air Batteries. Energy Environ. Sci. 2020, 13, 884–895. [Google Scholar] [CrossRef]
- Mao, Z.; Ding, C.; Liu, X.; Zhang, Q.; Qin, X.; Li, H.; Yang, F.; Li, Q.; Zhang, X.-G.; Zhang, J.; et al. Interstitial B-Doping in Pt Lattice to Upgrade Oxygen Electroreduction Performance. ACS Catal. 2022, 12, 8848–8856. [Google Scholar] [CrossRef]
- Shi, F.; Peng, J.; Li, F.; Qian, N.; Shan, H.; Tao, P.; Song, C.; Shang, W.; Deng, T.; Zhang, H.; et al. Design of Highly Durable Core-Shell Catalysts by Controlling Shell Distribution Guided by in-situ Corrosion Study. Adv. Mater. 2021, 33, 2101511. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Sasaki, K. Advanced Pt-Based Core-Shell Electrocatalysts for Fuel Cell Cathodes. Acc. Chem. Res. 2022, 55, 1226–1236. [Google Scholar] [CrossRef] [PubMed]
- Gong, S.; Zhang, Y.-X.; Niu, Z. Recent Advances in Earth-Abundant Core/Noble-Metal Shell Nanoparticles for Electrocatalysis. ACS Catal. 2020, 10, 10886–10904. [Google Scholar] [CrossRef]
- Li, M.; Zhao, Z.; Xia, Z.; Luo, M.; Zhang, Q.; Qin, Y.; Tao, L.; Yin, K.; Chao, Y.; Gu, L.; et al. Exclusive Strain Effect Boosts Overall Water Splitting in PdCu/Ir Core/Shell Nanocrystals. Angew. Chem. Int. Ed. 2021, 60, 8243–8250. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.-P.; Shan, S.; Zang, S.-Q.; Zhong, C.-J. Dynamic Core-Shell and Alloy Structures of Multimetallic Nanomaterials and Their Catalytic Synergies. Acc. Chem. Res. 2020, 53, 2913–2924. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, K.; Kuttiyiel, K.A.; Adzic, R.R. Designing High Performance Pt Monolayer Core-Shell Electrocatalysts for Fuel Cells. Curr. Opin. Electrochem. 2020, 21, 368–375. [Google Scholar] [CrossRef]
- Sasaki, K.; Naohara, H.; Choi, Y.; Cai, Y.; Chen, W.-F.; Liu, P.; Adzic, R.R. Highly Stable Pt Monolayer on PdAu Nanoparticle Electrocatalysts for the Oxygen Reduction Reaction. Nat. Commun. 2012, 3, 1115. [Google Scholar] [CrossRef]
- Zhao, F.; Zheng, L.; Yuan, Q.; Zhang, Q.; Sheng, T.; Yang, X.; Gu, L.; Wang, X. PtCu Subnanoclusters Epitaxial on Octahedral PtCu/Pt Skin Matrix as Ultrahigh Stable Cathode Electrocatalysts for Room-Temperature Hydrogen Fuel Cells. Nano Res. 2023, 16, 2252–2258. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, S.; Wu, W.; Chen, R.; Zhu, Y.; Jiang, H.; Yu, L.; Cheng, N. Tailored Lattice Compressive Strain of Pt-Skins by the L12-Pt3M Intermetallic Core for Highly Efficient Oxygen Reduction. Adv. Mater. 2023, 35, 2301310. [Google Scholar] [CrossRef]
- Wang, G.; Huang, B.; Xiao, L.; Ren, Z.; Chen, H.; Wang, D.; Abruña, H.D.; Lu, J.; Zhuang, L. Pt Skin on AuCu Intermetallic Substrate: A Strategy to Maximize Pt Utilization for Fuel Cells. J. Am. Chem. Soc. 2014, 136, 9643–9649. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Sun, Y.; Zhang, X.; Qin, Y.; Li, M.; Li, Y.; Li, C.; Yang, Y.; Wang, L.; Gao, P.; et al. Stable High-Index Faceted Pt Skin on Zigzag-Like PtFe Nanowires Enhances Oxygen Reduction Catalysis. Adv. Mater. 2018, 30, 1705515. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lyu, Z.; Chen, Z.; Zhu, S.; Shi, Y.; Chen, R.; Xie, M.; Yao, Y.; Chi, M.; Shao, M.; et al. Maximizing the Catalytic Performance of Pd@AuxPd1-x Nanocubes in H2O2 Production by Reducing Shell Thickness to Increase Compositional Stability. Angew. Chem. Int. Ed. 2021, 60, 19643–19647. [Google Scholar] [CrossRef] [PubMed]
- Kuttiyiel, K.A.; Sasaki, K.; Choi, Y.; Su, D.; Liu, P.; Adzic, R.R. Nitride Stabilized PtNi Core-Shell Nanocatalyst for High Oxygen Reduction Activity. Nano Lett. 2012, 12, 6266–6271. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Kuttiyiel, K.A.; Kim, K.-H.; Jang, J.; Lee, H.J.; Lee, J.M.; Seo, M.H.; Yang, T.-H.; Yim, S.-D.; Vargas, J.A.; et al. High Pressure Nitrogen-Infused Ultrastable Fuel Cell Catalyst for Oxygen Reduction Reaction. ACS Catal. 2021, 11, 5525–5531. [Google Scholar] [CrossRef]
- Guo, H.; Li, L.; Chen, Y.; Zhang, W.; Shang, C.; Cao, X.; Li, M.; Zhang, Q.; Tan, H.; Nie, Y.; et al. Precise Strain Tuning Boosts Electrocatalytic Hydrogen Generation. Adv. Mater. 2023, 35, 2302285. [Google Scholar] [CrossRef] [PubMed]
- Kluge, R.M.; Haid, R.W.; Riss, A.; Bao, Y.; Seufert, K.; Schmidt, T.O.; Watzele, S.A.; Barth, J.V.; Allegretti, F.; Auwärter, W.; et al. A Trade-Off Between Ligand and Strain Effects Optimizes the Oxygen Reduction Activity of Pt Alloys. Energy Environ. Sci. 2022, 15, 5181–5191. [Google Scholar] [CrossRef]
- Li, M.; Luo, M.; Xia, Z.; Yang, Y.; Huang, Y.; Wu, D.; Sun, Y.; Li, C.; Chao, Y.; Yang, W.; et al. Modulating the Surface Segregation of PdCuRu Nanocrystals for Enhanced All-pH Hydrogen Evolution Electrocatalysis. J. Mater. Chem. A 2019, 7, 20151–20157. [Google Scholar] [CrossRef]
- Adit Maark, T.; Peterson, A.A. Understanding Strain and Ligand Effects in Hydrogen Evolution over Pd(111) Surfaces. J. Phy. Chem. C 2014, 118, 4275–4281. [Google Scholar] [CrossRef]
- Liu, M.; Xin, H.; Wu, Q. Unusual Strain Effect of a Pt-Based L10 Face-Centered Tetragonal Core in Core/Shell Nanoparticles for the Oxygen Reduction Reaction. Phys. Chem. Chem. Phys. 2019, 21, 6477–6484. [Google Scholar] [CrossRef]
- Guo, L.-M.; Zhang, D.-F.; Guo, L. Structure Design Reveals the Role of Au for ORR Catalytic Performance Optimization in PtCo-Based Catalysts. Adv. Funct. Mater. 2020, 30, 2001575. [Google Scholar] [CrossRef]
- Luo, M.; Yang, Y.; Sun, Y.; Qin, Y.; Li, C.; Li, Y.; Li, M.; Zhang, S.; Su, D.; Guo, S. Ultrathin Two-Dimensional Metallic Nanocrystals for Renewable Energy Electrocatalysis. Mater. Today 2019, 23, 45–56. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, J.; Wei, Y.; Wu, Y.; Xia, D.; Gan, L. Engineering Nanoporous and Solid Core-Shell Architectures of Low-Platinum Alloy Catalysts for High Power Density PEM Fuel Cells. Nano Res. 2022, 15, 6148–6155. [Google Scholar] [CrossRef]
- Tao, L.; Huang, B.; Jin, F.; Yang, Y.; Luo, M.; Sun, M.; Liu, Q.; Gao, F.; Guo, S. Atomic PdAu Interlayer Sandwiched into Pd/Pt Core/Shell Nanowires Achieves Superstable Oxygen Reduction Catalysis. ACS Nano 2020, 14, 11570–11578. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zheng, C.Y.; Shen, B.; Mirkin, C.A. High-Index-Facet Metal-Alloy Nanoparticles as Fuel Cell Electrocatalysts. Adv. Mater. 2020, 32, 2002849. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Sun, Y.; Qin, Y.; Chen, S.; Li, Y.; Li, C.; Yang, Y.; Wu, D.; Xu, N.; Xing, Y.; et al. Surface and Near-Surface Engineering of PtCo Nanowires at Atomic Scale for Enhanced Electrochemical Sensing and Catalysis. Chem. Mater. 2018, 30, 6660–6667. [Google Scholar] [CrossRef]
- Jin, H.; Xu, Z.; Hu, Z.-Y.; Yin, Z.; Wang, Z.; Deng, Z.; Wei, P.; Feng, S.; Dong, S.; Liu, J.; et al. Mesoporous Pt@Pt-Skin Pt3Ni Core-Shell Framework Nanowire Electrocatalyst for Efficient Oxygen Reduction. Nat. Commun. 2023, 14, 1518. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, N.; Guo, S.; Zhang, X.; Li, J.; Yao, J.; Wu, T.; Lu, G.; Ma, J.-Y.; Su, D.; et al. Biaxially Strained PtPb/Pt Core/Shell Nanoplate Boosts Oxygen Reduction Catalysis. Science 2016, 354, 1410–1414. [Google Scholar] [CrossRef]
- Gao, P.; Pu, M.; Chen, Q.; Zhu, H. Pt-Based Intermetallic Nanocrystals in Cathode Catalysts for Proton Exchange Membrane Fuel Cells: From Precise Synthesis to Oxygen Reduction Reaction Strategy. Catalysts 2021, 11, 1050. [Google Scholar] [CrossRef]
- Li, J.; Sun, S. Intermetallic Nanoparticles: Synthetic Control and Their Enhanced Electrocatalysis. Acc. Chem. Res. 2019, 52, 2015–2025. [Google Scholar] [CrossRef]
- Lin, F.; Li, M.; Zeng, L.; Luo, M.; Guo, S. Intermetallic Nanocrystals for Fuel-Cells-Based Electrocatalysis. Chem. Rev. 2023, 123, 12507–12593. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, C.; Fang, J. Noble-Metal Based Random Alloy and Intermetallic Nanocrystals: Syntheses and Applications. Chem. Rev. 2021, 121, 736–795. [Google Scholar] [CrossRef]
- Xiao, W.; Lei, W.; Gong, M.; Xin, H.L.; Wang, D. Recent Advances of Structurally Ordered Intermetallic Nanoparticles for Electrocatalysis. ACS Catal. 2018, 8, 3237–3256. [Google Scholar] [CrossRef]
- Li, M.; Xia, Z.; Huang, Y.; Tao, L.; Chao, Y.; Yin, K.; Yang, W.; Yang, W.; Yu, Y.; Guo, S. Rh-Doped PdCu Ordered Intermetallics for Enhanced Oxygen Reduction Electrocatalysis with Superior Methanol Tolerance. Acta Phys.-Chin. Sin. 2020, 36, 1912049. [Google Scholar]
- Liao, Y.; Peng, L.; Wu, C.; Yan, Y.; Xie, H.; Chen, Y.; Wang, Y. Size and Near-Surface Engineering in Weak-Oxidative Confined Space to Fabricate 4 nm L10-PtCo@Pt Nanoparticles for Oxygen Reduction Reaction. Nano Res. 2023, 16, 6622–6631. [Google Scholar] [CrossRef]
- Feng, G.; Ning, F.; Pan, Y.; Chen, T.; Song, J.; Wang, Y.; Zou, R.; Su, D.; Xia, D. Engineering Structurally Ordered High-Entropy Intermetallic Nanoparticles with High-Activity Facets for Oxygen Reduction in Practical Fuel Cells. J. Am. Chem. Soc. 2023, 145, 11140–11150. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xi, Z.; Pan, Y.-T.; Spendelow, J.S.; Duchesne, P.N.; Su, D.; Li, Q.; Yu, C.; Yin, Z.; Shen, B.; et al. Fe Stabilization by Intermetallic L10-FePt and Pt Catalysis Enhancement in L10-FePt/Pt Nanoparticles for Efficient Oxygen Reduction Reaction in Fuel Cells. J. Am. Chem. Soc. 2018, 140, 2926–2932. [Google Scholar] [CrossRef]
- Li, J.; Sharma, S.; Liu, X.; Pan, Y.-T.; Spendelow, J.S.; Chi, M.; Jia, Y.; Zhang, P.; Cullen, D.A.; Xi, Z.; et al. Hard-Magnet L10-CoPt Nanoparticles Advance Fuel Cell Catalysis. Joule 2019, 3, 124–135. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Liang, J.; Li, S.; Lu, G.; Priest, C.; Wang, T.; Han, J.; Wu, G.; Wang, X.; et al. Inducing Covalent Atomic Interaction in Intermetallic Pt Alloy Nanocatalysts for High-Performance Fuel Cells. Angew. Chem. Int. Ed. 2023, 62, e202302134. [Google Scholar] [CrossRef]
- Kim, J.; Rong, C.; Liu, J.P.; Sun, S. Dispersible Ferromagnetic FePt Nanoparticles. Adv. Mater. 2009, 21, 906–909. [Google Scholar] [CrossRef]
- Wang, T.; Liang, J.; Zhao, Z.; Li, S.; Lu, G.; Xia, Z.; Wang, C.; Luo, J.; Han, J.; Ma, C.; et al. Sub-6 nm Fully Ordered L10-Pt-Ni-Co Nanoparticles Enhance Oxygen Reduction via Co Doping Induced Ferromagnetism Enhancement and Optimized Surface Strain. Adv. Energy Mater. 2019, 9, 1803771. [Google Scholar] [CrossRef]
- Cui, Z.; Chen, H.; Zhao, M.; Marshall, D.; Yu, Y.; Abruña, H.; DiSalvo, F.J. Synthesis of Structurally Ordered Pt3Ti and Pt3V Nanoparticles as Methanol Oxidation Catalysts. J. Am. Chem. Soc. 2014, 136, 10206–10209. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Ye, Y.; Jiang, W.; Li, J.; Tang, H.; Hu, J.; Du, L.; Cui, Z.; Liao, S. Mesoporous Carbon Confined Intermetallic Nanoparticles as Highly Durable Electrocatalysts for the Oxygen Reduction Reaction. J. Mater. Chem. A 2020, 8, 15822–15828. [Google Scholar] [CrossRef]
- Gong, Q.; Zhang, H.; Yu, H.; Jeon, S.; Ren, Y.; Yang, Z.; Sun, C.-J.; Stach, E.A.; Foucher, A.C.; Yu, Y.; et al. Amino-Tethering Synthesis Strategy Toward Highly Accessible Sub-3-nm L10-PtM Catalysts for High-Power Fuel Cells. Matter 2023, 6, 963–982. [Google Scholar] [CrossRef]
- Cheng, H.; Gui, R.; Yu, H.; Wang, C.; Liu, S.; Liu, H.; Zhou, T.; Zhang, N.; Zheng, X.; Chu, W.; et al. Subsize Pt-based Intermetallic Compound Enables Long-Term Cyclic Mass Activity for Fuel-Cell Oxygen Reduction. Proc. Natl. Acad. Sci. USA 2021, 118, e2104026118. [Google Scholar] [CrossRef]
- Liang, J.; Zhao, Z.; Li, N.; Wang, X.; Li, S.; Liu, X.; Wang, T.; Lu, G.; Wang, D.; Hwang, B.-J.; et al. Biaxial Strains Mediated Oxygen Reduction Electrocatalysis on Fenton Reaction Resistant L10-PtZn Fuel Cell Cathode. Adv. Energy Mater. 2020, 10, 2000179. [Google Scholar] [CrossRef]
- Yang, C.-L.; Wang, L.-N.; Yin, P.; Liu, J.; Chen, M.-X.; Yan, Q.-Q.; Wang, Z.-S.; Xu, S.-L.; Chu, S.-Q.; Cui, C.; et al. Sulfur-Anchoring Synthesis of Platinum Intermetallic Nanoparticle Catalysts for Fuel Cells. Science 2021, 374, 459–464. [Google Scholar] [CrossRef]
- Li, J.; Sharma, S.; Wei, K.; Chen, Z.; Morris, D.; Lin, H.; Zeng, C.; Chi, M.; Yin, Z.; Muzzio, M.; et al. Anisotropic Strain Tuning of L10 Ternary Nanoparticles for Oxygen Reduction. J. Am. Chem. Soc. 2020, 142, 19209–19216. [Google Scholar] [CrossRef]
- Yang, Z.; Yang, H.; Shang, L.; Zhang, T. Ordered PtFeIr Intermetallic Nanowires Prepared through a Silica-Protection Strategy for the Oxygen Reduction Reaction. Angew. Chem. Int. Ed. 2022, 61, e202113278. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, J.M.; Ha, Y.; Woo, J.; Byun, A.; Shin, T.J.; Park, K.H.; Jeong, H.Y.; Kim, H.; Kim, J.Y.; et al. Activity Origin and Multifunctionality of Pt-Based Intermetallic Nanostructures for Efficient Electrocatalysis. ACS Catal. 2019, 9, 11242–11254. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kwon, T.; Ha, Y.; Jun, M.; Baik, H.; Jeong, H.Y.; Kim, H.; Lee, K.; Joo, S.H. Intermetallic PtCu Nanoframes as Efficient Oxygen Reduction Electrocatalysts. Nano Lett. 2020, 20, 7413–7421. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Luo, M.; Sun, Y.; Li, C.; Huang, B.; Yang, Y.; Li, Y.; Wang, L.; Guo, S. Intermetallic hcp-PtBi/fcc-Pt Core/Shell Nanoplates Enable Efficient Bifunctional Oxygen Reduction and Methanol Oxidation Electrocatalysis. ACS Catal. 2018, 8, 5581–5590. [Google Scholar] [CrossRef]
- Lv, H.; Wang, Y.; Sun, L.; Yamauchi, Y.; Liu, B. A General Protocol for Precise Syntheses of Ordered Mesoporous Intermetallic Nanoparticles. Nat. Protoc. 2023, 18, 3126–3154. [Google Scholar] [CrossRef]
- Lv, H.; Zheng, Y.; Wang, Y.; Wang, J.; Liu, B.; Qiao, Z.-A. Ordered Mesoporous Intermetallic Ga-Pt Nanoparticles: Phase-Controlled Synthesis and Performance in Oxygen Reduction Electrocatalysis. Angew. Chem. Int. Ed. 2023, 62, e202304420. [Google Scholar] [CrossRef] [PubMed]
- Song, T.-W.; Chen, M.-X.; Yin, P.; Tong, L.; Zuo, M.; Chu, S.-Q.; Chen, P.; Liang, H.-W. Intermetallic PtFe Electrocatalysts for the Oxygen Reduction Reaction: Ordering Degree-Dependent Performance. Small 2022, 18, 2202916. [Google Scholar] [CrossRef]
- Chen, H.-Q.; Ze, H.; Yue, M.-F.; Wei, D.-Y.; Wu, Y.-F.; Dong, J.-C.; Zhang, Y.-J.; Zhang, H.; Tian, Z.-Q.; Li, J.-F.; et al. Unmasking the Critical Role of the Ordering Degree of Bimetallic Nanocatalysts on Oxygen Reduction Reaction by in situ Raman Spectroscopy. Angew. Chem. Int. Ed. 2022, 61, e202117834. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, N.; Liu, H.; Ma, W.; Hippalgaonkar, K.; Liu, Z.; Huang, Y. Ordering-Dependent Hydrogen Evolution and Oxygen Reduction Electrocatalysis of High-Entropy Intermetallic Pt4FeCoCuNi. Adv. Mater. 2023, 35, 2302067. [Google Scholar] [CrossRef]
- Shao, R.-Y.; Xu, X.-C.; Zhou, Z.-H.; Zeng, W.-J.; Song, T.-W.; Yin, P.; Li, A.; Ma, C.-S.; Tong, L.; Kong, Y.; et al. Promoting Ordering Degree of Intermetallic Fuel Cell Catalysts by Low-Melting-Point Metal Doping. Nat. Commun. 2023, 14, 5896. [Google Scholar] [CrossRef]
- Guan, J.; Yang, S.; Liu, T.; Yu, Y.; Niu, J.; Zhang, Z.; Wang, F. Intermetallic FePt@PtBi Core-Shell Nanoparticles for Oxygen Reduction Electrocatalysis. Angew. Chem. Int. Ed. 2021, 60, 21899–21904. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Wan, H.; Wu, T.; Shu, D.; Shen, L.; Wang, Y.; Ruterana, P.; Lund, P.D.; Wang, H. Core/Shell Cu/FePtCu Nanoparticles with Face-Centered Tetragonal Texture: An Active and Stable Low-Pt Catalyst for Enhanced Oxygen Reduction. Nano Energy 2018, 54, 280–287. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, Q.; Brozena, A.; Luo, J.; Miao, J.; Chi, M.; Wang, C.; Kevrekidis, I.G.; Ren, Z.J.; Greeley, J.; et al. High-Entropy Nanoparticles: Synthesis-Structure-Property Relationships and Data-Driven Discovery. Science 2022, 376, eabn3103. [Google Scholar] [CrossRef]
- Han, X.; Wu, G.; Zhao, S.; Guo, J.; Yan, M.; Hong, X.; Wang, D. Nanoscale High-Entropy Alloy for Electrocatalysis. Matter 2023, 6, 1717–1751. [Google Scholar] [CrossRef]
- Li, H.; Zhu, H.; Zhang, S.; Zhang, N.; Du, M.; Chai, Y. Nano High-Entropy Materials: Synthesis Strategies and Catalytic Applications. Small Struct. 2020, 1, 2000033. [Google Scholar] [CrossRef]
- Wang, X.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Electronic and Structural Engineering of Carbon-Based Metal-Free Electrocatalysts for Water Splitting. Adv. Mater. 2019, 31, 1803625. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Chen, Z.W.; Singh, C.V. Neural Network-Assisted Development of High-Entropy Alloy Catalysts: Decoupling Ligand and Coordination Effects. Matter 2020, 3, 1318–1333. [Google Scholar] [CrossRef]
- Ma, X.; Xin, H. Orbitalwise Coordination Number for Predicting Adsorption Properties of Metal Nanocatalysts. Phys. Rev. Lett. 2017, 118, 036101. [Google Scholar] [CrossRef] [PubMed]
- Calle-Vallejo, F.; Loffreda, D.; Koper, M.T.M.; Sautet, P. Introducing Structural Sensitivity into Adsorption-Energy Scaling Relations by Means of Coordination Numbers. Nat. Chem. 2015, 7, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Batchelor, T.A.A.; Pedersen, J.K.; Winther, S.H.; Castelli, I.E.; Jacobsen, K.W.; Rossmeisl, J. High-Entropy Alloys as a Discovery Platform for Electrocatalysis. Joule 2019, 3, 834–845. [Google Scholar] [CrossRef]
- Pedersen, J.K.; Clausen, C.M.; Krysiak, O.A.; Xiao, B.; Batchelor, T.A.A.; Löffler, T.; Mints, V.A.; Banko, L.; Arenz, M.; Savan, A.; et al. Bayesian Optimization of High-Entropy Alloy Compositions for Electrocatalytic Oxygen Reduction. Angew. Chem. Int. Ed. 2021, 60, 24144–24152. [Google Scholar] [CrossRef]
- Duan, J.; Wang, M.; Huang, R.; Miao, J.; Lu, Y.; Wang, T.; Li, T. A Novel High-Entropy Alloy with an Exceptional Combination of Soft Magnetic Properties and Corrosion Resistance. Sci. China Mater. 2023, 66, 772–779. [Google Scholar] [CrossRef]
- Yu, Y.; Xia, F.; Wang, C.; Wu, J.; Fu, X.; Ma, D.; Lin, B.; Wang, J.; Yue, Q.; Kang, Y. High-Entropy Alloy Nanoparticles as a Promising Electrocatalyst to Enhance Activity and Durability for Oxygen Reduction. Nano Res. 2022, 15, 7868–7876. [Google Scholar] [CrossRef]
- Zhang, W.; Feng, X.; Mao, Z.X.; Li, J.; Wei, Z. Stably Immobilizing Sub-3 nm High-Entropy Pt Alloy Nanocrystals in Porous Carbon as Durable Oxygen Reduction Electrocatalyst. Adv. Funct. Mater. 2022, 32, 2204110. [Google Scholar] [CrossRef]
- Zhan, C.; Xu, Y.; Bu, L.; Zhu, H.; Feng, Y.; Yang, T.; Zhang, Y.; Yang, Z.; Huang, B.; Shao, Q.; et al. Subnanometer High-Entropy Alloy Nanowires Enable Remarkable Hydrogen Oxidation Catalysis. Nat. Commun. 2021, 12, 6261. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhang, J.; Zhan, S.; Xia, F.; Wang, C.; Ma, D.; Yue, Q.; Wu, J.; Kang, Y. High-Entropy Alloy Nanosheets for Fine-Tuning Hydrogen Evolution. ACS Catal. 2022, 12, 11955–11959. [Google Scholar] [CrossRef]
- Jin, Z.; Lyu, J.; Zhao, Y.-L.; Li, H.; Lin, X.; Xie, G.; Liu, X.; Kai, J.-J.; Qiu, H.-J. Rugged High-Entropy Alloy Nanowires with in situ Formed Surface Spinel Oxide as Highly Stable Electrocatalyst in Zn-Air Batteries. ACS Mater. Lett. 2020, 2, 1698–1706. [Google Scholar] [CrossRef]
- Han, G.; Li, M.; Liu, H.; Zhang, W.; He, L.; Tian, F.; Liu, Y.; Yu, Y.; Yang, W.; Guo, S. Short-Range Diffusion Enables General Synthesis of Medium-Entropy Alloy Aerogels. Adv. Mater. 2022, 34, 2202943. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Sun, M.; Zhou, Y.; Luo, M.; Lv, F.; Li, M.; Zhang, Q.; Gu, L.; Huang, B.; Guo, S. A General Synthetic Method for High-Entropy Alloy Subnanometer Ribbons. J. Am. Chem. Soc. 2022, 144, 10582–10590. [Google Scholar] [CrossRef]
- Zhang, Q.; Shen, T.; Song, M.; Wang, S.; Zhang, J.; Huang, X.; Lu, S.; Wang, D. High-Entropy L12-Pt(FeCoNiCuZn)3 Intermetallics for Ultrastable Oxygen Reduction Reaction. J. Energy Chem. 2023, 86, 158–166. [Google Scholar] [CrossRef]
- Shao, Q.; Wang, P.; Huang, X. Opportunities and Challenges of Interface Engineering in Bimetallic Nanostructure for Enhanced Electrocatalysis. Adv. Funct. Mater. 2019, 29, 1806419. [Google Scholar] [CrossRef]
- Guo, X.; Li, M.; Qiu, L.; Tian, F.; He, L.; Geng, S.; Liu, Y.; Song, Y.; Yang, W.; Yu, Y. Engineering Electron Redistribution of Bimetallic Phosphates with CeO2 Enables High-Performance Overall Water Splitting. Chem. Eng. J. 2023, 453, 139796. [Google Scholar] [CrossRef]
- Gbadamasi, S.; Mohiuddin, M.; Krishnamurthi, V.; Verma, R.; Khan, M.W.; Pathak, S.; Kalantar-Zadeh, K.; Mahmood, N. Interface Chemistry of Two-Dimensional Heterostructures—Fundamentals to Applications. Chem. Soc. Rev. 2021, 50, 4684–4729. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Qian, G.; Chu, B.; Jiang, Z.; Tan, K.; Luo, L.; Li, B.; Yin, S. Tuning d-Band Center of Pt by PtCo-PtSn Heterostructure for Enhanced Oxygen Reduction Reaction Performance. Small 2022, 18, 2106773. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhou, P.; Zhao, Y.; Jiang, W.; Zhao, B.; Chen, X.; Li, M. Ultradurable Pt-Based Catalysts for Oxygen Reduction Electrocatalysis. Catalysts 2024, 14, 57. https://doi.org/10.3390/catal14010057
Li Z, Zhou P, Zhao Y, Jiang W, Zhao B, Chen X, Li M. Ultradurable Pt-Based Catalysts for Oxygen Reduction Electrocatalysis. Catalysts. 2024; 14(1):57. https://doi.org/10.3390/catal14010057
Chicago/Turabian StyleLi, Ziting, Peng Zhou, Yuxin Zhao, Wenyue Jiang, Bingxin Zhao, Xiaoshuang Chen, and Menggang Li. 2024. "Ultradurable Pt-Based Catalysts for Oxygen Reduction Electrocatalysis" Catalysts 14, no. 1: 57. https://doi.org/10.3390/catal14010057
APA StyleLi, Z., Zhou, P., Zhao, Y., Jiang, W., Zhao, B., Chen, X., & Li, M. (2024). Ultradurable Pt-Based Catalysts for Oxygen Reduction Electrocatalysis. Catalysts, 14(1), 57. https://doi.org/10.3390/catal14010057







