Increasing Al-Pair Abundance in SSZ-13 Zeolite via Zeolite Synthesis in the Presence of Alkaline Earth Metal Hydroxide Produces Hydrothermally Stable Co-, Cu- and Pd-SSZ-13 Materials
Abstract
1. Introduction
2. Results and Discussion
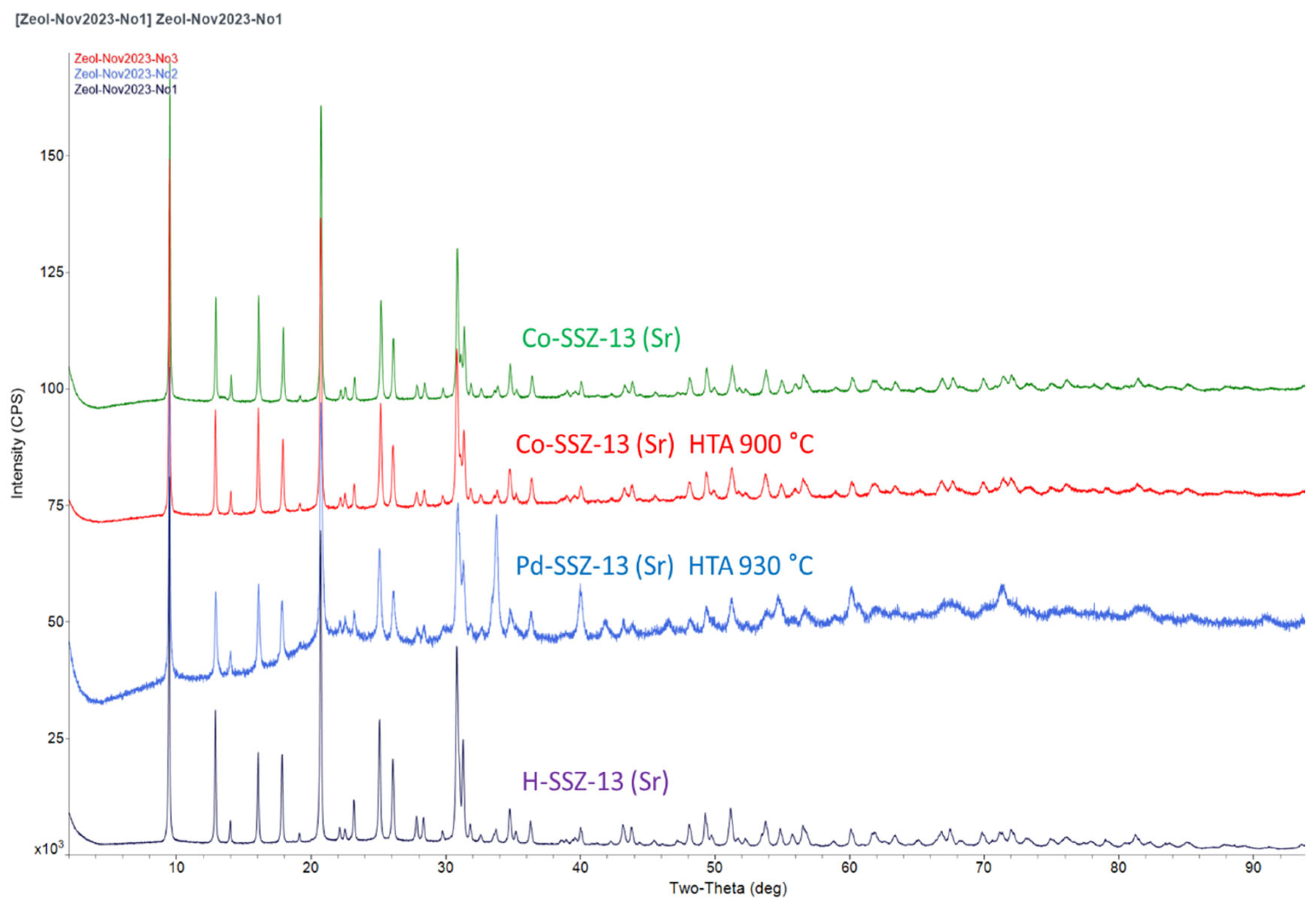
2.1. Formation of Highly Crystalline SSZ-13 Zeolite during Synthesis in the Presence of Sr(OH)2
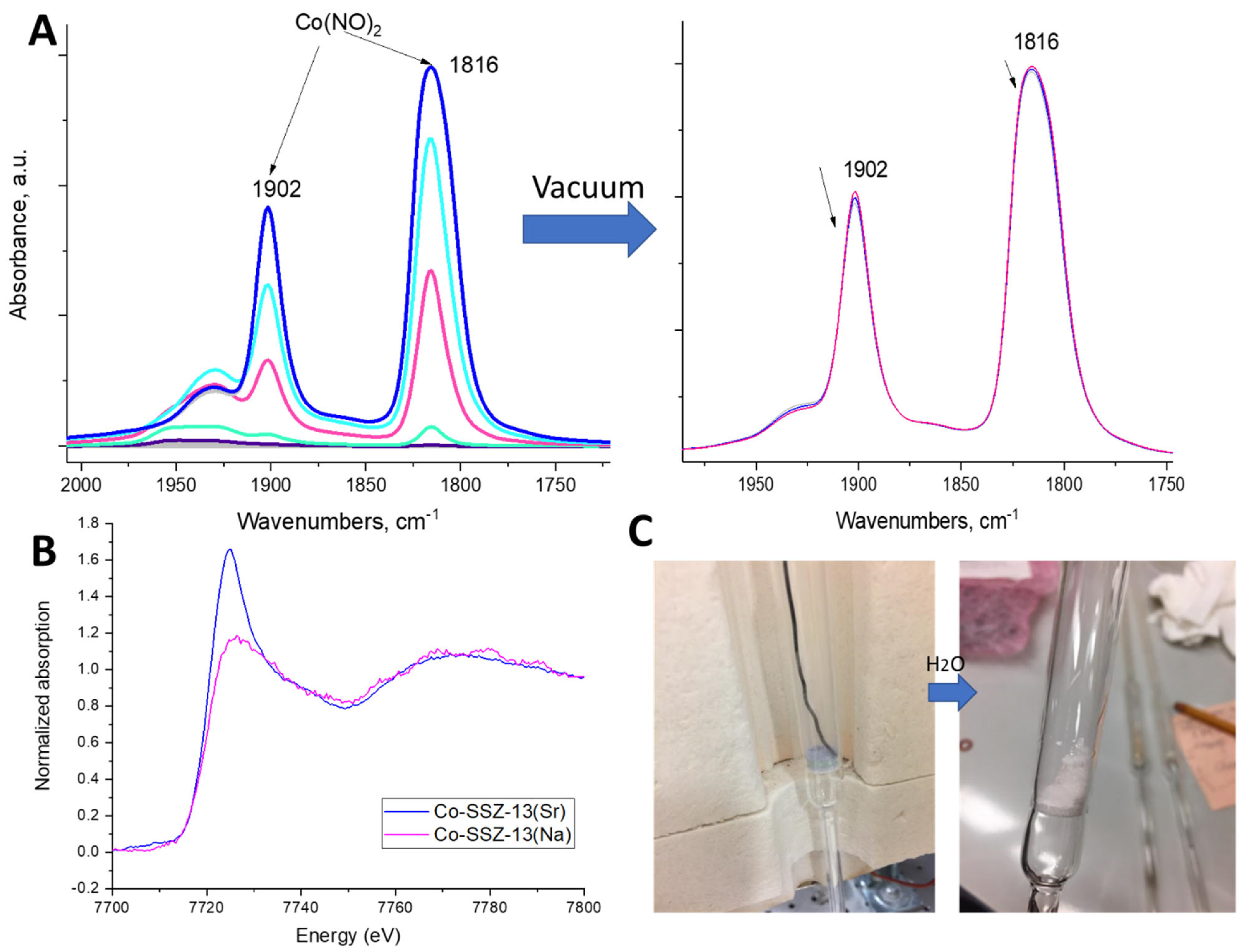
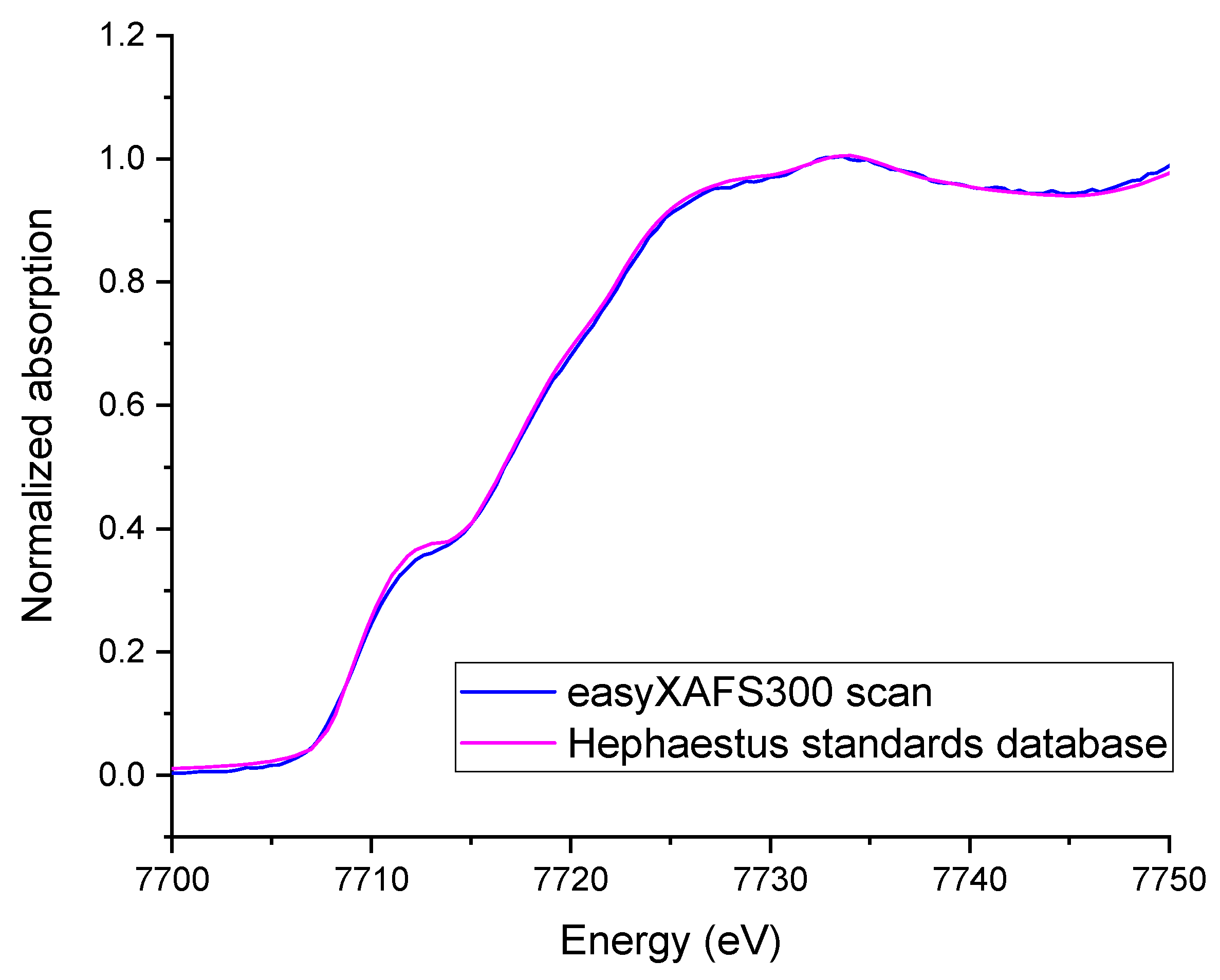
2.2. Probing Al Pairing with Co(II) Titration, NO Adsorption Measurement and Infra-Red Spectroscopy
2.3. Investigation of PNA Performance and Hydrothermal Stability of Co-SSZ-13 Materials
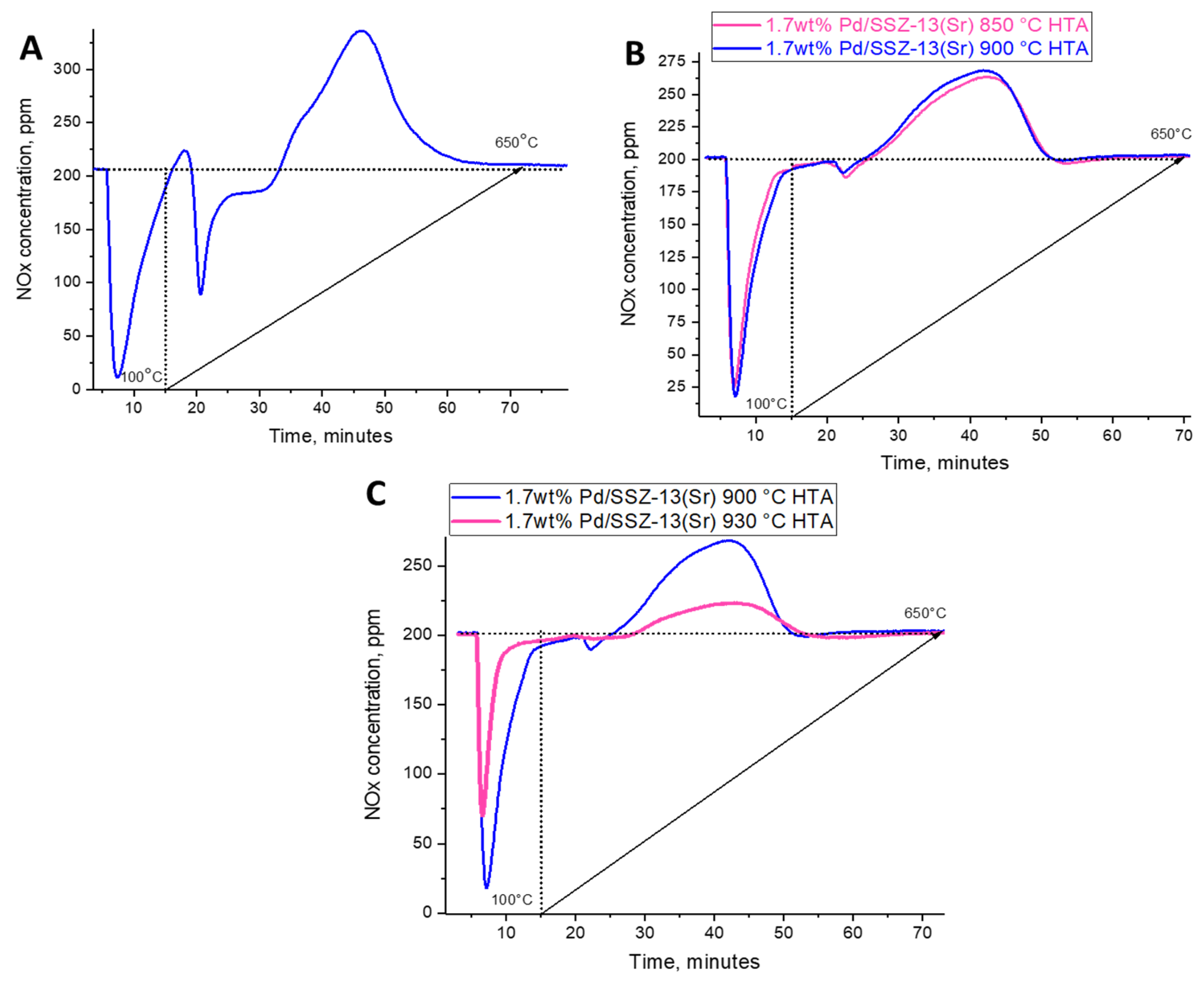
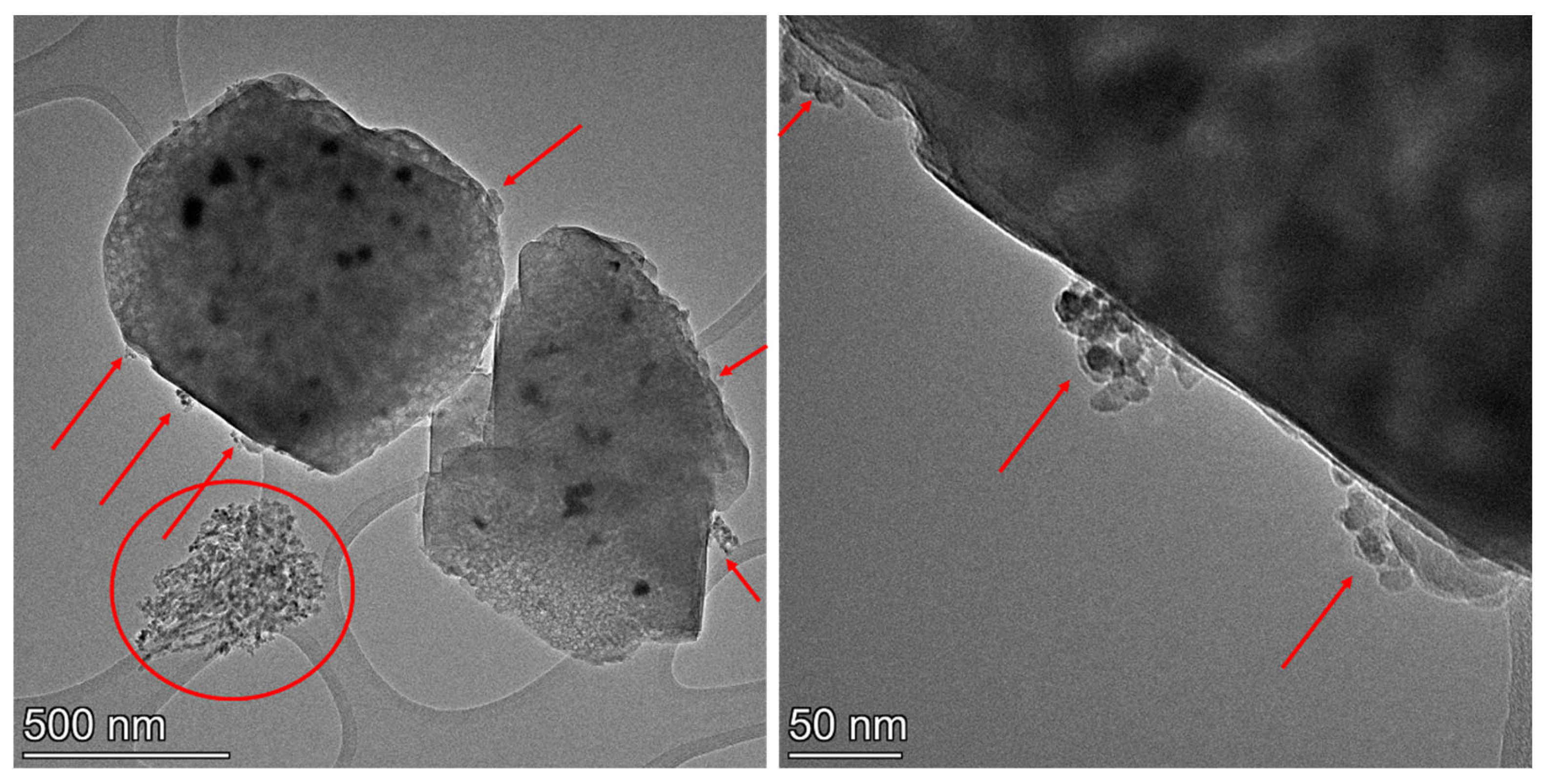
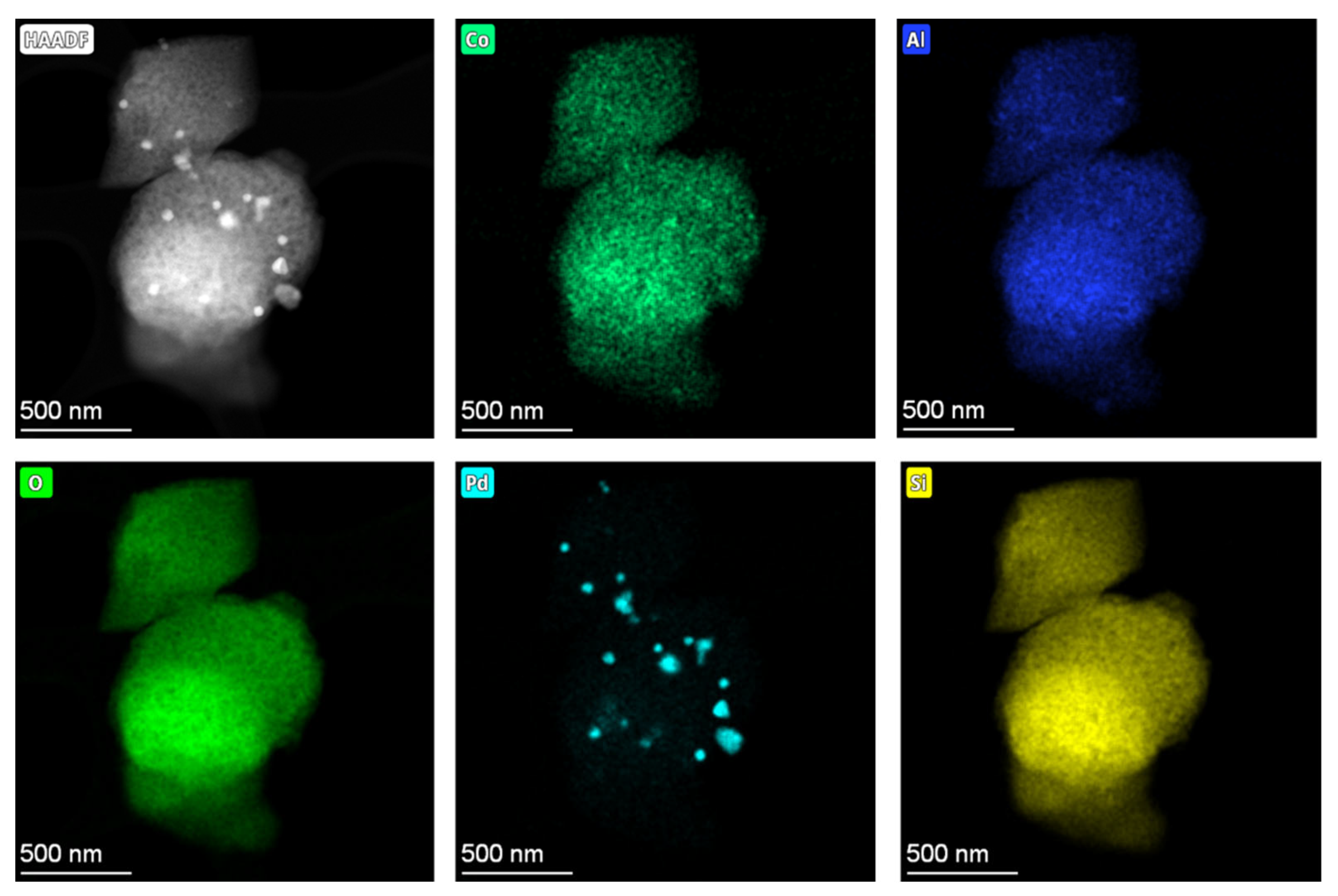
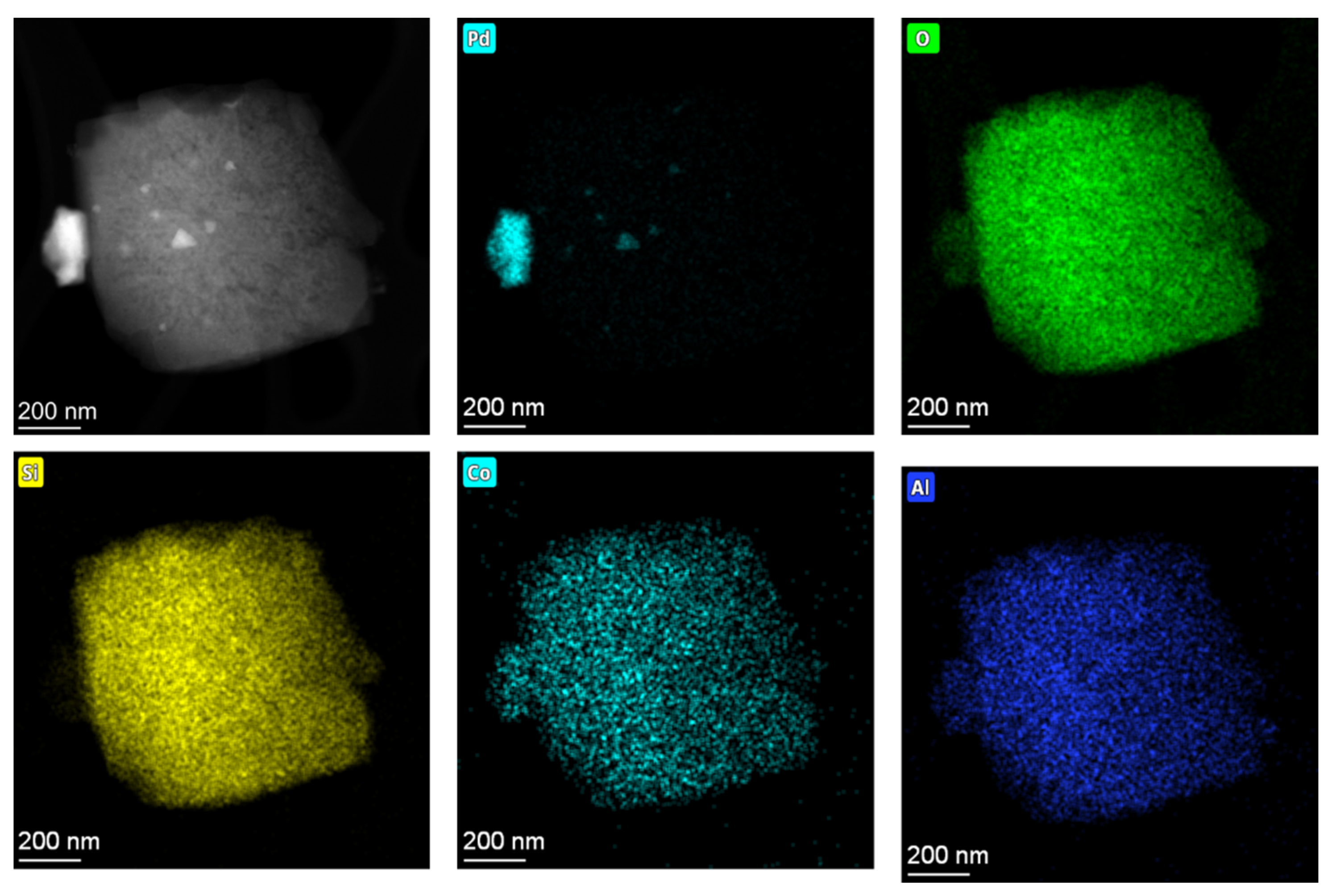
2.4. Investigation of Hydrothermal Stability and PNA Performance of Pd-Containing SSZ-13 Materials
2.5. Synthesis and Stability Evaluation of Cu/SSZ-13 Catalysts for SCR of Nitric Oxide
3. Experimental Methods
3.1. Surface Area and Pore Structure Characterization
3.2. Microscopy
3.3. X-ray Diffraction (XRD) Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F. Szanyi, Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials J. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Confining isolated atoms and clusters in crystalline porous materials for catalysis. Nat. Rev. Mater. 2020, 6, 244–263. [Google Scholar] [CrossRef]
- Zones, S.I. Zeolite SSZ-13 and Its Method of Preparation. US Patent 4544538, 1 October 1985. [Google Scholar]
- Kwak, J.-H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Bull, I.; Moini, A.; Koermer, G.; Patchett, J.; Jaglowski, W.; Roth, S. Zeolite Catalyst with Improved NOx Reduction in SCR. US Patent US20070134146A1, 2010. [Google Scholar]
- Fickel, D.W.; Lobo, R.F. Copper Coordination in Cu-SSZ-13 and Cu-SSZ-16 Investigated by Variable-Temperature XRD. J. Phys. Chem. C 2010, 114, 1633–1640. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Burton, S.D.; Szanyi, J.; Lee, J.H.; Peden, C.H.F. Effects of hydrothermal aging on NH3-SCR reaction over Cu/zeolites. J. Catal. 2012, 287, 203. [Google Scholar] [CrossRef]
- Song, I.; Koleva, I.Z.; Aleksandrov, H.A.; Chen, L.; Heo, J.; Li, D.; Wang, Y.; Szanyi, J.; Khivantsev, K. Ultrasmall Pd Clusters in FER Zeolite Alleviate CO Poisoning for Effective Low-Temperature Carbon Monoxide Oxidation. J. Am. Chem. Soc. 2023, 145, 50, 27493–27499 . [Google Scholar] [CrossRef]
- Royal College of Paediatrics and Child Health. Every Breath We Take—The Lifelong Impact of Air Pollution; Royal College of Paediatrics and Child Health: London, UK, 2016. [Google Scholar]
- Sachtler, W.M.H. Catalysis from Art to Science. In Surface Chemistry and Catalysis. Fundamental and Applied Catalysis; Carley, A.F., Davies, P.R., Hutchings, G.J., Spencer, M.S., Eds.; Springer: Boston, MA, USA, 2002. [Google Scholar]
- Khair, M.K.; Majewski, W.A. Diesel Emissions and Their Control; SAE International: Warrendale, PA, USA, 2006. [Google Scholar]
- Seiyama, T.; Arakawa, T.; Matsuda, T.; Yamazoe, N.; Takita, Y. Catalytic Reduction of Nitric Oxide with Ammonia over Transition Metal Ion-Exchanged Y Zeolites. Chem. Lett. 1975, 4, 781–784. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tran, D.; Szanyi, J.; Peden, C.H.F.; Lee, J.H. The Effect of Copper Loading on the Selective Catalytic Reduction of Nitric Oxide by Ammonia Over Cu-SSZ-13. Catal. Lett. 2012, 142, 295. [Google Scholar]
- Korhonen, S.T.; Fickel, D.W.; Lobo, R.F.; Weckhuysen, B.M.; Beale, A.M. Isolated Cu2+ ions: Active sites for selective catalytic reduction of NO. Chem. Commun. 2011, 47, 800–802. [Google Scholar] [CrossRef]
- Negri, C.; Selleri, T.; Borfecchia, E.; Martini, A.; Lomachenko, K.A.; Janssens, T.V.W.; Cutini, M.; Bordiga, S.; Berlier, G. Structureand Reactivity of Oxygen-Bridged Diamino Dicopper (II) Complexesin Cu-Ion-Exchanged Chabazite Catalyst for NH3-MediatedSelectiveCatalyticReduction. J. Am. Chem. Soc. 2020, 142, 15884–15896. [Google Scholar] [CrossRef] [PubMed]
- Kwak, J.H.; Varga, T.; Peden, C.H.F.; Gao, F.; Hanson, J.C.; Szanyi, J. Understanding NOx SCR Mechanisms and Activity on Cu/Chabazite Structures throughout the Catalyst Life Cycle. J. Catal. 2014, 314, 83–93. [Google Scholar] [CrossRef]
- Gao, F.; Mei, D.H.; Wang, Y.L.; Szanyi, J.; Peden, C.H.F. Selective Catalytic Reduction over Cu/SSZ-13: Linking Homo- and Heterogeneous Catalysis. J. Am. Chem. Soc. 2017, 139, 4935–4942. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Kollar, M.; Wang, Y.; Szanyi, J.; Peden CH, F. Understanding ammonia selective catalytic reduction kinetics over Cu/SSZ-13 from motion of the Cu ions. J. Catal. 2014, 319, 1–14. [Google Scholar] [CrossRef]
- Khivantsev, K.; Kwak, J.-H.; Jaegers, N.R.; Derewinski, M.A.; Szanyi, J. Identification of the Mechanism of NO Reduction with Ammonia (SCR) on Zeolite Catalysts. Chemrxiv 2020. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Aleksandrov, H.A.; Kovarik, L.; Derewinski, M.A.; Wang, Y.; Vayssilov, G.N.; Szanyi, J. Biomimetic CO oxidation below −100 °C by a nitrate-containing metal-free microporous system. Nat. Commun. 2021, 12, 6033. [Google Scholar] [CrossRef] [PubMed]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Derewinski, M.A.; Kwak, J.-H.; Szanyi, J. On the Nature of Extra-Framework Aluminum Species and Improved Catalytic Properties in Steamed Zeolites. Molecules 2022, 27, 2352. [Google Scholar] [CrossRef]
- Prodinger, S.; Derewinski, M.A.; Wang, Y.; Washton, N.M.; Walter, E.D.; Szanyi, J.; Gao, F.; Wang, Y.; Peden, C.H.F. Sub-micron Cu/SSZ-13: Synthesis and application as selective catalytic reduction (SCR) catalysts. Appl. Catal. B: Environ. 2017, 201, 461–469. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Collier, J.E.; Liu, D.; Mantarosie, L.; Durán-Martín, D.; Novák, V.; Rajaram, R.R.; Thompsett, D. Low Temperature NO Storage of Zeolite Supported Pd for Low Temperature Diesel Engine Emission Control. Catal. Lett. 2016, 146, 1706–1711. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Hanson, J.C.; Tao, F.; Tang, Y.; Zhang, X.; Koleva, I.Z.; Aleksandrov, H.A.; Vayssilov, G.N.; et al. Achieving Atomic Dispersion of Highly Loaded Transition Metals in Small-Pore Zeolite SSZ-13: High-Capacity and High-Efficiency Low-Temperature CO and Passive NOx Adsorbers. Angew. Chem. 2018, 130, 16914–16919. [Google Scholar]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Prodinger, S.; Derewinski, M.A.; Wang, Y.; Gao, F.; Szanyi, J. Palladium/Beta zeolite passive NOx adsorbers (PNA): Clarification of PNA chemistry and the effects of CO and zeolite crystallite size on PNA performance. Appl. Catal. A. Gen. 2019, 569, 141–148. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Koleva, I.Z.; Aleksandrov, H.A.; Kovarik, L.; Engelhard, M.; Gao, F.; Wang, Y.; Vayssilov, G.N.; Szanyi, J. Stabilization of Super Electrophilic Pd+2 Cations in Small-Pore SSZ-13 Zeolite/J. Phys. Chem. C 2020, 124, 309–321. [Google Scholar] [CrossRef]
- Khivantsev, K.; Gao, F.; Kovarik, L.; Wang, Y.; Szanyi, J. Molecular Level Understanding of How Oxygen and Carbon Monoxide Improve NOx Storage in Palladium/SSZ-13 Passive Nox Adsorbers: The Role of NO+ and Pd(II)(CO)(NO) Species. J. Phys. Chem. C 2018, 122, 10820–10827. [Google Scholar] [CrossRef]
- Moliner, M.; Corma, A. From metal-supported oxides to well-defined metal site zeolites: The next generation of passive NOx adsorbers for low-temperature control of emissions from diesel engines. React. Chem. Eng. 2019, 4, 223–234. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Hu, J.Z.; Gao, F.; Wang, Y.; Szanyi, J. Palladium/Zeolite Low Temperature Passive NOx Adsorbers (PNA): Structure-Adsorption Property Relationships for Hydrothermally Aged PNA Materials. Emiss. Control Sci. Technol. 2019, 6, 126–138. [Google Scholar] [CrossRef]
- Bello, E.; Margarit, V.J.; Gallego, E.M.; Schuetze, F.; Hengst, C.; Corma, A.; Molin, M. Deactivation and regeneration studies on Pd-containing medium pore zeolites as passive NOx adsorbers (PNAs) in cold-start applications. Microporous Mesoporous Mater. 2020, 302, 110222. [Google Scholar] [CrossRef]
- Khivantsev, K.; Jaegers, N.R.; Kovarik, L.; Wang, M.; Hu, J.Z.; Wang, Y.; Derewinski, M.A.; Szanyi, J. The superior hydrothermal stability of Pd/SSZ-39 in low temperature passive NOx adsorption (PNA) and methane combustion. Appl. Catal. B. 2021, 280, 119449. [Google Scholar] [CrossRef]
- Khivantsev, K.; Szanyi, J.; Jaegers, N.R.; Kovarik, L.; Gao, F.; Wang, Y. High-Capacity, Low-Temperature, Passive NOx and cd Adsorbers and Methods for Making Same. US Patent 16/546,641, 27 July 2021. [Google Scholar]
- Ryou, Y.S.; Lee, J.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D. Activation of Pd/SSZ-13 catalyst by hydrothermal aging treatment in passive NO adsorption performance at low temperature for cold start application. Appl. Catal. B Environ. 2017, 212, 140–149. [Google Scholar] [CrossRef]
- Ryou, Y.S.; Lee, J.; Lee, H.; Kim, C.H.; Kim, D.H. Effect of various activation conditions on the low temperature NO adsorption performance of Pd/SSZ-13 passive NOx adsorber. Catal. Today 2019, 320, 175–180. [Google Scholar] [CrossRef]
- Lee, J.; Ryou, Y.; Hwang, S.; Kim, Y.; Cho, S.J.; Lee, H.; Kim, C.H.; Kim, D.H. Comparative study of the mobility of Pd species in SSZ-13 and ZSM-5, and its implication for their activity as passive NOx adsorbers (PNAs) after hydro-thermal aging. Catal. Sci. Technol. 2019, 9, 163–173. [Google Scholar] [CrossRef]
- Kim, Y.; Hwang, S.; Lee, J.; Ryou, Y.S.; Lee, H.; Kim, C.H.; Kim, D.H. Comparison of NOx Adsorption/Desorption Behaviors over Pd/CeO2 and Pd/SSZ-13 as Passive NOx Adsorbers for Cold Start Application. Emiss. Control Sci. Technol. 2019, 5, 172–182. [Google Scholar] [CrossRef]
- Khivantsev, K.; Wei, X.; Kovarik, L.; Jaegers, N.R.; Waler, E.D.; Tran, P.; Wang, Y.; Szanyi, J. Palladium/Ferrierite versus Palladium/SSZ-13 Passive NOx Adsorbers: Adsorbate-Controlled Location of Atomically Dispersed Palladium(II) in Ferrierite Determines High Activity and Stability. Angew. Chem. Int. Ed. 2022, 61, e202107554. [Google Scholar] [CrossRef] [PubMed]
- Dedecek, J.; Sobalík, Z.; Wichterlova, B. Siting and Distribution of Framework Aluminium Atoms in Silicon-Rich Zeolites and Impact on Catalysis. Catal. Rev. Sci. Eng. 2012, 54, 135–223. [Google Scholar] [CrossRef]
- Pinar, A.B.; Gomez-Hortiguela, L.; Perez-Pariente, J. Cooperative Structure Directing Role of the Cage-Forming Tetramethylammonium Cation and the Bulkier Benzylmethylpyrrolidinium in the Synthesis of Zeolite Ferrierite. Chem. Mater. 2007, 19, 5617–5626. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Namba, S.; Kondo, J.N.; Tatsumi, T. Control of the Al Distribution in the Framework of ZSM-5 Zeolite and Its Evaluation by Solid-State NMR Technique and Catalytic Properties. J. Phys. Chem. C 2015, 119, 15303–15315. [Google Scholar] [CrossRef]
- Palčić, A.; Valtchev, V. Analysis and control of acid sites in zeolites. Appl. Catal. A Gen. 2020, 606, 117795. [Google Scholar] [CrossRef]
- Park, S.; Biligetu, T.; Wang, Y.; Nishitoba, T.; Kondo, J.N.; Yokoi, T. Acidic and catalytic properties of ZSM-5 zeolites with different Al distributions. Catal. Today 2018, 303, 64–70. [Google Scholar] [CrossRef]
- Yokoi, T.; Mochizuki, H.; Biligetu, T.; Wang, Y.; Tatsumi, T. Unique Al Distribution in the MFI Framework and Its Impact on Catalytic Properties. Chem. Lett. 2017, 46, 798–800. [Google Scholar] [CrossRef]
- Muraoka, K.; Chaikittisilp, W.; Okubo, T. Energy Analysis of Aluminosilicate Zeolites with Comprehensive Ranges of Framework Topologies, Chemical Compositions, and Aluminum Distributions. J. Am. Chem. Soc. 2016, 138, 6184–6193. [Google Scholar] [CrossRef]
- Dib, E.; Mineva, T.; Veron, E.; Sarou-Kanian, V.; Fayon, F.; Alonso, B. ZSM-5 Zeolite: Complete Al Bond Connectivity and Implications on Structure Formation from Solid-State NMR and Quantum Chemistry Calculations. J. Phys. Chem. Lett. 2018, 9, 19–24. [Google Scholar] [CrossRef]
- Biligetu, T.; Wang, Y.; Nishitoba, T.; Otomo, R.; Park, S.; Mochizuki, H.; Kondo, J.N.; Tatsumi, T.; Yokoi, T. Al distribution and catalytic performance of ZSM-5 zeolites synthesized with various alcohols. J. Catal. 2017, 353, 1–10. [Google Scholar] [CrossRef]
- Muraoka, K.; Chaikittisilp, W.; Yanaba, Y.; Yoshikawa, T.; Okubo, T. Directing Aluminum Atoms into Energetically Favorable Tetrahedral Sites in a Zeolite Framework by Using Organic Structure-Directing Agents. Angew. Chem. Int. Ed. 2018, 57, 3742–3746. [Google Scholar] [CrossRef] [PubMed]
- Pashkova, V.; Sklenak, S.; Klein, P.; Urbanova, M.; Dedecek, J. Location of Framework Al Atoms in the Channels of ZSM-5: Effect of the (Hydrothermal) Synthesis. J. Chem. Eur. J. 2016, 22, 3937–3941. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, J.; Beato, P.; Lundegaard, L.F.; Skibsted, J. Distribution of Aluminum over the Tetrahedral Sites in ZSM-5 Zeolites and Their Evolution after Steam Treatment. J Phys. Chem. C 2018, 122, 15595–15613. [Google Scholar] [CrossRef]
- Bucko, T.; Hafne, J. The role of spatial constraints and entropy in the adsorption and transformation of hydrocarbons catalyzed by zeolites. J. Catal. 2015, 329, 32–48. [Google Scholar] [CrossRef]
- Kwak, S.J.; Kwak, H.S.; Park, N.; Park, M.-J.; Lee, A.W.B. Recent progress on Al distribution over zeolite frameworks: Linking theories and experiments. Korean J. Chem. Eng. 2021, 38, 1117–1128. [Google Scholar] [CrossRef]
- Márquez-Alvarez, C.; Pinar, A.B.; García, R.; Grande-Casas, M.; Pérez-Pariente, J. Influence of Al Distribution and Defects Concentration of Ferrierite Catalysts Synthesized From Na-Free Gels in the Skeletal Isomerization of n-Butene. Top. Catal. 2009, 52, 1281–1291. [Google Scholar] [CrossRef]
- Nishitoba, T.; Yoshida, N.; Kondo, J.N.; Yokoi, T. Control of Al Distribution in the CHA-Type Aluminosilicate Zeolites and Its Impact on the Hydrothermal Stability and Catalytic Properties. Ind. Eng. Chem. Res. 2018, 57, 3914–3922. [Google Scholar] [CrossRef]
- Dědeček, J.; Kaucký, D.; Wichterlová, B.; Gonsiorová, O. Co2+ ions as probes of Al distribution in the framework of zeolites. ZSM-5 study. Phys. Chem. Chem. Phys. 2002, 4, 5406–5413. [Google Scholar] [CrossRef]
- Kharchenko, A.; Zholobenko, V.; Vicente, A.; Fernandez, C.; Vezin, H.; De Waele, V.; Mintova, S. Formation of copper nanoparticles in LTL nanosized zeolite: Spectroscopic characterization. Phys. Chem. Chem. Phys. 2018, 20, 2880–2889. [Google Scholar] [CrossRef]
- Ng, E.-P.; Zou, X.; Mintova, S. Chapter 12—Environmental Synthesis Concerns of Zeolites. In New and Future Developments in Catalysis; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 289–310. ISBN 9780444538765. [Google Scholar] [CrossRef]
- Pashkova, V.; Klein, P.; Dedecek, J.; Tokarova, V.; Wichterlova, B. Incorporation of Al at ZSM-5 hydrothermal synthesis. Tuning of Al pairs in the framework. Microporous Mesoporous Mater. 2015, 202, 138–146. [Google Scholar] [CrossRef]
- Gabova, V.; Dedecek, J.; Cejka, J. Control of Al distribution in ZSM-5 by conditions of zeolite synthesis. Chem. Commun. 2003, 1196–1197. [Google Scholar] [CrossRef] [PubMed]
- Dedecek, J.; Balgova, V.; Pashkova, V.; Klein, P.; Wichterlova, B. Synthesis of ZSM-5 Zeolites with Defined Distribution of Al Atoms in the Framework and Multinuclear MAS NMR Analysis of the Control of Al Distribution. Chem. Mater. 2012, 24, 3231–3239. [Google Scholar] [CrossRef]
- Burton, A.W.; Zones, S.I. Chapter 5 Organic molecules in zeolite synthesis: Their preparation and structure-directing effects. Stud. Surf. Sci. Catal. 2007, 168, 137–179. [Google Scholar]
- Lobo, R.F.; Zones, S.I.; Davis, M.E. Structure-direction in zeolite synthesis. J. Inclus. Phenom. Mol. 1995, 21, 47–78. [Google Scholar]
- Khivantsev, K.; Biancardi, A.; Fathizadeh, M.; Almalki, F.; Grant, J.; Tien, H.N.; Shakouri, N.; Blom, D.A.; Makris, T.; Regalbuto, J.R.; et al. Catalytic N−H Bond Activation and Breaking by a Well-Defined CoII1O4 Site of a Heterogeneous Catalyst. ChemCatChem 2018, 10, 736–742. [Google Scholar] [CrossRef]
- O’Day, P.A.; Parks, G.A.; Brown, G.E., Jr. Molecular Structure and Binding Sites of Cobalt(II) Surface Complexes on Kaolinite from X-Ray Absorption Spectroscopy. Clays Clay Miner. 1994, 42, 337–355. [Google Scholar] [CrossRef]
- Luo, J.; An, H.; Kamasamudram, K.; Currier, N.; Yezerets, A.; Thomas, W.; Allard, L. Impact of Accelerated Hydrothermal Aging on Structure and Performance of Cu-SSZ-13 SCR Catalysts. Int. J. Engines 2015, 8, 1181–1186. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Ivanova, E.; Daturi, M.; Saussey, J.; Lavalley, J.-C. Nitrosyl complexes on Co–ZSM-5: An FTIR spectroscopic study. Chem. Phys. Lett. 2003, 370, 712–718. [Google Scholar] [CrossRef]
- Mihaylova, A.; Hadjiivanov, K.; Che, M.J. Remarkable Effect of the Preparation Technique on the State of Cobalt Ions in BEA Zeolites Evidenced by FTIR Spectroscopy of Adsorbed CO and NO, TPR and XRD. Phys. Chem. B 2006, 110, 19530–19536. [Google Scholar] [CrossRef]
- Hadjiivanov, K.I. Identification of Neutral and Charged NxOy Surface Species by IR Spectroscopy. Catal. Rev. Sci. Eng. 2000, 42, 71–144. [Google Scholar] [CrossRef]
- Hadjiivanov, K.; Saussey, J.; Freysz, J.L.; Lavalley, J.C. FT-IR study of NO + O2 co-adsorption on H-ZSM-5: Re-assignment of the 2133 cm-1 band to NO+ species. Catal. Lett. 1998, 52, 103–108. [Google Scholar] [CrossRef]
- Tsyntsarski, B.; Avreyska, V.; Kolev, H.; Marinova, T.; Klissurski, D.; Hadjiivanov, K.J. FT-IR study of the nature and reactivity of surface NOx compounds formed after NO adsorption and NO + O2 coadsorption on zirconia- and sulfated zirconia-supported cobalt. Mol. Catal. A Chem. 2003, 193, 139–149. [Google Scholar] [CrossRef]
- Lunsford, J.H.; Hutta, P.J.; Lin, M.J.; Windhorst, K.A. Cobalt Nitrosyl Complexes in ZeolitesA, X, and Y. Inorg. Chem. 1978, 17, 606–610. [Google Scholar] [CrossRef]
- Windhorst, K.A.; Lunsford, J.H. Structure and Reactivity of Cobalt-Nitrosyl Complexes in Y-Type Zeolites. J. Am. Chem. Soc. 1975, 97, 1407–1412. [Google Scholar] [CrossRef]
- Chakarova, K.; Hadjiivanov, K. Coordination chemistry of cobalt ions in ferrierite: An FTIR spectroscopic study. Microporous Mesoporous Mater. 2009, 123, 23–128. [Google Scholar] [CrossRef]
- Usui, T.; Liu, Z.; Ibe, S.; Zhu, J.; Anand, C.; Igarashi, H.; Onaya, N.; Sasaki, Y.; Shiramata, Y.; Kusamoto, T.; et al. Improve the Hydrothermal Stability of Cu-SSZ-13 Zeolite Catalyst by Loading a Small Amount of Ce. ACS Catal. 2018, 8, 9165–9173. [Google Scholar] [CrossRef]
- González, J.M.; Villa, A.L. High Temperature SCR Over Cu-SSZ-13 and Cu-SSZ-13 + Fe-SSZ-13: Activity of Cu2+ and [CuOH]1+ Sites and the Apparent Promoting Effect of Adding Fe into Cu-SSZ-13 Catalyst. Catal. Lett. 2021, 151, 3011–3019. [Google Scholar] [CrossRef]
- Moliner, M.; Martínez, C.; Corma, A. Synthesis Strategies for Preparing Useful Small Pore Zeolites and Zeotypes for Gas Separations and Catalysis. Chem. Mater. 2014, 26, 246–258. [Google Scholar] [CrossRef]
- Sonoda, T.; Maruo, T.; Yamasaki, Y.; Tsunoji, N.; Takamitsu, Y.; Sadakane, M.; Sano, T.J. Synthesis of high-silica AEI zeolites with enhanced thermal stability by hydrothermal conversion of FAU zeolites, and their activity in the selective catalytic reduction of NOx with NH3. Mater. Chem. A 2015, 3, 857–865. [Google Scholar] [CrossRef]
- Kim, J.; Cho, S.J.; Kim, D.H. Facile Synthesis of KFI-type Zeolite and Its Application to Selective Catalytic Reduction of NOx with NH3. ACS Catal. 2017, 7, 6070–6081. [Google Scholar] [CrossRef]
- Ehrhardt, K.; Suckow, M.; Lutz, W. Hydrothermal decomposition of aluminosilicate zeolites and prediction of their long-term stability. Stud. Surf. Sci. Catal. 1995, 94, 179–186. [Google Scholar]
- Cruciani, G.J. Zeolites upon heating: Factors governing their thermal stability and structural changes. Phys. Chem. Solids 2006, 67, 1973–1994. [Google Scholar] [CrossRef]
- Seidler, G.T.; Mortensen, D.R.; Remesnik, A.J.; Pacold, J.I.; Ball, N.A.; Barry, N.; Styczinski, M.; Hoidn, O. A laboratory-based hard X-ray monochromator for high-resolution x-ray emission spectroscopy and x-ray absorption near edge structure measurements. Rev. Sci. Instrum. 2014, 85, 113906. [Google Scholar] [CrossRef] [PubMed]


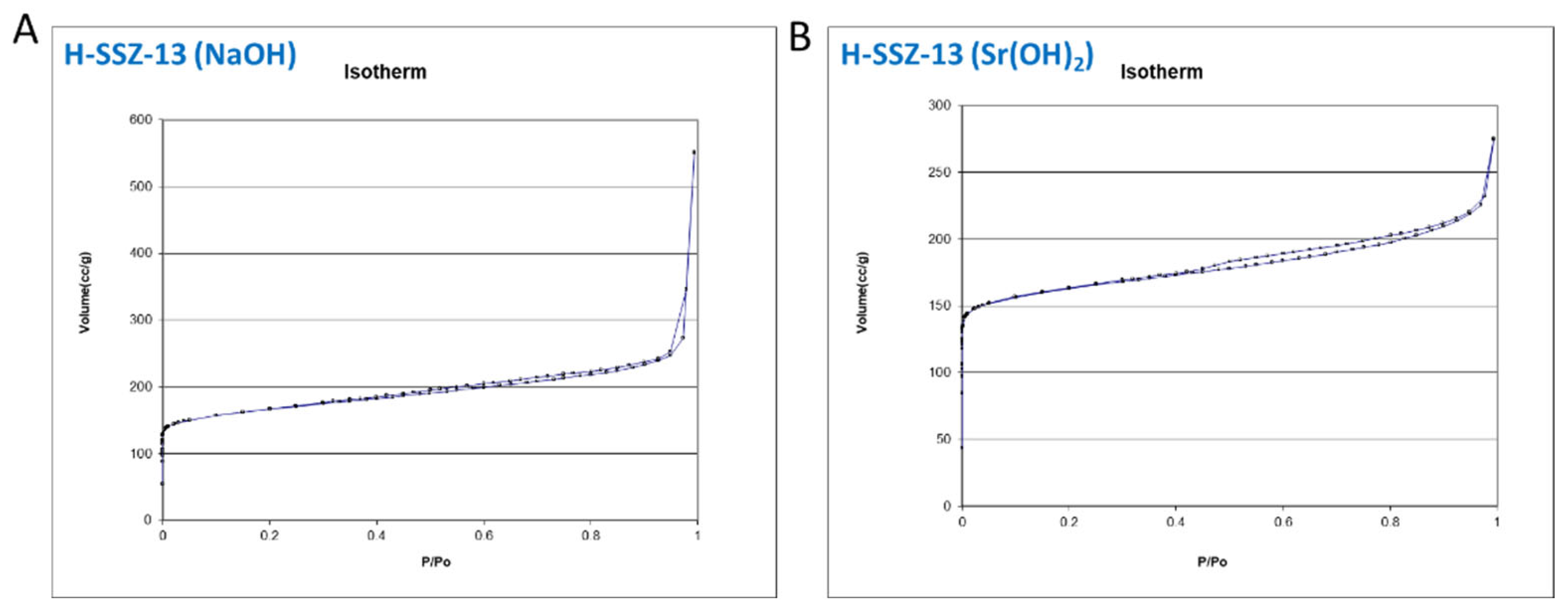
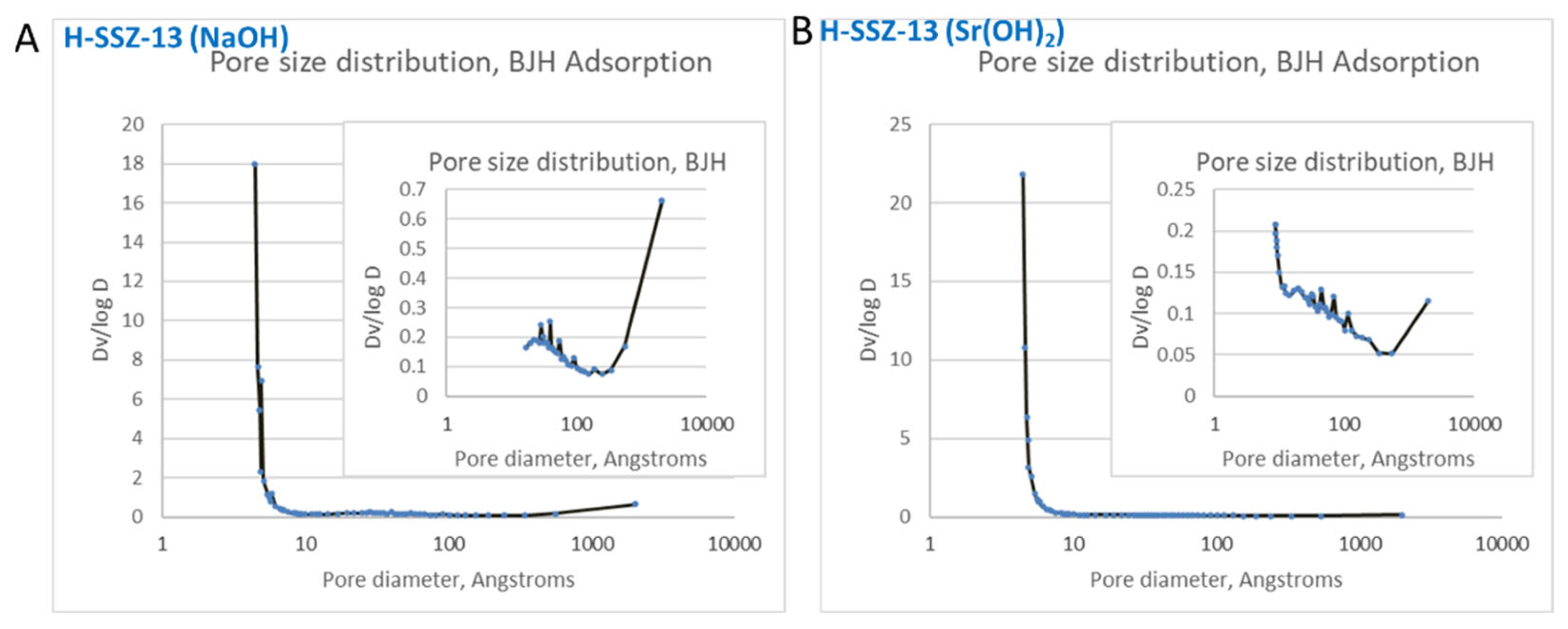


| Sample ID | Degas Temperature | BET Surface Area | T method Micropore Surface Area | Pore Volume | T method Micropore Surface Area |
|---|---|---|---|---|---|
| °C | m2/g | m2/g | cc/g | cc/g | |
| H-SSZ-13 (Na) | 250 | 623 | 409 | 0.85 | 0.16 |
| H-SSZ-13 (Sr) | 250 | 634 | 485 | 0.43 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khivantsev, K.; Derewinski, M.A.; Kovarik, L.; Bowden, M.; Li, X.S.; Jaegers, N.R.; Boglaienko, D.; Pereira-Hernandez, X.I.; Pearce, C.; Wang, Y.; et al. Increasing Al-Pair Abundance in SSZ-13 Zeolite via Zeolite Synthesis in the Presence of Alkaline Earth Metal Hydroxide Produces Hydrothermally Stable Co-, Cu- and Pd-SSZ-13 Materials. Catalysts 2024, 14, 56. https://doi.org/10.3390/catal14010056
Khivantsev K, Derewinski MA, Kovarik L, Bowden M, Li XS, Jaegers NR, Boglaienko D, Pereira-Hernandez XI, Pearce C, Wang Y, et al. Increasing Al-Pair Abundance in SSZ-13 Zeolite via Zeolite Synthesis in the Presence of Alkaline Earth Metal Hydroxide Produces Hydrothermally Stable Co-, Cu- and Pd-SSZ-13 Materials. Catalysts. 2024; 14(1):56. https://doi.org/10.3390/catal14010056
Chicago/Turabian StyleKhivantsev, Konstantin, Miroslaw A. Derewinski, Libor Kovarik, Mark Bowden, Xiaohong Shari Li, Nicholas R. Jaegers, Daria Boglaienko, Xavier I. Pereira-Hernandez, Carolyn Pearce, Yong Wang, and et al. 2024. "Increasing Al-Pair Abundance in SSZ-13 Zeolite via Zeolite Synthesis in the Presence of Alkaline Earth Metal Hydroxide Produces Hydrothermally Stable Co-, Cu- and Pd-SSZ-13 Materials" Catalysts 14, no. 1: 56. https://doi.org/10.3390/catal14010056
APA StyleKhivantsev, K., Derewinski, M. A., Kovarik, L., Bowden, M., Li, X. S., Jaegers, N. R., Boglaienko, D., Pereira-Hernandez, X. I., Pearce, C., Wang, Y., & Szanyi, J. (2024). Increasing Al-Pair Abundance in SSZ-13 Zeolite via Zeolite Synthesis in the Presence of Alkaline Earth Metal Hydroxide Produces Hydrothermally Stable Co-, Cu- and Pd-SSZ-13 Materials. Catalysts, 14(1), 56. https://doi.org/10.3390/catal14010056








