Comparative Study of Supported Ni and Co Catalysts Prepared Using the All-in-One Method in the Hydrogenation of CO2: Effects of Using (Poly)Vinyl Alcohol (PVA) as an Additive
Abstract
1. Introduction
2. Results and Discussion
2.1. Catalysts Characterization
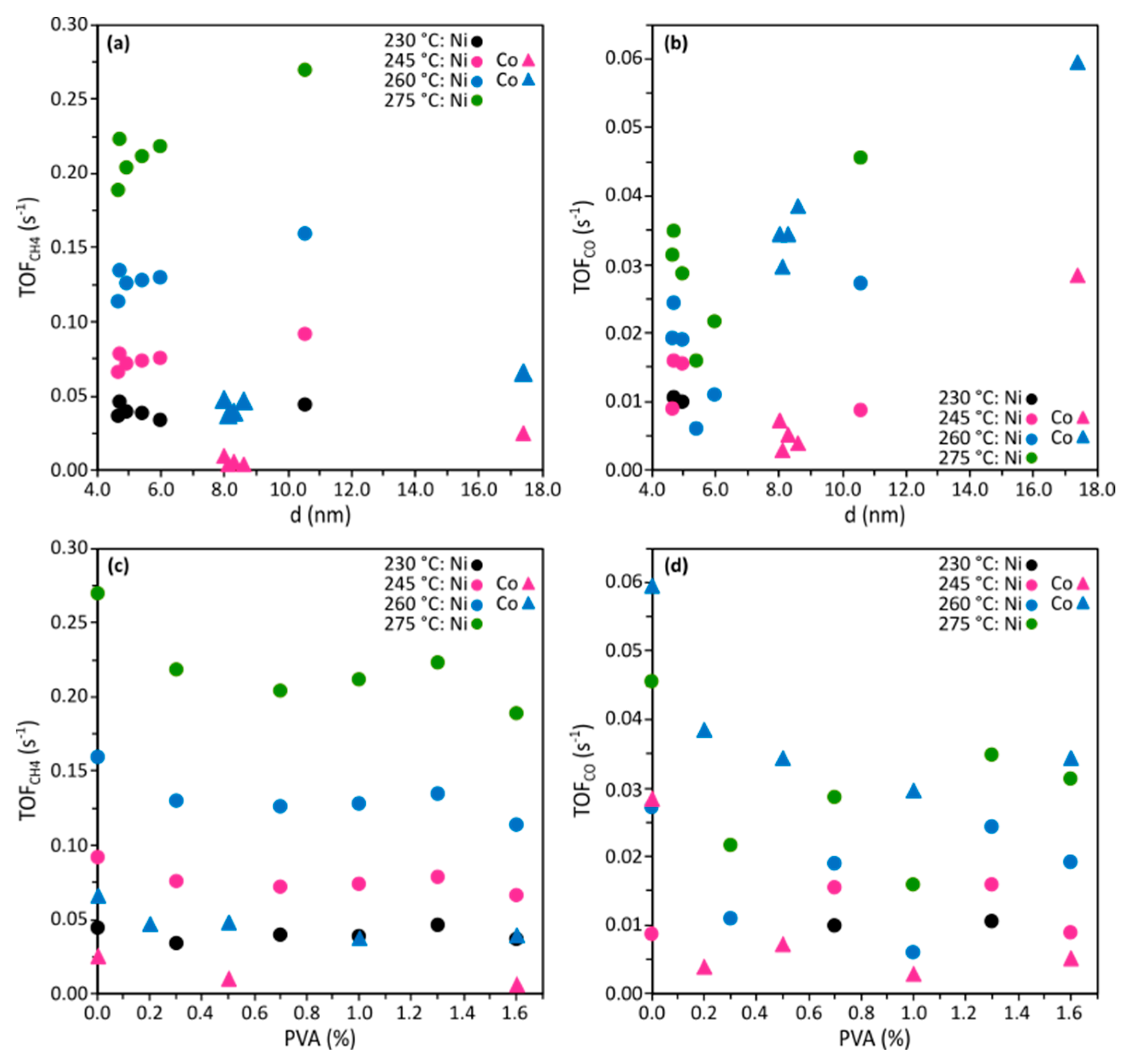
2.2. Catalytic Performance
3. Materials and Methods
3.1. Catalyst Preparation
3.2. Physicochemical Characterization
3.3. Catalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bailera, M.; Lisbona, P.; Romeo, L.M.; Espatolero, S. Power to Gas projects review: Lab, pilot and demo plants for storing renewable energy and CO2. Renew. Sust. Energy Rev. 2017, 69, 292–312. [Google Scholar] [CrossRef]
- Weatherbee, G.D.; Bartholomew, C.H. Hydrogenation of CO2 on Group VIII Metals: I. Specific Activity of Ni/SiO2. J. Catal. 1981, 68, 67–76. [Google Scholar] [CrossRef]
- Wang, X.; Shi, H.; Szanyi, J. Controlling selectivities in CO2 reduction through mechanistic understanding. Nat. Commun. 2017, 8, 513. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The renaissance of the Sabatier reaction and its applications on Earth and in space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- González-Castaño, M.; Dorneau, B.; Arellano-García, H. The reverse water gas shift reaction: A process systems engineering perspective. React. Chem. Eng. 2021, 6, 954–976. [Google Scholar] [CrossRef]
- Villora-Picó, J.J.; González-Arias, J.; Pastor-Pérez, L.; Odriozola, J.A.; Reina, T.R. A review on high-pressure heterogeneous catalytic processes for gas-phase CO2 valorization. Environ. Res. 2024, 240, 117520. [Google Scholar] [CrossRef] [PubMed]
- Mills, G.A.; Steffgen, F.W. Catalytic Methanation. Catal. Rev. 1973, 8, 159–210. [Google Scholar] [CrossRef]
- Wang, Y.; Winter, L.R.; Chen, J.G.; Yan, B. CO2 hydrogenation over heterogeneous catalysts at atmospheric pressure: From electronic properties to product selectivity. Green Chem. 2021, 23, 249–267. [Google Scholar] [CrossRef]
- Tommasi, M.; Degerli, S.N.; Ramis, G.; Rossetti, I. Advancements in CO2 Methanation: A Comprehensive Review of Catalysis, Reactor Design and Process Optimization. Chem. Eng. Res. Des. 2024, 201, 457–482. [Google Scholar] [CrossRef]
- Weatherbee, G.D.; Bartholomew, C.H. Hydrogenation of CO2 on Group VIII Metals. IV. Specific Activities and Selectivities of Silica-Supported Co, Fe and Ru. J. Catal. 1984, 87, 352–362. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Panagiotopoulou, P. Hydrogention of CO2 over supported noble metal catalysts. Appl. Catal. A Gen. 2017, 542, 63–70. [Google Scholar] [CrossRef]
- Ashok, J.; Pati, S.; Hongmanorom, P.; Tianxi, Z.; Junmei, C. A review of recent catalyst advances in CO2 methanation processes. Catal. Today 2020, 356, 471–489. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y. Recent trend in thermal catalytic low temperature CO2 methanation: A critical review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Tan, C.H.; Nomanbhay, S.; Shamsuddin, A.H.; Park, Y.-K.; Hernández-Cocoletzi, H.; Show, P.L. Current Developments in Catalytic Methanation of Carbon Dioxide—A Review. Front. Energy Res. 2022, 9, 795423. [Google Scholar] [CrossRef]
- Mutschler, R.; Moioli, E.; Luo, W.; Gallandat, N.; Züttel, A. CO2 hydrogenation reaction over pristine Fe, Co, Ni, Cu and Al2O3 supported Ru: Comparison and determination of the activation energies. J. Catal. 2018, 366, 139–149. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Huang, Q.; Jiang, M.; Askari, S.; Dewangan, N.; Kawi, S. State-of-art modifications of heterogeneous catalysts for CO2 methanation—Active sites, surface basicity and oxygen defects. Catal. Today 2022, 402, 88–103. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Sterk, E.B.; Palle, J.; Melcherts, A.E.M.; Zijlstra, B.; Groeneveld, E.; Berben, P.H.; Boereboom, J.M.; Hensen, E.J.M.; et al. Understanding carbon dioxide activation and carbon–carbon coupling over nickel. Nat. Commun. 2019, 10, 5330. [Google Scholar] [CrossRef]
- Quindimil, A.; De-La-Torre, U.; Pereda-Ayo, B.; Davó-Quiñonero, A.; Bailón-García, E.; Lozano-Castelló, D.; González-Marcos, J.A.; Bueno-López, A.; González-Velasco, J.R. Effect of metal loading on the CO2 methanation: A comparison between alumina supported Ni and Ru catalysts. Catal. Today 2020, 356, 419–432. [Google Scholar] [CrossRef]
- Shen, L.; Xu, J.; Zhu, M.; Han, Y.-F. Essential Role of the Support for Nickel-Based CO2 Methanation Catalysts. ACS Catal. 2020, 10, 14581–14591. [Google Scholar] [CrossRef]
- Pearce, B.B.; Twigg, M.V.; Woodward, C. Methanation. In Catalyst Handbook, 2nd ed.; Twigg, M.V., Ed.; Manson Publishing: Frome, UK, 1996; pp. 340–383. [Google Scholar]
- den Breejen, J.P.; Radstake, P.B.; Bezemer, G.L.; Bitter, J.H.; Frøseth, V.; Holmen, A.; de Jong, K.P. On the Origin of the Cobalt Particle Size Effects in Fischer-Tropsch Catalysis. J. Am. Chem. Soc. 2009, 131, 7197–7203. [Google Scholar] [CrossRef] [PubMed]
- Melaet, G.; Lindeman, A.E.; Somorjai, G.A. Cobalt Particle Size Effects in the Fischer–Tropsch Synthesis and in the Hydrogenation of CO2 Studied with Nanoparticle Model Catalysts on Silica. Top. Catal. 2014, 57, 500–507. [Google Scholar] [CrossRef]
- Iablokov, V.; Beaumont, S.K.; Alayoglu, S.; Pushkarev, V.V.; Specht, C.; Gao, J.; Alivisatos, A.P.; Kruse, N.; Somorjai, G.A. Size-Controlled Model Co Nanoparticle Catalysts for CO2 Hydrogenation: Synthesis, Characterization, and Catalytic Reactions. Nano Lett. 2012, 12, 3091–3096. [Google Scholar] [CrossRef] [PubMed]
- Srisawad, N.; Chaitree, W.; Mekasuwandumrong, O.; Shotipruk, A.; Jongsomjit, B.; Panpranot, J. CO2 hydrogenation over Co/Al2O3 catalysts prepared via a solid-state reaction of fine gibbsite and cobalt precursors. React. Kinet. Mech. Catal. 2012, 107, 179–188. [Google Scholar] [CrossRef]
- Zhou, G.; Wu, T.; Xie, H.; Zheng, X. Effects of structure on the carbon dioxide methanation performance of Co-based catalysts. Int. J. Hydrog. Energy 2013, 38, 10012–10018. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, M.S.; Lee, S.H.; Park, E.D. CO and CO2 Methanation over Supported Cobalt Catalysts. Top. Catal. 2017, 60, 714–720. [Google Scholar] [CrossRef]
- Díez-Ramírez, J.; Sánchez, P.; Kyriakou, V.; Zafeiratos, S.; Marnellos, G.E.; Konsolakis, M.; Dorado, F. Effect of support nature on the cobalt-catalyzed CO2 hydrogenation. J. CO2 Util. 2017, 21, 562–571. [Google Scholar] [CrossRef]
- Li, W.; Nie, X.; Jiang, X.; Zhang, A.; Ding, F.; Liu, M.; Liu, Z.; Guo, X.; Song, C. ZrO2 support imparts superior activity and stability of Co catalysts for CO2 methanation. Appl. Catal. B Environ. 2018, 220, 397–408. [Google Scholar] [CrossRef]
- Jimenez, J.D.; Wen, C.; Lauterbach, J. Design of highly active cobalt catalysts for CO2 hydrogenation via the tailoring of surface orientation of nanostructures. Catal. Sci. Technol. 2019, 9, 1970. [Google Scholar] [CrossRef]
- Yang, C.; Liu, S.; Wang, Y.; Song, J.; Wang, G.; Wang, S.; Zhao, Z.-J.; Mu, R.; Gong, J. The Interplay between Structure and Product Selectivity of CO2 Hydrogenation. Angew. Chem. Int. Ed. 2019, 58, 11242–11247. [Google Scholar] [CrossRef]
- Garbarino, G.; Cavattoni, T.; Riani, P.; Busca, G. Support effects in metal catalysis: A study of the behavior of unsupported and silica-supported cobalt catalysts in the hydrogenation of CO2 at atmospheric pressure. Catal. Today 2020, 345, 213–219. [Google Scholar] [CrossRef]
- Efremova, A.; Rajkumar, T.; Szamosvolgyi, Á.; Sápi, A.; Baán, K.; Szenti, I.; Gómez-Pérez, J.; Varga, G.; Kiss, J.; Halasi, G.; et al. Complexity of a Co3O4 System under Ambient-Pressure CO2 Methanation: Influence of Bulk and Surface Properties on the Catalytic Performance. J. Phys. Chem. C 2021, 125, 7130–7141. [Google Scholar] [CrossRef]
- Tu, J.; Wu, H.; Qian, Q.; Han, S.; Chu, M.; Jia, S.; Feng, R.; Zhai, J.; Hea, M.; Han, B. Low temperature methanation of CO2 over an amorphous cobalt-based catalyst. Chem. Sci. 2021, 12, 3937. [Google Scholar] [CrossRef] [PubMed]
- Villagra-Soza, F.; Godoy, S.; Karelovic, A.; Jiménez, R. Scrutinizing the mechanism of CO2 hydrogenation over Ni, Co and bimetallic NiCo surfaces: Isotopic measurements, operando-FTIR experiments and kinetics modelling. J. Catal. 2022, 414, 1–15. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, G.; Zhu, J.; Li, W.; Wang, J.; Bian, K.; Liu, Y.; Ding, F.; Song, C.; Guo, X. Unraveling the tunable selectivity on cobalt oxide and metallic cobalt sites for CO2 hydrogenation. Chem. Eng. J. 2022, 446, 137217. [Google Scholar] [CrossRef]
- Bredy, P.; Farrusseng, D.; Schuurman, Y.; Meunier, F.C. On the link between CO surface coverage and selectivity to CH4 during CO2 hydrogenation over supported cobalt catalysts. J. Catal. 2022, 411, 93–96. [Google Scholar] [CrossRef]
- Zhou, X.; Price, G.A.; Sunley, G.J.; Copéret, C. Small Cobalt Nanoparticles Favor Reverse Water-Gas Shift Reaction Over Methanation under CO2 Hydrogenation Conditions. Angew. Chem. Int. Ed. 2023, 62, e202314274. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Zhang, T.; Zhao, J.; Xiao, J.; Zhou, W. Tuning selectivity of CO2 hydrogenation over Co catalysts by surface decoration of Sn. J. Catal. 2024, 429, 115242. [Google Scholar] [CrossRef]
- Habazaki, H.; Yamasaki, M.; Zhang, B.-P.; Kawashima, A.; Kohno, S.; Takai, T.; Hashimoto, K. Co-methanation of carbon monoxide and carbon dioxide on supported nickel and cobalt catalysts prepared from amorphous alloys. Appl. Catal. A Gen. 1998, 172, 131–140. [Google Scholar] [CrossRef]
- Liang, C.; Tian, H.; Gao, g.; Zhang, s.; Liu, Q.; Dong, D.; Hu, X. Methanation of CO2 over alumina supported nickel or cobalt catalysts: Effects of the coordination between metal and support on formation of the reaction intermediates. Int. J. Hydrog. Energy 2020, 45, 531–543. [Google Scholar] [CrossRef]
- Liu, C.; Cundari, T.R.; Wilson, A.K. CO2 Reduction on Transition Metal (Fe, Co, Ni, and Cu) Surfaces: In Comparison with Homogeneous Catalysis. J. Phys. Chem. C 2012, 116, 5681–5688. [Google Scholar] [CrossRef]
- Xu, L.; Lian, X.; Chen, M.; Cui, Y.; Wang, F.; Li, W.; Huang, B. CO2 methanation over Co−Ni bimetal-doped ordered mesoporous Al2O3 catalysts with enhanced low-temperature activities. Int. J. Hydrog. Energy 2018, 43, 17172–17184. [Google Scholar] [CrossRef]
- Alrafei, B.; Polaert, I.; Ledoux, A.; Azzolina-Jury, F. Remarkably stable and efficient Ni and Ni-Co catalysts for CO2 methanation. Catal. Today 2020, 346, 23–33. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Modafferi, V.; Antonucci, V.; Antonucci, P.; Macario, A. Catalytic activity of Ni-Co supported metals in carbon dioxides methanation. Can. J. Chem. Eng. 2020, 98, 1924–1934. [Google Scholar] [CrossRef]
- Shafiee, P.; Alavi, S.M.; Rezaei, M. Solid-state synthesis method for the preparation of cobalt doped Ni–Al2O3 mesoporous catalysts for CO2 methanation. Int. J. Hydrog. Energy 2021, 46, 3933–3944. [Google Scholar] [CrossRef]
- Tsiotsias, A.I.; Charision, N.D.; Yentekakis, I.V.; Goula, M.A. Bimetallic Ni-Based Catalysts for CO2 Methanation: A Review. Nanomaterials 2021, 11, 28. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, G.; Wang, C.; Cavattoni, T.; Finocchio, E.; Riani, P.; Flytzani-Stephanopoulos, M.; Busca, G. A study of Ni/La-Al2O3 catalysts: A competitive system for CO2 methanation. Appl. Catal. B Environ. 2019, 248, 286–297. [Google Scholar] [CrossRef]
- Ho, P.H.; de Luna, G.S.; Angelucci, S.; Canciani, A.; Jones, W.; Decarolis, D.; Ospitali, F.; Aguado, E.R.; Rodríguez-Castellón, E.; Fornasari, G.; et al. Understanding structure-activity relationships in highly active La promoted Ni catalysts for CO2 methanation. Appl. Catal. B Environ. 2020, 278, 119256. [Google Scholar] [CrossRef]
- Ferreira, A.C.; Martinho, J.F.; Branco, J.B. Hydrogenation of CO2 over Cobalt-Lanthanide Bimetallic Oxide Nanofibers. ChemCatChem 2022, 14, e202101548. [Google Scholar] [CrossRef]
- Sousa Aguiar, E.F.; Costa, A.F.; Gandía Pascual, L.M.; Santos, I.B.; Arzamendi Manterola, M.C.; Almeida, L.C.; Montes Ramírez, M.; Odriozola Gordon, J.A. Method for Preparing Structured Catalytic Systems. World Patent WO 2014/085890 AI, 12 June 2014. [Google Scholar]
- Ribeiro, A.T.S.; Araújo, I.R.S.; da Silva, E.F.M.; Romano, P.N.; Almeida, J.M.A.R.; Sousa-Aguiar, E.F.; Tomovska, R.; Sanz, O.; Almeida, L.C. Improvement of Ni-based catalyst properties and activity for dry reforming of methane by application of all-in-one preparation method. J. Mater. Sci. 2023, 58, 3568–3581. [Google Scholar] [CrossRef]
- Cesteros, Y.Y.; Salagre, P.; Medina, F.; Sueiras, J.E. Preparation and Characterization of Several High-Area NiAl2O4 Spinels. Study of Their Reducibility. Chem. Mater. 2000, 12, 331–335. [Google Scholar] [CrossRef]
- González-Rangulan, V.V.; Reyero, I.; Bimbela, F.; Romero-Sarria, F.; Daturi, M.; Gandía, L.M. CO2 Methanation over Nickel Catalysts: Support Effects Investigated through Specific Activity and Operando IR Spectroscopy Measurements. Catalysts 2023, 13, 448. [Google Scholar] [CrossRef]
- Aljishi, A.; Veilleux, G.; Lalinde, J.A.H.; Kopyscinski, J. The effect of synthesis parameters on ordered mesoporous nickel alumina catalyst for CO2 methanation. Appl. Catal. A Gen. 2018, 549, 263–272. [Google Scholar] [CrossRef]
- Guilera, J.; Del Valle, J.; Alarcón, A.; Díaz, J.A.; Andreu, T. Metal-oxide promoted Ni/Al2O3 as CO2 methanation micro-size catalysts. J. CO2 Util. 2019, 30, 11–17. [Google Scholar] [CrossRef]
- Gandía, L.M.; Montes, M. Effect of the reduction temperature on the selectivity of the high temperature reaction of acetone and hydrogen over alumina and titania supported nickel and cobalt catalysts. J. Mol. Catal. 1994, 94, 347–367. [Google Scholar] [CrossRef]
- Italiano, C.; Llorca, J.; Pino, L.; Ferraro, M.; Antonucci, V.; Vita, A. CO and CO2 methanation over Ni catalysts supported on CeO2, Al2O3 and Y2O3 oxides. Appl. Catal. B Environ. 2020, 264, 118494. [Google Scholar] [CrossRef]
- Bentaleb, F.; Che, M.; Dubreuil, A.-C.; Thomazeau, C.; Marceau, E. Influence of organic additives on the properties of impregnation solutions and on nickel oxide particle size for Ni/Al2O3 catalysts. Catal. Today 2014, 235, 250–255. [Google Scholar] [CrossRef]
- Ryczkowski, J.; Grzegorczyk, W.; Nazimek, D. Support modification with organic reagents and its influence on the development of metal active surface areas in Ni/Al2O3 catalysts. Appl. Catal. A Gen. 1995, 126, 341–349. [Google Scholar] [CrossRef]
- Hatamie, S.; Ahadian, M.M.; Rashidi, A.; Karimi, A.; Akhavan, O. Novel synthesis of cobalt/poly vinyl alcohol/gamma alumina nanocomposite for catalytic application. Appl. Phys. A 2017, 123, 341. [Google Scholar] [CrossRef]
- Mateos-Pedrero, C.; Azenha, C.; Pacheco Tanaka, D.A.; Sousa, J.M.; Mendes, A. The influence of the support composition on the physicochemical and catalytic properties of Cu catalysts supported on Zirconia-Alumina for methanol steam reforming. Appl. Catal. B Environ. 2020, 277, 119243. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, V.; Kotok, V.; Zima, O.; Nafeev, R.; Verbitsky, V.; Melnyk, O. Definition of the Role of Polyvinylalcohol during Formation and in the Structure of Cathodic Synthesized Composite Electrochromic Nickel Hydroxide Layer: Template or Surfactant. East.-Eur. J. Enterp. Technol. 2022, 2, 6–14. [Google Scholar] [CrossRef]
- Simons, J.F.M.; de Heer, T.J.; van de Poll, R.C.J.; Muravev, V.; Kosinov, N.; Hensen, E.J.M. Structure Sensitivity of CO2 Hydrogenation on Ni Revisited. J. Am. Chem. Soc. 2023, 145, 20289–20301. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Calizzi, M.; Moioli, E.; Li, M.; Borsay, A.; Lombardo, L.; Mutschler, R.; Luo, W.; Züttel, A. Unraveling and optimizing the metal-metal oxide synergistic effect in a highly active Cox(CoO)1−x catalyst for CO2 hydrogenation. J. Energy Chem. 2021, 53, 241250. [Google Scholar] [CrossRef]
- Parastaev, A.; Valery Muravev, V.; Huertas Osta, E.; Kimpel, T.F.; Simons, J.F.M.; van Hoof, A.J.F.; Uslamin, E.; Zhang, L.; Struijs, J.J.C.; Burueva, D.B.; et al. Breaking structure sensitivity in CO2 hydrogenation by tuning metal–oxide interfaces in supported cobalt nanoparticles. Nat. Catal. 2022, 5, 1051–1060. [Google Scholar] [CrossRef]
- ten Have, I.C.; Kromwijk, J.J.G.; Monai, M.; Ferri, D.; Sterk, E.B.; Meirer, F.; Weckhuysen, B.M. Uncovering the reaction mechanism behind CoO as active phase for CO2 hydrogenation. Nat. Commun. 2022, 13, 324. [Google Scholar] [CrossRef] [PubMed]
- Beierlein, D.; Häussermann, D.; Pfeifer, M.; Schwarz, T.; Stöwe, K.; Traa, Y.; Klemm, E. Is the CO2 methanation on highly loaded Ni-Al2O3 catalysts really structure sensitive? Appl. Catal. B-Environ. 2019, 247, 200–219. [Google Scholar] [CrossRef]
- Vogt, C.; Groeneveld, E.; Kamsma, G.; Nachtegaal, M.; Lu, L.; Kiely, C.J.; Berben, P.H.; Meirer, F.; Weckhuysen, B.M. Unravelling structure sensitivity in CO2 hydrogenation over nickel. Nat. Catal. 2018, 1, 127–134. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Pannell, R.B.; Butler, J.L. Support and Crystallite Size Effects in CO Hydrogenation on Nickel. J. Catal. 1980, 65, 335–347. [Google Scholar] [CrossRef]
- Reuel, R.C.; Bartholomew, C.H. The Stoichiometries of H2 and CO adsorptions on Cobalt: Effects of Support and Preparation. J. Catal. 1984, 85, 63–77. [Google Scholar] [CrossRef]
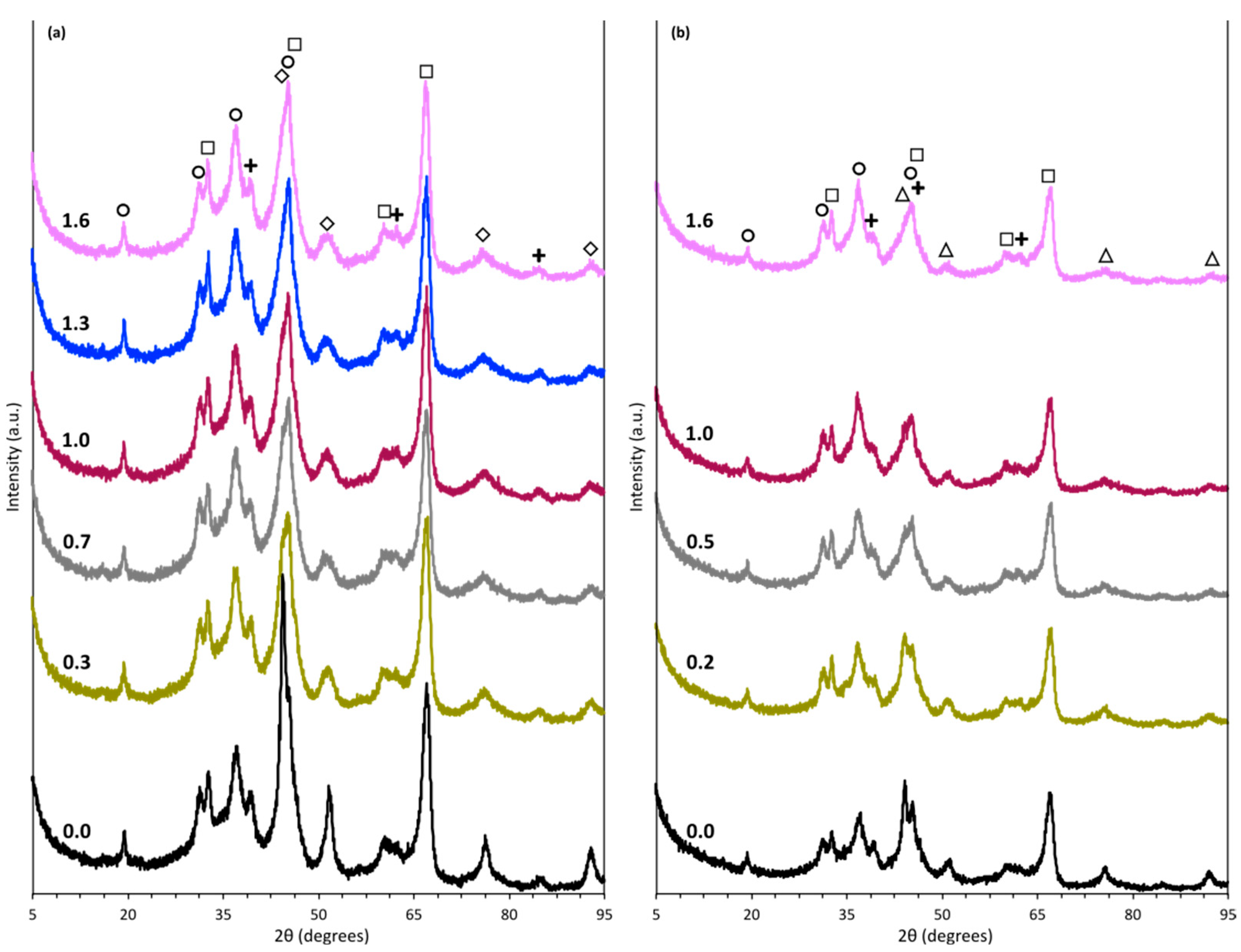
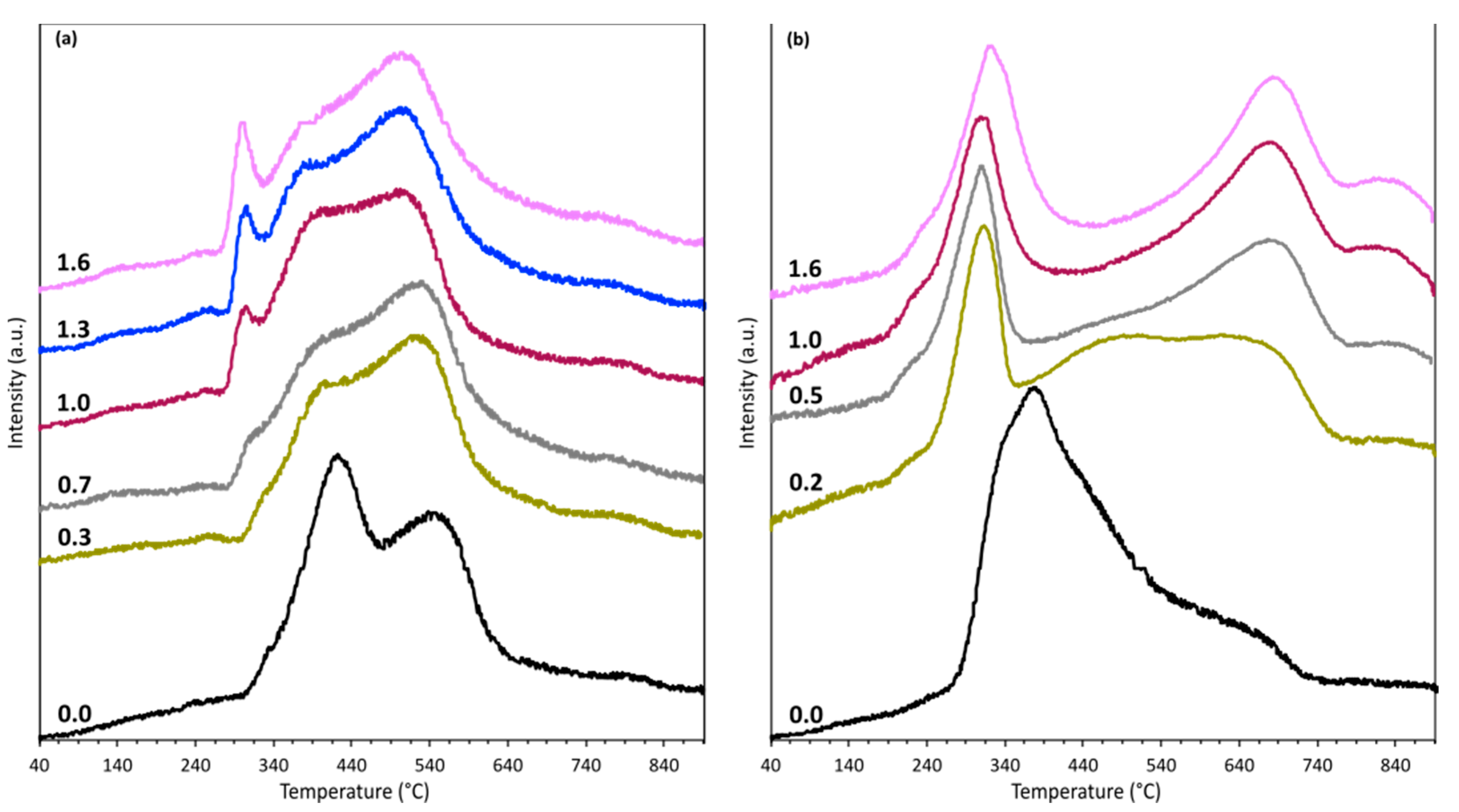
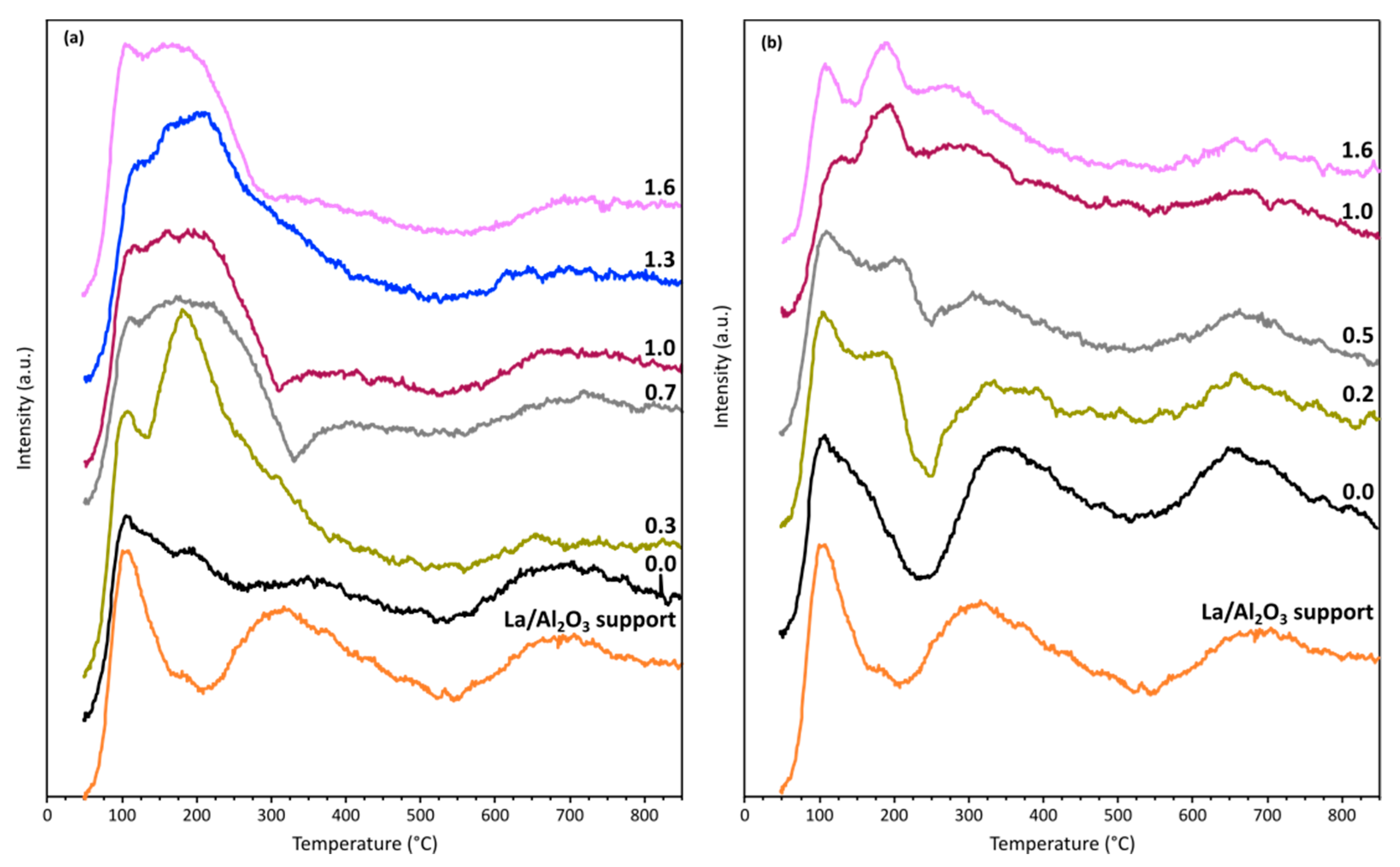


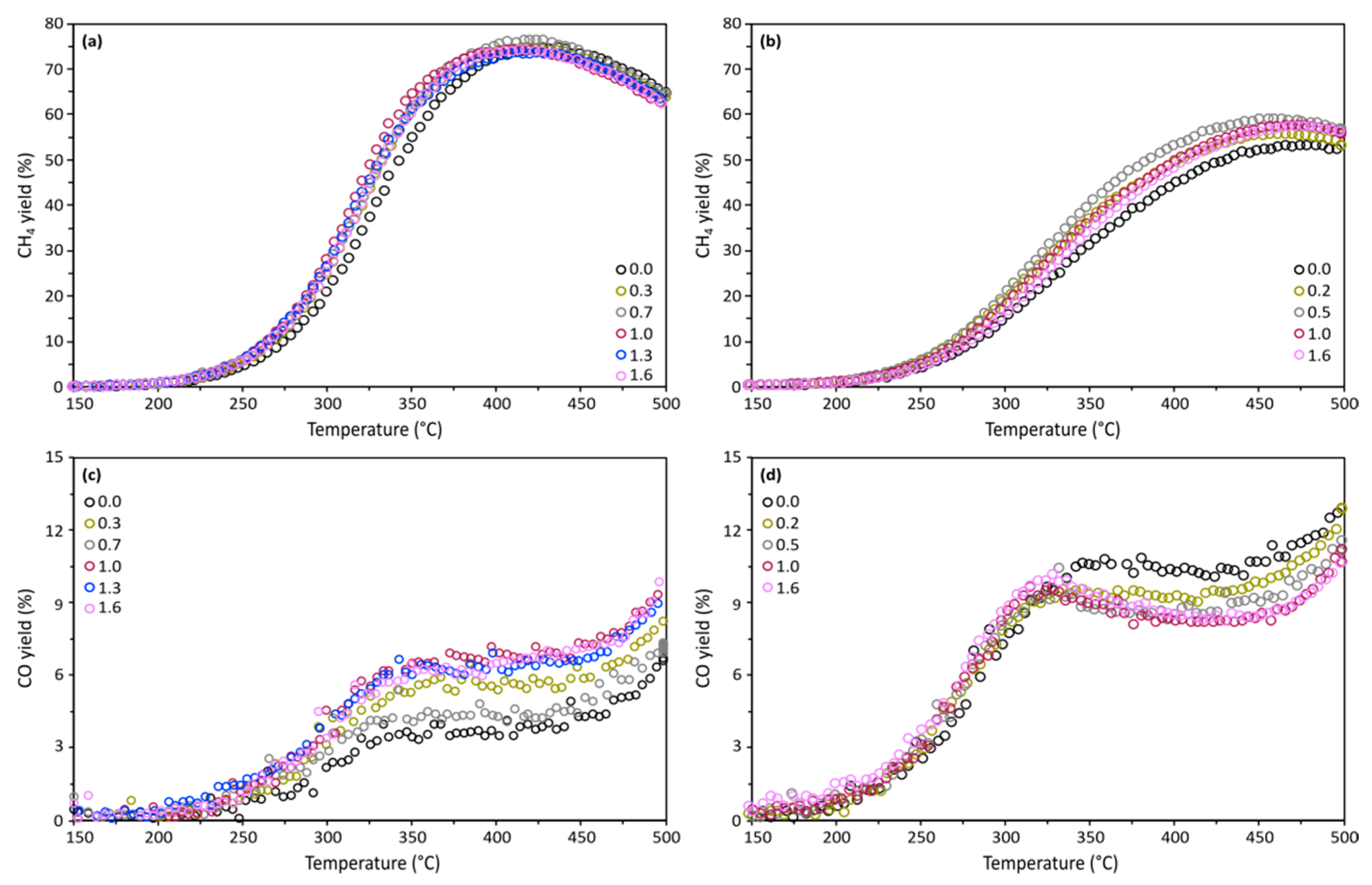

| Sample | Metal Content (wt. %) | SBET b (m2/g) | Vp c (cm3/g) | dp d (nm) | SM e (m2/gmetal) | D f (%) | DoR g (%) | dM h (nm) |
|---|---|---|---|---|---|---|---|---|
| La-Al2O3 | --- | 85 | 0.35 | 16 | -- | -- | -- | -- |
| Ni0/La-Al2O3 | 15.1 | 73 | 0.25 | 13 | 24 | 6.3 | 52 | 11 |
| Ni0.3/La-Al2O3 | 13.9 | 82 | 0.26 | 13 | 41 | 11 | 57 | 6.0 |
| Ni0.7/La-Al2O3 | 14.0 | 89 | 0.26 | 12 | 46 | 13 | 58 | 5.4 |
| Ni1.0/La-Al2O3 | 14.5 | 82 | 0.27 | 12 | 49 | 12 | 56 | 5.0 |
| Ni1.3/La-Al2O3 | 13.8 | 97 | 0.30 | 12 | 48 | 14 | 52 | 4.7 |
| Ni1.6/La-Al2O3 | 14.0 | 84 | 0.28 | 12 | 50 | 14 | 54 | 4.6 |
| Co0/La-Al2O3 | 14.6 | 64 | 0.24 | 14 | 21 | 3.1 | 91 | 17.4 |
| Co0.2/La-Al2O3 | 14.3 | 70 | 0.22 | 14 | 41 | 6.0 | 51 | 8.6 |
| Co0.5/La-Al2O3 | 14.3 | 81 | 0.24 | 12 | 47 | 7.0 | 55 | 8.0 |
| Co1.0/La-Al2O3 | 14.7 | 92 | 0.35 | 14 | 43 | 6.4 | 52 | 8.1 |
| Co1.6/La-Al2O3 | 14.3 | 76 | 0.28 | 14 | 42 | 6.3 | 52 | 8.3 |
| Sample | Weak | Medium–Strong | Total a | Total b |
|---|---|---|---|---|
| La-Al2O3 | 88 | 38 | 126 | 1.48 |
| Ni0/La-Al2O3 | 43 | 48 | 91 | 1.25 |
| Ni0.3/La-Al2O3 | 201 | 4 | 204 | 2.49 |
| Ni0.7/La-Al2O3 | 114 | 51 | 165 | 1.85 |
| Ni1.0/La-Al2O3 | 113 | 39 | 152 | 1.85 |
| Ni1.3/La-Al2O3 | 176 | 25 | 201 | 2.07 |
| Ni1.6/La-Al2O3 | 109 | 27 | 136 | 1.62 |
| Co0/La-Al2O3 | 50 | 241 | 291 | 4.55 |
| Co0.2/La-Al2O3 | 63 | 38 | 101 | 1.44 |
| Co0.5/La-Al2O3 | 56 | 35 | 91 | 1.12 |
| Co1.0/La-Al2O3 | 35 | 22 | 57 | 0.62 |
| Co1.6/La-Al2O3 | 35 | 19 | 54 | 0.71 |
| Active Metal | Ni | Co | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PVA Content (%) | 0.0 | 0.3 | 0.7 | 1.0 | 1.3 | 1.6 | 0.0 | 0.2 | 0.5 | 1.0 | 1.6 |
| (°C) | 337 | 328 | 327 | 321 | 326 | 327 | 382 | 365 | 355 | 368 | 374 |
| (°C) | 432 | 419 | 420 | 411 | 420 | 418 | 501 | 492 | 482 | 488 | 491 |
| (°C) | 428 | 419 | 424 | 415 | 412 | 414 | 480 | 471 | 465 | 471 | 478 |
| (%) | 78.4 | 80.2 | 80.5 | 80.9 | 80.0 | 80.7 | 64.8 | 66.1 | 68.4 | 66.8 | 66.5 |
| (%) | 74.6 | 74.8 | 76.3 | 74.2 | 73.5 | 74.1 | 52.9 | 55.5 | 58.9 | 57.5 | 57.1 |
| (%) | 6.6 | 8.5 | 7.3 | 9.5 | 9.5 | 9.5 | 13.0 | 12.9 | 11.5 | 10.6 | 10.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarrete, L.F.; Atienza-Martínez, M.; Reyero, I.; Urroz, J.C.; Amorrortu, O.; Sanz, O.; Montes, M.; Garcés, S.I.; Bimbela, F.; Gandía, L.M. Comparative Study of Supported Ni and Co Catalysts Prepared Using the All-in-One Method in the Hydrogenation of CO2: Effects of Using (Poly)Vinyl Alcohol (PVA) as an Additive. Catalysts 2024, 14, 47. https://doi.org/10.3390/catal14010047
Navarrete LF, Atienza-Martínez M, Reyero I, Urroz JC, Amorrortu O, Sanz O, Montes M, Garcés SI, Bimbela F, Gandía LM. Comparative Study of Supported Ni and Co Catalysts Prepared Using the All-in-One Method in the Hydrogenation of CO2: Effects of Using (Poly)Vinyl Alcohol (PVA) as an Additive. Catalysts. 2024; 14(1):47. https://doi.org/10.3390/catal14010047
Chicago/Turabian StyleNavarrete, Luisa F., María Atienza-Martínez, Inés Reyero, José Carlos Urroz, Oihana Amorrortu, Oihane Sanz, Mario Montes, Siby I. Garcés, Fernando Bimbela, and Luis M. Gandía. 2024. "Comparative Study of Supported Ni and Co Catalysts Prepared Using the All-in-One Method in the Hydrogenation of CO2: Effects of Using (Poly)Vinyl Alcohol (PVA) as an Additive" Catalysts 14, no. 1: 47. https://doi.org/10.3390/catal14010047
APA StyleNavarrete, L. F., Atienza-Martínez, M., Reyero, I., Urroz, J. C., Amorrortu, O., Sanz, O., Montes, M., Garcés, S. I., Bimbela, F., & Gandía, L. M. (2024). Comparative Study of Supported Ni and Co Catalysts Prepared Using the All-in-One Method in the Hydrogenation of CO2: Effects of Using (Poly)Vinyl Alcohol (PVA) as an Additive. Catalysts, 14(1), 47. https://doi.org/10.3390/catal14010047









