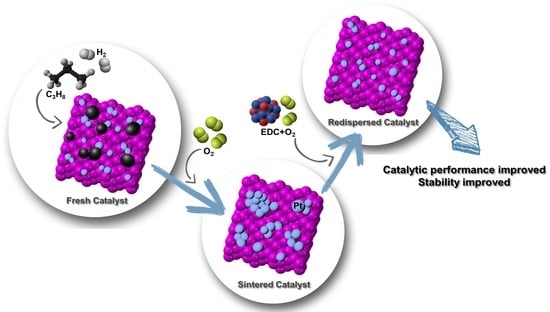
Structure Robustness of Highly Dispersed Pt/Al2O3 Catalyst for Propane Dehydrogenation during Oxychlorination Regeneration Process
Abstract
1. Introduction
2. Results
2.1. Characterization
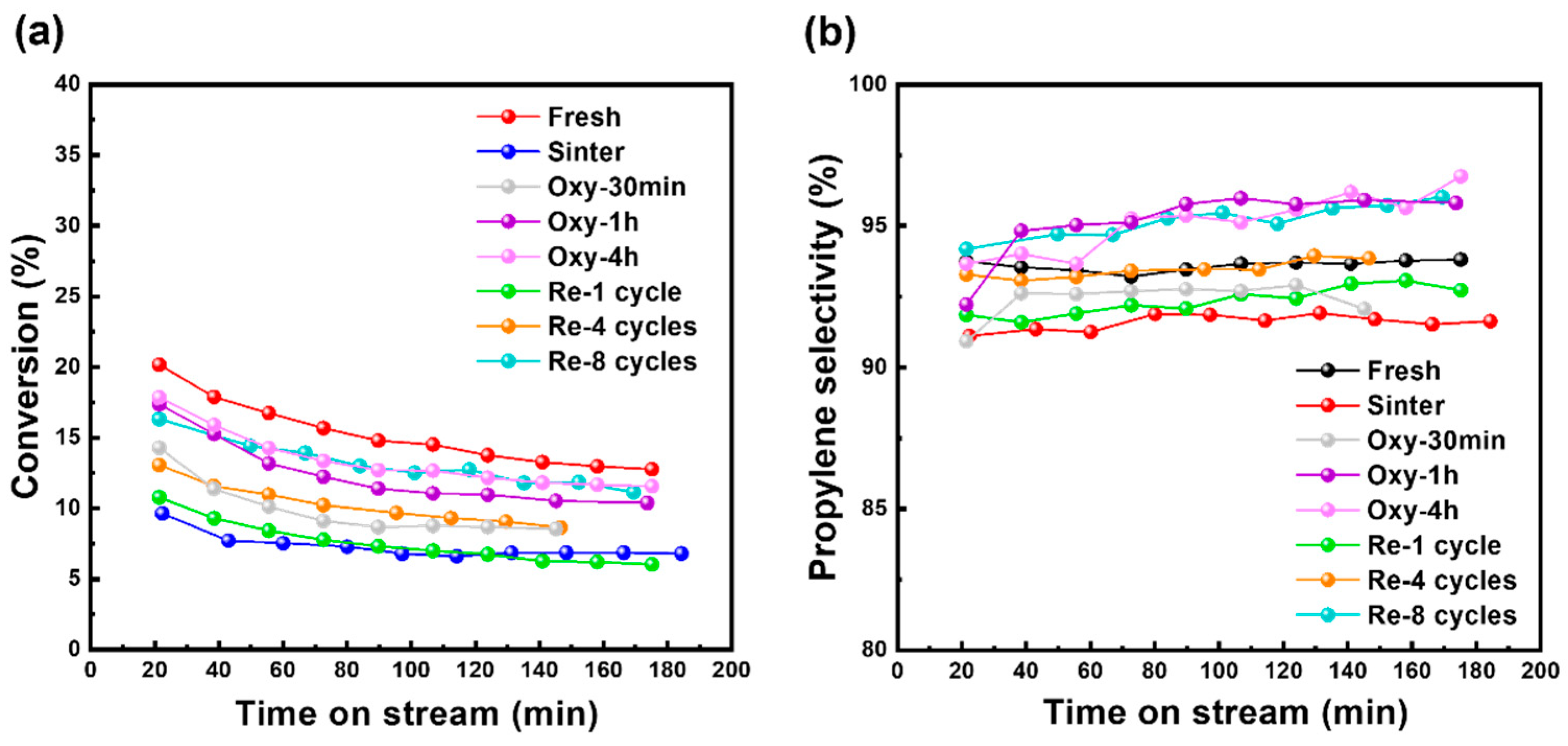
2.2. Catalytic Performance
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Otroshchenko, T.; Jiang, G.; Kondratenko, V.A.; Rodemerck, U.; Kondratenko, E.V. Current status and perspectives in oxidative, non-oxidative and CO2-mediated dehydrogenation of propane and isobutane over metal oxide catalysts. Chem. Soc. Rev. 2021, 50, 473–527. [Google Scholar] [CrossRef]
- Sattler, J.J.H.B.; Ruiz-Martinez, J.; Santillan-Jimenez, E.; Weckhuysen, B.M. Catalytic Dehydrogenation of Light Alkanes on Metals and Metal Oxides. Chem. Rev. 2014, 114, 10613–10653. [Google Scholar] [CrossRef]
- Dai, Y.; Gao, X.; Wang, Q.; Wan, X.; Zhou, C.; Yang, Y. Recent progress in heterogeneous metal and metal oxide catalysts for direct dehydrogenation of ethane and propane. Chem. Soc. Rev. 2021, 50, 5590–5630. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.H.; Jung, K.-D.; Kim, W.-I.; Um, B.-H.; Shin, C.-H.; Oh, K.; Koh, H.L. Effect of oxychlorination treatment on the regeneration of Pt-Sn/Al2O3catalyst for propane dehydrogenation. Res. Chem. Intermed. 2016, 42, 351–365. [Google Scholar] [CrossRef]
- Behafarid, F.; Roldan Cuenya, B. Towards the Understanding of Sintering Phenomena at the Nanoscale: Geometric and Environmental Effects. Top. Catal. 2013, 56, 1542–1559. [Google Scholar] [CrossRef]
- Chen, S.; Chang, X.; Sun, G.; Zhang, T.; Xu, Y.; Wang, Y.; Pei, C.; Gong, J. Propane dehydrogenation: Catalyst development, new chemistry, and emerging technologies. Chem. Soc. Rev. 2021, 50, 3315–3354. [Google Scholar] [CrossRef]
- Choi, Y.S.; Oh, K.; Jung, K.-D.; Kim, W.-I.; Koh, H.L. Regeneration of Pt-Sn/Al2O3 Catalyst for Hydrogen Production through Propane Dehydrogenation Using Hydrochloric Acid. Catalysts 2020, 10, 898. [Google Scholar] [CrossRef]
- Masoudian, S.K.; Sadighi, S.; Abbasi, A.; Salehirad, F.; Fazlollahi, A. Regeneration of a Commercial Catalyst for the Dehydrogenation of Isobutane to Isobutene. Chem. Eng. Technol. 2013, 36, 1593–1598. [Google Scholar] [CrossRef]
- Zhao, F.-C.; Yang, H.; Sui, Z.-J.; Zhu, Y.-A.; Chen, D.; Zhou, X.-G. Self-adaptive structure and catalytic performance of the Pt–Sn/Al2O3 propane dehydrogenation catalyst regenerated by dichloroethane oxychlorination. Catal. Sci. Technol. 2022, 12, 7171–7181. [Google Scholar] [CrossRef]
- Anstice, P.J.C.; Becker, S.M.; Rochester, C.H. The effects of Cl-induced alloying in Pt-Sn/Al2O3 catalysts on butane/H-2 reactions. Catal. Lett. 2001, 74, 9–15. [Google Scholar] [CrossRef]
- Rochlitz, L.; Pessemesse, Q.; Fischer, J.W.A.; Klose, D.; Clark, A.H.; Plodinec, M.; Jeschke, G.; Payard, P.-A.; Coperet, C. A Robust and Efficient Propane Dehydrogenation Catalyst from Unexpectedly Segregated Pt2Mn Nanoparticles. J. Am. Chem. Soc. 2022, 144, 13384–13393. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Zhen, S.; Sun, G.; Pei, C.; Zhao, Z.-J.; Gong, J. Support stabilized PtCu single-atom alloys for propane dehydrogenation. Chem. Sci. 2022, 13, 9537–9543. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, B.; Wang, X. High active and stable structure of PtBi0.5K4/Si-Beta catalyzing propane dehydrogenation. Chem. Eng. J. 2023, 474, 145894. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, Z.; Xing, S.; Zhang, J.; Zhao, S.; Xiong, M.; Luo, J.; Qin, Y.; Gao, Z. Small-Molecule Modification Provides Pt Nucleation Sites for Enhanced Propane Dehydrogenation Performance. J. Phys. Chem. C 2023, 127, 5754–5762. [Google Scholar] [CrossRef]
- Wang, G.; Yin, C.; Feng, F.; Zhang, Q.; Fu, H.; Bing, L.; Wang, F.; Han, D. Recent Progress on Catalyst Supports for Propane Dehydrogenation. Curr. Nanosci. 2023, 19, 473–483. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Liu, H.; Xiong, C.; Hu, P.; Wang, H.; Chen, S.; Ji, H. Enhanced performance for propane dehydrogenation through Pt clusters alloying with copper in zeolite. Nano Res. 2023, 16, 6537–6543. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, B.; Liu, G. Metal-based catalysts for the non-oxidative dehydrogenation of light alkanes to light olefins. React. Chem. Eng. 2021, 6, 9–26. [Google Scholar] [CrossRef]
- Wang, G.-D.; Jiang, J.-W.; Sui, Z.-J.; Zhu, Y.-A.; Zhou, X.-G. Kinetic Promotion Effect of Hydrogen and Dimethyl Disulfide Addition on Propane Dehydrogenation over the Pt–Sn–K/Al2O3 Catalyst. ACS Omega 2022, 7, 30773–30781. [Google Scholar] [CrossRef]
- Morgan, K.; Goguet, A.; Hardacre, C. Metal Redispersion Strategies for Recycling of Supported Metal Catalysts: A Perspective. ACS Catal. 2015, 5, 3430–3445. [Google Scholar] [CrossRef]
- Borgna, A.; Garetto, T.F.; Apesteguía, C.R.; Le Normand, F.; Moraweck, B. Sintering of Chlorinated Pt/γ-Al2O3 Catalysts: An In Situ Study by X-ray Absorption Spectroscopy. J. Catal. 1999, 186, 433–441. [Google Scholar] [CrossRef]
- Borgna, A.; Le Normand, F.; Garetto, T.F.; Apesteguía, C.R.; Moraweck, B. X-ray Absorption Spectroscopy: A powerful tool to investigate intermediate species during sintering-redispersion of metallic catalysts. In Studies in Surface Science and Catalysis; Delmon, B., Froment, G.F., Eds.; Elsevier: Amsterdam, The Netherlands, 1999; Volume 126, pp. 179–186. [Google Scholar]
- Hung, C.-C.; Yeh, C.-Y.; Shih, C.-C.; Chang, J.-R. Oxychlorination Redispersion of Pt Catalysts: Surface Species and Pt-Support Interactions Characterized by X-ray Absorption and FT-IR Spectroscopy. Catalysts 2019, 9, 362. [Google Scholar] [CrossRef]
- Dai, Y.; Lu, P.; Cao, Z.; Campbell, C.T.; Xia, Y. The physical chemistry and materials science behind sinter-resistant catalysts. Chem. Soc. Rev. 2018, 47, 4314–4331. [Google Scholar] [CrossRef]
- Monzón, A.; Garetto, T.F.; Borgna, A. Sintering and redispersion of Pt/γ-Al2O3 catalysts: A kinetic model. Appl. Catal. A Gen. 2003, 248, 279–289. [Google Scholar] [CrossRef]
- Susu, A.A.; Ogogo, E.O.; Ngomo, H.M. The effect of sintering-redispersion on the selective aromatic yield on supported platinum catalysts. Chem. Eng. Res. Des. 2006, 84, 664–676. [Google Scholar] [CrossRef]
- Gracia, F.J.; Miller, J.T.; Kropf, A.J.; Wolf, E.E. Kinetics, FTIR, and Controlled Atmosphere EXAFS Study of the Effect of Chlorine on Pt-Supported Catalysts during Oxidation Reactions. J. Catal. 2002, 209, 341–354. [Google Scholar] [CrossRef]
- Anderson, J.A.; Fernandez-Garcia, M. Use of IR and XANES to monitor catalysts during preparation, regeneration and reaction. Chem. Eng. Res. Des. 2000, 78, 935–942. [Google Scholar] [CrossRef]
- Arteaga, G.J.; Anderson, J.A.; Becker, S.M.; Rochester, C.H. Influence of oxychlorination treatment on the surface and bulk properties of a Pt–Sn/Al2O3 catalyst. J. Mol. Catal. A Chem. 1999, 145, 183–201. [Google Scholar] [CrossRef]
- Le Normand, F.; Borgna, A.; Garetto, T.F.; Apesteguia, C.R.; Moraweck, B. Redispersion of Sintered Pt/Al2O3 Naphtha Reforming Catalysts: An in Situ Study Monitored by X-ray Absorption Spectroscopy. J. Phys. Chem. 1996, 100, 9068–9076. [Google Scholar] [CrossRef]
- Rojek, J.; Nosewicz, S.; Maździarz, M.; Kowalczyk, P.; Wawrzyk, K.; Lumelskyj, D. Modeling of a Sintering Process at Various Scales. Procedia Eng. 2017, 177, 263–270. [Google Scholar] [CrossRef]
- Li, Z.; Ji, S.; Liu, Y.; Cao, X.; Tian, S.; Chen, Y.; Niu, Z.; Li, Y. Well-Defined Materials for Heterogeneous Catalysis: From Nanoparticles to Isolated Single-Atom Sites. Chem. Rev. 2020, 120, 623–682. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Sun, L.-L.; Sui, Z.-J.; Zhu, Y.-A.; Ye, G.-H.; Chen, D.; Zhou, X.-G.; Yuan, W.-K. Coke Formation on Pt–Sn/Al2O3 Catalyst for Propane Dehydrogenation. Ind. Eng. Chem. Res. 2018, 57, 8647–8654. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.Z.; Mei, D.; Yi, C.W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Szanyi, J. Coordinatively Unsaturated Al3+ Centers as Binding Sites for Active Catalyst Phases of Platinum on gamma-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef]
- Virnovskaia, A.; Morandi, S.; Rytter, E.; Ghiotti, G.; Olsbye, U. Characterization of Pt,Sn/Mg(Al)O catalysts for light alkane dehydrogenation by FT-IR Spectroscopy and catalytic measurements. J. Phys. Chem. C 2007, 111, 14732–14742. [Google Scholar] [CrossRef]
- Arteaga, G.J.; Anderson, J.A.; Rochester, C.H. FTIR study of CO adsorption on coked Pt-Sn/Al2O3 catalysts. Catal. Lett. 1999, 58, 189–194. [Google Scholar] [CrossRef]
- Kaylor, N.; Davis, R.J. Propane dehydrogenation over supported Pt-Sn nanoparticles. J. Catal. 2018, 367, 181–193. [Google Scholar] [CrossRef]
- Pieck, C.L.; Jablonski, E.L.; Parera, J.M. Sintering-redispersion of Pt-Re/Al2O3 during regeneration. Appl. Catal. 1990, 62, 47–60. [Google Scholar] [CrossRef]
- Serrano-Ruiz, J.C.; Huber, G.W.; Sánchez-Castillo, M.A.; Dumesic, J.A.; Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A. Effect of Sn addition to Pt/CeO2–Al2O3 and Pt/Al2O3 catalysts: An XPS, 119Sn Mössbauer and microcalorimetry study. J. Catal. 2006, 241, 378–388. [Google Scholar] [CrossRef]
- Lieske, H.; Lietz, G.; Spindler, H.; Völter, J. Reactions of platinum in oxygen- and hydrogen-treated Ptγ-Al2O3 catalysts: I. Temperature-programmed reduction, adsorption, and redispersion of platinum. J. Catal. 1983, 81, 8–16. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, H.; Jiang, J.; Sui, Z.; Zhu, Y.; Chen, D.; Zhou, X. Size Dependence of Pt Catalysts for Propane Dehydrogenation: From Atomically Dispersed to Nanoparticles. ACS Catal. 2020, 10, 12932–12942. [Google Scholar] [CrossRef]
- Sattler, A.; Paccagnini, M.; Liu, L.; Gomez, E.; Klutse, H.; Burton, A.W.; Corma, A. Assessment of metal-metal interactions and catalytic behavior in platinum-tin bimetallic subnanometric clusters by using reactive characterizations. J. Catal. 2021, 404, 393–399. [Google Scholar] [CrossRef]
- Campbell, C.T. The Energetics of Supported Metal Nanoparticles: Relationships to Sintering Rates and Catalytic Activity. Acc. Chem. Res. 2013, 46, 1712–1719. [Google Scholar] [CrossRef]
- Yang, M.L.; Zhu, Y.A.; Fan, C.; Sui, Z.J.; Chen, D.; Zhou, X.G. DFT study of propane dehydrogenation on Pt catalyst: Effects of step sites. Phys. Chem. Chem. Phys. 2011, 13, 3257–3267. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zong, X.; Patra, A.; Caratzoulas, S.; Vlachos, D.G. Propane Dehydrogenation on PtxSny (x, y ≤ 4) Clusters on Al2O3(110). Acs Catal. 2023, 13, 2802–2812. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, Y.; Wang, J. DFT study on the regeneration of Pt/gamma-Al2O3 catalyst: The effect of chlorine on the redispersion of metal species. Appl. Surf. Sci. 2021, 545, 148988. [Google Scholar] [CrossRef]
- Hu, S.; Li, W.-X. Sabatier principle of metal-support interaction for design of ultrastable metal nanocatalysts. Science 2021, 374, 1360–1365. [Google Scholar] [CrossRef]
- Kamiuchi, N.; Taguchi, K.; Matsui, T.; Kikuchi, R.; Eguchi, K. Sintering and redispersion of platinum catalysts supported on tin oxide. Appl. Catal. B Environ. 2009, 89, 65–72. [Google Scholar] [CrossRef]
- DeLaRiva, A.T.; Hansen, T.W.; Challa, S.R.; Datye, A.K. In situ Transmission Electron Microscopy of catalyst sintering. J. Catal. 2013, 308, 291–305. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, T.; Xu, Z.; Yue, Y.; Lin, M.; Zhu, H. Pt-Sn clusters anchored at Al-penta(3+) sites as a sinter-resistant and regenerable catalyst for propane dehydrogenation. J. Energy Chem. 2022, 65, 293–301. [Google Scholar] [CrossRef]
- Zhang, W.; Ma, H.; Wang, H.; Jiang, J.; Sui, Z.; Zhu, Y.-A.; Chen, D.; Zhou, X. Tuning partially charged Ptδ+ of atomically dispersed Pt catalysts toward superior propane dehydrogenation performance. Catal. Sci. Technol. 2021, 11, 7840–7843. [Google Scholar] [CrossRef]
- Zeeshan, M.; Chang, Q.-Y.; Zhang, J.; Hu, P.; Sui, Z.-J.; Zhou, X.-G.; Chen, D.; Zhu, Y.-A. Effects of Oxygen Vacancy and Pt Doping on the Catalytic Performance of CeO2 in Propane Dehydrogenation: A First-Principles Study. Chin. J. Chem. 2021, 39, 2391–2402. [Google Scholar] [CrossRef]
- Zhang, R.; Chang, Q.-Y.; Ma, F.; Zeeshan, M.; Yang, M.-L.; Sui, Z.-J.; Chen, D.; Zhou, X.-G.; Zhu, Y.-A. Enhanced catalytic performance of transition metal-doped Cr2O3 catalysts for propane dehydrogenation: A microkinetic modeling study. Chem. Eng. J. 2022, 446, 136913. [Google Scholar] [CrossRef]
- Fiedorow, R.M.J.; Wanke, S.E. The sintering of supported metal catalysts: I. Redispersion of supported platinum in oxygen. J. Catal. 1976, 43, 34–42. [Google Scholar] [CrossRef]
- Straguzzi, G.I.; Aduriz, H.R.; Gigola, C.E. Redispersion of platinum on alumina support. J. Catal. 1980, 66, 171–183. [Google Scholar] [CrossRef]
- Lee, T.J.; Kim, Y.G. Redispersion of supported platinum catalysts. J. Catal. 1984, 90, 279–291. [Google Scholar] [CrossRef]
- Otto, K.; Weber, W.H.; Graham, G.W.; Shyu, J.Z. Identification of dispersed platinum on -alumina by laser-Raman spectroscopy. Appl. Surf. Sci. 1989, 37, 250–257. [Google Scholar] [CrossRef]
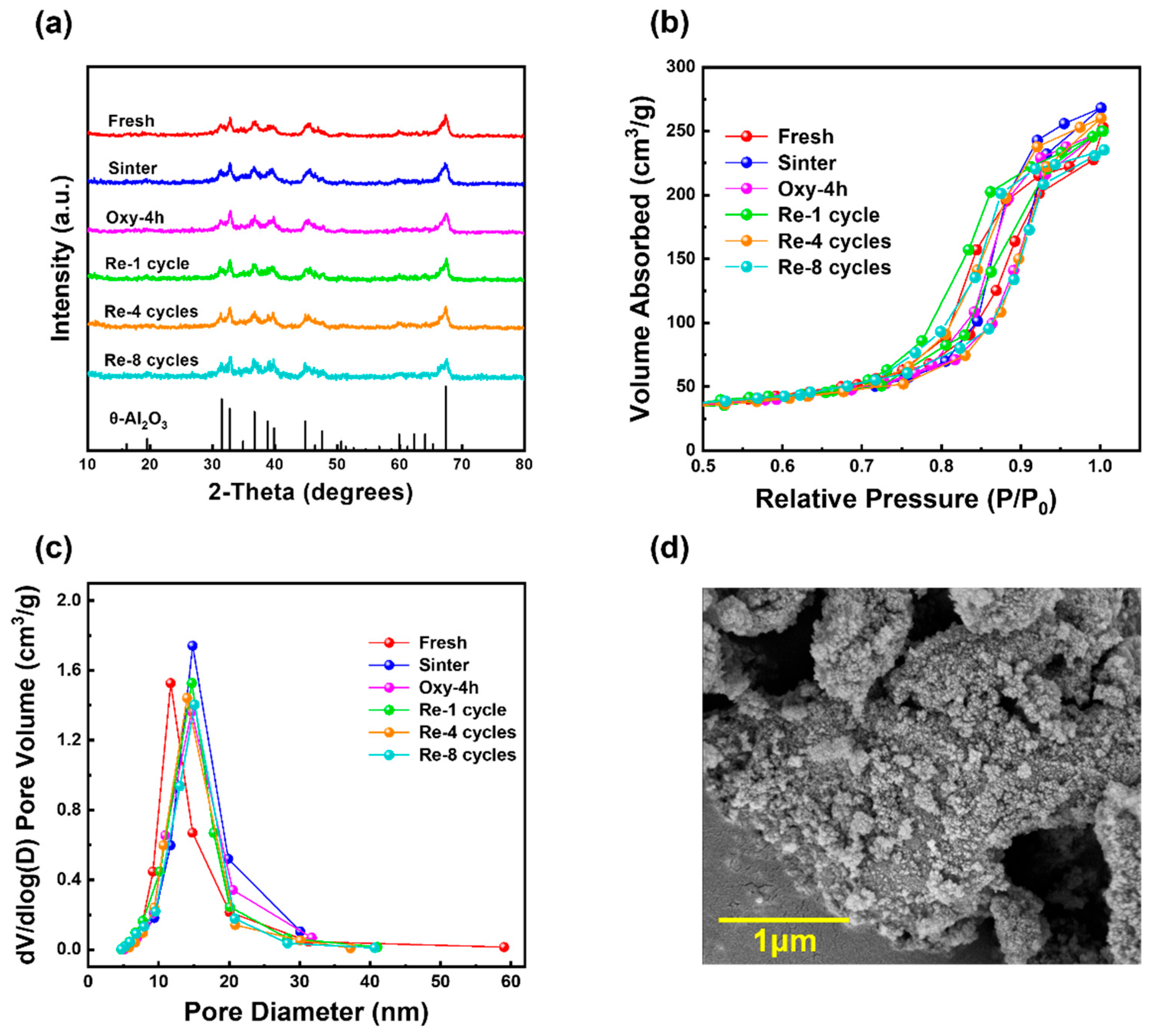
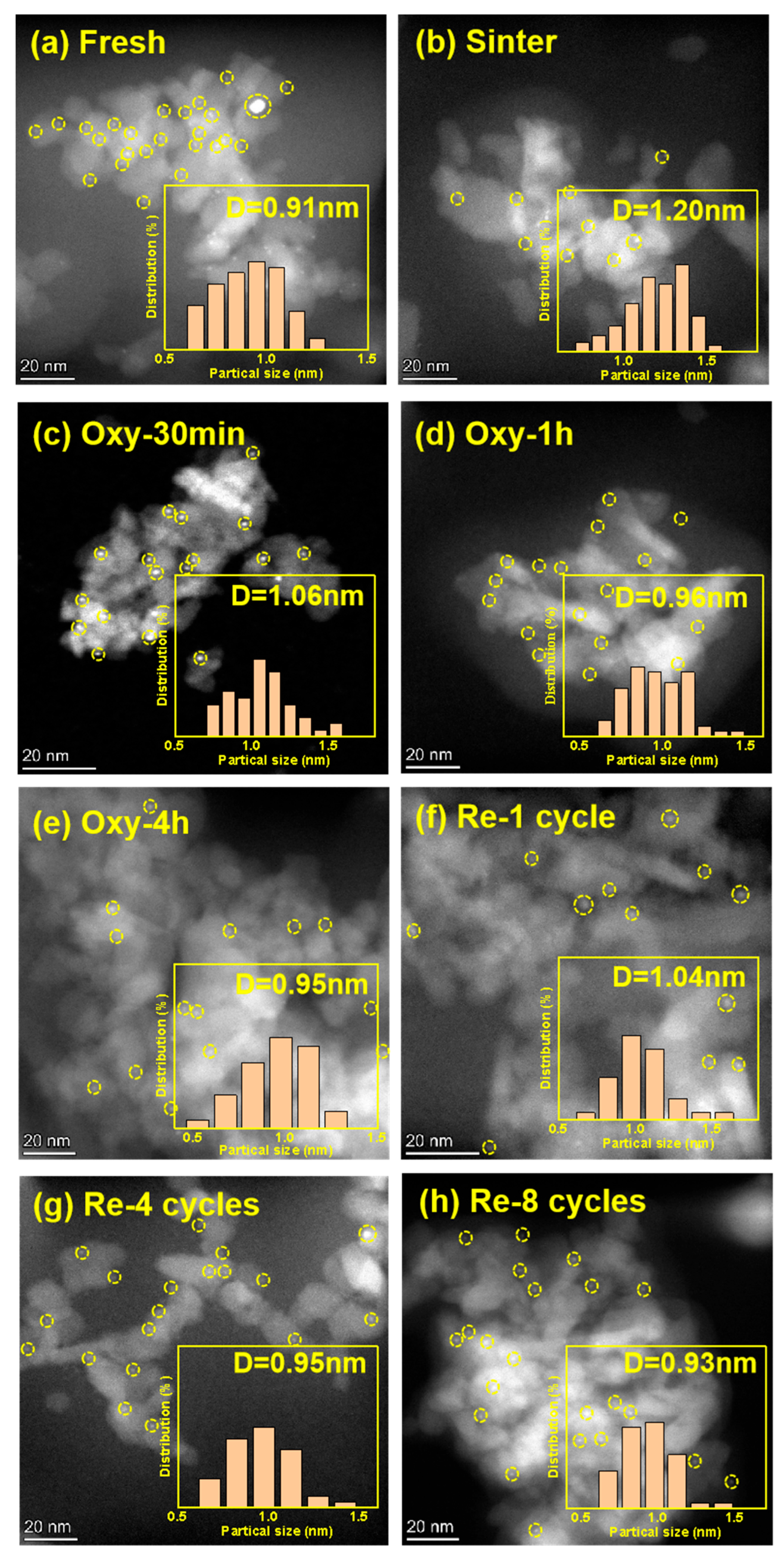

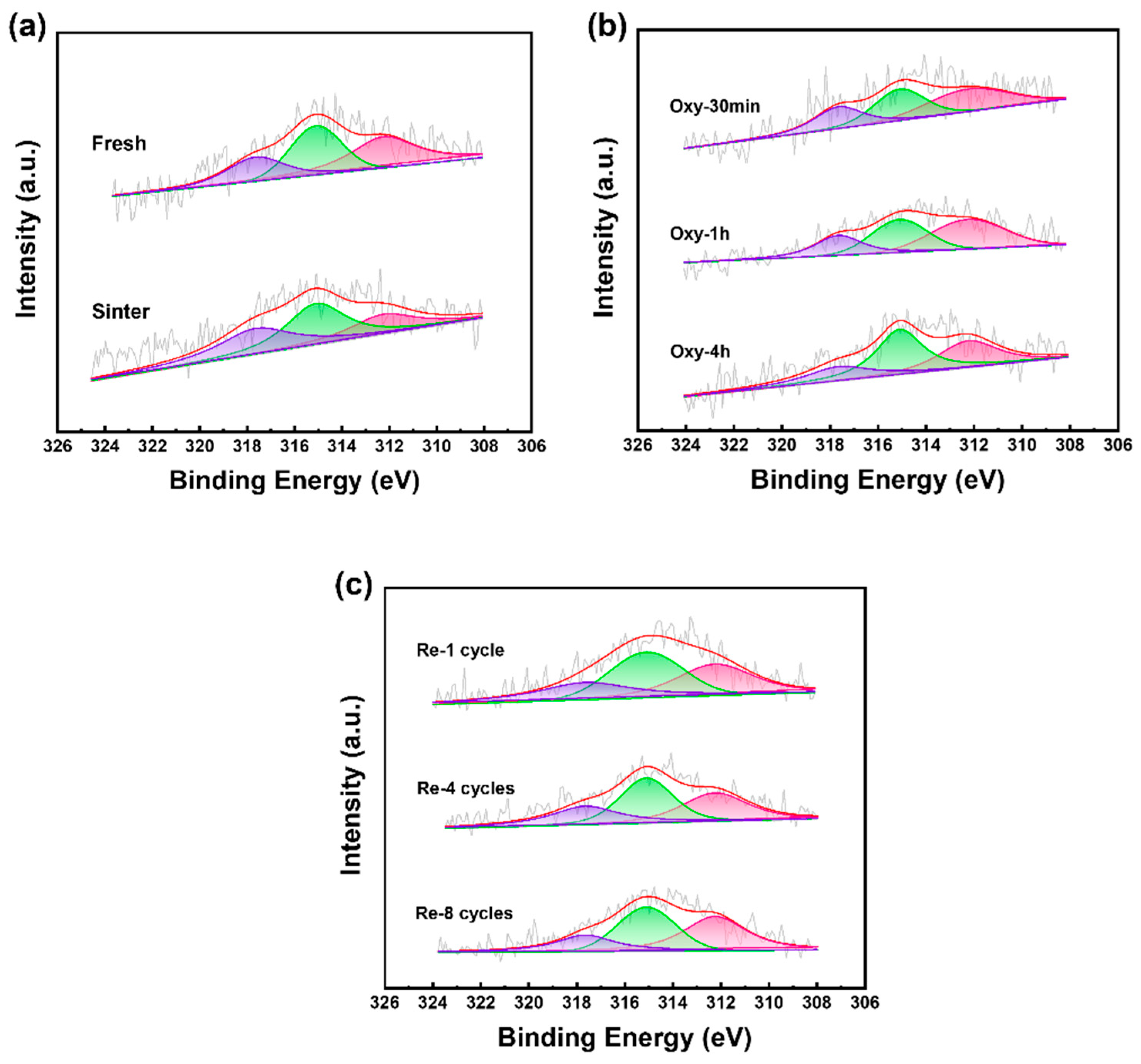
| Sample | SBET 1 (m2/g) | Dpore 1 (nm) | Vpore 1 (cm3/g) | Pt Dispersion 2 (%) | Pt4+ Ratio 3 (%) | TOF (s−1) |
|---|---|---|---|---|---|---|
| Fresh | 88.1 | 12.5 | 0.35 | 80.2 | 24.1 | 1.96 |
| Sintered | 78.9 | 14.8 | 0.36 | 48.3 | 35.3 | 1.58 |
| Oxy-30 min | 82.3 | 13.4 | 0.37 | 60.5 | 27.0 | 1.84 |
| Oxy-1 h | 85.1 | 14.0 | 0.36 | 70.8 | 23.3 | 1.88 |
| Oxy-4 h | 81.7 | 13.8 | 0.33 | 73.5 | 22.0 | 1.86 |
| Re-1 cycle | 87.5 | 14.3 | 0.39 | 59.5 | 28.0 | 1.42 |
| Re-4 cycles | 84.8 | 14.0 | 0.37 | 69.4 | 26.9 | 1.48 |
| Re-8 cycles | 83.4 | 14.2 | 0.39 | 72.4 | 21.1 | 1.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Sun, Y.; Zhou, Y.; Sui, Z.; Dai, Y.; Zhu, Y.; Zhou, X. Structure Robustness of Highly Dispersed Pt/Al2O3 Catalyst for Propane Dehydrogenation during Oxychlorination Regeneration Process. Catalysts 2024, 14, 48. https://doi.org/10.3390/catal14010048
Dong L, Sun Y, Zhou Y, Sui Z, Dai Y, Zhu Y, Zhou X. Structure Robustness of Highly Dispersed Pt/Al2O3 Catalyst for Propane Dehydrogenation during Oxychlorination Regeneration Process. Catalysts. 2024; 14(1):48. https://doi.org/10.3390/catal14010048
Chicago/Turabian StyleDong, Lu, Yitong Sun, Yifan Zhou, Zhijun Sui, Yunsheng Dai, Yian Zhu, and Xinggui Zhou. 2024. "Structure Robustness of Highly Dispersed Pt/Al2O3 Catalyst for Propane Dehydrogenation during Oxychlorination Regeneration Process" Catalysts 14, no. 1: 48. https://doi.org/10.3390/catal14010048
APA StyleDong, L., Sun, Y., Zhou, Y., Sui, Z., Dai, Y., Zhu, Y., & Zhou, X. (2024). Structure Robustness of Highly Dispersed Pt/Al2O3 Catalyst for Propane Dehydrogenation during Oxychlorination Regeneration Process. Catalysts, 14(1), 48. https://doi.org/10.3390/catal14010048