Insights into Photo/Electrocatalysts for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS) by Advanced Oxidation Processes
Abstract
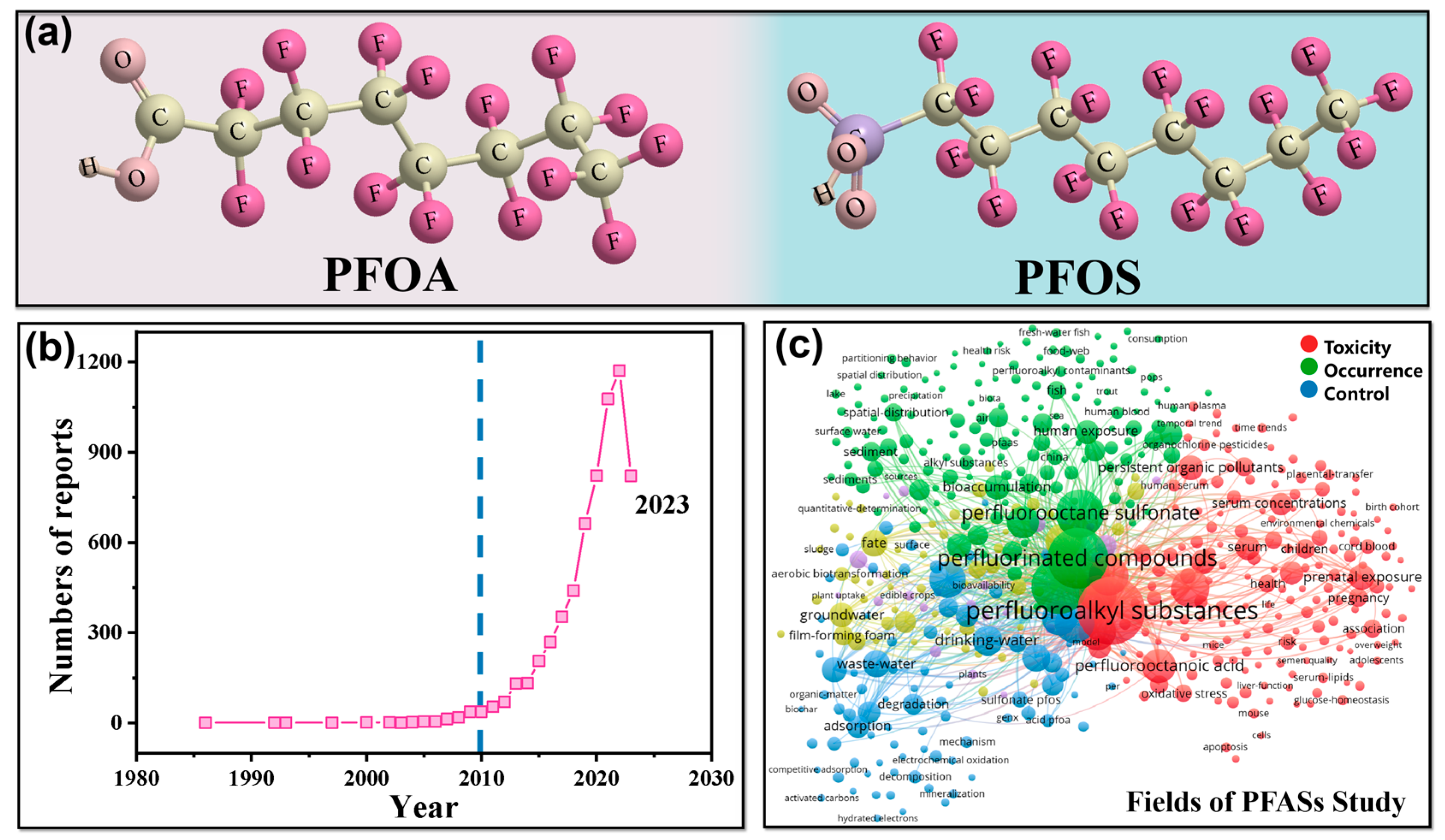
:1. Introduction
2. Fundamentals of PFAS Degradation by AOPs
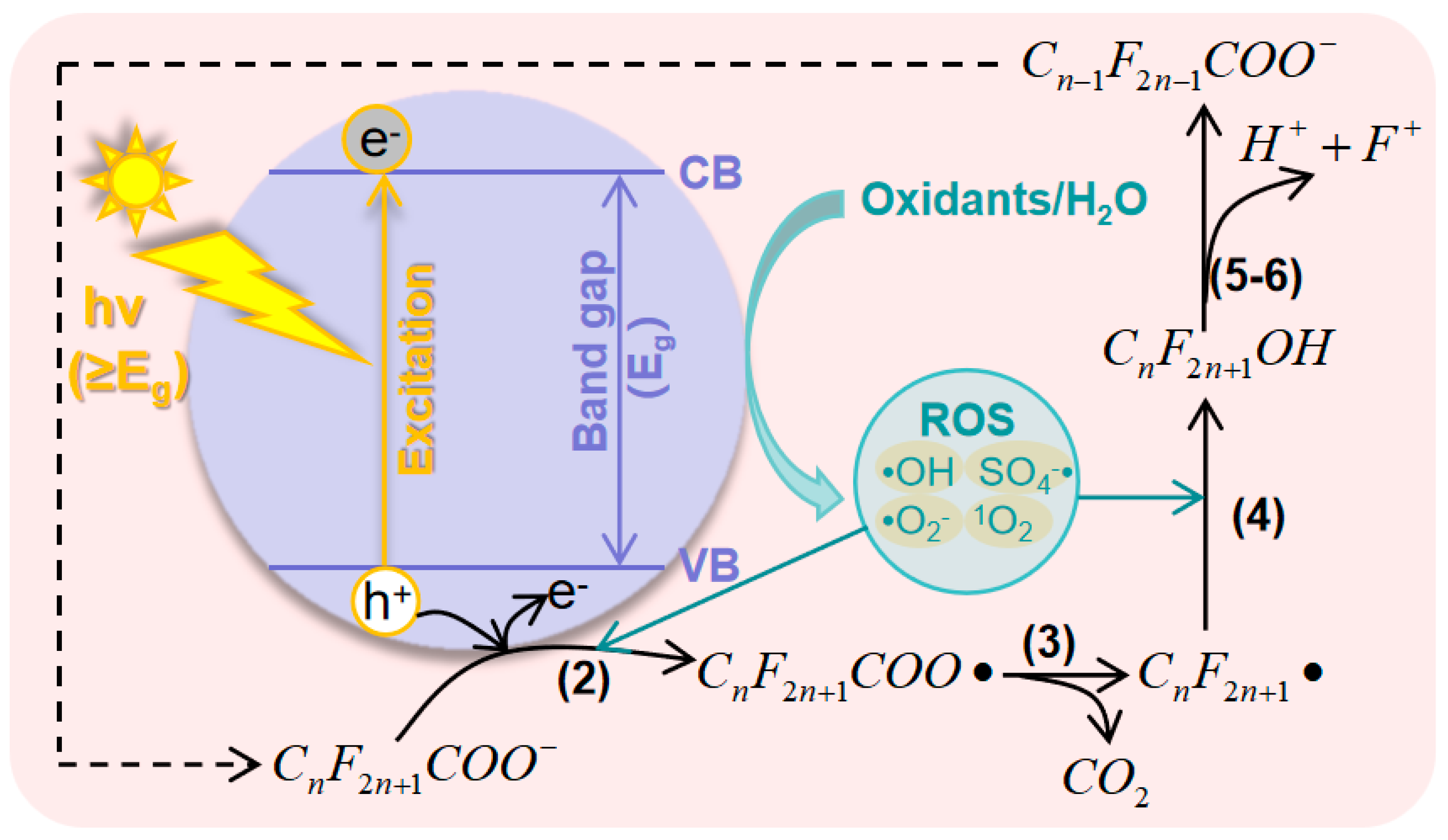
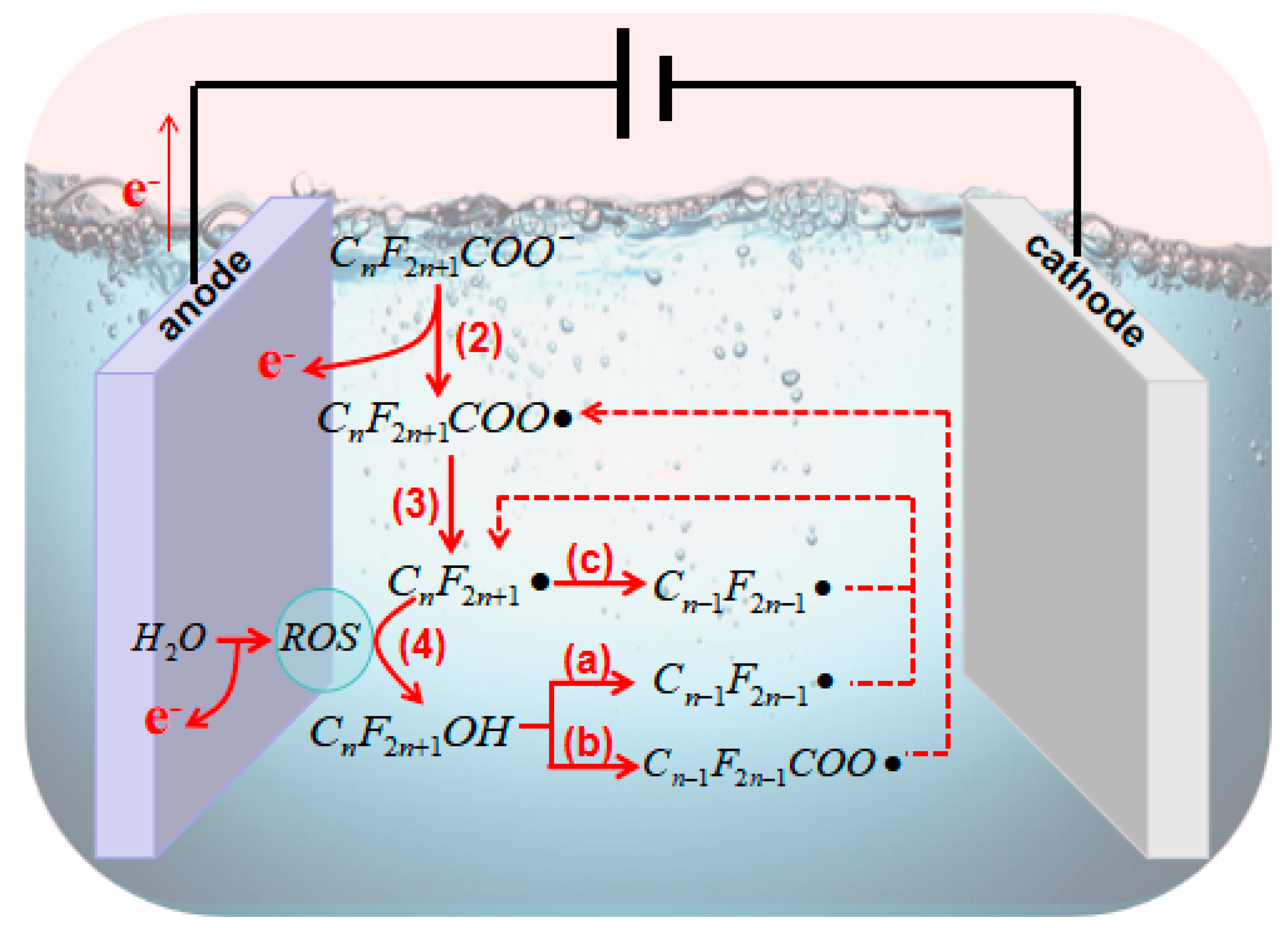
2.1. General Pathways and ROS for PFAS Degradation
2.2. Principles of Photocatalytic AOPs for PFAS
2.3. Principles of Electrocatalytic AOPs for PFASs
3. Photocatalysts in AOPs for PFAS Degradation
3.1. Metal Oxide-Based Materials
3.2. Bi-Based Materials
3.3. Other Compounds and Composites

- (1)
- The construction of defect sites through the introduction of oxygen vacancies and element doping. Defect sites serve as active sites for adsorption and catalytic reactions. Additionally, they broaden the light absorption range and enhance the light absorption capability by modifying the electronic and band structure.
- (2)
- The construction of heterojunctions by synthesizing composite materials. This involves combining two or more semiconductors with suitable band structures. The overlapping or coupling of these semiconductors enables the migration and separation of photo-generated charge carriers, effectively suppressing the recombination of electrons and holes. This leads to an enhanced photocatalytic efficiency.
- (3)
- The deposition of photocatalysts onto carbon material. The use of carbon nanomaterials, which possess a larger surface area and a porous structure, provides more active adsorption sites and improves catalyst stability. Furthermore, carbon materials facilitate interface charge transfer, prolong the lifetime of photo-generated charged carriers, and enhance visible light absorption by adjusting the band gap and acting as sensitizers.
- (4)
- The modulation of the crystal plane structure and electronic structure through the adjustment of the catalyst preparation methods. Exposing crystal planes with high surface energy and reactivity facilitates the separation of photo-generated electrons and holes, thus enhancing the photocatalytic activity of the catalysts.
| Photocatalysts | Target PFAS | [PFAS]0 | Experimental Conditions [Catalyst], Light Wavelength/Strength, pH | Removal Efficiency | Structural Engineering | Ref. | |
|---|---|---|---|---|---|---|---|
| Metal oxide-based materials | TiO2 | PFOA | 59 mg·L−1 | 0.25 g·L−1, 400~770 nm/300 W, pH = 3 TiO2/UV/PMS system, ([PMS] = 0.75 g·L−1) | 100% (8 h) | none | [75] |
| TiO2 nanotubes | PFOA | 50 mg·L−1 | 0.125 g·L−1, 254 nm/400 W, pH = 4 | 85% (24 h) | morphology control | [113] | |
| Pb−TiO2 | PFOA | 50 mg·L−1 | 0.5 g·L−1, 254 nm/400 W, pH = 5 | 50% (1.3 h) | metal-doping | [76] | |
| Pt−TiO2 | PFOA | 60 mg·L−1 | 0.5 g·L−1, 365 nm/125 W, pH = 3 | 100% (7 h) | metal-doping | [80] | |
| Cu−TiO2 | PFOA | 50 mg·L−1 | 0.5 g·L−1, 254 nm/100 W, pH = 5 | 91% (12 h) | metal-doping | [79] | |
| Sb2O3/TiO2 | PFOA | 10 mg·L−1 | 2.5 g·L−1, 200~280 nm/4 W, pH = 4.4 | 81.7% (2 h) | heterojunction | [71] | |
| BN/TiO2 | PFOA | 100 μM | 0.5 g·L−1, 254 nm/4 W, pH = 3.2 | 97.6% (4 h) | heterojunction | [28] | |
| PLA−3DP TiO2 | 11PFASs | ng·L−1~mg·L−1 | N/A, 280~400 nm/1.0 mW, pH = 7.1 ± 1 | 80% (24 h) | morphology control | [25] | |
| TiO2−MWCNTs | PFOA | 30 mg·L−1 | 1.6 g·L−1, 365 nm/300 W, pH = 3 | 94% (8 h) | carbon material loading | [84] | |
| MWCNTs/C−TiO2 | PFOA | 2 mg·L−1 | 1.0 g·L−1, 420 nm/300 W, pH = 4.65 | 90% (3.5 h) | morphology control | [85] | |
| rGO/TiO2 | PFOA | 100 mg·L−1 | 1.0 g·L−1, 200~600 nm/150 W, pH = 7 | 93 ± 7% (12 h) | morphology control | [89] | |
| Ti3C2/TiO2 | PFOA | 20 μM | 0.2 g·L−1, 254 nm/4.5 W, pH = 3 | >99.9% (16 h) | heterojunction | [114] | |
| In2O3 | PFOA | 100 μM | 0.5 g·L−1, 254 nm, pH = 4.2 | 80% (4 h) | none | [46] | |
| g-C3N4/In2O3 | PFOA | 200 mg·L−1 | 0.4 g·L−1, 254 nm/500 W, pH = N/A | 91% (1 h) | carbon material loading | [93] | |
| In2O3−GRs | PFOA | 30 mg·L−1 | 0.4 g·L−1, 254 nm/15 W, pH = N/A | 100% (3 h) | carbon material loading | [115] | |
| CeO2/In2O3 | PFOA | 100 mg·L−1 | 0.4 g·L−1, 254nm/500 W, pH = 2.84 | 100% (1 h) | heterojunction | [94] | |
| MnOx−In2O3 | PFOA | 50 mg·L−1 | 0.5 g·L−1, visual light/500 W, pH = 3.8 | 99.8% (3 h) | heterojunction | [74] | |
| β-Ga2O3 | PFOA | 10 mg·L−1 | 0.5 g·L−1, 254 nm/50 W, pH = 7 | 98.8% (1.5 h) | none | [77] | |
| In−Ga2O3 | PFOA | 20 mg·L−1 | 0.5 g·L−1, 320 nm/200 W, pH = 7 | 100% (1 h) | metal-doping | [95] | |
| ZnO | PFOA | 10 mg·L−1 | 0.2 g·L−1, 254 nm/28 W, pH = 4.5 ZnO/UV/O3 system | 70.5% (4 h) | none | [116] | |
| ZnO−rGO | PFOA | 10 mg·L−1 | 0.2 g·L−1, 254 nm, pH = N/A O3/UV/ZnO−rGO/S2O82− system | 99.2% (4 h) | carbon material loading | [117] | |
| Bi-based materials | BiOX/TiO2 | PFOA | 10 mg·L−1 | 0.2 g·L−1, 254 nm/30 W, pH = 7 | 100% (8 h) | carbon material loading | [26] |
| BiOCl nanosheets | PFOA | 0.02 mM | 0.5 g·L−1, 254 nm/10 W, pH = 4.8 | 59.3% (24 h) | morphology control | [99] | |
| BiOI0.95Br0.05 | PFOA | 20 mg·L−1 | 0.4 g·L−1, 254 nm/300 W, pH = 7 | 96% (2 h) | crystal facet control | [97] | |
| BiOI/Bi5O7I | PFOA | 15 mg·L−1 | 0.5 g·L−1, 400~760 nm/800 W, pH = 3.0 | 80% (6 h) | heterojunction | [73] | |
| BiOCl/BiPO4 | PFOA | 20 mg·L−1 | 0.5 g·L−1, 254 nm/2 W, pH = 7 | 100% (45 h) | heterojunction | [118] | |
| BiOHP | PFOA | 0.5 mg·L−1 | 1.8 g·L−1, 254 nm/18 W, pH = 4.0 | 70%(20 min) | morphology control | [102] | |
| BiOHP/CS | PFOA | 0.2 mg·L−1 | 1.0 g·L−1, 254 nm/18 W, pH = 7.0 | >90% (1 h) | carbon material loading | [103] | |
| Other composites | Fe−BEA35 | PFOA | 48 μM | 1 g·L−1, 365 nm/4 W, 254 nm/4 W, pH = 3 | >99% (24 h) | metal-doping + morphology control | [107] |
| Pt/IONRs | PFOA | 200 mg·L−1 | 0.4 g·L−1, 254 nm/500 W, pH = 1.85 | 98% (1 h) | metal-doping + morphology control | [119] | |
| Fe/TNTs@AC | PFOA | 0.1 mg·L−1 | 1.0 g·L−1, 254 nm/21 W, pH = 3.0 | 90% (4 h) | metal-doping + carbon material loading | [90] | |
| Ga/TNTs@AC | PFOS | 100 μg·L−1 | 1 g·L−1, nm/200 W, pH = 7.0 ± 0.1 | 75% (4 h) | metal-doping + carbon material loading | [112] | |
| Zn−AlLDHs −BiOCl | PFOA | 0.5 mg·L−1 | 0.5 g·L−1, <350 nm/50 W, pH = 2.0 | 90% (6 h) | metal-doping + morphology control | [101] | |
| Pb−BiFeO3 /rGO | PFOA | 50 mg·L−1 | 0.1·L−1, 254 nm/5 W, pH = 2.0 | 69.6% (8 h) | metal-doping + carbon material loading | [106] | |
4. Electrocatalysts in AOPs for PFAS Degradation
4.1. BDD-Based Materials
4.2. Metal Oxide-Based Materials
4.3. Other Compounds and Composites

- (1)
- Enhancing the yield of ROS on the electrode by doping a specific functional substance onto the surface. Doping helps optimize the crystalline phase of the catalytic electrodes, promoting a more compact arrangement of particles and effectively enhancing the electrode’s ability to generate physically adsorbed ROS species.
- (2)
- Improving the surface mass transfer efficiency of the electrode through metal loading and the implementation of three-dimensional nanostructures. Metal-doping is an important strategy for reducing and improving electron transfer. Constructing 3D nanostructures on the electrode surface increases the specific surface area and active sites, thereby enhancing the migration of PFASs toward the electrode and improving their adsorption efficiency.
- (3)
- Enhancing the stability of the electrode by introducing functional intermediate layers. Incorporating the intermediate layer strengthens the interaction between the active layer and the substrate, preventing the detachment of the active layer, improving electrode stability, and extending the electrode’s lifespan.
| Electrocatalysts | Target PFAS | [PFAS]0 (Electrolyte Solution) | Experimental Conditions Surface Area (S), Interelectrode Gap (L), Electric Current Density (J) | Removal Efficiency | Structural Engineering | Ref. | |
|---|---|---|---|---|---|---|---|
| BDD-based materials | BDD | PFOS | 0.4 mM (10 mM NaClO4) | S = 25 cm2, L = 2 mm, J = 20 mA·cm−2, T = 22 °C, pH = 4 | 50% (5.3 min) | none | [127] |
| BDD (high boron) | PFOA +PFOS | 0.1 mg L−1 (100 mM PBS) | S = 10.5 cm2, L = 2.5 cm, J = 75 mA·cm−2, T = 20~25 °C, pH = 7.8 | 80%PFOA, 78%PFOS | doping (B) | [11] | |
| BND | PFOA | 50 mg L−1 (0.05 M Na2SO4) | S = 10.5 cm2, L = 2.5 cm, J = 4 mA·cm−2, pH = 4.8 | 77.4% (3 h) | doping (N) | [120] | |
| Si/BDD | PFOA | 0.2 mg L−1 (0.4 g L−1 Na2SO4) | S = 81 cm2, L = 1 cm, J = 25 mA·cm−2, pH = 11 | >90% (1 h) | metal-doping | [129] | |
| Ti/BDD | PFCAs | 0.25 mM (10 mM NaClO4) | S = 25 cm2, L = 1.5 cm, J = 10 mA·cm−2, pH = 3 | >95% (3 h) | metal-doping | [66] | |
| Metal-oxide based materials | Ti3+/TiO2−NTA | PFOA | 50 mg L−1 (20 mM Na2SO4) | S = 25 cm2, L = 1 cm, J = 2 mA·cm−2, pH = 3~11 | 98.1% (1.5 h) | doping + morphology control | [21] |
| Ti/SnO2−Sb | PFOA | 100 mg L−1 (10 mM NaClO4) | L = 1 cm, J = 10 mA·cm−2, T = 25 °C, pH = 5 | 90.3% (1.5 h) | co-doping | [63] | |
| Ti/SnO2−Sb/PbO2 | 91.1% (1.5 h) | metal-doping +intercalation | |||||
| Ti/SnO2−Sb/MnO2 | 31.7% (1.5 h) | metal-doping +intercalation | |||||
| Ti/SnO2−Sb/Bi2O3 | PFOS | 20 mg L−1 (1.4 g L−1 NaClO4) | S = 17.64 cm2, J = 6.8 mA·cm−2, T = 32 °C, pH = 6.94 | 23.8% (3.5 h) | metal-doping +intercalation | [145] | |
| Ti/SnO2−F | PFOA | 100 mg L−1 (10 mM NaClO4) | S = 25 mm2, L = 1 cm, J = 100 mA·cm−2, T = 25 °C, pH = 7 | 96.5% (0.5 h) | co-doping | [122] | |
| SnO2−Sb/CA | PFOA | 100 mg L−1 (0.1 M Na2SO4) | S = 5 cm2, L = 1 cm, J = 20 mA·cm−2, T = 20 °C, pH = 7 | 91% (5 h) | metal-doping +carbon material loading | [110] | |
| Ti/SnO2−Sb−Bi | PFOA | 50 mg L−1 (1.4 g L−1 NaClO4) | S = 11.33 cm2, L = 10 mm, J = 22 mA·cm−2, pH = 4.71 | >99% (2 h) | co-doping | [65] | |
| Ti/Sn−Sb/SnO2−F −Sb | PFOA | 100 mg L−1 (10 mM NaClO4) | S = 25 mm2, L = 1 mm, J = 20 mA·cm−2, T = 25 ± 3 °C, pH = 2 | 99% (2 h) | co-doing +intercalation | [137] | |
| 3DG−PbO2 | PFOS | 50 mg mL−1 (0.05 M Na2SO4) | J = 30 mA·cm−2, T = 30 °C, pH = 7 | 96.17% (2 h) | carbon material loading | [140] | |
| Ceramic/PbO2 −PTFE | PFOA | 20 mg mL−1 (15 mM Na2SO4) | S = 20.6 cm2, L = 2 cm, J = 15 mA·cm−2, T = 25 °C, pH = 7 | 98.9% (5 h) | doping + loading | [22] | |
| Ti/TiO2NTs /Ag2O/PbO2 | PFOS | 0.0929 mM (1.4 g L−1 Na2SO4) | S = 12 cm2, L = 10 mm, J = 30 mA·cm−2, T = 30 ± 2 °C | 74.87% (1.5 h) | metal-doping +morphology control | [146] | |
| Magnéli phase TinO2n–1 | PFOS | 2.0 μM (100 mM Na2SO4) | S = 4 cm2, L = 10 cm, J = 10 mA·cm−2, T = 25 ± 1 °C, pH = 6 | 98.30 ± 0.51% (2 h) | morphology control | [141] | |
| Porous Ti4O7 | PFOA PFOS | 0.5 mM PFOA, 0.1 mM PFOS (0.25 M Na2SO4) | S = 1 cm2, L = 1.5 cm, J = 10 mA·cm−2, T = 25 ± 1 °C, | 99.9% PFOA 9.1% PFOS (3 h) | morphology control | [147] | |
| Ti4O7 REM | PFOA +PFOS | 10 μM (100 mM K2HPO4) | S = 0.5 cm2, anode potential = 3.6 V/SHE, pH = 7 | >99.9% | morphology control | [148] | |
| Ce-doped Ti4O7: (Ti1–xCex)4O7 | PFOS | 20 nM (10 mM Na2SO4) | S = 9 cm2, L = 5 mm, J = 20 mA·cm−2, pH = 7 | >83.3% (2 h) | metal-doping | [121] | |
| Pd/Ti4O7 | PFOA | 0.12 mM (50 mM Na2SO4) | S = 25 cm2, V = 30 mL, J = 10 mA·cm−2, T = 25 ± 1 °C, pH = 7.2 | >90% (1 h) | metal-doping | [142] | |
| Mixed metal oxide (MMO) | PFOA +PFOS | 5 mg L−1 (500 mg/L Na2SO4) | S = 100 cm2, L = 16 mm, J = 10 mA·cm−2, pH = 7.4 | >90% (8 h) | morphology control | [149] | |
| LaNixY1−xO3 (Y = Fe/Cu/Co/Sr) | PFOA | 0.25 mM (0.05 M Na2SO4) | L = 3 cm, J = 20 mA·cm−2, pH = 5, 1.0 mM FeSO4 | 90% (Y = Sr, 2.5 h) | crystal facet control | [150] | |
| Other composites | Mxene-based membrane | PFBA +PFOA | 1 μg/L (0.1 M Na2SO4) | S = 12.56 cm2, L = 10 mm, J = 10 mA·cm−2, pH = 7.00 ± 0.10 | >99% (3 h) | morphology control | [19] |
| Co−CN2−Fe2O3 | PFOA | 1.9 mg L−1 (0.05 M Na2SO4) | S = 0.2475 cm2, J = 2.2 mA·cm−2, T = 25 °C, pH = 2 | 70% (30 min) | metal-doping +morphology control | [20] | |
| Fe−Mn/CA | PFOA | 50 mg mL−1 (50 mM Na2SO4) | S = 7.0 cm2, L = 2 cm, J = 2.85 mA·cm−2, T = 25 °C, pH = 3 | 97% (4 h) | co-doping +morphology control | [143] | |
5. Conclusions and Perspectives
- (1)
- For catalysts in the photocatalytic oxidation of PFAS systems, current research primarily focuses on improving catalyst activity by addressing the rapid recombination of photogenerated e−−h+. Methods include introducing surface defects or oxygen vacancies, metal-doping, heterojunction construction, and crystal facet regulation [21,26,28,39,71,74,96,101]. Furthermore, material composites and morphology regulation have been utilized to enhance reaction probabilities between PFASs and active groups, effectively enhancing the efficiency of PFAS degradation [25,28,40,104].
- (2)
- For catalysts in the electrocatalytic oxidation of PFAS systems, current research mainly focuses on increasing the yield of active groups through various methods [21,85,94]. Additionally, enhancing the efficiency of electron transfer and mass transfer processes is achieved through metal loading and constructing nano 3D structures [43,85,124,140]. The stability of electrodes is also improved through the construction of intermediate layers [63,145].
- (1)
- Regarding catalysts: Research on photocatalytic materials aims to develop novel catalysts through the combination of various modification strategies. The goal is to enhance catalyst adsorption efficiency, increase the concentration of active components on the material surface, and improve light utilization, thereby promoting the efficient transfer and separation of photogenerated charges on the photocatalytic material surface [28,35,71,90]. Research on electrocatalytic materials mainly focuses on developing efficient, inexpensive, and highly stable anode materials to enhance the surface activity sites of electrodes, thereby effectively enhancing PFAS degradation.
- (2)
- Beyond catalysts: In-depth study of other factors in advanced oxidation processes aims to enhance degradation efficiency of PFAS through system optimization. This includes adjusting the solution chemistry [75], adopting various strategies for synergistic catalytic oxidation [83], and conducting research on photo/electrocatalytic treatment technology to improve the utilization of catalyst materials and reduce treatment costs [30].
- (3)
- Current research on photo/electrocatalytic oxidation for PFAS degradation is predominantly based on ideal reaction systems. However, it is necessary to consider the actual wastewater environment, including pH, natural organic matters, coexisting ions, and the competition adsorption of degradation intermediates on the catalyst surface. Research on the competitive reactions of PFASs and coexisting substances with effective ROS should be conducted to promote the practical application of photo/electrocatalytic degradation of PFASs [59,151,152].
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sadia, M.; Nollen, I.; Helmus, R.; ter Laak, T.L.; Béen, F.; Praetorius, A.; van Wezel, A.P. Occurrence, Fate, and Related Health Risks of PFAS in Raw and Produced Drinking Water. Environ. Sci. Technol. 2023, 57, 3062–3074. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Huang, F.; Deng, H.; Schaffrath, C.; Spencer, J.B.; O’Hagan, D.; Naismith, J.H. Crystal structure and mechanism of a bacterial fluorinating enzyme. Nature 2004, 427, 561–565. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Interim Updated PFOA and PFOS Health Advisories. 2022. Available online: https://www.epa.gov/sdwa/drinking-water-health-advisories-pfoa-and-pfos (accessed on 15 June 2022).
- Eurofins. PFAS in the Revised Drinking Water Directive (DWD). Available online: https://www.eurofins.se/tjaenster/miljoe-och-vatten/nyheter-miljo/pfas-in-the-revised-drinking-water-directive-dwd/ (accessed on 26 May 2023).
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef] [PubMed]
- Chow, S.J.; Croll, H.C.; Ojeda, N.; Klamerus, J.; Capelle, R.; Oppenheimer, J.; Jacangelo, J.G.; Schwab, K.J.; Prasse, C. Comparative investigation of PFAS adsorption onto activated carbon and anion exchange resins during long-term operation of a pilot treatment plant. Water Res. 2022, 226, 119198. [Google Scholar] [CrossRef]
- Bolan, N.; Sarkar, B.; Vithanage, M.; Singh, G.; Tsang, D.C.W.; Mukhopadhyay, R.; Ramadass, K.; Vinu, A.; Sun, Y.; Ramanayaka, S.; et al. Distribution, behaviour, bioavailability and remediation of poly- and per-fluoroalkyl substances (PFAS) in solid biowastes and biowaste-treated soil. Environ. Int. 2021, 155, 106600. [Google Scholar] [CrossRef]
- Bruton, T.K.; Sedlak, D.L. Treatment of Aqueous Film-Forming Foam by Heat-Activated Persulfate Under Conditions Representative of In Situ Chemical Oxidation. Environ. Sci. Technol. 2017, 51, 13878–13885. [Google Scholar] [CrossRef]
- Bao, Y.; Huang, J.; Cagnetta, G.; Yu, G. Removal of F-538 as PFOS alternative in chrome plating wastewater by UV/Sulfite reduction. Water Res. 2019, 163, 114907. [Google Scholar] [CrossRef]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Pierpaoli, M.; Szopińska, M.; Wilk, B.K.; Sobaszek, M.; Łuczkiewicz, A.; Bogdanowicz, R.; Fudala-Książek, S. Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes. J. Hazard. Mater. 2021, 403, 123606. [Google Scholar] [CrossRef]
- Wackett, L.P. Why Is the Biodegradation of Polyfluorinated Compounds So Rare? mSphere 2021, 6, 10–1128. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.-W.; Chapin, D.H. The Chemistry of Water Treatment Processes Involving Ozone, Hydrogen Peroxide and Ultraviolet Radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Yang, L.; Jiao, Y.; Xu, X.; Pan, Y.; Su, C.; Duan, X.; Sun, H.; Liu, S.; Wang, S.; Shao, Z. Superstructures with Atomic-Level Arranged Perovskite and Oxide Layers for Advanced Oxidation with an Enhanced Non-Free Radical Pathway. ACS Sustain. Chem. Eng. 2022, 10, 1899–1909. [Google Scholar] [CrossRef]
- Leonello, D.; Fendrich, M.A.; Parrino, F.; Patel, N.; Orlandi, M.; Miotello, A. Light-Induced Advanced Oxidation Processes as PFAS Remediation Methods: A Review. Appl. Sci. 2021, 11, 8468. [Google Scholar] [CrossRef]
- Broman, J.; Ceja, A.; Godoy, T.; Rivera, D.R.; Dionne, P.; Schipper, J.; Henkemeyer, S.; Cegielski, S.; Wong, G.; Kaur, A.; et al. Destruction of Per- and Polyfluoroalkyl Substances (PFAS) via Lacasse Enzymatic Degradation and Electrochemical Advanced Oxidation. In Proceedings of the 31st Waste-Management Education Research Conference (WERC), Las Cruces, NM, USA, 11–14 April 2021. [Google Scholar]
- Xiao, F.; Sasi, P.C.; Alinezhad, A.; Sun, R.; Ali, M.A. Thermal Phase Transition and Rapid Degradation of Forever Chemicals (PFAS) in Spent Media Using Induction Heating. ACS ES&T Eng. 2023, 3, 1370–1380. [Google Scholar]
- Horikoshi, S.; Sato, S.; Abe, M.; Serpone, N. A novel liquid plasma AOP device integrating microwaves and ultrasounds and its evaluation in defluorinating perfluorooctanoic acid in aqueous media. Ultrason. Sonochem. 2011, 18, 938–942. [Google Scholar] [CrossRef]
- Ma, Q.; Gao, J.; Moussa, B.; Young, J.; Zhao, M.; Zhang, W. Electrosorption, Desorption, and Oxidation of Perfluoroalkyl Carboxylic Acids (PFCAs) via MXene-Based Electrocatalytic Membranes. ACS Appl. Mater. Interfaces 2023, 15, 29149–29159. [Google Scholar] [CrossRef]
- Han, J.; Zhao, M.; Wu, K.; Hong, Y.; Huang, T.; Gu, X.; Wang, Z.; Liu, S.; Zhu, G. A bifunctional single-atom catalyst assisted by Fe2O3 for efficiently electrocatalytic perfluorooctanoic acid degradation by integrating reductive and oxidative processes. Chem. Eng. J. 2023, 470, 144270. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, T.; Yin, L.; Ni, C.; Ni, J.; Hou, L.-A. Enhanced perfluorooctane acid mineralization by electrochemical oxidation using Ti3+ self-doping TiO2 nanotube arrays anode. Chemosphere 2022, 286, 131804. [Google Scholar] [CrossRef]
- Lou, Z.; Wang, J.; Wang, S.; Xu, Y.; Wang, J.; Liu, B.; Yu, C.; Yu, J. Strong hydrophobic affinity and enhanced •OH generation boost energy-efficient electrochemical destruction of perfluorooctanoic acid on robust ceramic/PbO2-PTFE anode. Sep. Purif. Technol. 2022, 280, 119919. [Google Scholar] [CrossRef]
- Mojiri, A.; Zhou, J.L.; Ozaki, N.; KarimiDermani, B.; Razmi, E.; Kasmuri, N. Occurrence of per- and polyfluoroalkyl substances in aquatic environments and their removal by advanced oxidation processes. Chemosphere 2023, 330, 138666. [Google Scholar] [CrossRef]
- Chowdhury, N.; Choi, H. Photocatalytic degradation of perfluorooctanoic acid on Pb-doped TiO2 coated with reduced graphene oxide. Water Environ. Res. 2023, 95, e10871. [Google Scholar] [CrossRef] [PubMed]
- McQueen, A.D.; Tedrow, O.N.; Ballentine, M.L.; Kennedy, A.J. Demonstration of Photocatalytic Degradation of Per- and Polyfluoroalkyl Substances (PFAS) in Landfill Leachate Using 3D Printed TiO2 Composite Tiles. Water Air Soil Pollut. 2022, 233, 444. [Google Scholar] [CrossRef]
- Liu, X.; Duan, X.; Bao, T.; Hao, D.; Chen, Z.; Wei, W.; Wang, D.; Wang, S.; Ni, B.-J. High-performance photocatalytic decomposition of PFOA by BiOX/TiO2 heterojunctions: Self-induced inner electric fields and band alignment. J. Hazard. Mater. 2022, 430, 128195. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.; Xu, X.; Zheng, C.; Liu, X.; Zhao, D.; Qiu, W. Photocatalysis of aqueous PFOA by common catalysts of In2O3, Ga2O3, TiO2, CeO2 and CdS: Influence factors and mechanistic insights. Environ. Geochem. Health 2022, 44, 2943–2953. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Wang, B.; Heck, K.N.; Clark, C.A.; Wei, J.; Wang, M.; Metz, J.; Wu, G.; Tsai, A.-L.; Guo, S.; et al. Titanium oxide improves boron nitride photocatalytic degradation of perfluorooctanoic acid. Chem. Eng. J. 2022, 448, 137735. [Google Scholar] [CrossRef]
- Li, F.; Duan, J.; Tian, S.; Ji, H.; Zhu, Y.; Wei, Z.; Zhao, D. Short-chain per- and polyfluoroalkyl substances in aquatic systems: Occurrence, impacts and treatment. Chem. Eng. J. 2020, 380, 122506. [Google Scholar] [CrossRef]
- Lu, D.; Sha, S.; Luo, J.; Huang, Z.; Zhang Jackie, X. Treatment train approaches for the remediation of per- and polyfluoroalkyl substances (PFAS): A critical review. J. Hazard. Mater. 2020, 386, 121963. [Google Scholar] [CrossRef]
- Verma, S.; Varma, R.S.; Nadagouda, M.N. Remediation and mineralization processes for per- and polyfluoroalkyl substances (PFAS) in water: A review. Sci. Total Environ. 2021, 794, 148987. [Google Scholar] [CrossRef]
- Banks, D.; Jun, B.-M.; Heo, J.; Her, N.; Park, C.M.; Yoon, Y. Selected advanced water treatment technologies for perfluoroalkyl and polyfluoroalkyl substances: A review. Sep. Purif. Technol. 2020, 231, 115929. [Google Scholar] [CrossRef]
- Nzeribe, N.; Crimi, M.; Thagard, S.M.; Holsen, T.M. Physico-Chemical Processes for the Treatment of Per- And Polyfluoroalkyl Substances (PFAS): A review Blossom. Crit. Rev. Environ. Sci. Technol. 2019, 49, 866–915. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Bartosiewicz, I.; Kulisa, K. Advanced Oxidation/Reduction Processes treatment for aqueous perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS)—A review of recent advances. Chem. Eng. J. 2018, 336, 170–199. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Boffito, D.C. Mechanisms and pathways of PFAS degradation by advanced oxidation and reduction processes: A critical review. Chem. Eng. J. 2022, 450, 138352. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Reactive species in advanced oxidation processes: Formation, identification and reaction mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Yu, G.; Wang, Y.; Cao, H.; Zhao, H.; Xie, Y. Reactive Oxygen Species and Catalytic Active Sites in Heterogeneous Catalytic Ozonation for Water Purification. Environ. Sci. Technol. 2020, 54, 5931–5946. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, J.L.; Altaee, A.; Ahmed, M.B.; Johir, M.A.H.; Ren, J.; Li, X. Improved photocatalysis of perfluorooctanoic acid in water and wastewater by Ga2O3/UV system assisted by peroxymonosulfate. Chemosphere 2020, 239, 124722. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C.; Zhang, Y.; Zhang, Y.; Zhu, L. Underneath mechanisms into the super effective degradation of PFOA by BiOF nanosheets with tunable oxygen vacancies on exposed (101) facets. Appl. Catal. B 2021, 286, 119911. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; ElKady, M.; Gar Alalm, M. CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 2021, 406, 127152. [Google Scholar] [CrossRef]
- Cao, M.H.; Wang, B.B.; Yu, H.S.; Wang, L.L.; Yuan, S.H.; Chen, J. Photochemical decomposition of perfluorooctanoic acid in aqueous periodate with VUV and UV light irradiation. J. Hazard. Mater. 2010, 179, 1143–1146. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Shao, T.; Li, X. In2O3 nanoporous nanosphere: A highly efficient photocatalyst for decomposition of perfluorooctanoic acid. Appl. Catal. B 2012, 125, 350–357. [Google Scholar] [CrossRef]
- Liu, X.; Wei, W.; Xu, J.; Wang, D.; Song, L.; Ni, B.-J. Photochemical decomposition of perfluorochemicals in contaminated water. Water Res. 2020, 186, 116311. [Google Scholar] [CrossRef]
- Song, Z.; Tang, H.; Wang, N.; Zhu, L. Reductive defluorination of perfluorooctanoic acid by hydrated electrons in a sulfite-mediated UV photochemical system. J. Hazard. Mater. 2013, 262, 332–338. [Google Scholar] [CrossRef]
- Bentel, M.J.; Yu, Y.; Xu, L.; Li, Z.; Wong, B.M.; Men, Y.; Liu, J. Defluorination of Per- and Polyfluoroalkyl Substances (PFASs) with Hydrated Electrons: Structural Dependence and Implications to PFAS Remediation and Management. Environ. Sci. Technol. 2019, 53, 3718–3728. [Google Scholar] [CrossRef]
- Li, X.; Zhang, P.; Jin, L.; Shao, T.; Li, Z.; Cao, J. Efficient Photocatalytic Decomposition of Perfluorooctanoic Acid by Indium Oxide and Its Mechanism. Environ. Sci. Technol. 2012, 46, 5528–5534. [Google Scholar] [CrossRef] [PubMed]
- Banayan Esfahani, E.; Asadi Zeidabadi, F.; Zhang, S.; Mohseni, M. Photo-chemical/catalytic oxidative/reductive decomposition of per- and poly-fluoroalkyl substances (PFAS), decomposition mechanisms and effects of key factors: A review. Environ. Sci. Water Res. Technol. 2022, 8, 698–728. [Google Scholar] [CrossRef]
- Eberson, L. Electron-Transfer Reactions in Organic Chemistry. Adv. Phys. Org. Chem. 1982, 18, 79–185. [Google Scholar]
- Zhang, H.; Xie, C.; Chen, L.; Duan, J.; Li, F.; Liu, W. Different reaction mechanisms of SO4•− and •OH with organic compound interpreted at molecular orbital level in Co(II)/peroxymonosulfate catalytic activation system. Water Res. 2023, 229, 119392. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Activation of persulfate (PS) and peroxymonosulfate (PMS) and application for the degradation of emerging contaminants. Chem. Eng. J. 2018, 334, 1502–1517. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical advanced oxidation processes: A review on their application to synthetic and real wastewaters. Appl. Catal. B 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Yadav, S.; Ibrar, I.; Al-Juboori, R.A.; Singh, L.; Ganbat, N.; Kazwini, T.; Karbassiyazdi, E.; Samal, A.K.; Subbiah, S.; Altaee, A. Updated review on emerging technologies for PFAS contaminated water treatment. Chem. Eng. Res. Des. 2022, 182, 667–700. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, P. Effects of pH on photochemical decomposition of perfluorooctanoic acid in different atmospheres by 185nm vacuum ultraviolet. J. Environ. Sci. 2014, 26, 2207–2214. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.R.; Ozaki, H.; Okada, T.; Taniguchi, S.; Takanami, R. Factors influencing UV photodecomposition of perfluorooctanoic acid in water. Chem. Eng. J. 2012, 180, 197–203. [Google Scholar] [CrossRef]
- Wang, S.; Yang, Q.; Chen, F.; Sun, J.; Luo, K.; Yao, F.; Wang, X.; Wang, D.; Li, X.; Zeng, G. Photocatalytic degradation of perfluorooctanoic acid and perfluorooctane sulfonate in water: A critical review. Chem. Eng. J. 2017, 328, 927–942. [Google Scholar] [CrossRef]
- Phan Thi, L.-A.; Do, H.-T.; Lee, Y.-C.; Lo, S.-L. Photochemical decomposition of perfluorooctanoic acids in aqueous carbonate solution with UV irradiation. Chem. Eng. J. 2013, 221, 258–263. [Google Scholar] [CrossRef]
- Qanbarzadeh, M.; Wang, D.; Ateia, M.; Sahu, S.P.; Cates, E.L. Impacts of Reactor Configuration, Degradation Mechanisms, and Water Matrices on Perfluorocarboxylic Acid Treatment Efficiency by the UV/Bi3O(OH)(PO4)2 Photocatalytic Process. ACS ES&T Eng. 2021, 1, 239–248. [Google Scholar]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M. MIL-53(Al)/ZnO coated plates with high photocatalytic activity for extended degradation of trimethoprim via novel photocatalytic reactor. Sep. Purif. Technol. 2020, 249, 117173. [Google Scholar] [CrossRef]
- Fouad, M.; Gar Alalm, M.; El-Etriby, H.K.; Boffito, D.C.; Ookawara, S.; Ohno, T.; Fujii, M. Visible-light-driven photocatalytic disinfection of raw surface waters (300–5000 CFU/mL) using reusable coated Ru/WO3/ZrO2. J. Hazard. Mater. 2021, 402, 123514. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Mollah, M.Y.A.; Susan, M.A.B.H.; Islam, M.M. Role of in situ electrogenerated reactive oxygen species towards degradation of organic dye in aqueous solution. Electrochim. Acta 2020, 344, 136146. [Google Scholar] [CrossRef]
- Liu, X.Y.; Zhang, Y.Q.; Xia, X.H.; Shi, S.J.; Lu, Y.; Wang, X.L.; Gu, C.D.; Tu, J.P. Self-assembled porous NiCo2O4 hetero-structure array for electrochemical capacitor. J. Power Sour. 2013, 239, 157–163. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Direct and Mediated Anodic Oxidation of Organic Pollutants. Chem. Rev. 2009, 109, 6541–6569. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Ding, S.; Zhang, L. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Ti/SnO2–Sb, Ti/SnO2–Sb/PbO2 and Ti/SnO2–Sb/MnO2 anodes. Water Res. 2012, 46, 2281–2289. [Google Scholar] [CrossRef]
- Niu, J.; Lin, H.; Gong, C.; Sun, X. Theoretical and Experimental Insights into the Electrochemical Mineralization Mechanism of Perfluorooctanoic Acid. Environ. Sci. Technol. 2013, 47, 14341–14349. [Google Scholar] [CrossRef]
- Zhuo, Q.; Deng, S.; Yang, B.; Huang, J.; Yu, G. Efficient Electrochemical Oxidation of Perfluorooctanoate Using a Ti/SnO2-Sb-Bi Anode. Environ. Sci. Technol. 2011, 45, 2973–2979. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Niu, J.; Xu, J.; Huang, H.; Li, D.; Yue, Z.; Feng, C. Highly Efficient and Mild Electrochemical Mineralization of Long-Chain Perfluorocarboxylic Acids (C9–C10) by Ti/SnO2–Sb–Ce, Ti/SnO2–Sb/Ce–PbO2, and Ti/BDD Electrodes. Environ. Sci. Technol. 2013, 47, 13039–13046. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Lin, H.; Xu, J.; Wu, H.; Li, Y. Electrochemical Mineralization of Perfluorocarboxylic Acids (PFCAs) by Ce-Doped Modified Porous Nanocrystalline PbO2 Film Electrode. Environ. Sci. Technol. 2012, 46, 10191–10198. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, L.; Cui, W.; Li, R.; Song, T.; Cui, Z. Electrochemical degradation of perfluorooctanoic acid (PFOA) by Yb-doped Ti/SnO2–Sb/PbO2 anodes and determination of the optimal conditions. RSC Adv. 2015, 5, 84856–84864. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Wang, W.; Ji, Y.; Niu, J. Enhanced perfluorooctanoic acid degradation by electrochemical activation of peroxymonosulfate in aqueous solution. Environ. Int. 2020, 137, 105562. [Google Scholar] [CrossRef]
- Estrellan, C.R.; Salim, C.; Hinode, H. Photocatalytic decomposition of perfluorooctanoic acid by iron and niobium co-doped titanium dioxide. J. Hazard. Mater. 2010, 179, 79–83. [Google Scholar] [CrossRef]
- Yao, X.; Zuo, J.; Wang, Y.-J.; Song, N.-N.; Li, H.-H.; Qiu, K. Enhanced Photocatalytic Degradation of Perfluorooctanoic Acid by Mesoporous Sb2O3/TiO2 Heterojunctions. Front. Chem. 2021, 9, 690520. [Google Scholar] [CrossRef]
- Ryou, H.; Yoo, T.H.; Yoon, Y.; Lee, I.G.; Shin, M.; Cho, J.; Cho, B.J.; Hwang, W.S. Hydrothermal Synthesis and Photocatalytic Property of Sn-doped β-Ga2O3 Nanostructure. ECS J. Solid State Sci. Technol. 2020, 9, 045009. [Google Scholar] [CrossRef]
- Wang, J.; Cao, C.; Wang, Y.; Wang, Y.; Sun, B.; Zhu, L. In situ preparation of p-n BiOI@Bi5O7I heterojunction for enhanced PFOA photocatalytic degradation under simulated solar light irradiation. Chem. Eng. J. 2020, 391, 123530. [Google Scholar] [CrossRef]
- Wu, Y.; Li, Y.; Fang, C.; Li, C. Highly Efficient Degradation of Perfluorooctanoic Acid over a MnOx-Modified Oxygen-Vacancy-Rich In2O3 Photocatalyst. ChemCatChem 2019, 11, 2297–2303. [Google Scholar] [CrossRef]
- Xu, B.; Ahmed, M.B.; Zhou, J.L.; Altaee, A. Visible and UV photocatalysis of aqueous perfluorooctanoic acid by TiO2 and peroxymonosulfate: Process kinetics and mechanistic insights. Chemosphere 2020, 243, 125366. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Kuo, J.; Wu, C.-H. Decomposition of perfluorooctanoic acid by ultraviolet light irradiation with Pb-modified titanium dioxide. J. Hazard. Mater. 2016, 303, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, X.; Yang, L.; Wang, F.; Li, J.; Xia, W.; Li, W.; Zhou, L.; Zhao, C. ß-Ga2O3 Nanorod Synthesis with a One-step Microwave Irradiation Hydrothermal Method and its Efficient Photocatalytic Degradation for Perfluorooctanoic Acid. Photochem. Photobiol. 2015, 91, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Chen, M.-J.; Lo, S.-L.; Lee, Y.-C.; Huang, C.-C. Photocatalytic decomposition of perfluorooctanoic acid by transition-metal modified titanium dioxide. J. Hazard. Mater. 2015, 288, 168–175. [Google Scholar] [CrossRef]
- Li, M.; Yu, Z.; Liu, Q.; Sun, L.; Huang, W. Photocatalytic decomposition of perfluorooctanoic acid by noble metallic nanoparticles modified TiO2. Chem. Eng. J. 2016, 286, 232–238. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, Z.-b.; Zhang, R.-h.; Li, M.-j.; Chen, Y.; Wang, L.; Kuang, Y.; Zhang, B.; Zhu, Y.-h. Photocatalytic Degradation of Perfluorooctanoic Acid by Pd-TiO2 Photocatalyst. Huanjing Kexue 2015, 36, 2138–2146. [Google Scholar]
- Tian, A.; Wu, Y.; Mao, K. Enhanced Performance of Surface Modified TiO2 Nanotubes for the Decomposition of Perfluorooctanoic Acid. In Proceedings of the International Conference on Materials Science, Resource and Environmental Engineering (MSREE), Xian, China, 10–11 December 2016. [Google Scholar]
- Peng, Y.-P.; Chen, H.; Huang, C.P. The Synergistic Effect of Photoelectrochemical (PEC) Reactions Exemplified by Concurrent Perfluorooctanoic acid (PFOA) Degradation and Hydrogen Generation over Carbon and Nitrogen codoped TiO2 Nanotube Arrays (C-N-TNTAs) photoelectrode. Appl. Catal. B-Environ. 2017, 209, 437–446. [Google Scholar] [CrossRef]
- Song, C.; Chen, P.; Wang, C.; Zhu, L. Photodegradation of perfluorooctanoic acid by synthesized TiO2–MWCNT composites under 365 nm UV irradiation. Chemosphere 2012, 86, 853–859. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, C.; Luo, P.; Hu, Q. Removal of perfluorooctanoic acid by MWCNT-modified carbon-doped titanium dioxide in a peroxymonosulfate/simulated sunlight system. Appl. Surf. Sci. 2023, 614, 156251. [Google Scholar] [CrossRef]
- Panchangam, S.C.; Yellatur, C.S.; Yang, J.-S.; Loka, S.S.; Lin, A.Y.C.; Vemula, V. Facile fabrication of TiO2-graphene nanocomposites (TGNCs) for the efficient photocatalytic oxidation of perfluorooctanoic acid (PFOA). J. Environ. Chem. Eng. 2018, 6, 6359–6369. [Google Scholar] [CrossRef]
- Yang, J.-S.; Lai, W.W.-P.; Panchangam, S.C.; Lin, A.Y.-C. Photoelectrochemical degradation of perfluorooctanoic acid (PFOA) with GOP25/FTO anodes: Intermediates and reaction pathways. J. Hazard. Mater. 2020, 391, 122247. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xu, J.; Song, S.; Wang, J.; Li, Y.; Liu, R.; Shen, Y. TiO2 quantum dots loaded sulfonated graphene aerogel for effective adsorption-photocatalysis of PFOA. Sci. Total Environ. 2020, 698, 134275. [Google Scholar] [CrossRef]
- Gomez-Ruiz, B.; Ribao, P.; Diban, N.; Rivero, M.J.; Ortiz, I.; Urtiaga, A. Photocatalytic degradation and mineralization of perfluorooctanoic acid (PFOA) using a composite TiO2 −rGO catalyst. J. Hazard. Mater. 2018, 344, 950–957. [Google Scholar] [CrossRef]
- Li, F.; Wei, Z.; He, K.; Blaney, L.; Cheng, X.; Xu, T.; Liu, W.; Zhao, D. A concentrate-and-destroy technique for degradation of perfluorooctanoic acid in water using a new adsorptive photocatalyst. Water Res. 2020, 185, 116219. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Shao, T.; Wang, J.; Jin, L.; Li, X. Different nanostructured In2O3 for photocatalytic decomposition of perfluorooctanoic acid (PFOA). J. Hazard. Mater. 2013, 260, 40–46. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Li, J.; Shao, T.; Wang, J.; Jin, L. Synthesis of In2O3 porous nanoplates for photocatalytic decomposition of perfluorooctanoic acid (PFOA). Catal. Commun. 2014, 43, 42–46. [Google Scholar] [CrossRef]
- Xu, C.; Qiu, P.; Chen, H.; Jiang, F.; Wang, X. Fabrication of two-dimensional indium oxide nanosheets with graphitic carbon nitride nanosheets as sacrificial templates. Mater. Lett. 2019, 242, 24–27. [Google Scholar] [CrossRef]
- Jiang, F.; Zhao, H.; Chen, H.; Xu, C.; Chen, J. Enhancement of photocatalytic decomposition of perfluorooctanoic acid on CeO2/In2O3. RSC Adv. 2016, 6, 72015–72021. [Google Scholar] [CrossRef]
- Tan, X.; Chen, G.; Xing, D.; Ding, W.; Liu, H.; Li, T.; Huang, Y. Indium-modified Ga2O3 hierarchical nanosheets as efficient photocatalysts for the degradation of perfluorooctanoic acid. Environ. Sci. Nano 2020, 7, 2229–2239. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Yao, H.; Dang, L.; Li, Z. Efficient decomposition of organic compounds and reaction mechanism with BiOI photocatalyst under visible light irradiation. J. Mol. Catal. A Chem. 2011, 334, 116–122. [Google Scholar] [CrossRef]
- Li, T.; Wang, C.; Wang, T.; Zhu, L. Highly efficient photocatalytic degradation toward perfluorooctanoic acid by bromine doped BiOI with high exposure of (001) facet. Appl. Catal. B 2020, 268, 118442. [Google Scholar] [CrossRef]
- Zhang, K.-L.; Liu, C.-M.; Huang, F.-Q.; Zheng, C.; Wang, W.-D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Song, Z.; Dong, X.; Wang, N.; Zhu, L.; Luo, Z.; Fang, J.; Xiong, C. Efficient photocatalytic defluorination of perfluorooctanoic acid over BiOCl nanosheets via a hole direct oxidation mechanism. Chem. Eng. J. 2017, 317, 925–934. [Google Scholar] [CrossRef]
- Sun, Y.; Li, G.; Wang, W.; Gu, W.; Wong, P.K.; An, T. Photocatalytic defluorination of perfluorooctanoic acid by surface defective BiOCl: Fast microwave solvothermal synthesis and photocatalytic mechanisms. J. Environ. Sci. 2019, 84, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, Z.; Yang, M.; Chen, J.; Li, C.; Zhang, C.; Zhang, X. In-situ fabrication of a spherical-shaped Zn-Al hydrotalcite with BiOCl and study on its enhanced photocatalytic mechanism for perfluorooctanoic acid removal performed with a response surface methodology. J. Hazard. Mater. 2020, 399, 123070. [Google Scholar] [CrossRef]
- Sahu, S.P.; Qanbarzadeh, M.; Ateia, M.; Torkzadeh, H.; Maroli, A.S.; Cates, E.L. Rapid Degradation and Mineralization of Perfluorooctanoic Acid by a New Petitjeanite Bi3O(OH)(PO4)2 Microparticle Ultraviolet Photocatalyst. Environ. Sci. Technol. Lett. 2018, 5, 533–538. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, Y.; Duan, J.; Xia, Y.; Tong, T.; Zhang, L.; Zhao, D. Enhanced photocatalytic degradation of perfluorooctanoic acid using carbon-modified bismuth phosphate composite: Effectiveness, material synergy and roles of carbon. Chem. Eng. J. 2020, 395, 124991. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, G.; Gao, Z.; Cao, J.; Li, D.; Yun, H.; Zeng, T. Enhanced visible-light-driven photocatalytic activity of BiFeO3 via electric-field control of spontaneous polarization. J. Alloys Compd. 2019, 783, 943–951. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Zhang, W.; Zheng, H.; Zhu, W.; Sun, N.; Zheng, Y.; Wang, P. Microwave enhanced Fenton-like process for degradation of perfluorooctanoic acid (PFOA) using Pb-BiFeO3/rGO as heterogeneous catalyst. Chem. Eng. J. 2017, 326, 756–764. [Google Scholar] [CrossRef]
- Shang, E.; Li, Y.; Niu, J.; Li, S.; Zhang, G.; Wang, X. Photocatalytic degradation of perfluorooctanoic acid over Pb-BiFeO3/rGO catalyst: Kinetics and mechanism. Chemosphere 2018, 211, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; Georgi, A.; Gonzalez-Olmos, R.; Kopinke, F.-D. Degradation of perfluorooctanoic acid adsorbed on Fe-zeolites with molecular oxygen as oxidant under UV-A irradiation. Appl. Catal. B Environ. 2020, 278, 119283. [Google Scholar] [CrossRef]
- Luo, D.; Yuan, J.; Zhou, J.; Zou, M.; Xi, R.; Qin, Y.; Shen, Q.; Hu, S.; Xu, J.; Nie, M.; et al. Synthesis of samarium doped ferrite and its enhanced photocatalytic degradation of perfluorooctanoic acid (PFOA). Opt. Mater. 2021, 122, 111636. [Google Scholar] [CrossRef]
- Xu, J.; Wu, M.; Yang, J.; Wang, Z.; Chen, M.; Teng, F. Efficient photocatalytic degradation of perfluorooctanoic acid by a wide band gap p-block metal oxyhydroxide InOOH. Appl. Surf. Sci. 2017, 416, 587–592. [Google Scholar] [CrossRef]
- Zhao, H.; Gao, J.; Zhao, G.; Fan, J.; Wang, Y.; Wang, Y. Fabrication of novel SnO2-Sb/carbon aerogel electrode for ultrasonic electrochemical oxidation of perfluorooctanoate with high catalytic efficiency. Appl. Catal. B 2013, 136–137, 278–286. [Google Scholar] [CrossRef]
- Duan, L.; Wang, B.; Heck, K.; Guo, S.; Clark, C.A.; Arredondo, J.; Wang, M.; Senftle, T.P.; Westerhoff, P.; Wen, X.; et al. Efficient Photocatalytic PFOA Degradation over Boron Nitride. Environ. Sci. Technol. Lett. 2020, 7, 613–619. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, T.; Zhao, D.; Li, F.; Liu, W.; Wang, B.; An, B. Adsorption and solid-phase photocatalytic degradation of perfluorooctane sulfonate in water using gallium-doped carbon-modified titanate nanotubes. Chem. Eng. J. 2021, 421, 129676. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Lo, S.-L.; Kuo, J. Effects of titanate nanotubes synthesized by a microwave hydrothermal method on photocatalytic decomposition of perfluorooctanoic acid. Water Res. 2011, 45, 4131–4140. [Google Scholar] [CrossRef]
- Song, H.; Wang, Y.; Ling, Z.; Zu, D.; Li, Z.; Shen, Y.; Li, C. Enhanced photocatalytic degradation of perfluorooctanoic acid by Ti3C2 MXene-derived heterojunction photocatalyst: Application of intercalation strategy in DESs. Sci. Total Environ. 2020, 746, 141009. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, P.; Li, J.; Shao, T.; Jin, L. Synthesis of In2O3-graphene composites and their photocatalytic performance towards perfluorooctanoic acid decomposition. J. Photochem. Photobiol. A Chem. 2013, 271, 111–116. [Google Scholar] [CrossRef]
- Xu, C.; Qiu, P.; Chen, H.; Jiang, F. Platinum modified indium oxide nanorods with enhanced photocatalytic activity on degradation of perfluorooctanoic acid (PFOA). J. Taiwan Inst. Chem. Eng. 2017, 80, 761–768. [Google Scholar] [CrossRef]
- Wu, D.; Li, X.; Tang, Y.; Lu, P.; Chen, W.; Xu, X.; Li, L. Mechanism insight of PFOA degradation by ZnO assisted-photocatalytic ozonation: Efficiency and intermediates. Chemosphere 2017, 180, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, X.; Zhang, J.; Chen, W.; Lu, P.; Tang, Y.; Li, L. Efficient PFOA degradation by persulfate-assisted photocatalytic ozonation. Sep. Purif. Technol. 2018, 207, 255–261. [Google Scholar] [CrossRef]
- Bacha, A.-U.-R.; Nabi, I.; Fu, Z.; Li, K.; Cheng, H.; Zhang, L. A comparative study of bismuth-based photocatalysts with titanium dioxide for perfluorooctanoic acid degradation. Chin. Chem. Lett. 2019, 30, 2225–2230. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, X.; Quan, X.; Fan, Y.; Chen, S.; Zhao, X. Enhanced Perfluorooctanoic Acid Degradation by Electrochemical Activation of Sulfate Solution on B/N Codoped Diamond. Environ. Sci. Technol. 2019, 53, 5195–5201. [Google Scholar] [CrossRef]
- Lin, H.; Xiao, R.; Xie, R.; Yang, L.; Tang, C.; Wang, R.; Chen, J.; Lv, S.; Huang, Q. Defect Engineering on a Ti4O7 Electrode by Ce3+ Doping for the Efficient Electrooxidation of Perfluorooctanesulfonate. Environ. Sci. Technol. 2021, 55, 2597–2607. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, C.; Yu, G.; Zhuo, Q.; Deng, S.; Wu, J.; Zhang, H. Highly efficient electrochemical degradation of perfluorooctanoic acid (PFOA) by F-doped Ti/SnO2 electrode. J. Hazard. Mater. 2015, 299, 417–424. [Google Scholar] [CrossRef]
- Sukeesan, S.; Boontanon, N.; Boontanon, S.K. Improved electrical driving current of electrochemical treatment of Per- and Polyfluoroalkyl Substances (PFAS) in water using Boron-Doped Diamond anode. Environ. Technol. Innov. 2021, 23, 101655. [Google Scholar] [CrossRef]
- Song, S.; Zhan, L.; He, Z.; Lin, L.; Tu, J.; Zhang, Z.; Chen, J.; Xu, L. Mechanism of the anodic oxidation of 4-chloro-3-methyl phenol in aqueous solution using Ti/SnO2–Sb/PbO2 electrodes. J. Hazard. Mater. 2010, 175, 614–621. [Google Scholar] [CrossRef]
- Duan, T.; Wen, Q.; Chen, Y.; Zhou, Y.; Duan, Y. Enhancing electrocatalytic performance of Sb-doped SnO2 electrode by compositing nitrogen-doped graphene nanosheets. J. Hazard. Mater. 2014, 280, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Iizuka, Y.; Nakata, K.; Murakami, T.; Tryk, D.A.; Fujishima, A.; Koide, Y.; Morito, Y. Efficient electrochemical decomposition of perfluorocarboxylic acids by the use of a boron-doped diamond electrode. Diamond Relat. Mater. 2011, 20, 64–67. [Google Scholar] [CrossRef]
- Carter, K.E.; Farrell, J. Oxidative Destruction of Perfluorooctane Sulfonate Using Boron-Doped Diamond Film Electrodes. Environ. Sci. Technol. 2008, 42, 6111–6115. [Google Scholar] [CrossRef]
- Zhi, J.-F.; Wang, H.-B.; Nakashima, T.; Rao, T.N.; Fujishima, A. Electrochemical Incineration of Organic Pollutants on Boron-Doped Diamond Electrode. Evidence for Direct Electrochemical Oxidation Pathway. J. Phys. Chem. B 2003, 107, 13389–13395. [Google Scholar] [CrossRef]
- Barisci, S.; Suri, R. Electrooxidation of short and long chain perfluorocarboxylic acids using boron doped diamond electrodes. Chemosphere 2020, 243, 125349. [Google Scholar] [CrossRef] [PubMed]
- Guan, B.; Zhi, J.; Zhang, X.; Murakami, T.; Fujishima, A. Electrochemical route for fluorinated modification of boron-doped diamond surface with perfluorooctanoic acid. Electrochem. Commun. 2007, 9, 2817–2821. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Lei, Y.; Lv, B.; Gao, J.; Zhang, Y.; Li, D. Fabrication and Electrochemical Treatment Application of a Novel Lead Dioxide Anode with Superhydrophobic Surfaces, High Oxygen Evolution Potential, and Oxidation Capability. Environ. Sci. Technol. 2010, 44, 1754–1759. [Google Scholar] [CrossRef]
- Zhuo, Q.; Xiang, Q.; Yi, H.; Zhang, Z.; Yang, B.; Cui, K.; Bing, X.; Xu, Z.; Liang, X.; Guo, Q.; et al. Electrochemical oxidation of PFOA in aqueous solution using highly hydrophobic modified PbO2 electrodes. J. Electroanal. Chem. 2017, 801, 235–243. [Google Scholar] [CrossRef]
- Aquino, J.M.; Rocha-Filho, R.C.; Ruotolo, L.A.M.; Bocchi, N.; Biaggio, S.R. Electrochemical degradation of a real textile wastewater using β-PbO2 and DSA® anodes. Chem. Eng. J. 2014, 251, 138–145. [Google Scholar] [CrossRef]
- Mukimin, A.; Vistanty, H.; Zen, N. Oxidation of textile wastewater using cylinder Ti/β-PbO2 electrode in electrocatalytic tube reactor. Chem. Eng. J. 2015, 259, 430–437. [Google Scholar] [CrossRef]
- Zhong, C.; Wei, K.; Han, W.; Wang, L.; Sun, X.; Li, J. Electrochemical degradation of tricyclazole in aqueous solution using Ti/SnO2–Sb/PbO2 anode. J. Electroanal. Chem. 2013, 705, 68–74. [Google Scholar] [CrossRef]
- Song, S.; Fan, J.; He, Z.; Zhan, L.; Liu, Z.; Chen, J.; Xu, X. Electrochemical degradation of azo dye C.I. Reactive Red 195 by anodic oxidation on Ti/SnO2–Sb/PbO2 electrodes. Electrochim. Acta 2010, 55, 3606–3613. [Google Scholar] [CrossRef]
- Yang, B.; Wang, J.; Jiang, C.; Li, J.; Yu, G.; Deng, S.; Lu, S.; Zhang, P.; Zhu, C.; Zhuo, Q. Electrochemical mineralization of perfluorooctane sulfonate by novel F and Sb co-doped Ti/SnO2 electrode containing Sn-Sb interlayer. Chem. Eng. J. 2017, 316, 296–304. [Google Scholar] [CrossRef]
- Xu, Z.; Yu, Y.; Liu, H.; Niu, J. Highly efficient and stable Zr-doped nanocrystalline PbO2 electrode for mineralization of perfluorooctanoic acid in a sequential treatment system. Sci. Total Environ. 2017, 579, 1600–1607. [Google Scholar] [CrossRef] [PubMed]
- Ellis, D.A.; Denkenberger, K.A.; Burrow, T.E.; Mabury, S.A. The Use of 19F NMR to Interpret the Structural Properties of Perfluorocarboxylate Acids: A Possible Correlation with Their Environmental Disposition. J. Phys. Chem. A 2004, 108, 10099–10106. [Google Scholar] [CrossRef]
- Duan, X.; Wang, W.; Wang, Q.; Sui, X.; Li, N.; Chang, L. Electrocatalytic degradation of perfluoroocatane sulfonate (PFOS) on a 3D graphene-lead dioxide (3DG-PbO2) composite anode: Electrode characterization, degradation mechanism and toxicity. Chemosphere 2020, 260, 127587. [Google Scholar] [CrossRef]
- Shi, H.; Wang, Y.; Li, C.; Pierce, R.; Gao, S.; Huang, Q. Degradation of Perfluorooctanesulfonate by Reactive Electrochemical Membrane Composed of Magnéli Phase Titanium Suboxide. Environ. Sci. Technol. 2019, 53, 14528–14537. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Niu, J.; Chu, C.; Weon, S.; Zhu, Q.; Lu, J.; Stavitski, E.; Kim, J.-H. Amorphous Pd-Loaded Ti4O7 Electrode for Direct Anodic Destruction of Perfluorooctanoic Acid. Environ. Sci. Technol. 2020, 54, 10954–10963. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, M.; Zhao, H.; Chen, Y.; Xiao, F.; Chu, W.; Zhao, G. Efficiently degradation of perfluorooctanoic acid in synergic electrochemical process combining cathodic electro-Fenton and anodic oxidation. Chem. Eng. J. 2019, 378, 122071. [Google Scholar] [CrossRef]
- Abdelghafar, F.; Xu, X.; Jiang, S.P.; Shao, Z. Designing single-atom catalysts toward improved alkaline hydrogen evolution reaction. Mater. Rep. Energy 2022, 2, 100144. [Google Scholar] [CrossRef]
- Zhuo, Q.; Li, X.; Yan, F.; Yang, B.; Deng, S.; Huang, J.; Yu, G. Electrochemical oxidation of 1H,1H,2H,2H-perfluorooctane sulfonic acid (6:2 FTS) on DSA electrode: Operating parameters and mechanism. J. Environ. Sci. 2014, 26, 1733–1739. [Google Scholar] [CrossRef]
- Zhuo, Q.; Luo, M.; Guo, Q.; Yu, G.; Deng, S.; Xu, Z.; Yang, B.; Liang, X. Electrochemical Oxidation of Environmentally Persistent Perfluorooctane Sulfonate by a Novel Lead Dioxide Anode. Electrochim. Acta 2016, 213, 358–367. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Liang, S.; Wang, C.; Wang, Y.; Jin, F.; Luo, Q.; Huang, Q. Development of macroporous Magnéli phase Ti4O7 ceramic materials: As an efficient anode for mineralization of poly- and perfluoroalkyl substances. Chem. Eng. J. 2018, 354, 1058–1067. [Google Scholar] [CrossRef]
- Le, T.X.H.; Haflich, H.; Shah, A.D.; Chaplin, B.P. Energy-Efficient Electrochemical Oxidation of Perfluoroalkyl Substances Using a Ti4O7 Reactive Electrochemical Membrane Anode. Environ. Sci. Technol. Lett. 2019, 6, 504–510. [Google Scholar] [CrossRef]
- Schaefer, C.E.; Andaya, C.; Urtiaga, A.; McKenzie, E.R.; Higgins, C.P. Electrochemical treatment of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonic acid (PFOS) in groundwater impacted by aqueous film forming foams (AFFFs). J. Hazard. Mater. 2015, 295, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Guo, D.; Dong, X.; Li, Y.; Huang, Y.; Chen, H.; Li, S. Electrocatalytic degradation of perfluorooctanoic acid by LaNixY1-xO3 (Y=Fe, Cu, Co, Sr) gas dispersion electrode. J. Fluorine Chem. 2021, 242, 109700. [Google Scholar] [CrossRef]
- Zhang, Y.; Thomas, A.; Apul, O.; Venkatesan, A.K. Coexisting ions and long-chain per- and polyfluoroalkyl substances (PFAS) inhibit the adsorption of short-chain PFAS by granular activated carbon. J. Hazard. Mater. 2023, 460, 132378. [Google Scholar] [CrossRef]
- Xiong, X.; Shang, Y.; Bai, L.; Luo, S.; Seviour, T.W.; Guo, Z.; Ottosen, L.D.M.; Wei, Z. Complete defluorination of perfluorooctanoic acid (PFOA) by ultrasonic pyrolysis towards zero fluoro-pollution. Water Res. 2023, 235, 119829. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yuan, T.; Yang, X.; Ding, S.; Ma, M. Insights into Photo/Electrocatalysts for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS) by Advanced Oxidation Processes. Catalysts 2023, 13, 1308. https://doi.org/10.3390/catal13091308
Chen X, Yuan T, Yang X, Ding S, Ma M. Insights into Photo/Electrocatalysts for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS) by Advanced Oxidation Processes. Catalysts. 2023; 13(9):1308. https://doi.org/10.3390/catal13091308
Chicago/Turabian StyleChen, Xiaoyan, Taoyue Yuan, Xinyu Yang, Shunke Ding, and Mengtao Ma. 2023. "Insights into Photo/Electrocatalysts for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS) by Advanced Oxidation Processes" Catalysts 13, no. 9: 1308. https://doi.org/10.3390/catal13091308
APA StyleChen, X., Yuan, T., Yang, X., Ding, S., & Ma, M. (2023). Insights into Photo/Electrocatalysts for the Degradation of Per- and Polyfluoroalkyl Substances (PFAS) by Advanced Oxidation Processes. Catalysts, 13(9), 1308. https://doi.org/10.3390/catal13091308






