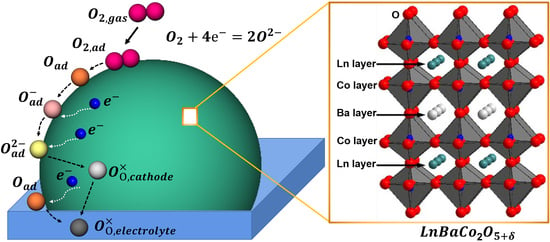
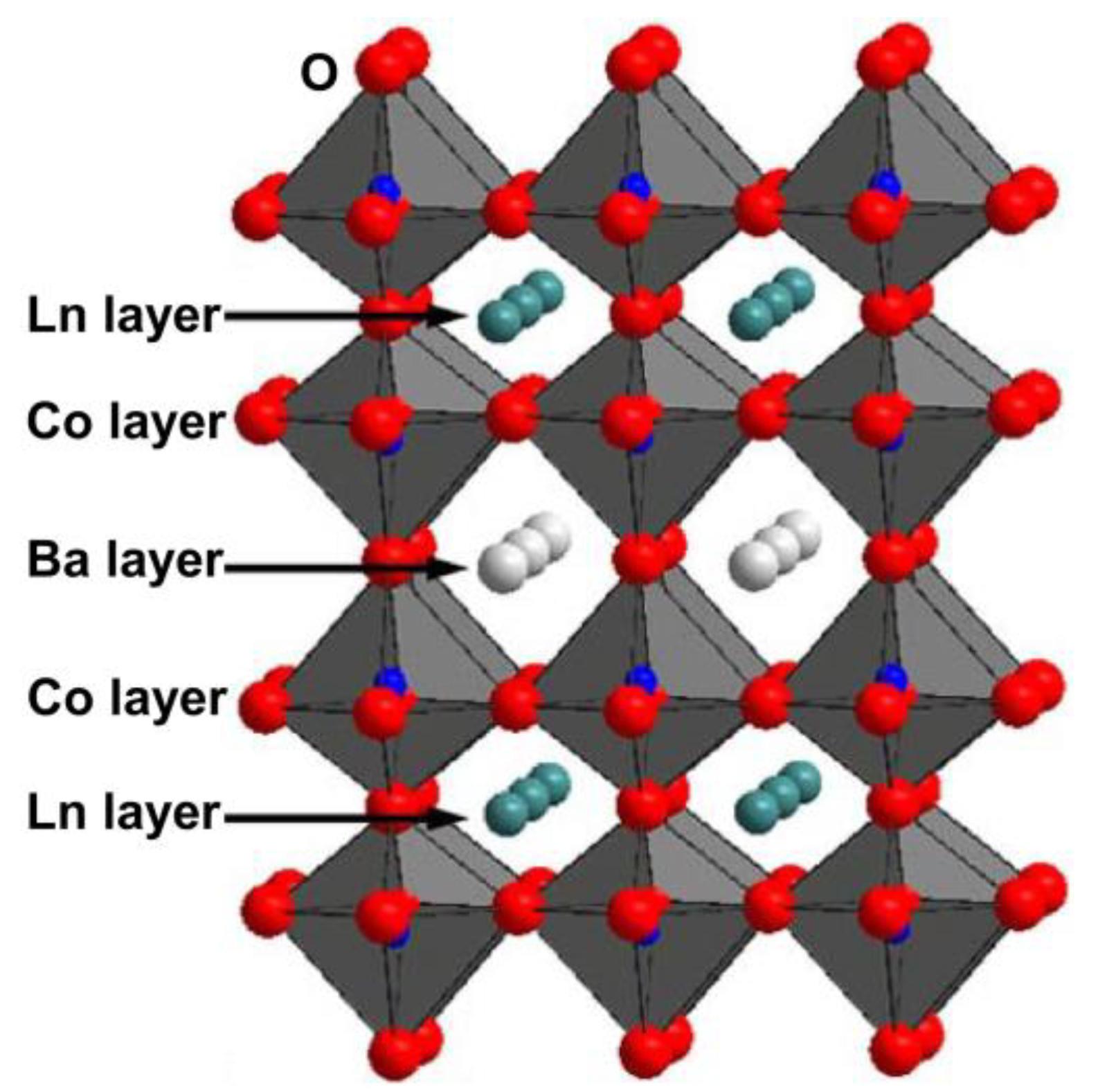
Progress in Developing LnBaCo2O5+δ as an Oxygen Reduction Catalyst for Solid Oxide Fuel Cells
Abstract
:1. Introduction
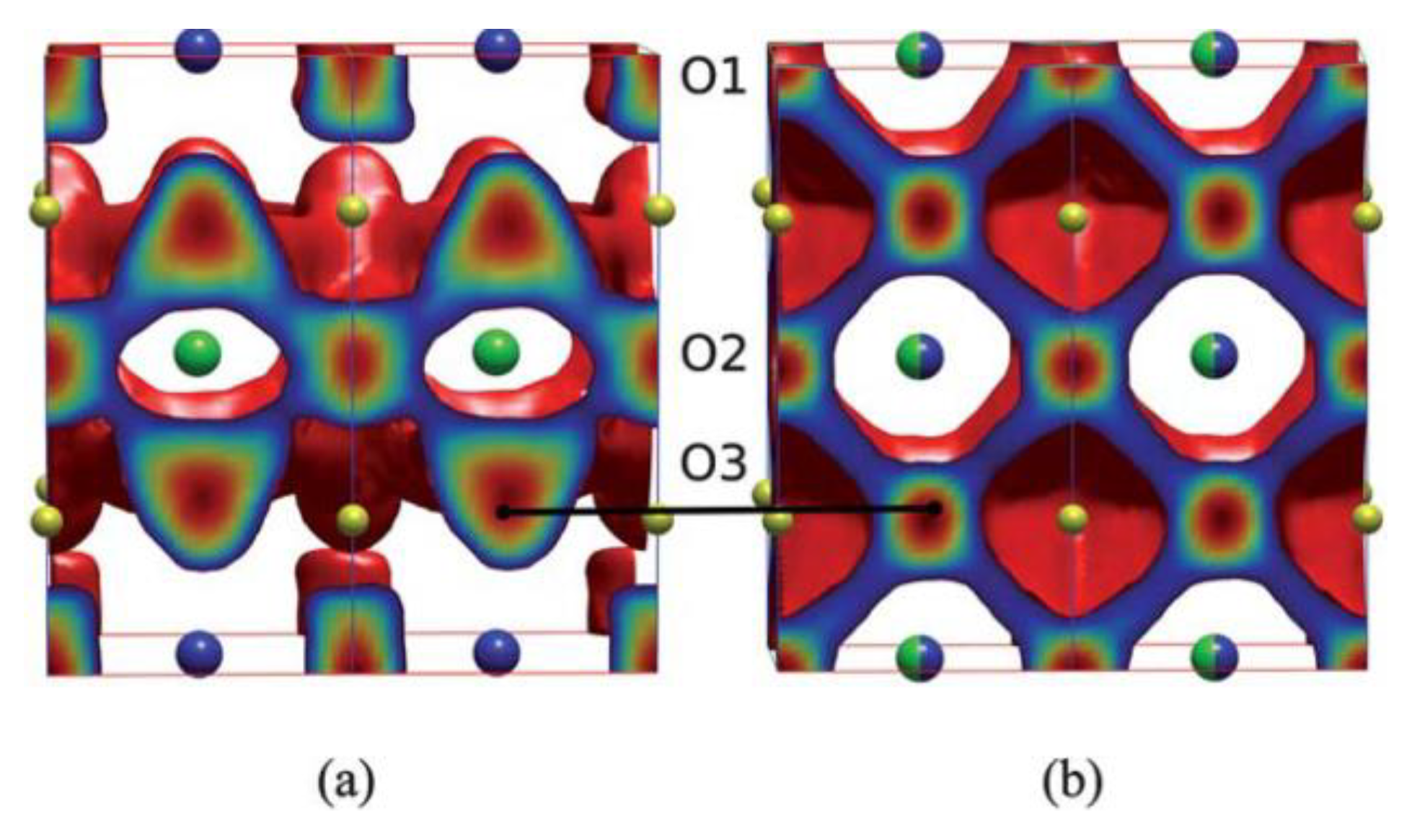
2. Physicochemical Properties of LnBaCo2O5+δ
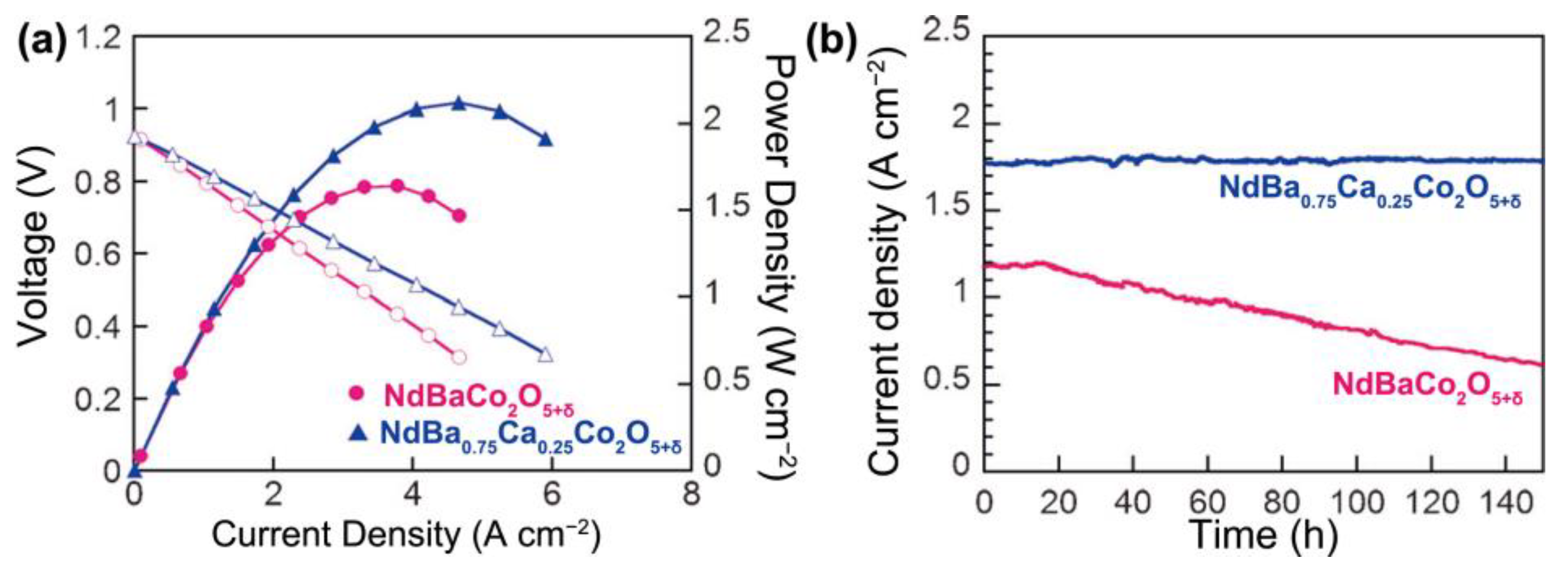
3. Compositional Optimization of LnBaCo2O5+δ
4. Nanostructure and Nanoscience of LnBaCo2O5+δ
5. Conclusions and Outlook
- (1)
- Surface Chemistry of LnBaCo2O5+δ Under Operating Conditions
- (2)
- Microstructure of LnBaCo2O5+δ Cathode Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wachsman, E.D.; Lee, K.T. Lowering the temperature of solid oxide fuel cells. Science 2011, 334, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Wachsman, E.; Ishihara, T.; Kilner, J. Low-temperature of solid-oxide fuel cells. MRS Bulletin 2014, 39, 773–779. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Lang, Y.; Meng, G. Recent advances to the development of low-temperature solid oxide fuel cells. Fuel Cells 2004, 4, 41–47. [Google Scholar] [CrossRef]
- Xia, C.R.; Xia, M.L. Novel cathodes for low-temperature solid oxide fuel cells. Adv. Mater. 2002, 14, 521–523. [Google Scholar] [CrossRef]
- Kilner, J.A.; Burriel, M. Intermediate-temperature solid-oxide fuel cells. Annu. Rev. Mater. Res. 2014, 44, 365–393. [Google Scholar] [CrossRef]
- Zhou, W.; Sunarso, J.; Zhao, M.; Liang, F.; Klande, T.; Feldhoff, A. A highly active perovskites electrode for the oxygen reduction reaction below 600 °C. Angew. Chem. Int. Ed. 2013, 52, 14036–14040. [Google Scholar] [CrossRef]
- Jiang, S.P. Development of lanthanum strontium manganite perovskite cathode materials of solid oxide fuel cells: A review. J. Mater. Sci. 2008, 43, 6799–6833. [Google Scholar] [CrossRef]
- Adler, S.B. Factors governing oxygen reduction in solid oxide fuel cell cathodes. Chem. Rev. 2004, 104, 4791–4843. [Google Scholar] [CrossRef]
- Adler, S.B. Mechanism and kinetics of oxygen reduction on porous La1-xSrxCoO3-δ electrodes. Solid State Ionics 1998, 111, 125–134. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.A.; Steele, B.C.H. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Hwang, H.J.; Moon, J.-W.; Lee, S.; Lee, E.A. Electrochemical performance of LSCF-based composite cathodes for intermediate temperature SOFCs. J. Power Sources 2005, 145, 243–248. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Wang, Q.; Su, Z.X. A comparative study of electrochemical performance of La0.5Ba0.5CoO3-δ and La0.5Ba0.5CoO3-δ-Gd0.1Ce0.9O1.95 cathodes. Int. J. Hydrogen Energy 2012, 37, 2157–2165. [Google Scholar] [CrossRef]
- Steele, B.C.H. Appraisal of Ce1-yGdyO2-y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ionics 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Bao, S.; Pang, S.; Wang, W.; Chen, J.; Chen, M.; Ma, J.; Nan, C.-W.; Chen, C. Ca doping effect on the magnetic and electronic transport properties in double perovskite PrBaCo2O5+δ films. Appl. Phys. Lett. 2017, 111, 232406. [Google Scholar] [CrossRef]
- Wu, J.; Guzman, R.; Bao, S.; Zhang, Y.; Chen, Y.; Shen, S.; Yu, P.; Nan, C.-W.; Zhou, W.; Chen, C.; et al. Mosaic growth induced magnetic anisotropy in double perovskite PrBaCo2O5+δ thin films. Acta Materialia 2022, 234, 118040. [Google Scholar] [CrossRef]
- Kudyakova, V.S.; Shalamova, A.M.; Politov, B.V.; Suntsov, A.Y. Specific interrelations of magnetic, thermodynamic and structural properties in highly non-stoichiometric PrBaMnFeO6−δ double perovskite. J. Alloys Compd. 2021, 886, 161133. [Google Scholar] [CrossRef]
- Choi, S.; Kucharczyk, C.J.; Liang, Y.; Zhang, X.; Takeuchi, I.; Ji, H.-I.; Haile, S.M. Exceptional power density and stability at intermediate temperatures in protonic ceramic fuel cells. Nat. Energy 2018, 3, 202–210. [Google Scholar] [CrossRef]
- Wang, N.; Tang, C.; Du, L.; Zhu, R.; Xing, L.; Song, Z.; Yuan, B.; Zhao, L.; Aoki, Y.; Ye, S. Advanced cathode materials for protonic ceramic fuel cells: Recent progress and future perspectives. Adv. Energy Mater. 2022, 12, 2201882. [Google Scholar] [CrossRef]
- Teketel, B.S.; Beshiwork, B.A.; Luo, X.; Tian, D.; Zhu, S.; Desta, H.G.; Yang, Q.; Chen, Y.; Lin, B. A-site doping enabled higher-oxygen-vacancy cobalt-free layered perovskite cathode for higher-performing protonic ceramic fuel cells. Ceram. Int. 2022, 48, 37232–37241. [Google Scholar] [CrossRef]
- Islam, M.S.; Wang, S.; Nolan, A.M.; Mo, Y. First-principles computational design and discovery of novel double-perovskite proton conductors. Chem. Mater. 2021, 33, 8278–8288. [Google Scholar] [CrossRef]
- Cao, J.; Jia, Y.; Shao, Z. Perovskites for protonic ceramic fuel cells: A review. Energy Environ. Sci. 2022, 15, 2200–2232. [Google Scholar] [CrossRef]
- Teketel, B.S.; Beshiwork, B.A.; Tian, D.; Zhu, S.; Desta, H.G.; Kashif, K.; Chen, Y.; Lin, B. Promoted performance of layered perovskite PrBaFe2O5+δ cathode for protonic ceramic fuel cells by Zn doping. Catalysts 2022, 12, 488. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, K.; He, F.; Zhou, Y.; Sasaki, K.; Zhao, B.; Choi, Y.; Liu, M.; Chen, Y. Surface regulating of a double-perovskite electrode for protonic ceramic fuel cells to enhance oxygen reduction activity and contaminants poisoning tolerance. Adv. Energy Mater. 2022, 12, 2200761. [Google Scholar] [CrossRef]
- Malyshkin, D.; Novikov, A.; Ivanov, I.; Sereda, V.; Tsvetkov, D.; Zuev, A. The origin of triple conductivity and water uptake in layered double perovskites: A case study on lanthanum-substituted GdBaCo2O6-δ. J. Alloys Compd. 2020, 845, 156309. [Google Scholar] [CrossRef]
- Kim, B.-J.; Fabbri, E.; Castelli, I.E.; Borlaf, M.; Graule, T.; Nachtegaal, M.; Schmidt, T.J. Fe-doping in double perovskite PrBaCo2(1-x)Fe2xO6-δ: Insights into structural and electronic effects to enhance oxygen evolution catalyst stability. Catalysts 2019, 9, 263. [Google Scholar] [CrossRef]
- Xing, L.; Xia, T.; Li, Q.; Zhao, H.; Sun, L.; Huo, L.-H. High-Performance and CO2-durable composite cathodes toward electrocatalytic oxygen reduction: Ce0.8Sm0.2O1.9 Nanoparticle-decorated double perovskite EuBa0.5Sr0.5Co2O5+δ. ACS Sust. Chem. Eng. 2019, 7, 17907–17918. [Google Scholar] [CrossRef]
- Baral, A.K.; Sankar, K.V.; Matatyaho, A.; Kushnir, G.; Tsur, Y. Tri-functional double perovskite oxide catalysts for fuel cells and electrolyzers. ChemSusChem 2020, 13, 5671–5682. [Google Scholar] [CrossRef]
- Shin, T.H.; Myung, J.-H.; Verbraeken, M.; Kim, G.; Irvine, J.T.S. Oxygen deficient layered double perovskite as an active cathode for CO2 electrolysis using a solid oxide conductor. Faraday Discuss. 2015, 182, 227. [Google Scholar] [CrossRef]
- Wang, H.; Enriquez, E.; Collins, G.; Ma, C.; Liu, M.; Zhang, Y.; Dong, C.; Chen, C. Anomalous redox properties and ultrafast chemical sensing behavior of double perovskite CaBaCo2O5+δ thin films. J. Materiomics 2015, 1, 113–117. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, X.; Xia, Y.; Pang, S.; Xu, F.; Whangbo, M.-H.; Sun, L.; Chen, C. Anomaly negative resistance phenomena in highly epitaxial PrBa0.7Ca0.3Co2O5+δ thin films induced from superfast redox reactions. Catalysts 2021, 11, 1441. [Google Scholar] [CrossRef]
- Bao, S.; Ma, C.; Chen, G.; Xu, X.; Enriquez, E.; Chen, C.; Zhang, Y.; Bettis, J.L.; Whangbo, M.-H.; Dong, C.; et al. Ultrafast atomic layer-by-layer oxygen vacancy-exchange diffusion in double-perovskite LnBaCo2O5.5+δ thin films. Sci. Rep. 2014, 4, 4726. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, I.L.; Zakiryanov, P.O.; Sereda, V.V.; Mazurin, M.O.; Malyshkin, D.A.; Zuev, A.Y.; Tsvetkov, D.S. Nonstoichiometry, Defect chemistry and oxygen transport in Fe-doped layered double perovskite cobaltite PrBaCo2-xFexO6-δ (x = 0–0.6) membrane materials. Membranes 2022, 12, 1200. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Ge, L.; Ran, R.; Shao, Z.; Liu, S. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater. 2008, 56, 4876–4889. [Google Scholar] [CrossRef]
- Yagovitin, R.E.; Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Sereda, V.V.; Zuev, A.Y. Thermodynamics of Formation and Disordering of YBaCo2O6-δ Double Perovskite as a Base for Novel Dense Ceramic Membrane Materials. Membranes 2023, 13, 10. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, L.; Zhen, D.; Yoo, S.; Ding, Y.; Chen, D.; Chen, Y.; Zhang, Q.; Doyle, B.; Xiong, X.; et al. A tailored double perovskite nanofiber catalyst enables ultrafast oxygen evolution. Nat. Commun. 2017, 8, 14586. [Google Scholar] [CrossRef]
- Hua, B.; Zhang, Y.-Q.; Yan, N.; Li, M.; Sun, Y.-F.; Chen, J.; Li, J.; Luo, J.-L. The excellence of both worlds: Developing effective double perovskite oxide catalyst of oxygen reduction reaction for room and elevated temperature applications. Adv. Funct. Mater. 2016, 26, 4106–4112. [Google Scholar] [CrossRef]
- Fang, F.; Feng, N.; Zhao, P.; Chen, C.; Li, X.; Meng, J.; Liu, G.; Chen, L.; Wan, H.; Guan, G. In situ exsolution of Co/CoOx core-shell nanoparticles on double perovskite porous nanotubular webs: A synergistically active catalyst for soot efficient oxidation. Chem. Eng. J. 2019, 372, 752–764. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Chen, Y.; Yang, L.; Wang, Y.; Wei, M. A-site cation-ordered double perovskite PrBaCo2O5+δ oxide as an anion-inserted pseudocapacitor electrode with outstanding stability. J. Alloys Compd. 2019, 810, 151830. [Google Scholar] [CrossRef]
- Zhang, R.; Hu, S.; Lu, C.; Xu, Z. Bandgap engineering of Gd0.8Ca0.2BaCo2O5+δ double perovskite for photocatalysis applications. Ceram. Int. 2018, 44, 15483–15489. [Google Scholar] [CrossRef]
- Lu, Y.; Dai, T.; Lu, C.; Cao, C.; Zhang, W.; Xu, W.; Min, H.; Yang, X. Fabrication of doped SmBaCo2O5+δ double perovskites for enhanced solar driven interfacial evaporation. Ceram. Int. 2019, 45, 24903–24908. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, R.; Wei, L.; Lu, C.; Ni, Y.; Xu, Z. Specific features of spectral and electrical properties of double-perovskite LnBaCo2O5+δ (Ln=lanthanides) under solar irradiation. Ceram. Int. 2017, 43, 1186–1192. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2O5+δ perovskite cathodes for solid oxide fuel cells: An overview and perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Tarancόn, A.; Burriel, M.; Santiso, J.; Skinner, S.J.; Kilner, J.A. Advances in layered oxide cathodes for intermediate temperature solid oxide fuel cells. J. Mater. Chem. 2010, 20, 3799–3813. [Google Scholar] [CrossRef]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Achieving fast oxygen diffusion in perovskites by cation ordering. Appl. Phys. Lett. 2005, 86, 091910. [Google Scholar] [CrossRef]
- Taskin, A.A.; Lavrov, A.N.; Ando, Y. Transport and magnetic properties of GdBaCo2O5+δ single crystals: A cobalt oxide with square-lattice CoO2 planes over a wide range of electron and hole doping. Phys. Rev. B 2005, 71, 134414. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Reimus, L.; Brodersen, P.; Mims, C.A. Rapid oxygen ion diffusion and surface exchange kinetics in PrBaCo2O5+x with a perovskite related structure and ordered A cations. J. Mater. Chem. 2007, 17, 2500–2505. [Google Scholar] [CrossRef]
- Kim, G.; Wang, S.; Jacobson, A.J.; Yang, Z.; Donner, W.; Chen, C.L.; Reimus, L.; Brodersen, P.; Mims, C.A. Oxygen exchange kinetics of epitaxial PrBaCo2O5+δ thin films. Appl. Phys. Lett. 2006, 88, 024103. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ananjev, M.V.; Eremin, V.A.; Zuev, A.Y.; Kurumchin, E.K. Oxygen nonstoichiometry, defect structure and oxygen diffusion in the double perovskite GdBaCo2O6−δ. Dalton Trans. 2014, 43, 15937–15943. [Google Scholar] [CrossRef]
- Sayers, R.; De Souza, R.A.; Kilner, J.A.; Skinner, S.J. Low temperature diffusion and oxygen stoichiometry in lanthanum nickelate. Solid State Ionics 2010, 181, 386–391. [Google Scholar] [CrossRef]
- Munnings, C.N.; Skinner, S.J.; Amow, G. Whitfield, P.S;. Davidson, I.J. Oxygen transport in the La2Ni1−xCoxO4+δ system. Solid State Ionics 2005, 176, 1895–1901. [Google Scholar] [CrossRef]
- Tarancón, A.; Morata, A.; Dezanneau, G.; Skinner, S.J.; Kilner, J.A.; Estradé, S.; Hernández-Ramírez, F.; Peiró, F.; Morante, J.R. GdBaCo2O5+x layered perovskite as an intermediate temperature solid oxide fuel cell cathode. J. Power Sources 2007, 174, 255–263. [Google Scholar] [CrossRef]
- Seymour, I.D.; Chroneos, A.; Kilner, J.A.; Grimes, R.W. Defect processes in orthorhombic LnBaCo2O5.5 double perovskites. Phys. Chem. Chem. Phys. 2011, 13, 15305–15310. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, D.; Chroneos, A.; Tarancόn, A.; Kilner, J.A. Oxygen ion diffusion in cation ordered/disordered GdBaCo2O5+δ. J. Mater. Chem. 2011, 21, 2183–2186. [Google Scholar] [CrossRef]
- Tarancόn, A.; Chroneos, A.; Parfitt, D.; Kilner, J. Oxygen diffusion in ordered/disordered double perovskites. Ecs Trans. 2011, 35, 1151–1154. [Google Scholar] [CrossRef]
- Hermet, J.; Geneste, G.; Dezanneau, G. Molecular dynamics simulations of oxygen diffusion in GdBaCo2O5.5. Appl. Phys. Lett. 2010, 97, 174102. [Google Scholar] [CrossRef]
- Shiiba, H.; Nakayama, M.; Kasuga, T.; Grimes, R.W.; Kilner, J.A. Calculation of arrangement of oxygen ions and vacancies in double perovskite GdBaCo2O5+δ by first-principles DFT with monte carlo simulations. Phys. Chem. Chem. Phys. 2013, 15, 10494–10499. [Google Scholar] [CrossRef]
- Zapata, J.; Burriel, M.; Carcía, P.; Kilner, J.A.; Santiso, J. Anisotropic O18 tracer diffusion in epitaxial films of GdBaCo2O5+δ cathode material with different orientations. J. Mater. Chem. A 2013, 1, 7408–7414. [Google Scholar] [CrossRef]
- Seymour, I.D.; Tarancόn, A.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W. Anisotropic oxygen diffusion in PrBaCo2O5.5 double perovskites. Solid State Ionics 2012, 216, 41–43. [Google Scholar] [CrossRef]
- Burriel, M.; Peña-Martínez, J.; Chater, R.J.; Fearn, S.; Berenov, A.V.; Skinner, S.J.; Kilner, J.A. Anisotropic oxygen ion diffusion in layered PrBaCo2O5+δ. Chem. Mater. 2012, 24, 613–621. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yashima, M.; Peña-Martínez, J.; Kilner, J.A. Experimental visualization of the diffusional pathway of oxide ions in a layered perovskite-type cobaltite PrBaCo2O5+δ. Chem. Mater. 2013, 25, 2638–2641. [Google Scholar] [CrossRef]
- Cox-Galhotra, R.A.; Huq, A.; Hodges, J.P.; Yu, C.; Wang, X.; Gong, W.; Jacobson, A.J.; McIntosh, S. An in-situ neutron diffraction study of the crystal structure of PrBaCo2O5+δ at high temperature and controlled oxygen partial pressure. Solid State Ionics 2013, 249–250, 34–40. [Google Scholar] [CrossRef]
- Hu, Y.; Hernandez, O.; Broux, T.; Bahout, M.; Hermet, J.; Ottochian, A.; Ritter, C.; Geneste, G.; Dezanneau, G. Oxygen diffusion mechanism in the mixed ion-electron conductor NdBaCo2O5+x. J. Mater. Chem. 2012, 22, 18744–18747. [Google Scholar] [CrossRef]
- Cox-Galhotra, R.A.; Huq, A.; Hodges, J.P.; Kim, J.-H.; Yu, C.; Wang, X.; Jacobson, A.J.; Mcintosh, S. Visualizing oxygen anion transport pathways in NdBaCo2O5+δ by in situ neutron diffraction. J. Mater. Chem. 2013, 1, 3091–3100. [Google Scholar] [CrossRef]
- Hermet, J.; Dupé, B.; Dezanneau, G. Simulations of REBaCo2O5.5 (RE = Gd, La, Y) cathode materials through energy minimization and molecular dynamics. Solid State Ionics 2012, 216, 50–53. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Defect structure and charge transfer in the double perovskite GdBaCo2O6−δ. Solid State Ionics 2011, 192, 215–219. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.-A.; Grande, T. Effect of A-site cation ordering on chemical stability, oxygen stoichiometry and electrical conductivity in layered LaBaCo2O5 double perovskite. Materials 2016, 9, 154. [Google Scholar] [CrossRef]
- Anjum, U.; Khan, T.S.; Agarwal, M.; Haider, M.A. Identifying the origin of the limiting process in a double perovskite PrBa0.5Sr0.5Co1.5Fe0.5O5+δ Thin-film electrode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2019, 11, 25243–25253. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Sereda, V.V.; Zuev, A.Y. Oxygen nonstoichiometry and defect structure of the double perovskite GdBaCo2O6−δ. Solid State Ionics 2010, 180, 1620–1625. [Google Scholar] [CrossRef]
- Pang, S.; Wang, W.; Su, Y.; Shen, X.; Wang, Y.; Xu, K.; Chen, C. Synergistic effect of A-site cation ordered-disordered perovskite as a cathode material for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2017, 164, F775–F780. [Google Scholar] [CrossRef]
- Zhou, Y.; Lü, Z.; Xu, S.; Wei, B.; Xu, D.; Yang, Z. The electronic structure and the oxygen adsorption at BaO terminated surface of GdBaCo2O5.5: A first principles study. Solid State Commun. 2020, 311, 113871. [Google Scholar] [CrossRef]
- Wang, H.B.; Bao, S.Y.; Liu, J.; Collins, G.; Ma, C.R.; Liu, M.; Chen, C.L.; Dong, C.; Whangbo, M.-H.; Guo, H.M.; et al. Ultrafast chemical dynamic behavior in highly epitaxial LaBaCo2O5+δ thin films. J. Mater. Chem. C 2014, 2, 5660–5666. [Google Scholar] [CrossRef]
- Subardi, A.; Liao, K.Y.; Fu, Y.P. Oxygen transport, thermal and electrochemical properties of NdBa0.5Sr0.5Co2O5+δ cathode for SOFCs. J. Eur. Ceram. Soc. 2019, 39, 30–40. [Google Scholar] [CrossRef]
- Chen, D.J.; Ran, R.; Zhang, K.; Wang, J.; Shao, Z.P. Intermediate-temperature electrochemical performance of a polycrystalline PrBaCo2O5+δ cathode on samarium-doped ceria electrolyte. J. Power Sources 2009, 188, 96–105. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. LnBaCo2O5+δ oxides as cathodes for intermediate-temperature solid oxide fuel cells. J. Electrochem. Soc. 2008, 155, B385–B390. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Su, Z.X.; Xu, H.X.; Xu, Q.L.; Chen, C.L. Characterization of cation-ordered perovskite oxide LaBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 6836–6843. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Wang, Q.X.N.; Zhang, Q.Y. Structural stability and high-temperature electrical properties of cation-oredered/disordered perovskite LaBaCoO. Mater. Chem. Phys. 2012, 131, 642–646. [Google Scholar] [CrossRef]
- Chen, D.J.; Ran, R.; Shao, Z.P. Effect of firing temperature on the microstructure and performance of PrBaCo2O5+δ cathodes on Sm0.2Ce0.8O1.9 electrolytes fabricated by spray deposition-firing processes. J. Power Sources 2010, 195, 4667–4675. [Google Scholar] [CrossRef]
- Téllez, H.; Druce, J.; Ju, Y.-W.; Kilner, J.; Ishihara, T. Surface chemistry evolution in LnBaCo2O5+δ double perovskites for oxygen electrodes. Int. J. Hydrogen Energy 2014, 39, 20856–20863. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Pérez-Coll, D.; Peña-Martínez, J.; Garcia-Martín, S. New insights into the GdBaCo2O5+δ material: Crystal structure, electrical and electrochemical properties. J. Power Sources 2014, 263, 90–97. [Google Scholar] [CrossRef]
- Ishizawa, N.; Asaka, T.; Kudo, T.; Fukuda, K.; Yasuhara, A.; Abe, N.; Arima, T. Structural evolution of GdBaCo2O5+δ (δ = 7/18) at elevated temperatures. Chem. Mater. 2014, 26, 6503–6517. [Google Scholar] [CrossRef]
- Aksenova, T.V.; Gavrilova, L.Y.; Yaremchenko, A.A.; Cherepanov, V.A.; Kharton, V.V. Oxygen nonstoichiometry, thermal expansion and high-temperature electrical properties of layered NdBaCo2O5+δ and SmBaCo2O5+δ. Mater. Res. Bull. 2010, 45, 1288–1292. [Google Scholar] [CrossRef]
- Shi, Z.; Xia, T.; Meng, F.; Wang, J.; Lian, J.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C.; Meng, J. A layered perovskite EuBaCo2O5+δ for intermediate-temperature solid oxide fuel cell cathode. Fuel Cells 2013, 14, 979–990. [Google Scholar] [CrossRef]
- Tsvetkov, D.; Tsvetkova, N.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. PrBaCo2O6-δ-Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells: Stability and cation interdiffusion. Energies 2019, 12, 417. [Google Scholar] [CrossRef]
- Marrero-Jerez, J.; Peña-Martínez, J.; Nñez, P. Study of the oxygen desorption from GdBa1-xSrxCo2O5+δ (x = 0, 0.25, 0.5 and 1): Effect of the Sr-content on the oxidation state of cobalt ions. J. Alloys Compd. 2014, 606, 269–272. [Google Scholar] [CrossRef]
- Kim, J.-H.; Prado, F.; Manthiram, A. Characterization of GdBa1-xSrxCo2O5+δ (0 ≤ x ≤ 1.0) double perovskites as cathodes for solid oxide fuel cells. J. Electrochem. Soc. 2008, 155, B1023–B1028. [Google Scholar] [CrossRef]
- Kim, J.-H.; Cassidy, M.; Irvine, J.T.S.; Bae, J. Advanced electrochemical properties of LnBa0.5Sr0.5Co2O5+δ (Ln = Pr, Sm, and Gd) as cathode materials for IT-SOFC. J. Electrochem. Soc. 2009, 156, B682–B689. [Google Scholar] [CrossRef]
- Subardi, A.; Cheng, M.-H.; Fu, Y.-P. Chemical bulk diffusion and electrochemical properties of SmBa0.6Sr0.4Co2O5+δ cathode for intermediate solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 20783–20790. [Google Scholar] [CrossRef]
- Jun, A.; Kim, J.; Shin, J.; Kim, G. Optimization of Sr content in layered SmBa1-xSrxCo2O5+δ perovskite cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 18381–18388. [Google Scholar] [CrossRef]
- Lü, S.Q.; Meng, X.W.; Ji, Y.; Fu, C.W.; Sun, C.C.; Zhao, H.Y. Electrochemical performances of NdBa0.5Sr0.5Co2O5+x as potential cathode material for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 8094–8096. [Google Scholar] [CrossRef]
- Yoo, S.; Choi, S.; Kim, J.; Shin, J.; Kim, G. Investigation of layered perovskite type NdBa1-xSrxCo2O5+δ (x = 0, 0.25, 0.5, 0.75, and 1.0) cathodes for intermediate-temperature solid oxide fuel cells. Electrochimica Acta 2013, 100, 44–50. [Google Scholar] [CrossRef]
- Ding, H.P.; Xue, X.J. PrBa0.5Sr0.5Co2O5+δ layered perovskite cathode for intermediate temperature solid oxide fuel cells. Electrochimica Acta 2010, 55, 3812–3816. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Xu, H.X.; Jiang, L.; Xu, Q.L.; Shi, Y.C.; Zhang, Q.Y. Structure and properties of layered-perovskite LaBa1-xCo2O5+δ (x = 0-0.15) as intermediate-temperature cathode material. J. Power Sources 2013, 240, 54–59. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Wang, Q.; Su, Z.X. Characterization of Ba-deficient PrBa1-xCo2O5+δ as cathode material for intermediate temperature solid oxide fuel cells. J. Power Sources 2012, 204, 53–59. [Google Scholar] [CrossRef]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Wang, Q.; Su, Z.X.; Zhang, Q.Y. Highly enhanced electrochemical performance of PrBa0.92Co2O5+δ cathode by introducing Ba cationic-deficiency. Int. J. Hydrogen Energy 2012, 37, 3998–4001. [Google Scholar] [CrossRef]
- Jiang, L.; Li, F.; Wei, T.; Zeng, R.; Huang, Y.H. Evaluation of Pr1+xBa1-xCo2O5+δ (x = 0 − 0.3) as cathode materials for solid-oxide fuel cells. Electrochimica Acta 2014, 133, 364–372. [Google Scholar] [CrossRef]
- Jiang, X.N.; Shi, Y.C.; Zhou, W.L.; Li, X.N.; Su, Z.X.; Pang, S.L.; Jiang, L. Effects of Pr3+-deficiency on structure and properties of PrBaCo2O5+δ cathode material-A comparison with Ba2+-deficiency case. J. Power Sources 2014, 272, 371–377. [Google Scholar] [CrossRef]
- Wang, J.P.; Meng, F.C.; Xia, T.; Shi, Z.; Lian, J.; Xu, C.B.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C. Superior electrochemical performance and oxygen reduction kinetics of layered perovskite. Int. J. Hydrogen Energy 2014, 39, 18392–18404. [Google Scholar] [CrossRef]
- Yoo, S.; Jun, A.; Ju, Y.-W.; Odkhuu, D.; Hyodo, J.; Jeong, H.Y.; Park, N.; Shin, J.; Ishihara, T.; Kim, G. Development of Double-perovskite compounds as cathode materials for low-temperature solid oxide fuel cells. Angew. Chem. Int. Ed. 2014, 53, 13064–13067. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, J.C.; He, B.B.; Chen, F.L.; Xia, C.R. Synthesis, characterization and evaluation of PrBaCo2-xFexO5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 3658–3665. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, J.-H.; Manthiram, A. Effects of Fe substitution on the structure and properties of LnBaCo2-xFexO5+δ (Ln = Nd and Gd) cathodes. J. Power Sources 2010, 195, 6411–6419. [Google Scholar] [CrossRef]
- Jun, A.; Lim, T.-H.; Shin, J.; Kim, G. Electrochemical properties of B-site Ni doped layered perovskite cathodes for IT-SOFCs. Int. J. Hydrogen Energy 2014, 39, 20791–20798. [Google Scholar] [CrossRef]
- Hu, Y.; Bogicevic, C.; Bouffanais, Y.; Giot, M.; Hernandez, O.; Dezanneau, G. Synthesis, physical-chemical characterization and electrochemical performance of GdBaCo2-xNixO5 (x = 0-0.8) as cathode materials for IT-SOFC application. J. Power Sources 2013, 242, 50–56. [Google Scholar] [CrossRef]
- Wei, B.; Lü, Z.; Jia, D.C.; Huang, X.Q.; Zhang, Y.H.; Su, W.H. Thermal expansion and electrochemical properties of Ni-doped GdBaCo2O5+δ double-perovskite type oxides. Int. J. Hydrogen Energy 2010, 35, 3775–3782. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2-xNixO5+δ (0 ≤ x ≤1.0) perovskite cathodes for intermediate temperature solid oxide fuel cells. J. Electrochem. Soc. 2011, 158, B276–B282. [Google Scholar] [CrossRef]
- Lü, S.Q.; Long, G.H.; Ji, Y.; Meng, X.W.; Zhao, H.Y.; Sun, C.C. SmBaCoCuO5+x as cathode material based on GDC electrolyte for intermediate-temperature solid oxide fuel cells. J. Alloy Compd. 2011, 509, 2824–2828. [Google Scholar] [CrossRef]
- Jo, S.H.; Muralidharan, P.; Kim, D.K. Enhancement of electrochemical performance and thermal compatibility of GdBaCo2/3Fe2/3Cu2/3O5+δ cathode on Ce1.9Gd0.1O1.95 electrolyte for IT-SOFCs. Electrochem. Commun. 2009, 11, 2085–2088. [Google Scholar] [CrossRef]
- Choi, S.; Yoo, S.; Kim, J.; Park, S.; Jun, A.; Sengodan, S.; Kim, J.; Shin, J.; Jeong, H.Y.; Choi, Y.; et al. Highly efficient and robust cathode materials for low-temperature solid oxide fuel cells: PrBa0.5Sr0.5Co2-xFexO5+δ. Sci. Rep. 2013, 3, 2426. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jun, A.; Shin, J.; Kim, G. Effect of Fe Doping on Layered GdBa0.5Sr0.5Co2O5+δ Perovskite cathodes for intermediate temperature solid oxide fuel cells. J. Am. Ceram. Soc. 2014, 97, 651–656. [Google Scholar] [CrossRef]
- Kim, J.; Choi, S.; Park, S.; Kim, C.; Shin, J.; Kim, G. Effect of Mn on the electrochemical properties of a layered perovskite NdBa0.5Sr0.5Co2-xMnxO5+δ (x = 0, 0.25, and 0.5) for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2013, 112, 712–718. [Google Scholar] [CrossRef]
- Pramana, S.S.; Cavallaro, A.; Li, C.; Handoko, A.D.; Chan, K.W.; Walker, R.J.; Regoutz, A.; Herrin, J.S.; Yeo, B.S.; Payne, D.J.; et al. Crystal structure and surface characteristics of Sr-doped GdBaCo2O6-δ double perovskites: Oxygen evolution reaction and conductivity. J. Mater. Chem. A 2018, 6, 5335–5345. [Google Scholar] [CrossRef]
- Subardi, A.; Chen, C.C.; Cheng, M.H.; Chang, W.K.; Fu, Y.P. Electrical, thermal and electrochemical properties of SmBa1-xSrxCo2O5+δ cathode materials for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2016, 204, 118–127. [Google Scholar] [CrossRef]
- Xue, J.; Shen, Y.; He, T. Performance of double-perovskite YBa0.5Sr0.5Co2O5+δ as cathode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 6894–6898. [Google Scholar] [CrossRef]
- Subardi, A.; Indra, A.; Setiawan, J.; Fu, Y.-P. Structural and Electrochemical Analysis of SmBa0.8Sr0.2Co2O5+δ Cathode Oxide for IT-SOFCs. Int. J. Integr. Eng. 2023, 15, 173–179. [Google Scholar] [CrossRef]
- Zan, J.; Wang, S.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Characterization and functional application of PrBa0.5Sr0.5Co1.5Fe0.5O5+δ cathode material for IT-SOFC. Mater. Res. Bull. 2021, 137, 111173. [Google Scholar] [CrossRef]
- Costilla-Aguilar, S.U.; Escudero, M.J.; Cienfuegos-Pelaes, R.F.; Aguilar-Martinez, J.A. Double perovskite La1.8Sr0.2CoFeO5+δ as a cathode material for intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2021, 862, 158025. [Google Scholar] [CrossRef]
- Liu, Y.; Cao, Y.; Sun, S.; Lu, C.; Wang, B.; Liu, G.; Gao, S.; Niu, B. Novel CO2-tolerant Co-based double perovskite cathode for intermediate temperature solid oxide fuel cells. J. Eur. Ceram. Soc. 2023, 43, 1028–1038. [Google Scholar] [CrossRef]
- Li, M.; Chen, K.; Hua, B.; Luo, J.-l.; Rickard, W.D.A.; Li, J.; Irvine, J.T.S.; Jiang, S.P. Smart utilization of cobaltite-based double perovskite cathodes on barrier-layer-free zirconia electrolyte of solid oxide fuel cells. J. Mater. Chem. A 2016, 4, 19019–19025. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, L.Z.; Han, M.-F. A-site deficient Ba1−xCo0.7Fe0.2Ni0.1O3-δ cathode for intermediate temperature SOFC. J. Power Sources 2011, 196, 868–871. [Google Scholar] [CrossRef]
- Chen, T.; Pang, S.; Shen, X.; Jiang, X.; Wang, W. Evaluation of Ba-deficient PrBa1-xFe2O5+δ oxides as cathode materials for intermediate-temperature solid oxide fuel cells. RSC Adv. 2016, 6, 13829–13836. [Google Scholar] [CrossRef]
- Yao, C.; Yang, J.; Zhang, H.; Chen, S.; Lang, X.; Meng, J.; Cai, K. Evaluation of A-site Ba-deficient PrBa0.5-xSr0.5Co2O5+δ (x = 0, 0.04 and 0.08) as cathode materials for solid oxide fuel cells. J. Alloys Compd. 2021, 883, 160759. [Google Scholar] [CrossRef]
- Idrees, A.; Jiang, X.; Jiang, L.; Zhang, Q. Properties of composite cathodes composed of Pr3+-deficient perovskite oxide and ionic conductor Ce0.8Sm0.2O1.9. Ceram. Int. 2020, 46, 17532–17539. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Sun, N.; Li, J.; Shen, Y.; Wang, F.; Li, J. Nd3+-deficiency double perovskite Nd1-xBaCo2O5+δ and performance optimization as cathode materials for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2021, 47, 33886–33896. [Google Scholar] [CrossRef]
- Yi, K.; Sun, L.; Li, Q.; Xia, T.; Huo, L.; Zhao, H.; Li, J.; Lü, Z.; Bassat, J.-M.; Rougier, A.; et al. Effect of Nd-deficiency on electrochemical properties of NdBaCo2O6-δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 10228–10238. [Google Scholar] [CrossRef]
- Lü, S.; Zhu, Y.; Fu, X.; Huang, R.; Guo, Y.; Zhang, W.; Li, H.; Hou, L.; Meng, X. A-site deficient Fe-based double perovskite oxides PrxBaFe2O5+δ as cathodes for solid oxide fuel cells. J. Alloys Compd. 2022, 911, 165002. [Google Scholar] [CrossRef]
- Nie, Z.; Wang, J.; Xia, T.; Wang, G. A-site Ca-doped layered double perovskite Pr1-xCaxBa0.94Co2O5+δ as high-performance and stable cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2022, 905, 164191. [Google Scholar] [CrossRef]
- Yao, C.; Yang, J.; Zhang, H.; Lang, X.; Cai, K. Ca-doped PrBa1-xCaxCoCuO5+δ (x = 0–0.2) as cathode materials for solid oxide fuel cells. Ceram. Int. 2022, 48, 7652–7662. [Google Scholar] [CrossRef]
- Asensio, A.M.; Clematis, D.; Cademartori, D.; Carpanese, M.P.; Viviani, M.; Carbone, C.; Barbucci, A. Calcium doping in double perovskite SmBa1−xCaxCo2O5+δ to enhance the electrochemical activity of solid oxide cell reversible oxygen electrode. J. Alloys Compd. 2023, 933, 167731. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Liu, X.; Sun, N.; Li, J.; Shen, Y.; Wang, F.; Yang, L.; Chu, X.; Xu, M.; et al. Effect of calcium doping on Sm1–xCaxBaCo2O5+δ cathode materials for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2021, 390, 138830. [Google Scholar] [CrossRef]
- Jin, F.; Liu, X.; Chu, X.; Shen, Y.; Li, J. Effect of nonequivalent substitution of Pr3+/4+ with Ca2+ in PrBaCoFeO5+δ as cathodes for IT-SOFC. J. Mater. Sci. 2021, 56, 1147–1161. [Google Scholar] [CrossRef]
- Du, Z.; Yan, C.; Zhao, H.; Zhang, Y.; Yang, C.; Yi, S.; Lu, Y.; Świerczek, K. Effective Ca-doping in Y1-xCaxBaCo2O5+δ cathode materials for intermediate temperature solid oxide fuel cells. J. Mater. Chem. A 2017, 5, 25641–25651. [Google Scholar] [CrossRef]
- Pang, S.; Su, Y.; Yang, G.; Shen, X.; Zhu, M.; Wu, X.; Li, S.; Yang, X.; Xi, X. Enhanced electrochemical performance of Ca-doped NdBa1-xCaxCoCuO5+δ as cathode material for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2018, 44, 21902–21907. [Google Scholar] [CrossRef]
- Xia, W.; Liu, X.; Jin, F.; Jia, X.; Shen, Y.; Li, J. Evaluation of calcium codoping in double perovskite PrBaCo2O5+δ as cathode material for IT-SOFCs. Electrochim. Acta 2020, 364, 137274. [Google Scholar] [CrossRef]
- Li, J.; Sun, N.; Liu, X.; Shen, Y.; Wang, F.; Li, J.; Shi, K.; Jin, F. Investigation on Nd1–xCaxBaCo2O5+δ double perovskite as new oxygen electrode materials for reversible solid oxide cells. J. Alloys Compd. 2022, 913, 165245. [Google Scholar] [CrossRef]
- Wang, L.; Xie, P.; Bian, L.; Liu, X.; Chou, K. Performance of Ca-doped GdBa1-xCaxFe2O5+δ (x=0, 0.1) as cathode materials for IT-SOFC application. Catal. Today 2018, 318, 132–136. [Google Scholar] [CrossRef]
- Xiang, W.; Wang, J.; Li, S.; Xia, T.; Wang, G. Positive effects of calcium-doping on the cathode performance of layered perovskite Eu1-xCaxBaCo2O5+δ for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2019, 801, 220–228. [Google Scholar] [CrossRef]
- Wang, W.; Pang, S.; Su, Y.; Shen, X.; Wang, Y.; Xu, K.; Xi, X.; Xiang, J. The effect of calcium on the properties of SmBa1−xCaxCoCuO5+δ as a cathode material for intermediate-temperature solid oxide fuel cells. J. Eur. Ceram. Soc. 2017, 37, 1557–1562. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, C.; Chen, X.; Chen, F.; Xiong, X.; Liu, Y.; Yan, J.; Fujita, T. A-site double-lanthanide-doped La1-xPrxBaCo2O5+δ cathode materials for intermediate-temperature solid oxide fuel cells. J. Mater. Sci. 2022, 57, 14398–14412. [Google Scholar] [CrossRef]
- Bangwal, A.S.; Jha, P.K.; Chauhan, M.; Singh, S.; Sinha, A.S.K.; Jha, P.A.; Singh, P. Compositional effect on oxygen reduction reaction in Pr excess double perovskite Pr1-xBa1-xCo2O6-δ cathode materials. Int. J. Hydrogen Energy 2020, 45, 23378–23390. [Google Scholar] [CrossRef]
- Chen, F.; Zhou, D.; Xiong, X.; Pan, J.; Cai, D.; Wei, Z.; Chen, X.; Liu, Y.; Luo, N.; Yan, J.; et al. Doping strategy on improving the overall cathodic performance of double perovskite LnBaCo2O5+δ (Ln=Pr, Gd) as potential SOFC cathode materials. J. Mater. 2023, 9, 825–837. [Google Scholar] [CrossRef]
- Zhu, F.; He, F.; Xu, K.; Chen, Y. Enhancing the oxygen reduction reaction activity and durability of a double-perovskite via an A-site tuning. Sci. China Mater. 2022, 65, 3043–3052. [Google Scholar] [CrossRef]
- Wang, S.; Zan, J.; Qiu, W.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Evaluation of perovskite oxides LnBaCo2O5+δ (Ln = La, Pr, Nd and Sm) as cathode materials for IT-SOFC. J. Electroanal. Chem. 2021, 886, 115144. [Google Scholar] [CrossRef]
- Yang, Q.; Tian, D.; Liu, R.; Wu, H.; Chen, Y.; Ding, Y.; Lu, X.; Lin, B. Exploiting rare-earth-abundant layered perovskite cathodes of LnBa0.5Sr0.5Co1.5Fe0.5O5+δ (Ln=La and Nd) for SOFCs. Int. J. Hydrogen Energy 2021, 46, 5630–5641. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, B.; Hao, X.; Wang, Y.; Liu, J.; Jiang, P.; He, T. Layered oxygen-deficient double perovskite GdBaFe2O5+δ as electrode material for symmetrical solid-oxide fuel cells. Electrochim. Acta 2021, 370, 137807. [Google Scholar] [CrossRef]
- Saccoccio, M.; Jiang, C.; Gao, Y.; Chen, D.; Ciucci, F. Nb-substituted PrBaCo2O5+δ as a cathode for solid oxide fuel cells: A systematic study of structural, electrical, and electrochemical properties. Int. J. Hydrogen Energy 2017, 42, 19204–19215. [Google Scholar] [CrossRef]
- Akande, S.O.; Chroneos, A.; Schwingenschlögl, U. O vacancy formation in (Pr/Gd)BaCo2O5.5 and the role of antisite defects. Phys. Chem. Chem. Phys. 2017, 19, 11455–11459. [Google Scholar] [CrossRef] [PubMed]
- Anjum, U.; Vashishtha, S.; Agarwal, M.; Tiwari, P.; Sinha, N.; Agrawal, A.; Basu, S.; Haider, M.A. Oxygen anion diffusion in double perovskite GdBaCo2O5+δ and LnBa0.5Sr0.5Co2-xFexO5+δ (Ln = Gd, Pr, Nd) electrodes. Int. J. Hydrogen Energy 2016, 41, 7631–7640. [Google Scholar] [CrossRef]
- Ananyev, M.V.; Eremin, V.A.; Tsvetkov, D.S.; Porotnikova, N.M.; Farlenkov, A.S.; Zuev, A.Y.; Fetisov, A.V.; Kurumchin, E.K. Oxygen isotope exchange and diffusion in LnBaCo2O6−δ (Ln = Pr, Sm, Gd) with double perovskite structure. Solid State Ionics 2017, 304, 96–106. [Google Scholar] [CrossRef]
- Zhou, Q.; Wang, F.; Shen, Y.; He, T. Performances of LnBaCo2O5+x–Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2010, 195, 2174–2181. [Google Scholar] [CrossRef]
- Malyshkin, D.; Novikov, A.; Tsvetkov, D.; Zuev, A. Preparation, oxygen nonstoichiometry and defect structure of double perovskite LaBaCo2O6–δ. Mater. Lett. 2018, 229, 324–326. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, W.; Li, Y.; Yu, B. REBaCo2O5+δ (RE = Pr, Nd, and Gd) as promising oxygen electrodes for intermediate-temperature solid oxide electrolysis cells. RSC Adv. 2017, 7, 16332–16340. [Google Scholar] [CrossRef]
- Politov, B.V.; Suntsov, A.Y.; Leonidov, I.A.; Patrakeev, M.V.; Kozhevnikov, V.L. Thermodynamic analysis of defect equilibration in double perovskites based on PrBaCo2O6–δ cobaltite. J. Solid State Chem. 2017, 249, 108–113. [Google Scholar] [CrossRef]
- Jin, F.; Liu, J.; Shen, Y.; He, T. Improved electrochemical performance and thermal expansion compatibility of LnBaCoFeO5+δ-Sm0.2Ce0.8O1.9 (Ln=Pr and Nd) composite cathodes for IT-SOFCs. J. Alloys Compd. 2016, 685, 483–491. [Google Scholar] [CrossRef]
- Zając, W.; Świerczek, K.; Molenda, J. Thermochemical compatibility between selected (La,Sr)(Co,Fe,Ni)O3 cathodes and rare earth doped ceria electrolytes. J. Power Sources 2007, 173, 675–680. [Google Scholar] [CrossRef]
- Zhu, J.H.; Geng, S.J.; Ballard, D.A. Evaluation of several low thermal expansion Fe–Co–Ni alloys as interconnect for reduced-temperature solid oxide fuel cell. Int. J. Hydrogen Energy 2007, 32, 3682–3688. [Google Scholar] [CrossRef]
- Señarís-Rodríguez, M.A.; Goodenough, J.B. Magnetic and Transport Properties of the System La1-xSrxCoO3-δ (0 < x ≤ 0.50). J. Solid State Chem. 1995, 118, 323–336. [Google Scholar] [CrossRef]
- Huang, K.; Lee, H.Y.; Goodenough, J.B. Sr- and Ni- Doped LaCoO3 and LaFeO3 perovskites: New cathode materials for solid-oxide fuel cells. J. Electrochem. Chem. 1998, 145, 3220–3227. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Zuev, A.Y. Crystal structure and oxygen content of the double perovskites GdBaCo2-xFexO6-δ. J. Solid State Chem. 2013, 199, 154–159. [Google Scholar] [CrossRef]
- Xue, J.; Shen, Y.; He, T. Double-perovskites YBaCo2−xFexO5+δ cathodes for intermediate-temperature solid oxide fuel cells. J. Power Sources 2011, 196, 3729–3735. [Google Scholar] [CrossRef]
- Zou, J.; Park, J.; Kwak, B.; Yoon, H.; Chung, J. Effect of Fe doping on PrBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Solid State Ionics 2012, 206, 112–119. [Google Scholar] [CrossRef]
- Joo, S.; Kim, J.; Shin, J.; Lim, T.-H.; Kim, G. Investigation of a layered perovskite for IT-SOFC cathodes: B-site Fe-doped YBa0.5Sr0.5Co2-xFexO5+δ. J. Electrochem. Soc. 2016, 163, F1489–F1495. [Google Scholar] [CrossRef]
- Son, S.J.; Kim, D.; Park, H.J.; Joo, J.H. Investigation of oxygen ion transport and surface exchange properties of PrBaFe2O5+δ. J. Eur. Ceram. Soc. 2021, 41, 2691–2698. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Yang, J.-X.; Wang, P.-F.; Yao, C.-G.; Yu, X.-D.; Shi, F.-N. Novel cobalt-free perovskite PrBaFe1.9Mo0.1O5+δ as a cathode material for solid oxide fuel cells. Solid State Ionics 2023, 391, 116144. [Google Scholar] [CrossRef]
- Politov, B.V.; Suntsov, A.Y.; Leonidov, I.A.; Patrakeev, M.V.; Kozhevnikov, V.L. High-temperature defect thermodynamics of nickel substituted double-perovskite cobaltite PrBaCo2-xNixO6-δ (x = 0.2). J. Alloys Compd. 2017, 727, 778–784. [Google Scholar] [CrossRef]
- Xia, L.-N.; You, J.; He, Z.-P.; Huang, X.-W.; Yu, Y. Performances of nickel-doped SmBaCo2O5+δ-Sm0.2Ce0.8O1.9 composite cathodes for IT-SOFC. Int. J. Hydrogen Energy 2016, 41, 1176–1186. [Google Scholar] [CrossRef]
- Xia, L.-N.; He, Z.-P.; Huang, X.W.; Yu, Y. Synthesis and properties of SmBaCo2-xNixO5+δ perovskite oxide for IT-SOFC cathodes. Ceram. Int. 2016, 42, 1272–1280. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Sayagués, M.J.; Gotor, F.J. A Novel, Simple and highly efficient route to obtain PrBaMn2O5+δ double perovskite: Mechanochemical synthesis. Nanomaterials 2021, 11, 380. [Google Scholar] [CrossRef]
- Huang, X.; Feng, J.; Abdellatif, H.R.S.; Zou, J.; Zhang, G.; Ni, C. Electrochemical evaluation of double perovskite PrBaCo2-xMnxO5+δ (x = 0, 0.5, 1) as promising cathodes for IT-SOFCs. Int. J. Hydrogen Energy 2018, 43, 8962–8971. [Google Scholar] [CrossRef]
- Ren, R.; Wang, Z.; Meng, X.; Xu, C.; Qiao, J.; Sun, W.; Sun, K. Boosting the electrochemical performance of Fe-based layered double perovskite cathodes by Zn2+ doping for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2020, 12, 23959–23967. [Google Scholar] [CrossRef]
- Sun, C.; Kong, Y.; Niu, Y.; Yin, X.; Zhang, N. Probing Zr substituting effects on the oxygen reduction reaction of Fe-based double perovskite cathodes for solid oxide fuel cells. ACS Appl. Energy Mater. 2022, 5, 4486–4495. [Google Scholar] [CrossRef]
- Sun, C.; Kong, Y.; Shao, L.; Zhang, Q.; Wu, X.; Zhang, N.; Sun, K. Significant zirconium substitution effect on the oxygen reduction activity of the cathode material NdBaCo2O5+δ for solid oxide fuel cells. ACS Sust. Chem. Eng. 2019, 7, 11603–11611. [Google Scholar] [CrossRef]
- Zhang, B.; Wan, Y.; Hua, Z.; Tang, K.; Xia, C. Tungsten-doped PrBaFe2O5+δ double perovskite as a high-performance electrode material for symmetrical solid oxide fuel cells. ACS Appl. Energy Mater. 2021, 4, 8401–8409. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, J.; Kwon, O.; Lim, C.; Sengodan, S.; Shin, J.; Kim, G. Scandium doping effect on a layered perovskite cathode for low-temperature solid oxide fuel cells (LT-SOFCs). Appl. Sci. 2018, 8, 2217. [Google Scholar] [CrossRef]
- Xu, J.; Cai, H.; Hao, G.; Zhang, L.; Song, Z.; Long, W.; Zhang, L.; Wang, L. Characterization of high-valence Mo-doped PrBaCo2O5+δ cathodes for IT-SOFCs. J. Alloys Compd. 2020, 842, 155600. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, S.; Han, H.; Tang, K.; Xia, C. Cobalt-free double perovskite oxide as a promising cathode for solid oxide fuel cells. ACS Appl. Mater. Interfaces 2023, 15, 8253–8262. [Google Scholar] [CrossRef]
- Bao, X.; Su, X.; Wang, S.; Pan, B.; Wang, L.; Zhang, L.; Song, Z.; Long, W.; Li, C. Effects of Bi-doping on structure and properties of YBaCo2O5+δ layered perovskite cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2023, 965, 171391. [Google Scholar] [CrossRef]
- Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G. Cobalt based layered perovskites as cathode material for intermediate temperature solid oxide fuel cells: A brief review. J. Power Sources 2015, 298, 46–67. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, Y.; Yu, F.; Pan, Z.; Yang, H.; Guo, L. Ca and Fe co-doped SmBaCo2O5+δ layered perovskite as an efficient cathode for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2017, 696, 964–970. [Google Scholar] [CrossRef]
- Tian, Y.; Liu, Y.; Wang, W.; Jia, L.; Pu, J.; Chi, B.; Li, J. High performance and stability of double perovskite-type oxide NdBa0.5Ca0.5Co1.5Fe0.5O5+δ as an oxygen electrode for reversible solid oxide electrochemical cell. J. Energy Chem. 2020, 43, 108–115. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. Novel PrBa0.9Ca0.1Co2-xZnxO5+δ double-perovskite as an active cathode material for high-performance proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 31009–31016. [Google Scholar] [CrossRef]
- Dong, F.F.; Ni, M.; Chen, Y.B.; Chen, D.J.; Tadé, M.O.; Shao, Z.P. Structural and oxygen-transport studies of double perovskites PrBa1-xCo2O5+δ (x = 0.00, 0.05, and 0.10) toward their application as superior oxygen reduction electrodes. J. Mater. Chem. A 2014, 2, 20520–20529. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.X.; Lai, S.Y.; Gerdes, K.; Liu, M.L. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552–575. [Google Scholar] [CrossRef]
- Choi, Y.; Choi, S.; Jeong, H.Y.; Liu, M.L.; Kim, B.-S.; Kim, G. Highly efficient layer-by-layer-assisted infiltration for high-performance and cost-effective fabrication of nanoelectrodes. ACS Appl. Mater. Interfaces 2014, 6, 17352–17357. [Google Scholar] [CrossRef]
- Han, D.; Wu, H.; Li, J.L.; Wang, S.R.; Zhan, Z.L. Nanostructuring of SmBa0.5Sr0.5Co2O5+δ cathodes for reduced-temperature solid oxide fuel cells. J. Power Sources 2014, 246, 409–416. [Google Scholar] [CrossRef]
- Ding, H.P.; Xue, X.J. An Interfacial nanospike-structured cathode for low temperature solid oxide fuel cells. Adv. Mater. Interfaces 2014, 1, 1400008. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, D.J.; Chen, C.; Shao, Z.P.; Ciucci, F. Oriented PrBaCo2O5+δ thin films for solid oxide fuel cells. J. Power Sources 2015, 278, 623–629. [Google Scholar] [CrossRef]
- Liu, J.; Collins, G.; Liu, M.; Chen, C.L. Superfast oxygen exchange kinetics on highly epitaxial LaBaCo2O5+δ thin films for intermediate temperature solid oxide fuel cells. APL Mater. 2013, 1, 031101. [Google Scholar] [CrossRef]
- Liu, J.; Collins, G.; Liu, M.; Chen, C.L.; He, J.; Jiang, J.C.; Meletis, E.I. Ultrafast oxygen exchange kinetics on highly epitaxial PrBaCo2O5+δ thin films. Appl. Phys. Lett. 2012, 100, 193903. [Google Scholar] [CrossRef]
- Pang, S.; Long, C.; Tang, X.; Fang, T.; Ke, L.; Yang, G.; Song, Y.; Chen, C. Highly active and robust biomimetic ceramic catalyst for oxygen reduction reaction: Inspired by plant leaves. Ceram. Int. 2023, 49, 20273–20280. [Google Scholar] [CrossRef]
- Kim, S.; Jun, A.; Kwon, O.; Kim, J.; Yoo, S.; Jeong, H.Y.; Shin, J.; Kim, G. Nanostructured double perovskite cathode with low sintering temperature for intermediate temperature solid oxide fuel cells. ChemSusChem 2015, 8, 3153–3158. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Zuev, A.Y. Oxygen content, cobalt oxide exsolution and defect structure of the double perovskite PrBaCo2O6-δ. J. Mater. Chem. A 2016, 4, 1962–1969. [Google Scholar] [CrossRef]
- Pang, S.; Song, Y.; Cui, M.; Tang, X.; Long, C.; Ke, L.; Yang, G.; Fang, T.; Guan, Y.; Chen, C. Rapid and durable oxygen reduction reaction enabled by a perovskite oxide with self-cleaning surface. J. Energy Chem. 2023, 83, 333–340. [Google Scholar] [CrossRef]
- Fu, M.; Lin, X.; Tan, L.; Zhang, P.; Xie, H.; Tao, Z. Self-assembled Fe-doped PrBaCo2O5+δ composite cathodes with disorder transition region for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2023, 48, 15229–15237. [Google Scholar] [CrossRef]
- Jiang, X.N.; Xu, H.X.; Wang, Q.; Jiang, L.; Li, X.N.; Xu, Q.L.; Shi, Y.C.; Zhang, Q.Y. Fabrication of GdBaCo2O5+δ cathode using electrospun composite nanofibers and its improved electrochemical performance. J. Alloys Compd. 2013, 557, 184–189. [Google Scholar] [CrossRef]
- Hedayat, N.; Du, Y.; Ilkhani, H. Review on fabrication techniques for porous electrodes of solid oxide fuel cells by sacrificial template methods. Renew. Sust. Energ. Rev. 2017, 77, 1221–1239. [Google Scholar] [CrossRef]
- Chen, Y.; Bu, Y.; Zhao, B.; Zhang, Y.; Ding, D.; Hu, R.; Wei, T.; Rainwater, B.; Ding, Y.; Chen, F.; et al. A durable, high-performance hollow-nanofiber cathode for intermediate-temperature fuel cells. Nano Energy 2016, 26, 90–99. [Google Scholar] [CrossRef]
- Fan, L.; Zhu, B.; Su, P.-C.; He, C. Nanomaterials and technologies for low temperature solid oxide fuel cells: Recent advances, challenges and opportunities. Nano Energy 2018, 45, 148–176. [Google Scholar] [CrossRef]
- Zhang, Y.; Knibbe, R.; Sunarso, J.; Zhong, Y.; Zhou, W.; Shao, Z.; Zhu, Z. Recent progress on advanced materials for solid-oxide fuel cells operating below 500 °C. Adv. Mater. 2017, 29, 1700132. [Google Scholar] [CrossRef]
- Xu, X.; Wang, W.; Zhou, W.; Shao, Z. Recent advances in novel nanostructuring methods of perovskite electrocatalysts for energy-related applications. Small Methods 2018, 2, 1800071. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, G.; Wang, G.; Irvine, J.T.S. Synthesis and applications of nanoporous perovskite metal oxides. Chem. Sci. 2018, 9, 3623–3637. [Google Scholar] [CrossRef]
- Zhen, D.; Zhao, B.; Shin, H.-C.; Bu, Y.; Ding, Y.; He, G.; Liu, M. Electrospun porous perovskite La0.6Sr0.4Co1–xFexO3–δ nanofibers for efficient oxygen evolution reaction. Adv. Mater. Interfaces 2017, 4, 1700146. [Google Scholar] [CrossRef]
- Wang, Y.; Arandiyan, H.; Tahini, H.A.; Scott, J.; Tan, X.; Dai, H.; Gale, J.D.; Rohl, A.L.; Smith, S.C.; Amal, R. The controlled disassembly of mesostructured perovskites as an avenue to fabricating high performance nanohybrid catalysts. Nat. Commun. 2017, 8, 15553. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Liu, M.; Wang, G.Q.; Lu, H.L.; Yang, T.Z.; Guo, H.M.; Ma, C.R.; Xu, X.; Zhang, M.H.; Jiang, J.C.; et al. Step terrace tuned anisotropic transport properties of highly epitaxial LaBaCo2O5.5+δ thin films on vicinal SrTiO3 substrates. ACS Appl. Mater. Interfaces 2014, 6, 6704–6708. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, M.; Collins, G.; Chen, C.L.; Jiang, X.N.; Gong, W.Q.; Jacobson, A.J.; He, J.; Jiang, J.C.; Meletis, E.I. Epitaxial nature and transport properties in (LaBa)Co2O5+δ thin films. Chem. Mater. 2010, 22, 799–802. [Google Scholar] [CrossRef]
- Druce, J.; Téllez, H.; Burriel, M.; Sharp, M.D.; Fawcett, L.J.; Cook, S.N.; Mcphail, D.S.; Ishihara, T.; Brongersma, H.H.; Kilner, J.A. Surface termination and subsurface restructuring of perovskite-based solid oxide electrode materials. Energy Environ. Sci. 2014, 7, 3593–3599. [Google Scholar] [CrossRef]
- Lee, W.; Yildiz, B. Factors that influence cation segregation at the surfaces of perovskite oxides. ECS Trans. 2013, 57, 2115–2123. [Google Scholar] [CrossRef]
- Lee, W.; Han, J.W.; Chen, Y.; Cai, Z.H.; Yildiz, B. Cation size mismatch and charge interactions drive dopant segregation at the surfaces of manganite perovskites. J. Am. Chem. Soc. 2013, 135, 7909–7925. [Google Scholar] [CrossRef]
- Mebane, D.S. A variational approach to surface cation segregation in mixed conducting perovskites. Comput. Mater. Sci. 2014, 103, 231–236. [Google Scholar] [CrossRef]
- Pang, S.; Xu, J.; Su, Y.; Yang, G.; Zhu, M.; Cui, M.; Shen, X.; Chen, C. The role of A-site cation size mismatch in tune the catalytic activity and durability of double perovskite oxides. Appl. Catal. B Environ. 2020, 270, 118868. [Google Scholar] [CrossRef]
- Anjum, U.; Agarwal, M.; Khan, T.S.; Haider, M.A. Mechanistic elucidation of surface cation segregation in double perovskite PrBaCo2O5+δ material using MD and DFT simulations for solid oxide fuel cells. Ionics 2020, 26, 1307–1314. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, B.; Lü, Z.; Feng, J.; Xu, L.; Gao, H.; Zhang, Y.; Huang, X. Performance degradation of double-perovskite PrBaCo2O5+δ oxygen electrode in CO2 containing atmospheres. Appl. Surf. Sci. 2017, 416, 649–655. [Google Scholar] [CrossRef]
- Wei, B.; Chen, K.; Wang, C.C.; Lü, Z.; Jiang, S.P. Performance degradation of SmBaCo2O5+δ cathode induced by chromium deposition for solid oxide fuel cells. Electrochim. Acta 2015, 174, 327–331. [Google Scholar] [CrossRef]
- Kim, J.H.; Yoo, S.; Murphy, R.; Chen, Y.; Ding, Y.; Pei, K.; Zhao, B.; Kim, G.; Choi, Y.M.; Liu, M. Promotion of oxygen reduction reaction on a double perovskite electrode by a water-induced surface modification. Energy Environ. Sci. 2021, 14, 1506–1516. [Google Scholar] [CrossRef]
- Wei, B.; Schroeder, M.; Martin, M. Surface cation segregation and chromium deposition on the double perovskite oxide PrBaCo2O5+δ. ACS Appl. Mater. Interfaces 2018, 10, 8621–8629. [Google Scholar] [CrossRef] [PubMed]
- Druce, J.; Téllez, H.; Hyodo, J. Surface segregation and poisoning in materials for low-temperature SOFCs. MRS Bull. 2014, 39, 810–815. [Google Scholar] [CrossRef]
- Lu, F.; Xia, T.; Li, Q.; Wang, J.; Huo, L.; Zhao, H. Heterostructured simple perovskite nanorod-decorated double perovskite cathode for solid oxide fuel cells: Highly catalytic activity, stability and CO2-durability for oxygen reduction reaction. Appl. Catal. B Environ. 2019, 249, 19–31. [Google Scholar] [CrossRef]
- Ke, L.; Pang, S.; Long, C.; Fang, T.; Yang, G.; Song, Y.; He, X.; Ma, S.; Qian, Y.; Shen, X.; et al. Quenching-induced surface reconstruction of perovskite oxide for rapid and durable oxygen catalysis. Chem. Eng. J. 2023, 463, 142509. [Google Scholar] [CrossRef]
- Yang, G.; Xu, J.; Pang, S.; Cui, M.; Shen, X. Tuning interfacial chemistry and electrochemical properties of solid oxide cells via cation interdiffusion. Ceram. Int. 2020, 46, 12044–12049. [Google Scholar] [CrossRef]


| Electrical Conductivity (S cm−1) | TEC (10−6 K−1) | ASR (Ω cm2) | Refs | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 600 | 700 | 800 | 600 | 700 | 800 | Electrolyte | |||
| LaBaCo2O5+δ | 558 | 447 | 355 | - | 0.195 | 0.039 | 0.010 | GDC | [93] |
| LaBa0.9Co2O5+δ | 483 | 386 | 306 | - | 0.118 | 0.023 | 0.007 | GDC | [93] |
| PrBaCo2O5+δ | 208 | 164 | 128 | 23.4 (30–900 °C) | 0.181 | 0.038 | 0.009 | GDC | [94] |
| 212 | 170 | 138 | 24.6 (30–800 °C) | - | 0.078 | SDC | [100] | ||
| PrBa0.92Co2O5+δ | 233 | 187 | 146 | 22.8 (30–900 °C) | 0.093 | 0.024 | 0.007 | GDC | [94] |
| PrBa0.5Sr0.5Co2O5+δ | - | - | - | - | 0.688 | 0.154 | - | GDC | [87] |
| PrBaCoFeO5+δ | 91 | 68 | 62 | 24.9 (30–800 °C) | - | 0.105 | - | SDC | [100] |
| NdBa0.5Sr0.5Co2O5+δ | 695 | 556 | 442 | - | - | 0.139 | 0.039 | LSGM | [90] |
| 263 | 204 | 191 | 25.2 (100–800 °C) | 2.800 | 0.676 | 0.086 | SDC | [73] | |
| SmBaCo2O5+δ | 560 | 440 | - | - | 0.192 | - | - | GDC | [89] |
| SmBa0.5Sr0.5Co2O5+δ | 1000 | 810 | - | - | 0.141 | - | - | GDC | [89] |
| - | - | - | - | 0.631 | 0.092 | - | GDC | [87] | |
| SmBaCoCuO5+δ | 27 | 28 | 31 | 15.5 (30–850 °C) | - | 0.382 | 0.086 | GDC | [107] |
| GdBaCo2O5+δ | 311 | 249 | 196 | - | - | - | - | GDC | [85] |
| - | - | - | 20.0 (30–900 °C) | 0.4 | - | - | GDC | [104] | |
| 472 | 374 | 305 | 16.6 (80–900 °C) | - | - | - | GDC | [86] | |
| GdBa0.5Sr0.5Co2O5+δ | 591 | 492 | 409 | - | - | - | - | - | [85] |
| - | - | - | - | 1.260 | 0.561 | - | GDC | [87] | |
| GdBa0.4Sr0.6Co2O5+δ | 1099 | 930 | 591 | 19.5 (80–900 °C) | - | - | - | GDC | [86] |
| GdBaCo1.7Ni0.3O5+δ | - | - | - | 15.5 (30–900 °C) | 0.54 | 0.297 | - | GDC | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, F.; Pang, S. Progress in Developing LnBaCo2O5+δ as an Oxygen Reduction Catalyst for Solid Oxide Fuel Cells. Catalysts 2023, 13, 1288. https://doi.org/10.3390/catal13091288
Zheng F, Pang S. Progress in Developing LnBaCo2O5+δ as an Oxygen Reduction Catalyst for Solid Oxide Fuel Cells. Catalysts. 2023; 13(9):1288. https://doi.org/10.3390/catal13091288
Chicago/Turabian StyleZheng, Fa, and Shengli Pang. 2023. "Progress in Developing LnBaCo2O5+δ as an Oxygen Reduction Catalyst for Solid Oxide Fuel Cells" Catalysts 13, no. 9: 1288. https://doi.org/10.3390/catal13091288
APA StyleZheng, F., & Pang, S. (2023). Progress in Developing LnBaCo2O5+δ as an Oxygen Reduction Catalyst for Solid Oxide Fuel Cells. Catalysts, 13(9), 1288. https://doi.org/10.3390/catal13091288