1. Introduction
Graphite (G) has gained significant attention due to its excellent intercalation properties, and also for its role as a precursor for graphene oxide (GO) preparation. GO can be produced through strong oxidation of graphite using chemicals like KMnO
4, H
2SO
4, or HClO
4, resulting in an oxygen-rich carbonaceous material [
1]. This material contains various oxygen groups, such as epoxides, hydroxyl, and carboxyl groups, making GO hydrophilic [
2]. Intercalated GO, in nanocomposites, allows water molecules to become incorporated into its interlayer structure. The versatile properties of GO are explored for various applications in sensors, catalysts, and as an effective adsorbent, owing to its layered structure and the presence of functional groups [
3].
Industries like textiles, printing, and tanning release wastewater containing numerous dye substances, posing significant threats to humans, animals, and the environment. While various methods are employed to remove contaminants from water, adsorption using activated carbon has proven effective due to its simplicity and cost-effectiveness [
4]. However, the high production and regeneration costs of activated carbon, coupled with limited adsorption capacity at times, highlight the need for alternative solutions.
Graphene-based nanocomposites have emerged as promising candidates for overcoming these limitations. The unique properties of graphene-based materials, including high surface area, chemical inertness, mechanical strength, and excellent electrical and thermal conductivity make them suitable for diverse applications, from sensing to energy storage, and heterogeneous catalysis [
5]. Graphene serves as an ideal support framework for functional nanoparticles (NPs), presenting immense research opportunities [
6,
7].
Chemical oxidation of graphite to GO, followed by reduction to produce graphene nanosheets, is a widely used method due to its low cost, simplicity, and high yield [
8]. The presence of sp
3-hybridized carbon atoms decorated with oxygen-containing functional groups makes GO chemically active and gives it hydrophilic properties. Consequently, these functional groups act as anchoring sites for adsorbing diverse metal oxide NPs, extending the scope of graphene-based composites in electronics, photocatalysis, and photovoltaic devices [
9,
10].
One key advantage of graphene-based materials is their ability to enhance charge transport in various devices, thanks to its unique structure with abundant delocalized electrons within the conjugated sp
2-bonded graphitic carbon network, conferring remarkable conductivity. Several metal oxide–RGO nanocomposites, including palladium, silver, gold, TiO
2, Co
3O
4, and CdSe particles, have already been reported [
11,
12]. Moreover, the utilization of graphene-based nanocomposites in photocatalytic reactions offers a safer and greener approach, as it does not involve the use of toxic substances and does not require high-temperature conditions. The exploration of graphene-based nanocomposites presents a promising avenue for addressing water purification challenges and advancing various technological applications across multiple industries [
13].
Graphene-based nanomaterials have been extensively studied for their antimicrobial properties against various human pathogens, such as
Listeria monocytogenes,
Staphylococcus aureus,
Pseudomonas aeruginosa,
Salmonella typhimurium,
Streptococcus mutans,
Escherichia coli,
Staphylococcus epidermidis,
Vibrio harveyi, and
Enterococcus faecalis [
14,
15]. Numerous research studies have reported the promising antibacterial properties of graphene [
16].
Graphene oxide (GO) demonstrates its antibacterial efficacy by damaging the cell membrane through chemical reactions, with the shape and type of bacteria influencing the extent of its bactericidal properties [
17].
Klebsiella pneumoniae is responsible for a significant proportion of all Gram-negative infections, including urinary tract infections, cystitis, pneumonia, and pyogenic liver abscesses [
18,
19]. Such infections can lead to hospitalization and even mortality [
20].
In our study, we investigated the antimicrobial properties of the nickel(II) complex-modified GO. Our findings demonstrate that the modified GO exhibits potent antimicrobial activity, offering a promising avenue for combating bacterial infections caused by pathogens such as Klebsiella pneumoniae.
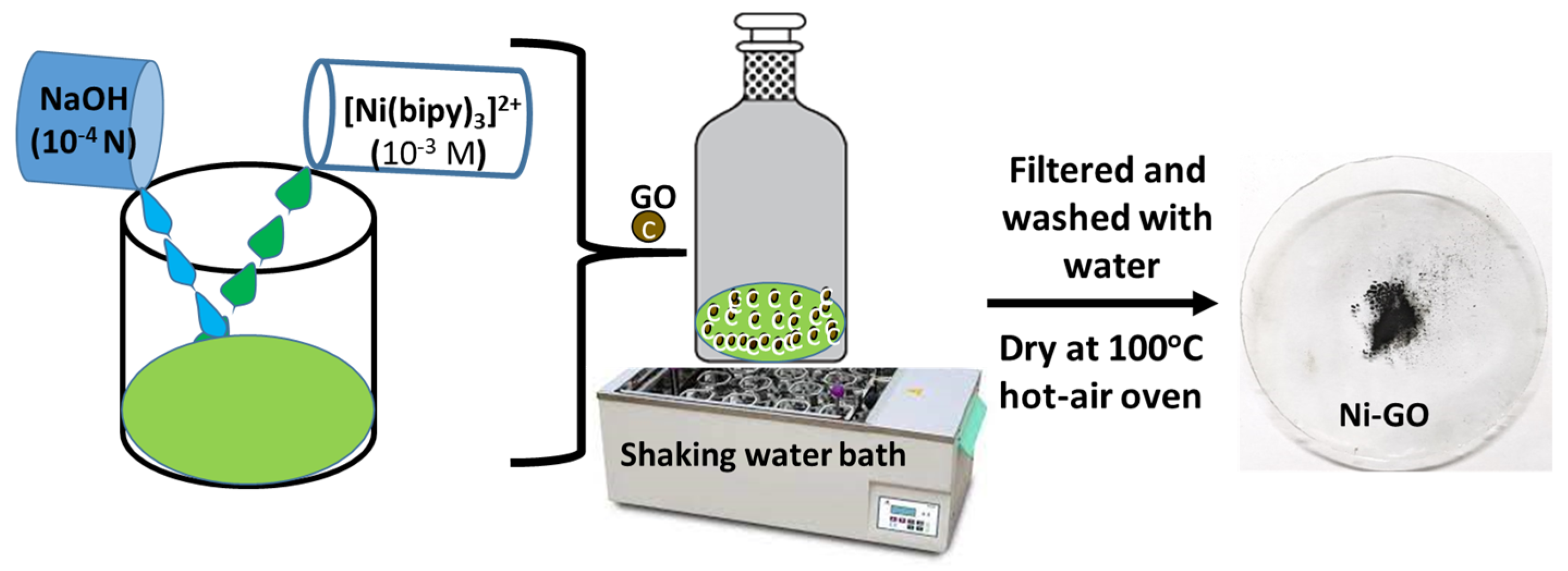
In this study, we have successfully synthesized nanostructured composites through a one-step simple adsorption approach, involving the anchoring of a nickel(II) complex onto the surface of graphene oxide (GO). The significance of the method employed lies in its ease of incorporation, as the oxide functional groups present in GO facilitate the binding of the nickel complex to the GO surface matrix. To comprehensively characterize the nanocomposites, we conducted textural, structural, and morphological analyses using several complementary techniques. The obtained data are crucial for optimizing the materials’ properties and exploring their potential applications, particularly as sunlight-active materials in photocatalysis processes.
Our findings demonstrate that the Nickel(II)-Bipyridine Complex-Modified Graphene Oxide (Ni-GO) nanocomposite exhibits remarkable photocatalytic activity under sunlight irradiation, owing to its efficient utilization of solar energy and the presence of surface-active species. These features make the GO nanocomposite a promising candidate for various photocatalytic applications, particularly in environmental remediation. Moreover, we investigated the application of Ni-modified GO in the adsorption of dyes in an aqueous phase under sunlight irradiation. The results revealed the nanocomposite’s ability to effectively degrade dyes, highlighting its potential as an eco-friendly and efficient material for wastewater treatment and dye removal.
Overall, this study presents a valuable contribution to the field of nanocomposite materials with potential applications in photocatalysis and environmental remediation. The successful synthesis and comprehensive characterization of the Ni-GO nanocomposite pave the way for future research and applications in various areas, promoting sustainable and green technologies.
2. Results and Discussion
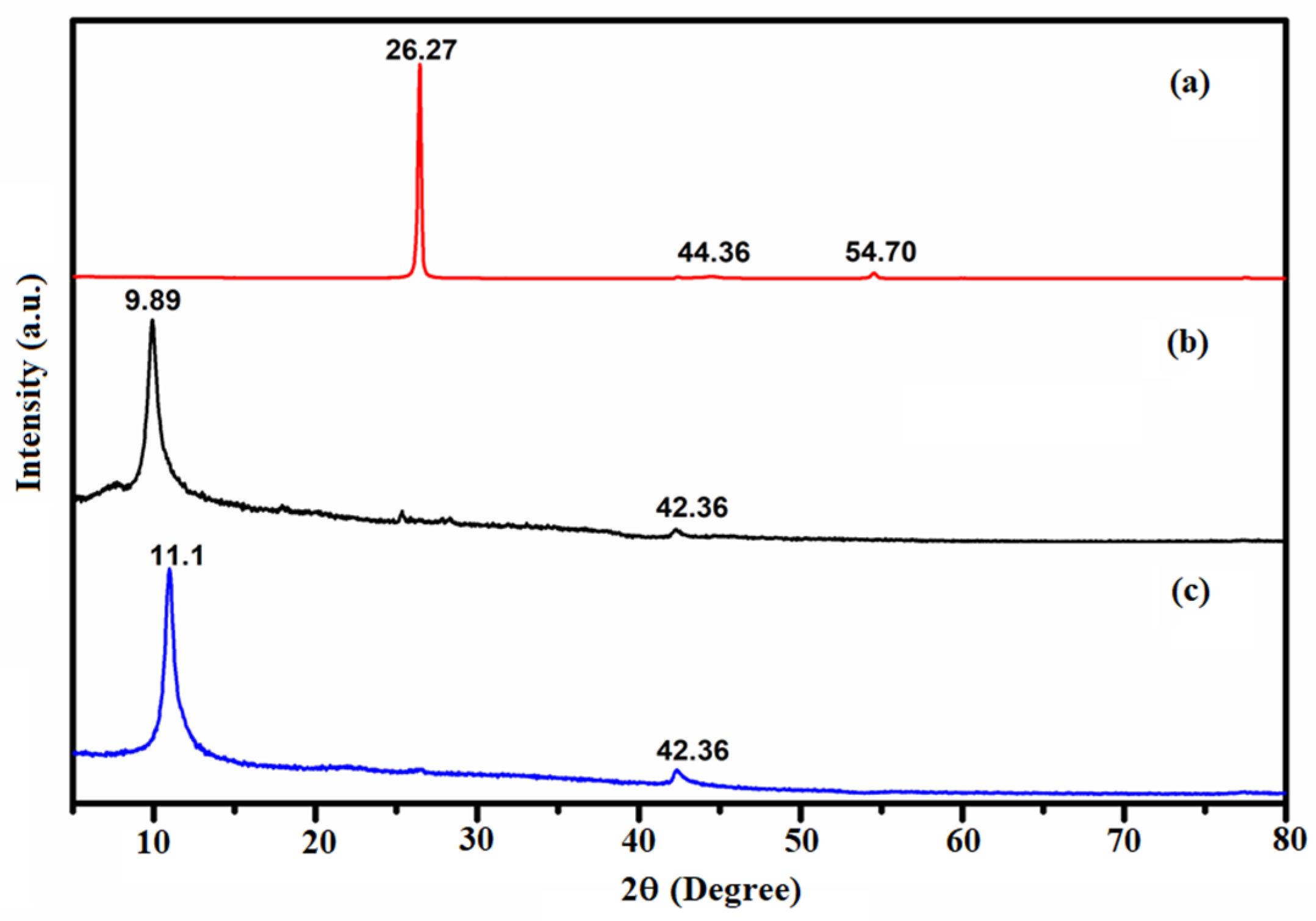
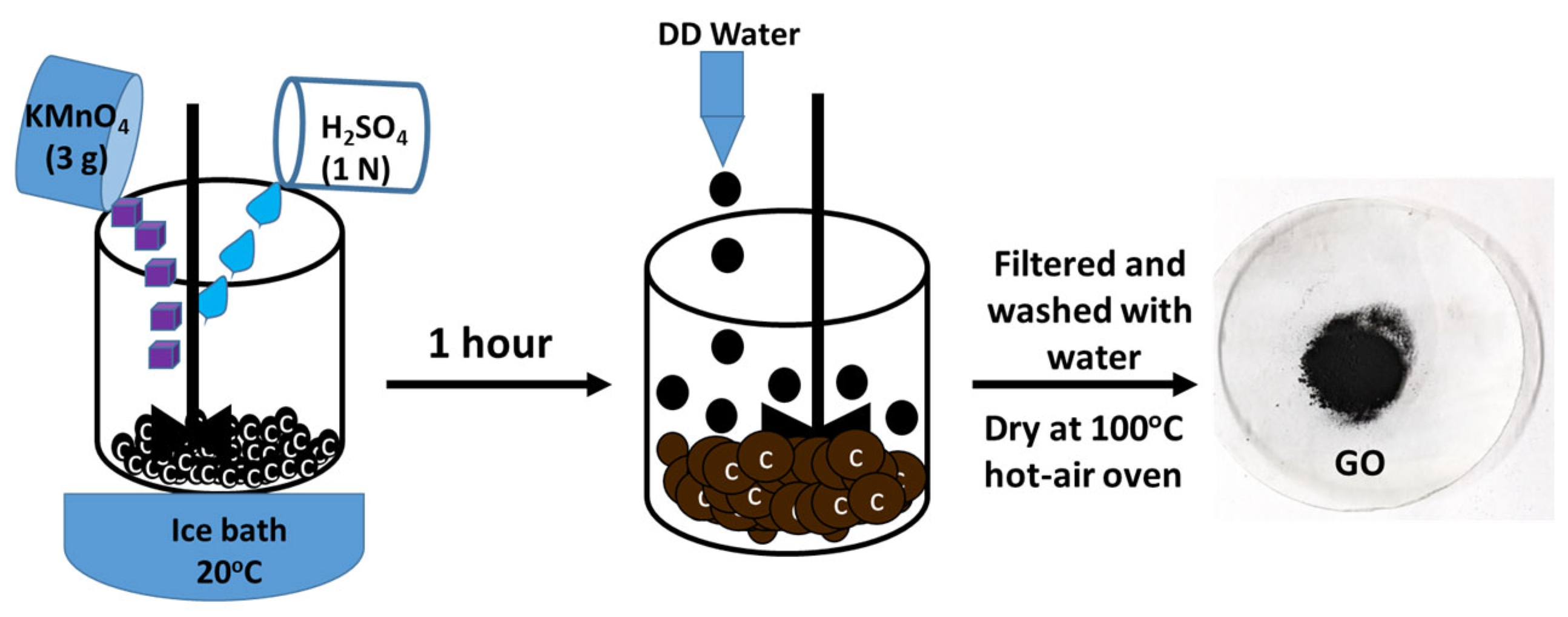
Besides the structural arrangement, the extent of oxidation and purity of the materials were examined by the XRD characterization technique. Pusty et al. reported a peak at 25.6° corresponding to the (002) planes of interlayer distance of 3.47 Å [
21]. Li et al. reported a peak (002) of GO at 11.1° and is ascribed to the introduction of oxygenated functional groups attached on both the sides and edges of carbon sheets [
22]. Allen et al. reported a diffraction peak centered at 2θ = 26.19°, and a clear (002) orientation of pure G with an interlayer spacing of 0.34 nm was found [
23]. In our study, the formation of GO was confirmed by the appearance of the new peak at 2θ = 12.68° with an interlayer distance of 0.699 nm corresponding to the (002) lattice plane.
The recorded powder XRD patterns of the G, the as-prepared GO, and the Ni-GO samples were recorded as shown in
Figure 1 and the data are presented in
Tables S1–S3. A predominant peak was recorded at 2θ = 26.27° for the diffraction pattern of the graphite. The other short peaks were recorded at 2θ = 44.36° and 54.7°, as shown in
Figure 1a. The diffraction pattern of the as-prepared sample of GO showed a predominant peak at a 2θ value of 9.89°; short peaks were observed at 26.34° and 42.36° as shown in
Figure 1b. The disappearance of the GO peaks at 26.27° in graphite and the appearance of a new peak at 9.89° indicated the complete oxidation of G to form GO with an interlayer distance of 0.88 nm, which corresponds to the GO. The peak (002) of GO at 11.1° was ascribed to the introduction of oxygenated functional groups attached on both the sides and edges of the carbon sheets. The disappearance of the graphitic peak ensured the complete oxidation of G into GO [
24]. The X-ray diffraction pattern of the as-prepared Ni-GO sample was observed as shown in
Figure 1c. Meanwhile, the diffraction pattern of the Ni-GO composite is similar to those of GO, but a little peak shift was observed from 9.89° (GO) to 11.1° (Ni-GO). The nickel complex deposited on the GO was confirmed by the appearance of small bumps at 17.93°, 25.41°, and 42.36°. This indicated the Ni deposition, the removal of a large number of oxygen-containing groups, and the formation of much more disordered graphene sheets [
25]. No other peaks of impurities were observed.
The crystallite size was calculated by the XRD peak broadened using Scherrer’s formula D = Kλ/βcos θ. The crystallite sizes of G, GO, and Ni-GO samples were 59.47 nm, 19.34 nm, and 17.15 nm, respectively (
Table 1), which were calculated from the measured values for the spacing of the different planes as in 2θ, and the values were 26.43°, 12.87°, and 10.98° respectively. The crystallite sizes decrease in the following order: G > GO > Ni-GO. Hence, the XRD result indicates that the precursor complex of Nickel can modify the GO to form amorphous Ni-GO nanocomposites.
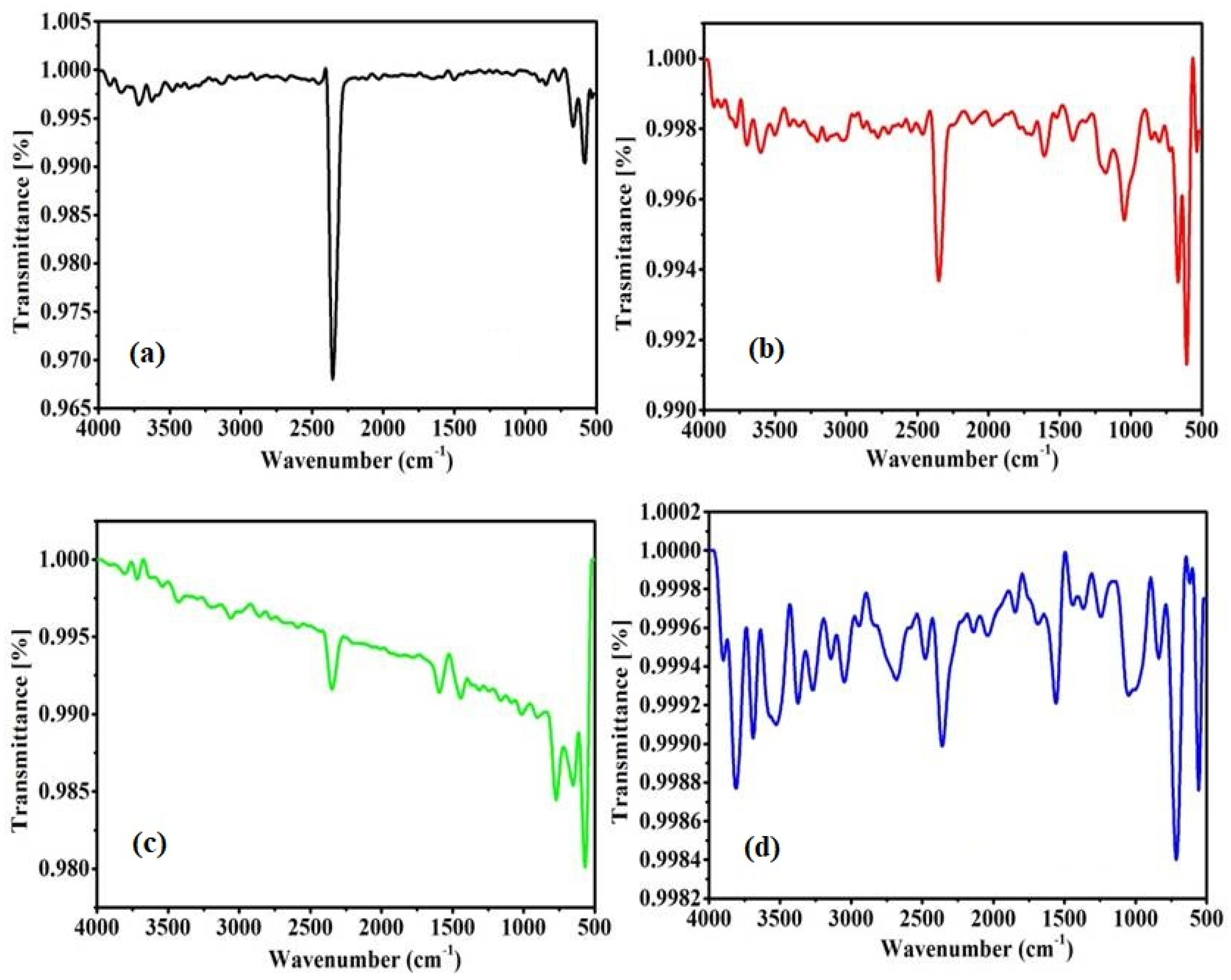
The types of chemical bonds present on the prepared samples were verified using the FTIR characterization technique.
Figure 2 shows the FTIR spectrum obtained for the G, GO, precursor complex, and the modified GO powder.
Figure 2a shows a very sharp peak at 2348 cm
−1, which corresponds to a free O=C–O bond. There are two broad bands observed in the range from 3500 cm
−1, and a band at 1646 cm
−1 (
Figure 2b); these are typical bands of both stretching and bending of O–H bonds, and are attributed to the interaction of adsorbed water molecules on the GO surface [
26]. This indicates the presence of hydroxyl groups in the GO network. Furthermore, a weak band was observed at 2336 cm
−1, corresponding to O–C=O bonding, which are the bonds present in the hexagonal GO [
21]. Some of the peaks were observed at 1610, 1172, and 1040 cm
−1, attributed to the presence of a C=C strain on the aromatic ring of GO, the C–O bond of the epoxy groups, and C–OH strain for the alkoxy groups in the GO sheets, respectively. The other weak bands localized at 2900, 2100, and 1638 cm
−1 can be assigned to the vibrations of C–H bond, the C=O strain, and the vibration of C=C conjugates of the GO, respectively [
22].
Figure 2c shows peaks at 640, 1227, 1646, 1706, and 3427 cm
−1 of the nickel precursor complex, which corresponds to the previously reported work [
23].
Figure 2d shows peaks at 640 cm
−1 and a broad peak at 3427 cm
−1. A peak at 2348 cm
−1 in graphite completely disappeared, indicating the modification of a small amount of nickel complex with GO to form Ni-GO.
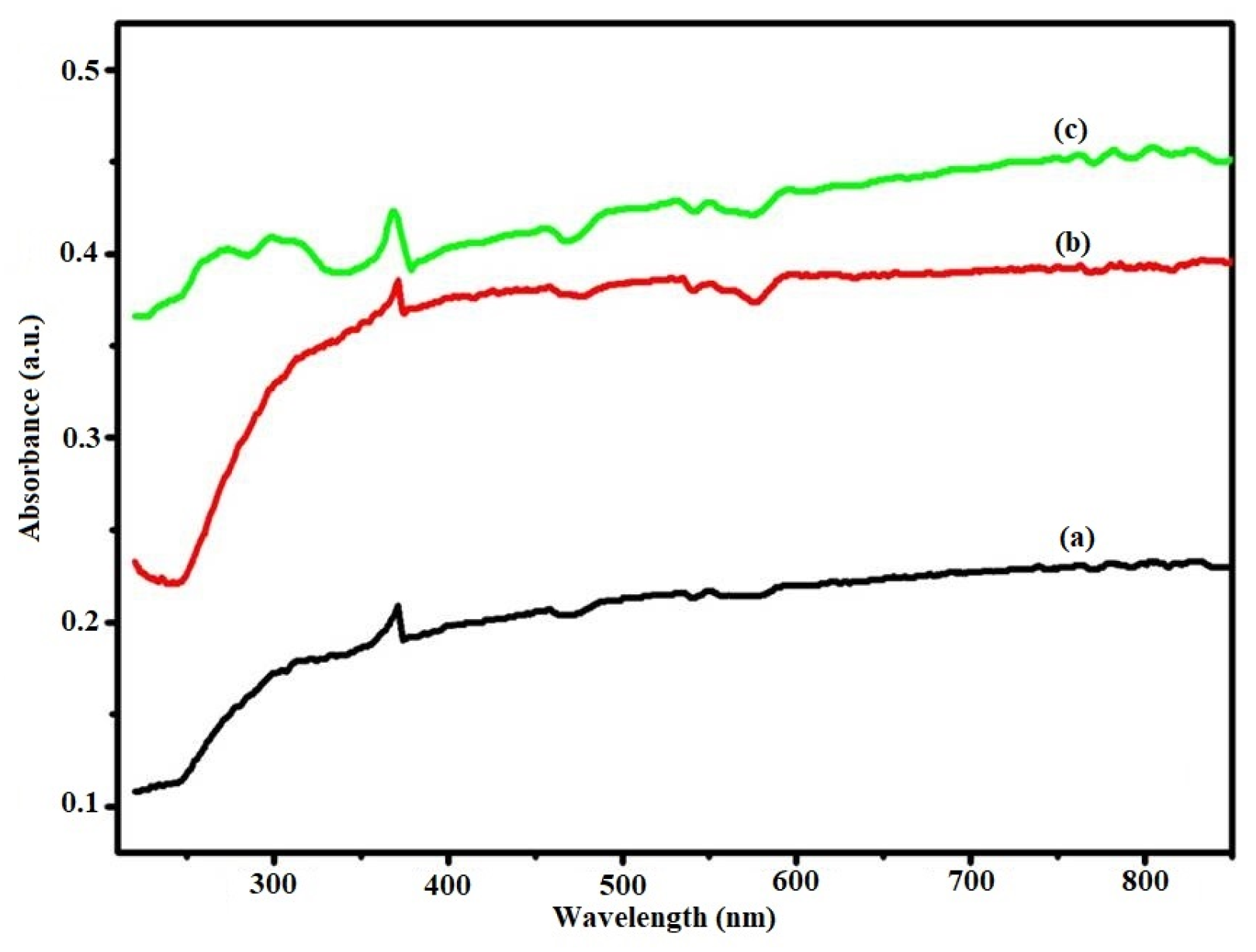
Figure 3 shows the diffuse reflectance spectra of graphite, GO, and the Ni-GO composite. A very weak absorbance spectra was observed, indicating that graphite does not possess optical characters. Then, the peaks at 300 nm in the UV-vis spectrum of GO, which is characteristic of π-π* transition in the sp
2 basal plane (C=C), and a shoulder peak at around 370 nm, which is attributed to n-π* transitions of the oxygen functional groups, were consistent with the UV-vis results. The spectra showed that the nickel-bipyridine complex significantly affects the optical properties of GO. A red shift of the adsorption edge was observed in the nickel-modified GO composite. The results indicated that our prepared sample containing nickel(II) precursor complex can absorb electromagnetic radiation with a wavelength higher than GO; absorption increased the comparison with graphite and GO, which indicated that our nickel complex-modified GO could narrow the band gap of pristine photocatalysts. For the sample sensitized with nickel complex, a significant shift in the light absorption towards lengths greater than 400 nm was observed.
UV–visible absorption measurement is one of the most important methods to evaluate the optical properties of semiconductors.
Figure 3 shows the UV–visible absorption spectrum of the G, GO, and Ni-GO nanocomposites. Two broad absorption peaks can be seen around 372 nm and 458 nm in sample G, and two small sharp peaks exist at 272 nm and 296 nm. One broad peak was observed in the range of 453 nm to 462 nm in the sample GO. Two clear broad peaks were observed around 343–361 nm, 420–430 nm, and one small sharp peak was observed at 592 nm in Ni-GO sample.
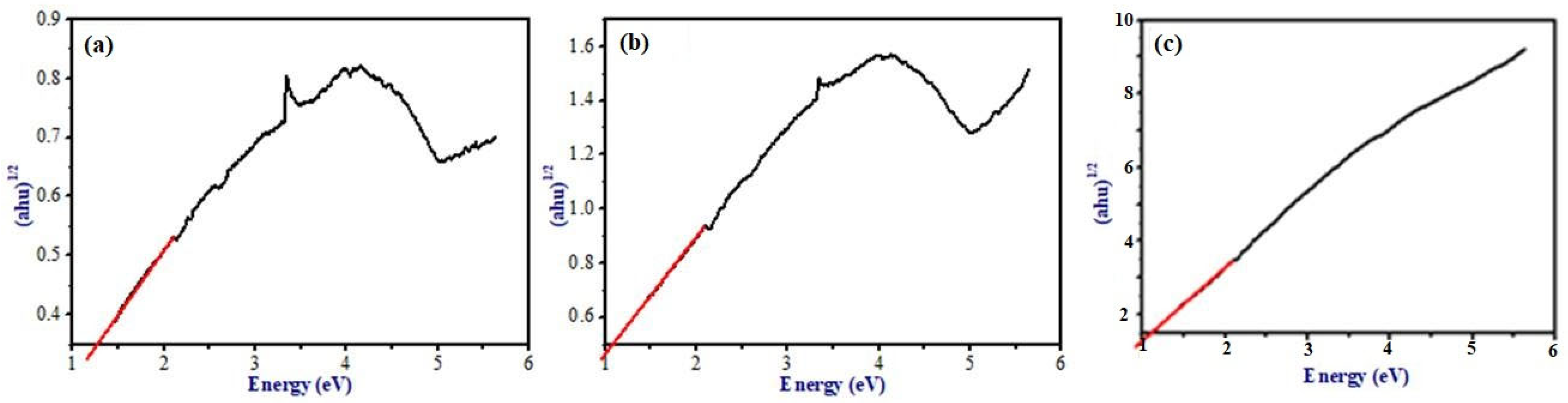
In DRS analysis, our prepared nanocomposite was observed in the visible light region, therefore this composite may remain active in visible-light photocatalysis. For more discussion on the optical properties of the prepared samples, band gap energies were calculated. The band gap energy of the samples can be determined from the Eg measurements using Tauc plot (
Figure 4). A graph was plotted between (αhν)
1/2 vs. E; the intercept value represents the band gap energy. The calculated value of the band gap of G, GO, and Ni-GO are 1.264 eV, 1.08 eV, and 1.09 eV, respectively, which is similar to the value of GO that lies in between (1.00–2.00 eV). The band gap energy decreased for GO and Ni-GO compared to G powder. Therefore, our preparation method is effective for the preparation of visible-light active photocatalyst. Hence, we performed our photocatalytic studies under sunlight irradiation.
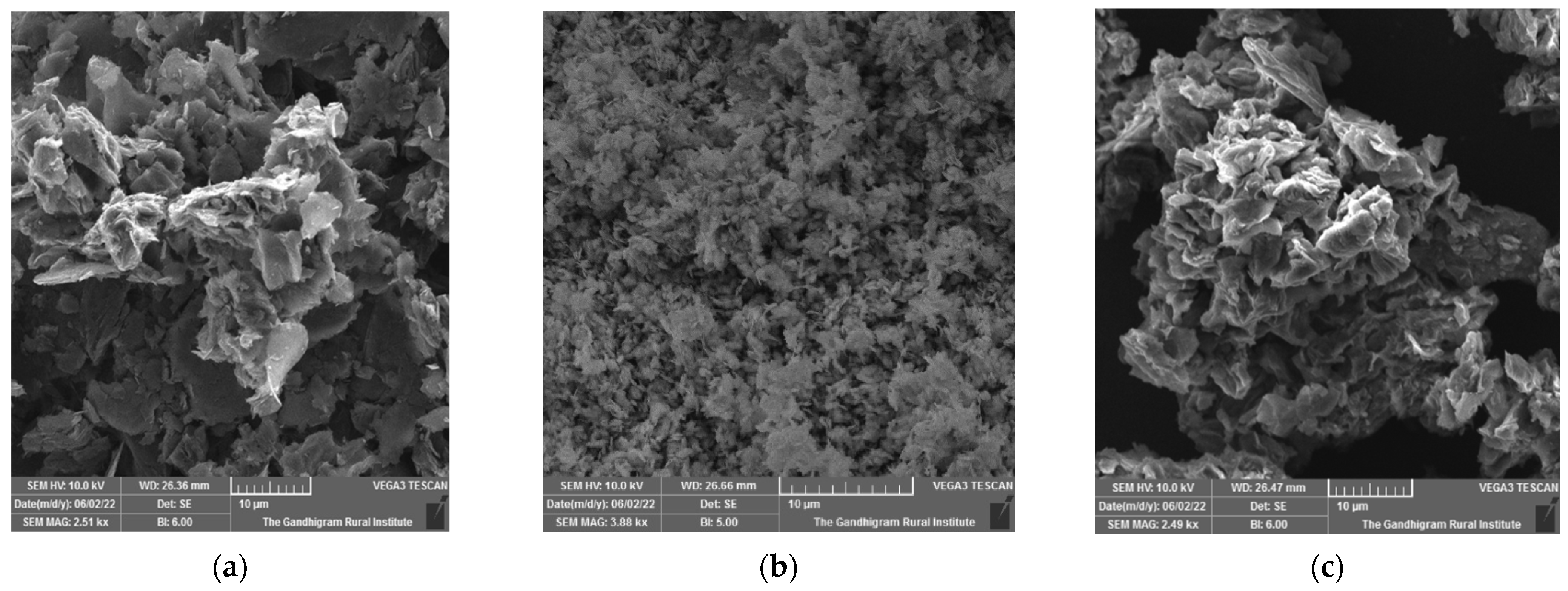
The morphologies and microstructures of G, GO, and Ni-GO composites were investigated via SEM and shown in
Figure 5a–c, respectively. The compact-wrinkled-like GO sheet with surface defects confirmed the formation of the 2D structure, which clearly indicated that the graphene oxide has been well modified with nickel complex during the adsorption process with the nickel precursor complex in water. Surface concentrations of Ni on GO are shown in
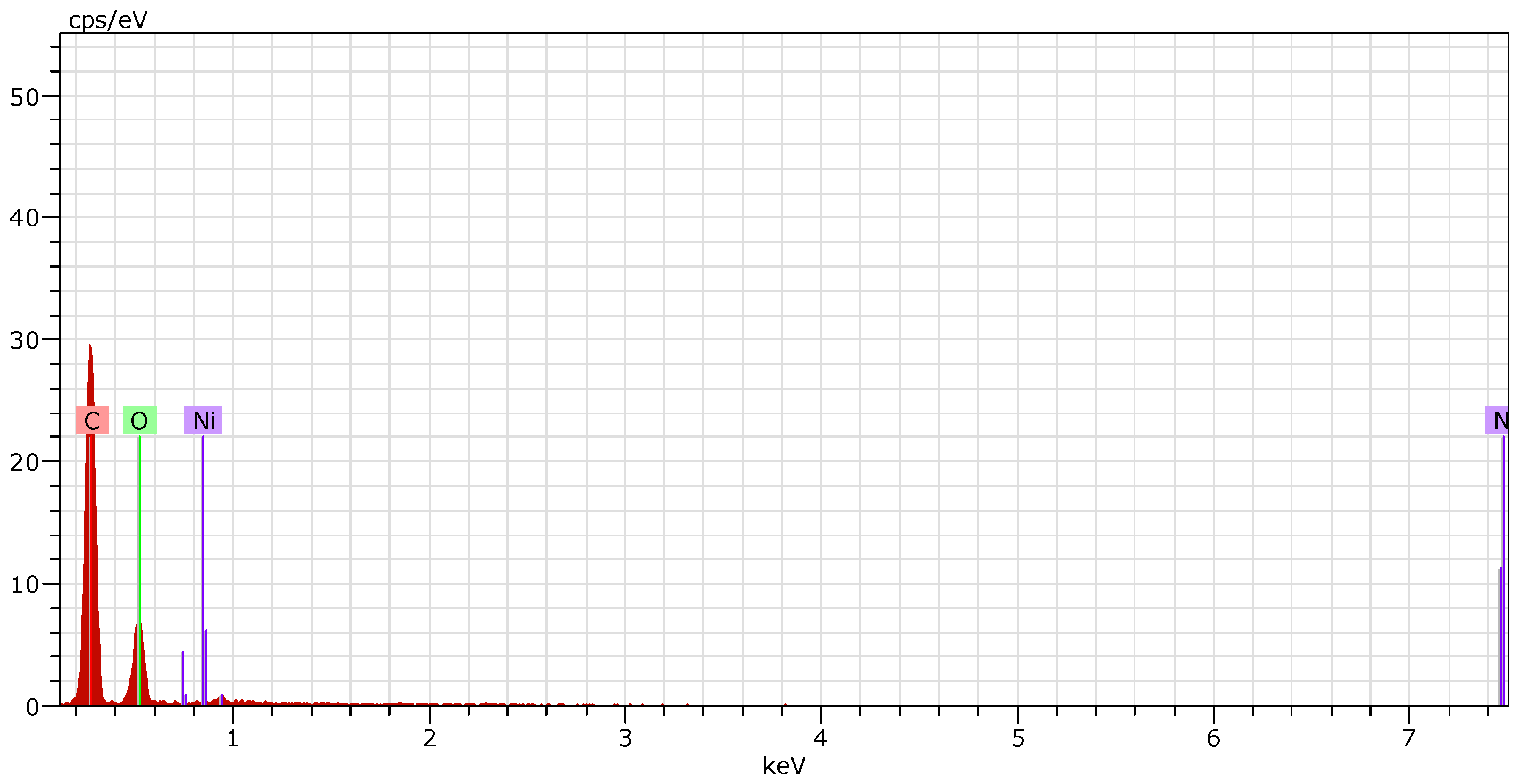
Figure 6 via the EDAX spectra of Ni-GO composites. The EDAX spectra of G and GO are shown in
Figures S6 and S7. The Ni-GO composite of Ni on GO were calculated from EDAX in
Table S4, in which the concentration of nickel loaded on the solid was calculated using EDAX studies as the concentration of nickel loaded on the solid GO. The EDAX spectra confirmed the existence of nickel in the GO powder. EDAX results showed the presence of carbon and oxygen in the GO sample; however, the presence of a small quantity of nickel (1.57 At%) was evident in the final sample. It could be an evidence for the introduction of nickel complex onto the GO surface. Hence, the formation of Ni-GO composites was confirmed due to the surface-induced reaction from the complex—the surface adduct in adsorption process.
Figure 7a illustrates an XPS survey scan spectra of Ni
2+-modified GO. The two strong peaks at about 284 and 532 eV correspond to the binding energies that are assigned as C 1s and O 1s orbitals, respectively. GO was confirmed in
Figure 7b,c similarly to the reported work [
27,
28,
29,
30,
31,
32]. The peaks in
Figure 7d depict high-resolution spectra of Ni 2p orbitals of Ni
2+ ions in our prepared sample. The peaks at 857 and 874 eV correspond to the binding energies of Ni 2p
3/2 and Ni 2p
1/2. Four electronic signals were seen from the high-resolution spectrum of Ni 2p before reduction at 856, 838, 865, and 863 eV. These peaks were assigned as the satellite peaks of Ni 2p orbital. The high resolution XPS spectrum did not show any peaks at 854 and 873 eV. These results indicated that Ni present in +2 oxidation alone has no impurity of the zero-valent Ni species.
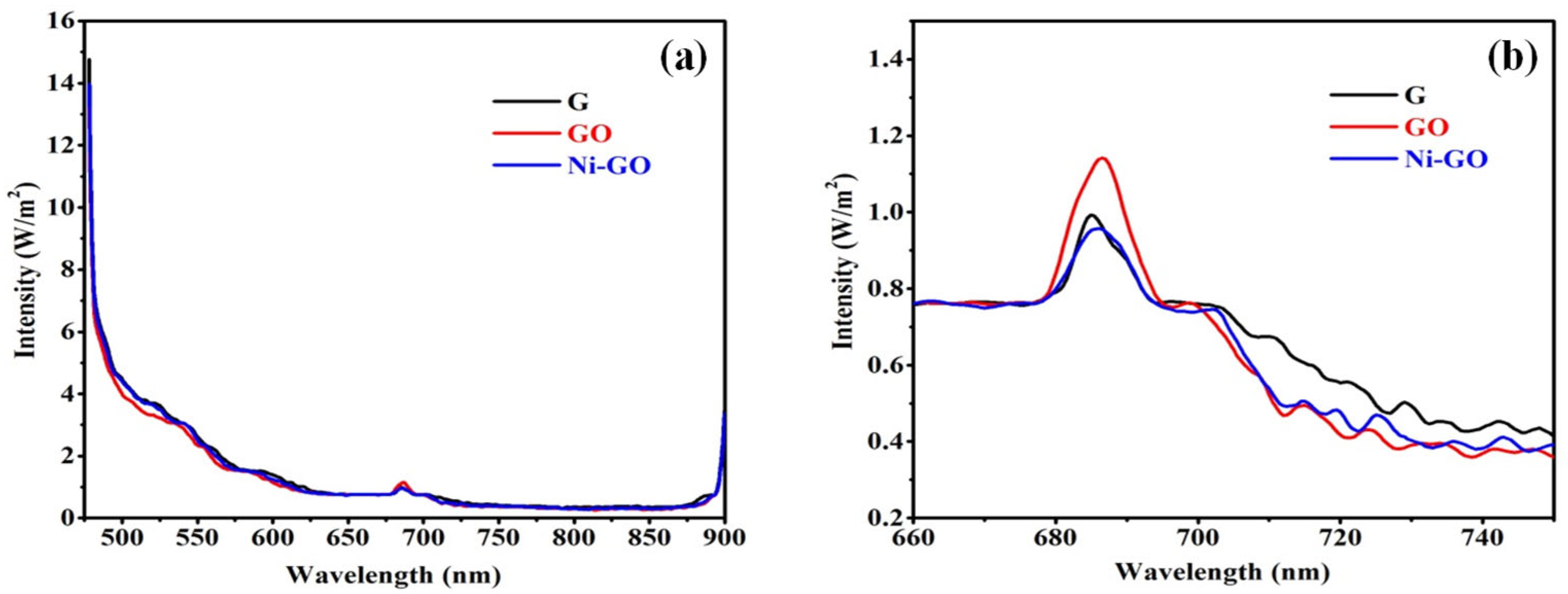
The photoluminescence spectra have been widely used to investigate the efficiency of charge carrier trapping, immigration, transfer, and to understand the fate of electron-hole pairs in photocatalysts. It can be seen that pure graphite, GO, and Ni-GO as-prepared samples showed emission peaks at 521 nm and 685 nm, and weaker emission peaks at 592 nm and 700 nm, as presented in
Figure 8. The surface showed a fluorescence emission spectrum at 685 nm, with an additional peak at 700 nm, corresponding to green emissions [
33].
The GO-based photocatalysts have been widely studied for the degradation of organic pollutants, including Rhodamine B (RhB) under sunlight irradiation. The unique properties of GO, such as its high surface area, excellent electron transport properties, and strong light absorption capabilities, make it a promising candidate for photocatalytic applications. Several research articles have reported the successful use of GO-based photocatalysts for RhB degradation under sunlight irradiation. In a study by Li et al., a reduced graphene oxide-TiO
2 nanocomposite exhibited enhanced photocatalytic activity for RhB degradation under simulated sunlight, attributed to efficient charge separation and transfer at the GO-TiO
2 interface [
34]. Xu et al. investigated the photocatalytic degradation of RhB using a nitrogen-doped graphene oxide (NGO) photocatalyst under natural sunlight. The GO exhibited excellent photocatalytic performance due to its increased visible light absorption and efficient separation of charge carriers [
35]. Zhu et al. prepared a bismuth oxide (Bi
2O
3) and reduced graphene oxide (rGO) nanocomposite for RhB degradation under natural sunlight. The combination of Bi
2O
3 and rGO enhanced the light absorption and charge transfer, leading to efficient photocatalytic degradation [
36]. These studies collectively demonstrate the promising potential of GO-based photocatalysts for the degradation of Rhodamine B and other organic pollutants under sunlight irradiation. The synergy between GO and other photocatalytic materials can significantly enhance the photocatalytic performance, making them valuable candidates for environmental remediation and sustainable wastewater treatment processes. The presence of RhB in water causes severe environmental and public health issues. The release of RhB into water without proper pre-treatment leads to carcinogenicity, dermal problems on contact, and optical damage.
In this study, a comparative analysis of previous research findings is essential to assess the effectiveness of the nickel(II) complex-loaded GO photocatalytic composite regarding the photo degradation of Rhodamine B (RhB) in an aqueous medium under sunlight irradiation. To better understand the unique properties and advantages of the synthesized composite, we compared its photocatalytic performance with that of other materials commonly used in photodegradation studies, such as graphite and graphene oxide.
The photocatalytic activities of the different samples, including graphite, graphene oxide, and nickel-loaded graphene oxide, were evaluated by monitoring the degradation yields of the RhB substrate under identical experimental conditions. The blank RhB solution, which did not contain any catalyst, was used as a reference for comparison.
By analyzing and comparing the photodegradation yields of the RhB substrate in the presence of these different catalysts, we can determine the relative efficiency and superiority of the nickel(II) complex-loaded GO photocatalytic composite. Additionally, this comparative study allows us to identify any synergistic effects resulting from the incorporation of the nickel(II) complex onto the GO surface, which may contribute to enhanced photocatalytic performance.
The Inclusion of previous research findings in our study provides valuable insights into the state-of-the-art photocatalytic materials and offers a benchmark to gauge the advancement and potential applications of the synthesized nickel(II) complex-loaded GO nanocomposite. Moreover, it enables us to establish the significance of our research and its contribution to the field of advanced photocatalysis, paving the way for the development of efficient and sustainable materials for environmental remediation and other relevant applications.
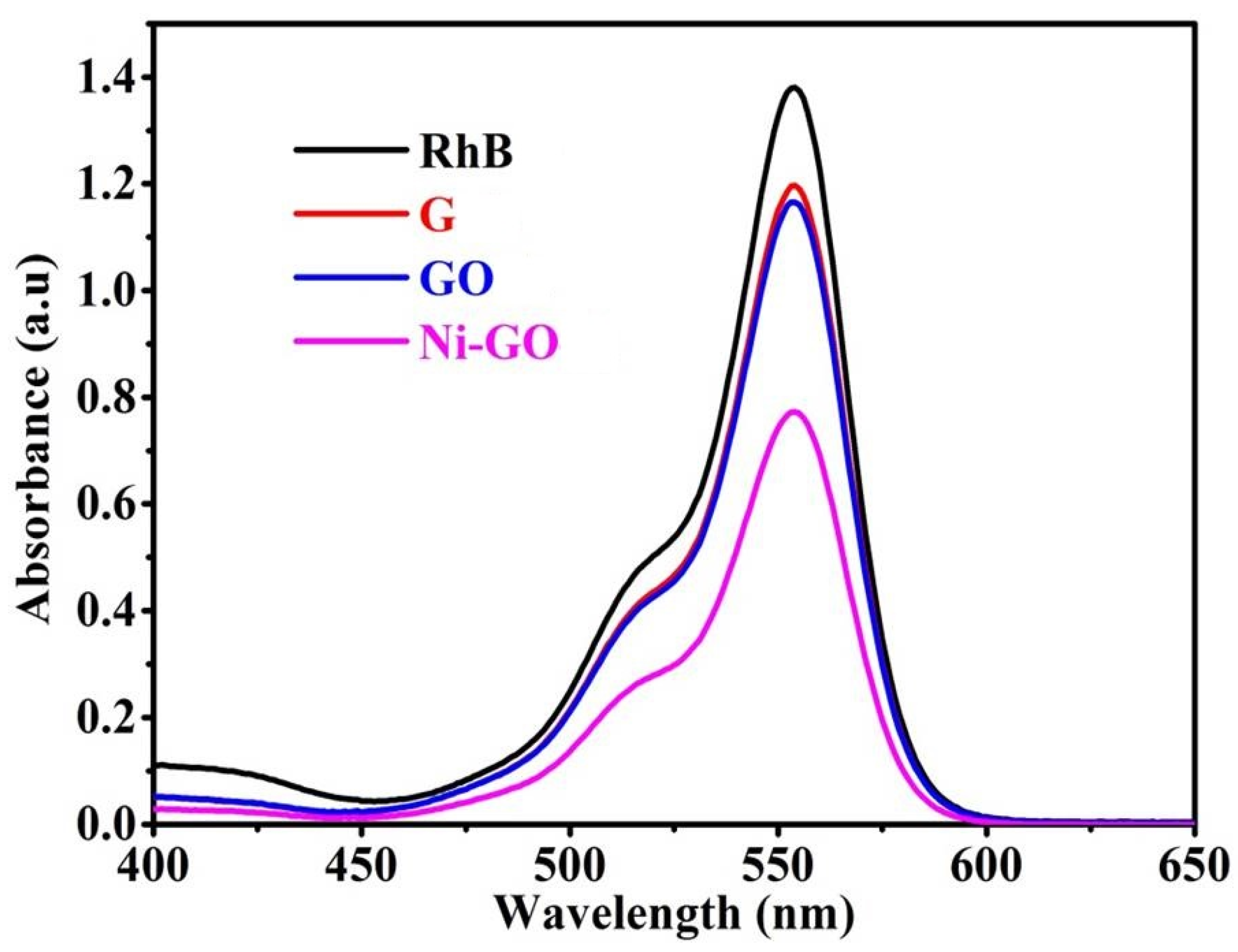
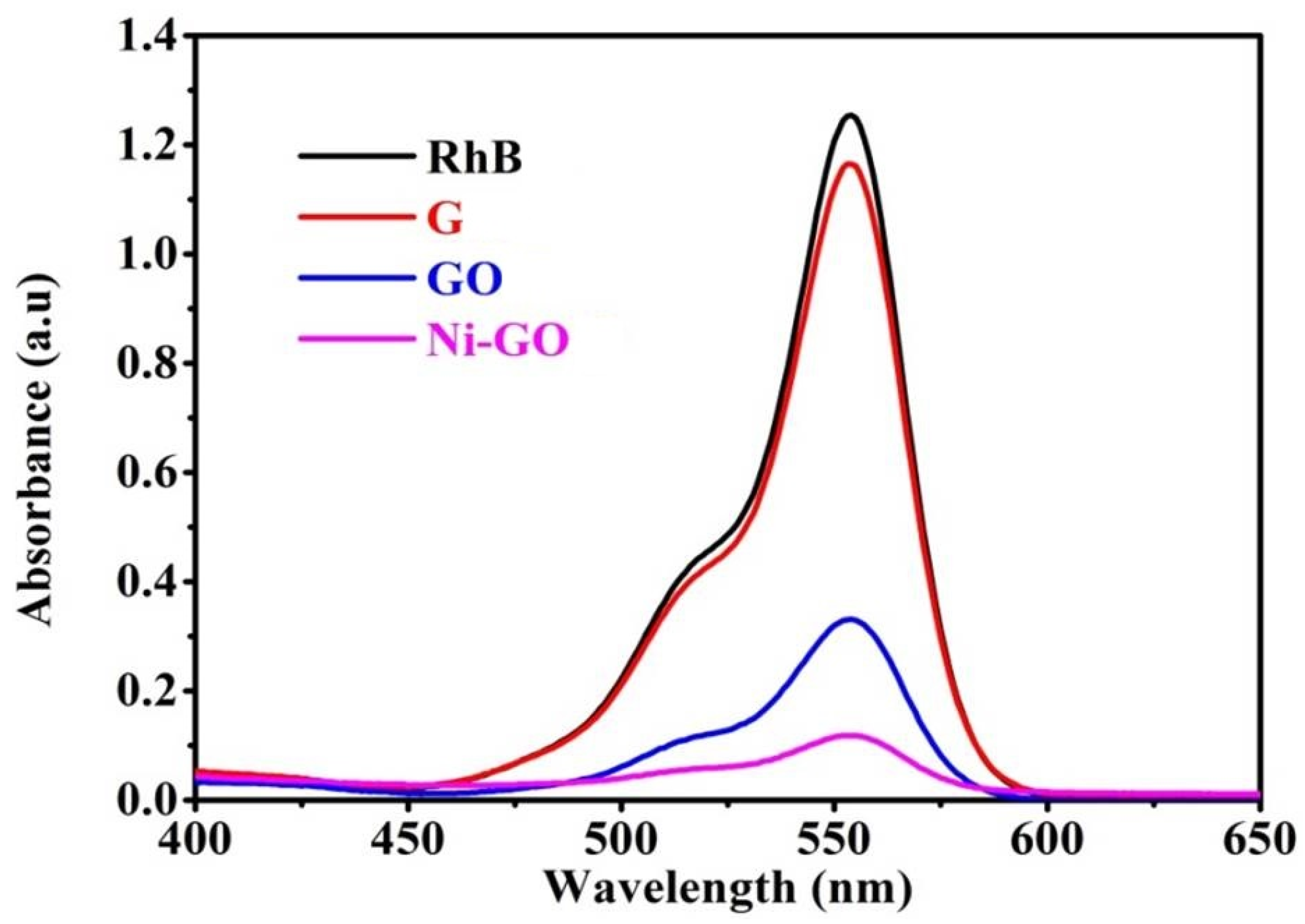
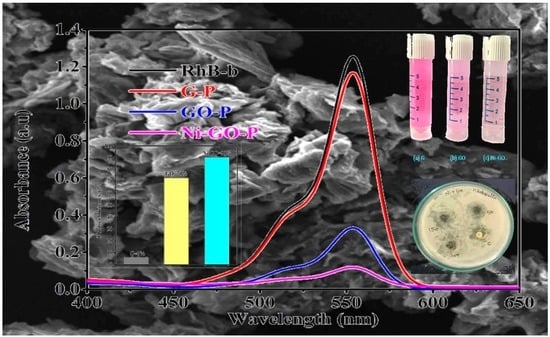
UV-visible spectra were obtained as shown in
Figure 9, where the gradual decrease of the absorption bands of RhB was found, at the point where the various catalysts were used at 90 min contact time intervals due to adsorption/degradation of the RhB molecules.
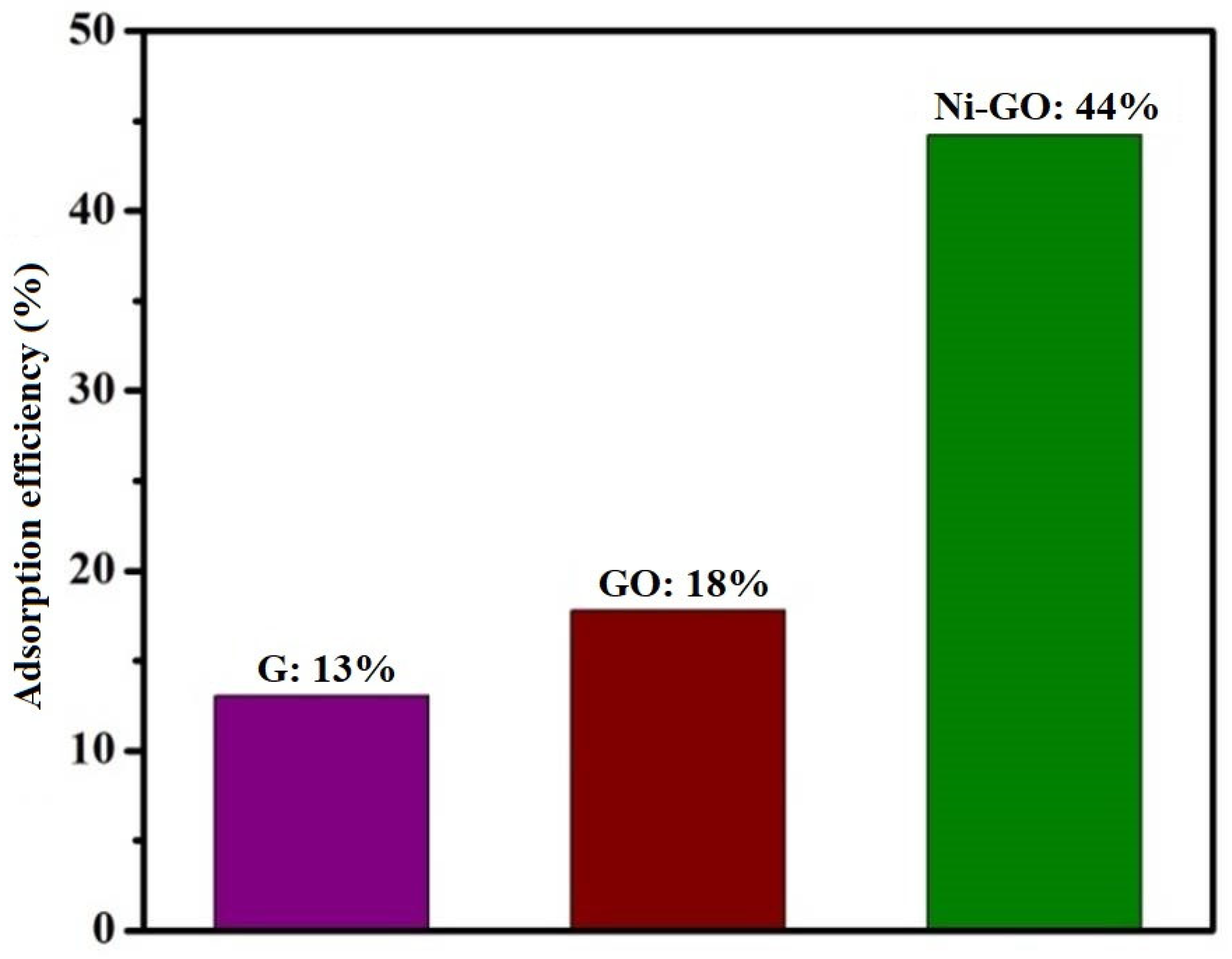
Graphite and modified-GO showed minimal degradation of RhB in dark conditions, in which the degradation efficiency was measured as 13% and 18% (as shown in
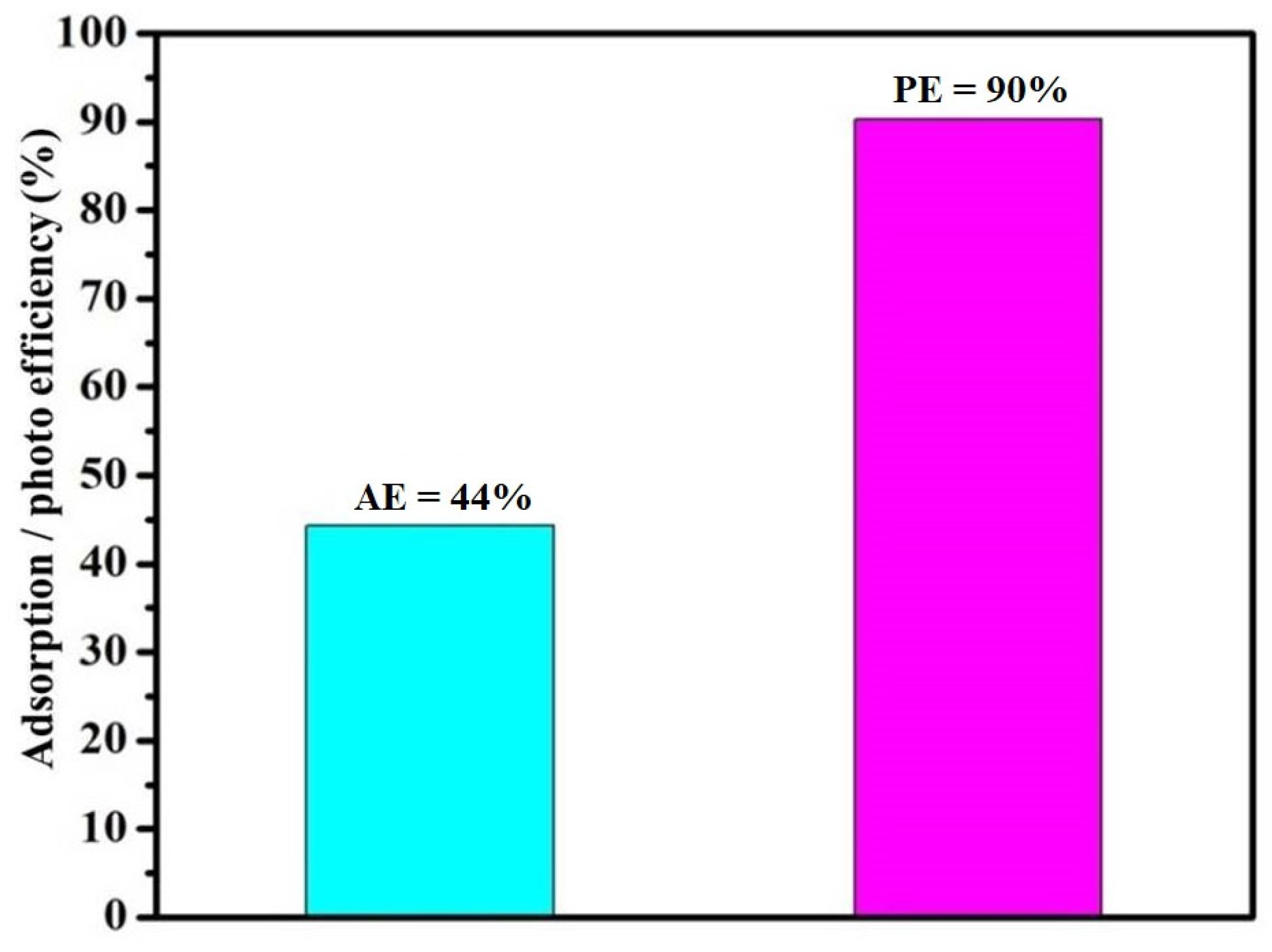
Figure 10), respectively. Adsorption efficiency of the as-prepared Ni-GO composite was 44%. This indicated that RhB can easily interact with the Ni-GO composite surface. This adsorption capacity may influence the degradation efficiency in light irradiating systems. Therefore, we expected high photo catalytic efficiency for the Ni-GO sample.
Figure 11 represents the repetitive scan spectra where a gradual decrease of the adsorption bands of RhB was found, whereas the irradiation time increased due to oxidation of the RhB molecules. This indicated an increase in the electron deficiency and break-down of the RhB molecules.
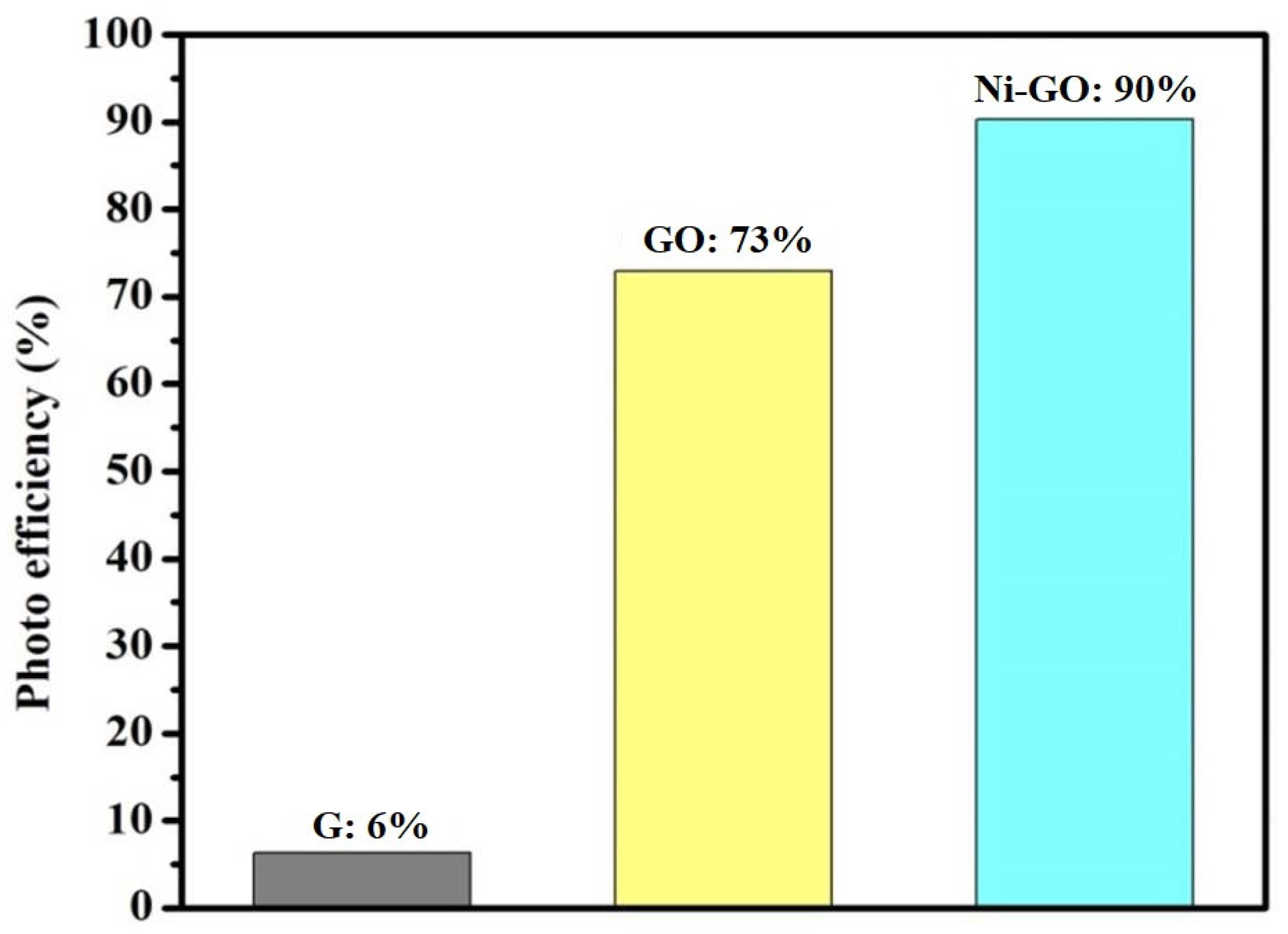
Graphite presented fair activity for the degradation of RhB under sunlight irradiation, in which the degradation efficiency was 6%. Prepared GO showed more activity, nearly 73%, and the Ni-GO composite showed 90% degradation of RhB under sunlight irradiation. The differences were nearly 84% and 17% compared to G and GO samples, respectively, even with an irradiation time of 90 min.
Of all the prepared samples, the Ni-GO composite showed the best efficiency for the degradation of RhB; the PDE reached up to 90% after 90 min of irradiation. In general, it was observed that the photo degradation efficiency of the nickel-supported catalysts was found to follow an order (
Figure 12): G (6%) < GO (73%) < Ni-GO (73.8%). With respect to operational convenience, it is important to consider that this elimination protocol does not require any heating or cooling for all the components employed in this study (substrates, catalysts, and solvents), is commercially available, and is also cost-effective. Photo degradation efficiency was high for Ni-GO under sunlight compared to adsorption efficiency in dark conditions (
Figure 13). It was evident that as-prepared Ni-GO acts as a very good sunlight-activated photo catalyst. The UV-visible spectra of the RhB irradiated solution at different time intervals were recorded as shown in
Figure S8. The PDE of RhB versus irradiation time was shown in
Figure S9.
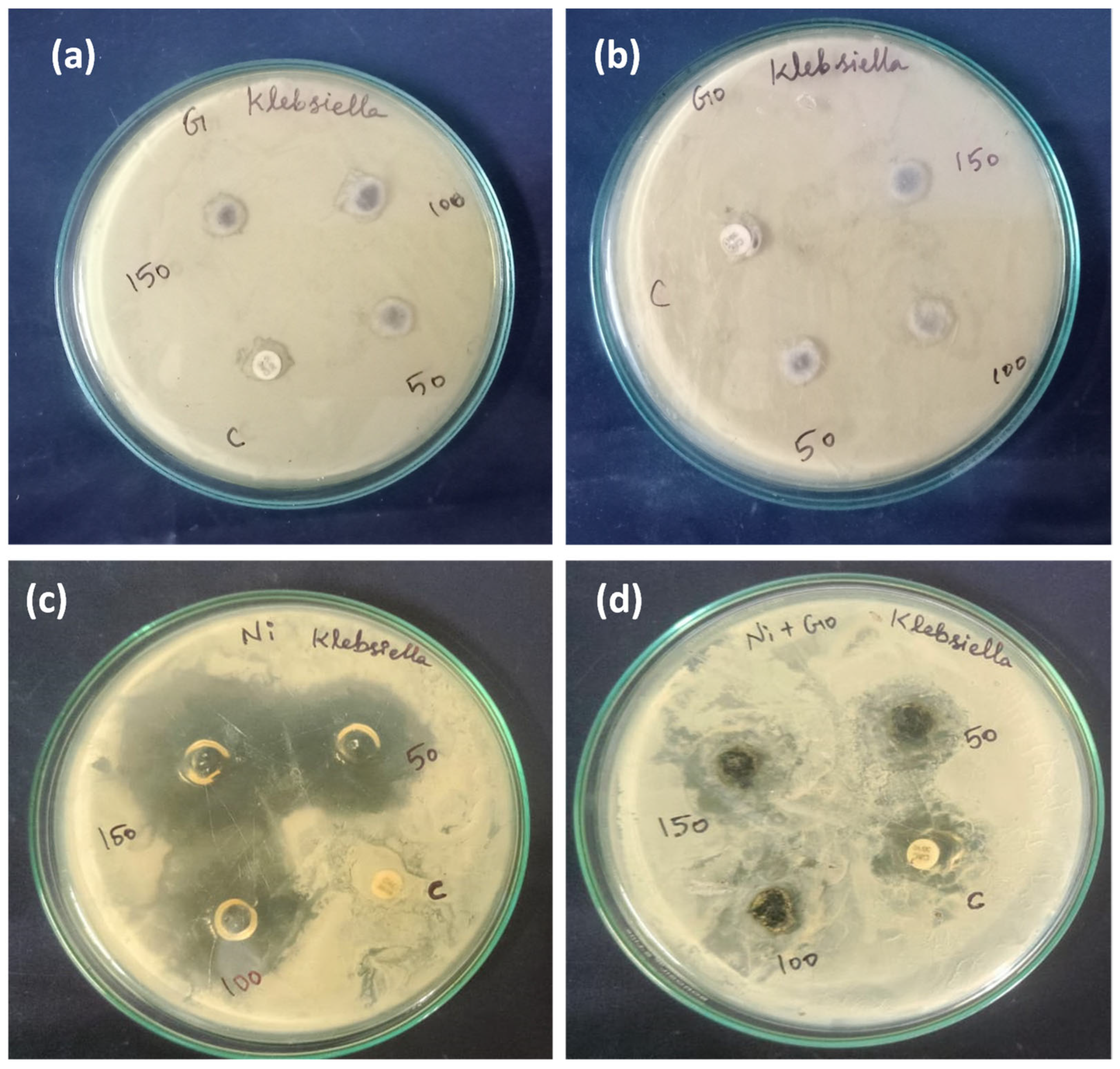
Antimicrobial activity refers to the ability of certain agents to inhibit the growth of bacteria and prevent the formation of microbial colonies by destroying microorganisms. The antimicrobial activity and mechanisms of a specific compound, the nickel(II)-bipyridine complex and its nanocomposite with graphene oxide (Ni-GO), against
Klebsiella were investigated [
37].
The researchers conducted colony-forming capability assays and observed images that demonstrated the ability of the as-prepared samples to effectively kill the bacteria in a concentration-dependent manner. This suggests that both the nickel complex and Ni-GO nanocomposite exhibit promising antibacterial properties.
Further analysis of the antimicrobial mechanism revealed that the bactericidal effect of Ni-GO on Klebsiella is associated with a reactive oxygen species (ROS)-mediated pathway. ROS are highly reactive molecules that can cause significant damage to bacterial cells, leading to their destruction. This mechanism could be one of the key factors contributing to the antimicrobial activity observed in the study.
These findings hold significant implications for the development of novel antimicrobial materials with excellent optical properties. The potential of the nickel complex and Ni-GO nanocomposite to be formulated as effective antimicrobial agents could pave the way for their application in various fields, such as medical devices, coatings, and surface treatments, to prevent bacterial growth and biofilm formation.
It is worth mentioning that the study is a part of a broader scientific effort to explore the antimicrobial properties of nanoparticles. Numerous studies have been conducted on various nanoparticles with antimicrobial activities, many of which have shown promising results against
Klebsiella [
38,
39,
40,
41]. These nanoparticles have been found to accumulate in the bacterial membrane, disrupting its integrity and leading to bacterial death.
As research in this field continues, it is essential to explore the potential of these novel antimicrobial materials while also ensuring their safety and efficacy in real-world applications. The development of effective and eco-friendly antimicrobial agents is crucial in the fight against antibiotic-resistant bacteria and the prevention of infections in healthcare and industrial settings.
In our current study, we conducted an investigation into the antimicrobial activity of our compounds. The antimicrobial screening of the complex and the nanocomposite against the pathogenic microorganism
Klebsiella, commonly associated with various diseases, revealed that the complex displayed moderate activity (
Figure 14).
We evaluated several metal complexes, including [Ni(bipy)
3]Cl
2·5H
2O, graphite, graphene oxide, and nickel-modified GO, for their antimicrobial activity against
Klebsiella, and the results are presented in
Table 2. Among all the samples tested, the Ni(II)-bipyridine complex exhibited higher biological activity compared to the other nanocomposites. The order of biological activity was as follows: Ni(II)-complex > Ni-GO > G > GO.
The findings from our antimicrobial study indicate that both the Ni(II) precursor complex and the nickel(II) complex-modified GO possess significant antibacterial properties. These materials hold promise as potential agents for combating bacterial infections.
Nonetheless, there is still ample scope for further exploration of the biological activity of our compounds. Further research is needed to fully understand their mechanisms of action and to optimize their effectiveness as antimicrobial agents. This study opens up exciting possibilities for the development of novel antibacterial materials with potential applications in various fields, including medicine and biotechnology.
The superior photocatalytic performance and disease resistance of the bulk system on the Ni-GO nanocomposite can be attributed to several physical mechanisms:
Enhanced photocatalytic activity: The incorporation of the Ni(II)-complex on the surface of GO creates new active sites that facilitate the generation and separation of electron-hole pairs upon light absorption. The Ni(II) ions can act as electron acceptors, promoting efficient charge transfer and reducing recombination of charge carriers. This enhanced electron transfer process leads to higher photocatalytic activity, enabling the bulk system to effectively degrade pollutants or organic compounds under light irradiation. In our photocatalytic study, we observed a significant enhancement in the photocatalytic activity of our Ni-GO nanocomposite.
Increased light absorption: The introduction of the Ni(II)-complex modifies the electronic band structure of GO, leading to a broadened absorption range in the visible and UV regions. This increased light absorption allows the bulk system to harness a broader spectrum of light energy, enhancing its photocatalytic performance by utilizing a wider range of wavelengths for photo-induced reactions. In our study, UV-vis absorption studies revealed an increase in absorbance within the visible light region and a reduction in the band-gap energy.
Surface area and reactivity: GO itself possesses a high surface area due to its layered structure. The modification with Ni(II)-complex may further increase the surface area and create more active sites, providing additional opportunities for pollutants or pathogens to interact with the photocatalyst. This increased surface area and reactivity contribute to the improved performance of the bulk system in both photocatalysis and disease resistance. Further exploration is warranted in the study of surface area and the reactivity of our catalyst.
Antibacterial properties: The incorporation of Ni(II)-complex on GO may introduce antibacterial or antimicrobial properties to the bulk system. Metal ions, such as Ni(II), can exhibit bactericidal or biocidal effects, leading to improved disease resistance. The antibacterial properties of the bulk system help to inhibit the growth of bacteria and other harmful microorganisms, making it more effective in applications where disease prevention is crucial. In our study, both Ni(II)-complex and Ni-GO nanocomposites exhibited an enhanced antibacterial activity compared to pristine GO.
Synergistic effects: The combination of Ni(II)-complex and GO may result in synergistic effects that amplify the overall photocatalytic performance and disease resistance of the bulk system. The interaction between the metal complex and the GO matrix can lead to cooperative behaviors that enhance the catalytic and antimicrobial activities, making the bulk system more efficient in multiple applications.
Overall, the physical mechanism behind the superior photocatalytic performance and disease resistance of the bulk system on Ni(II)-complex-modified GO involves a combination of enhanced charge transfer, increased light absorption, higher surface area, potential antibacterial properties, and synergistic effects between the components. These features make the bulk system a promising candidate for various environmental and biomedical applications.