Removal of Organic Sulfur Pollutants from Gasification Gases at Intermediate Temperature by Means of a Zinc–Nickel-Oxide Sorbent for Integration in Biofuel Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Removal of Thiophene, Effect on Main Operating Parameters
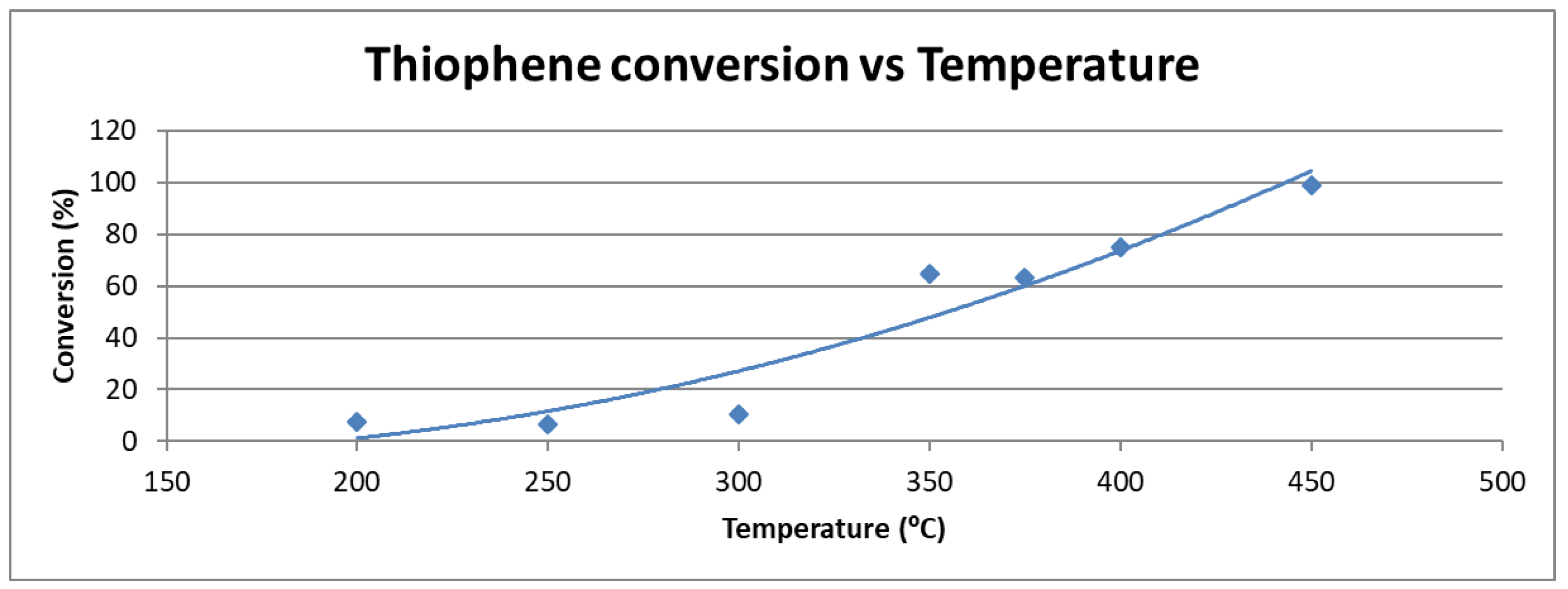
2.1.1. Effect of Temperature
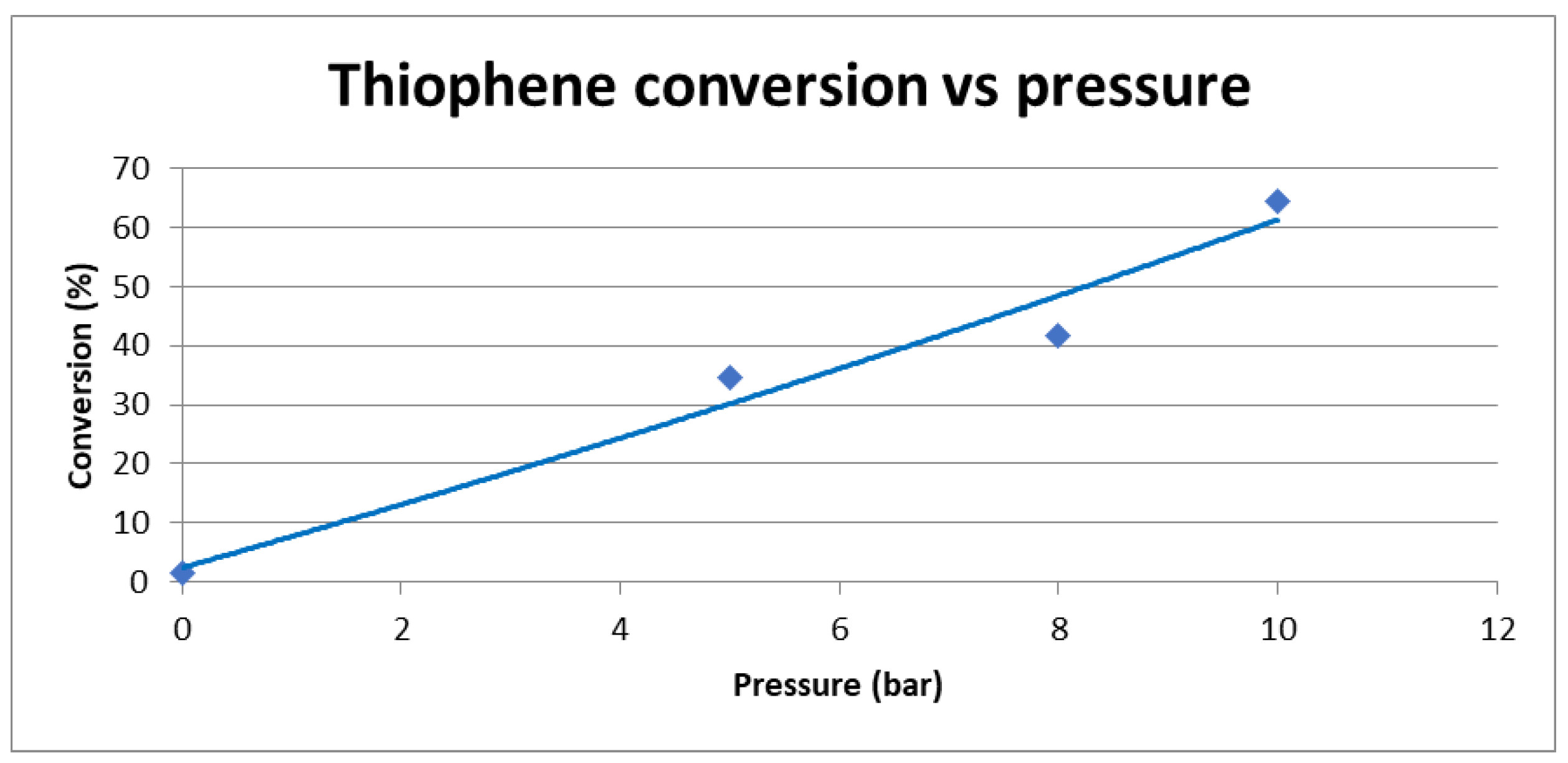
2.1.2. Effect of Pressure
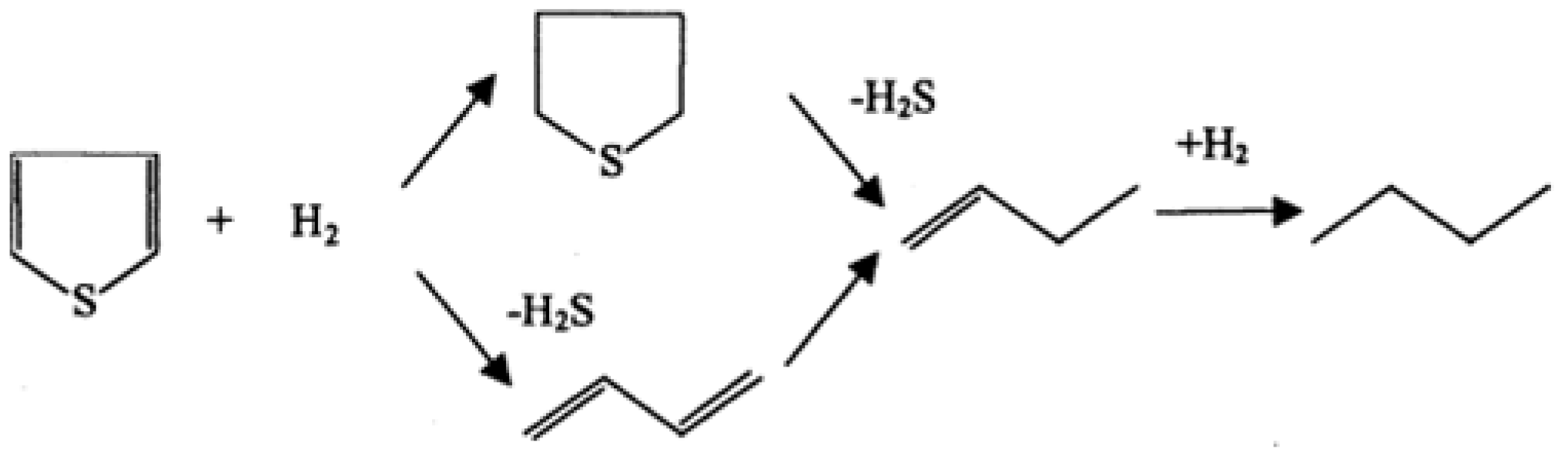
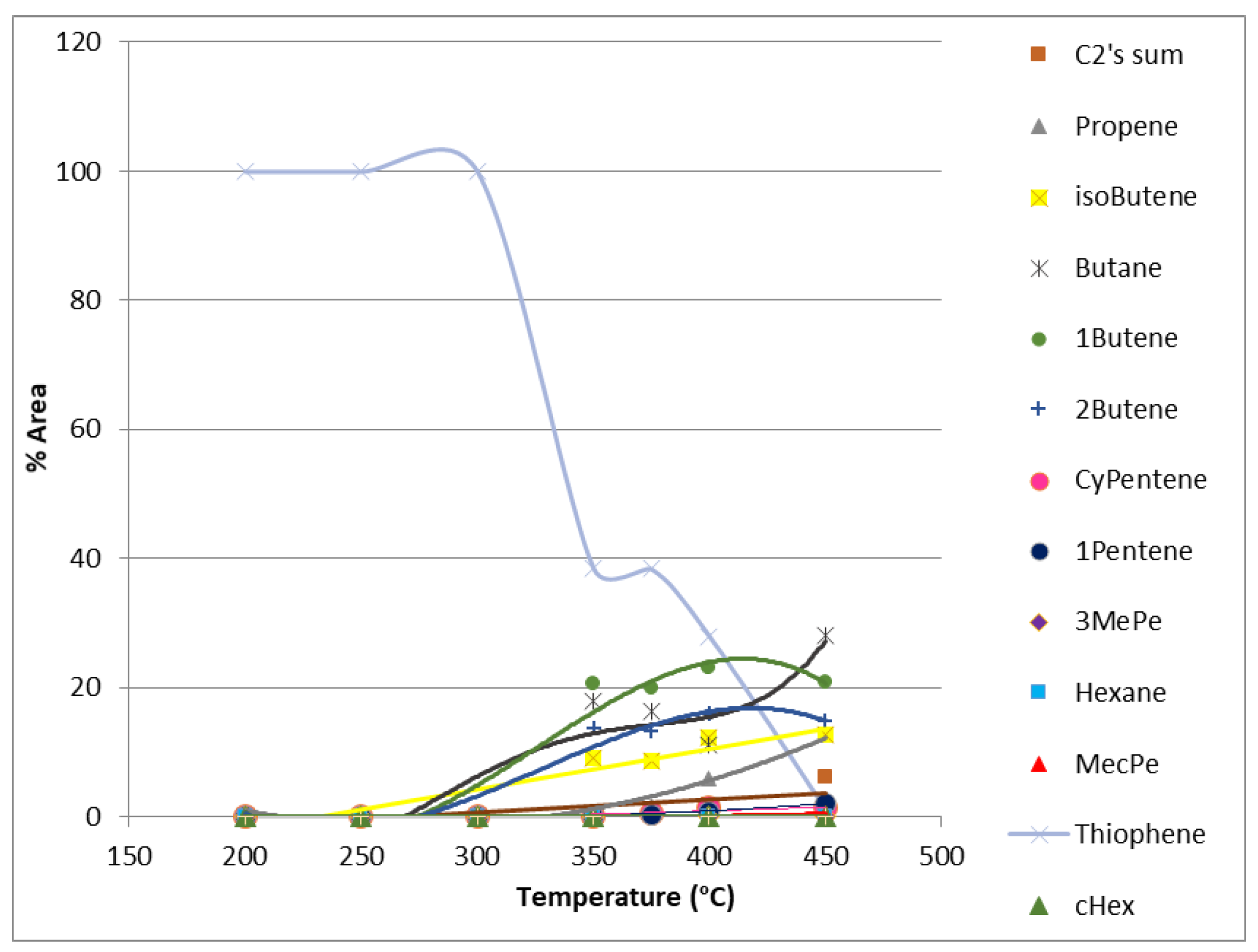
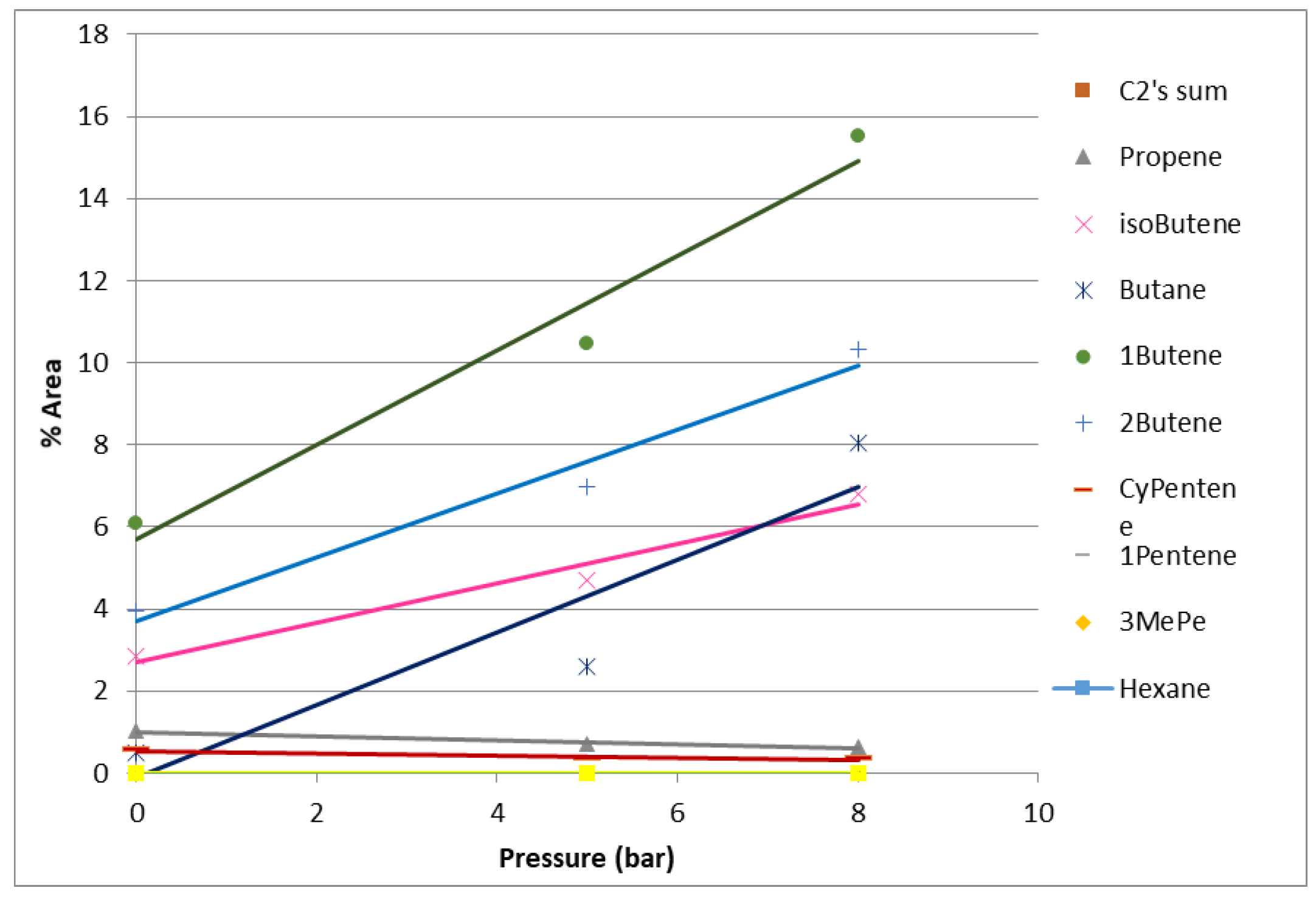
2.1.3. Product Distribution
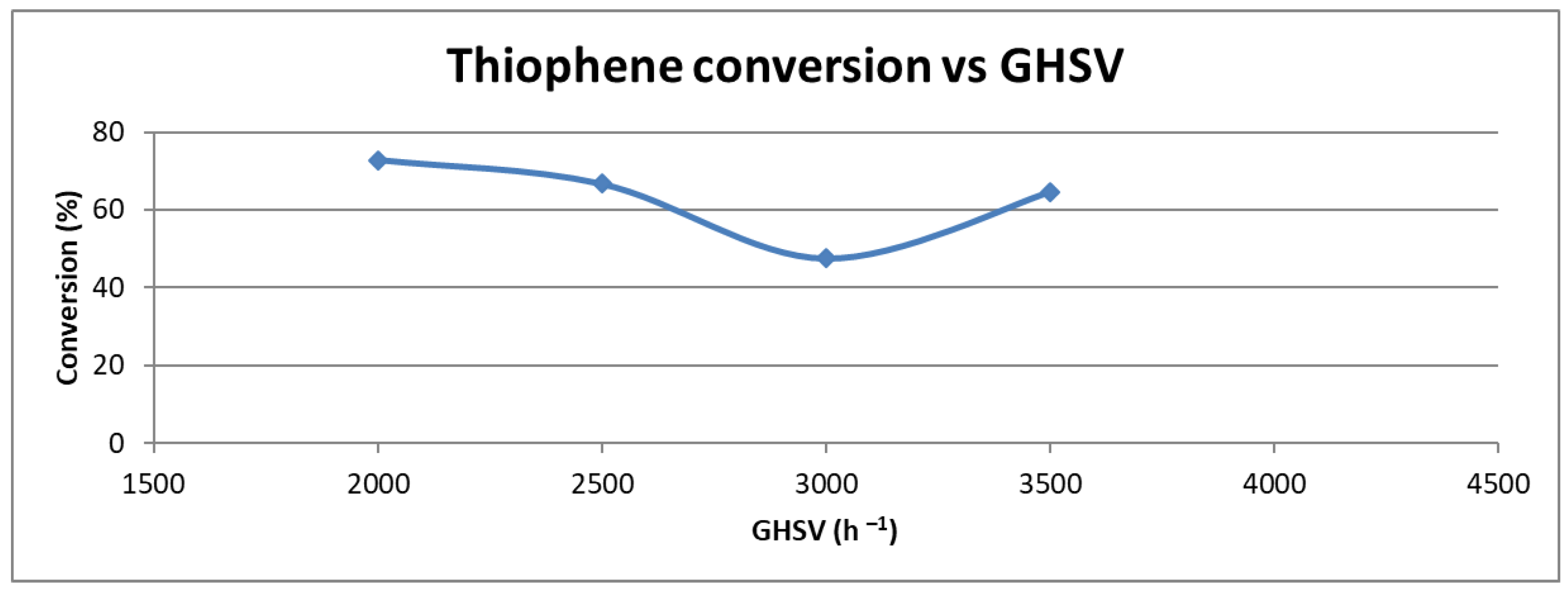
2.1.4. Effect of Gas Hourly Space Velocity
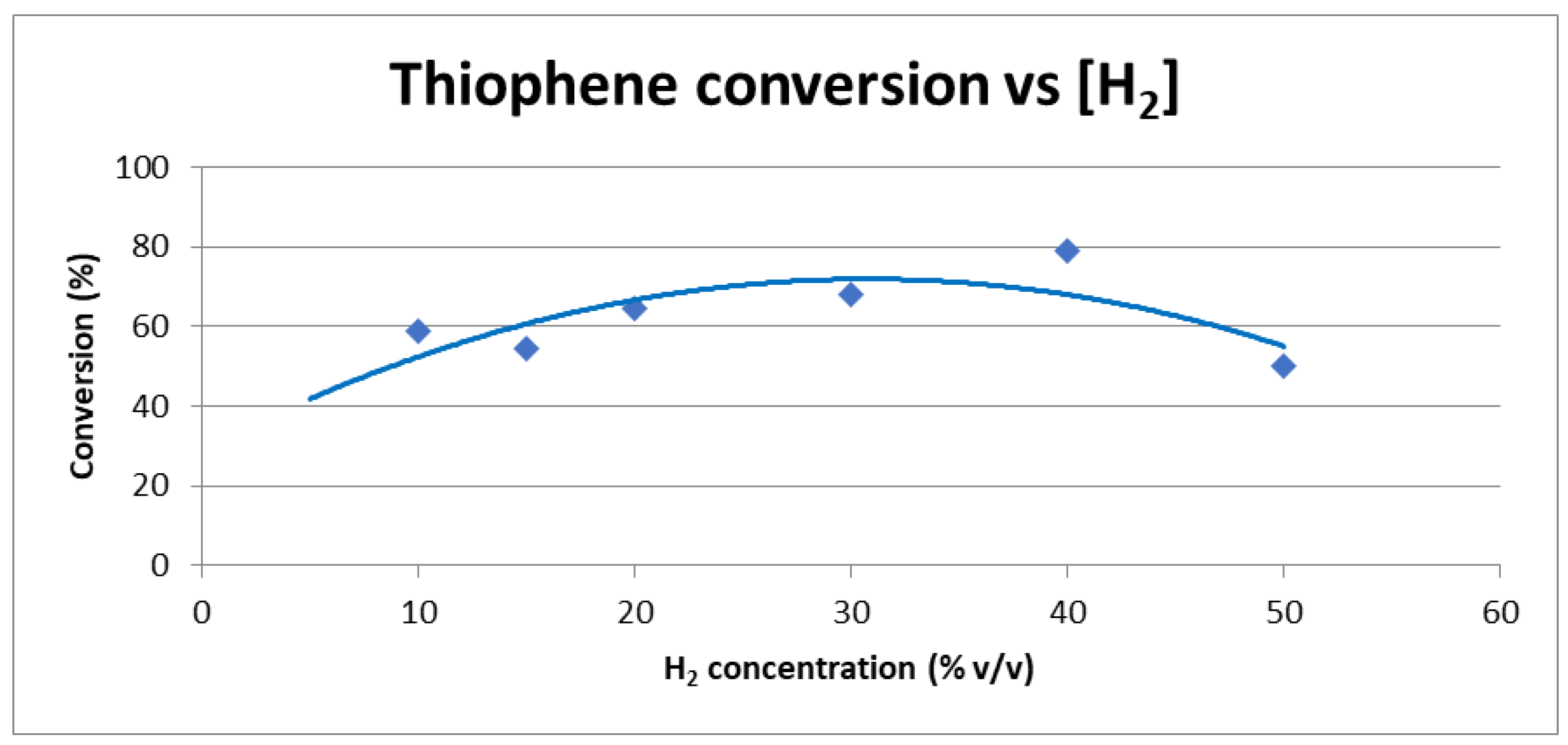
2.1.5. Effect of Hydrogen
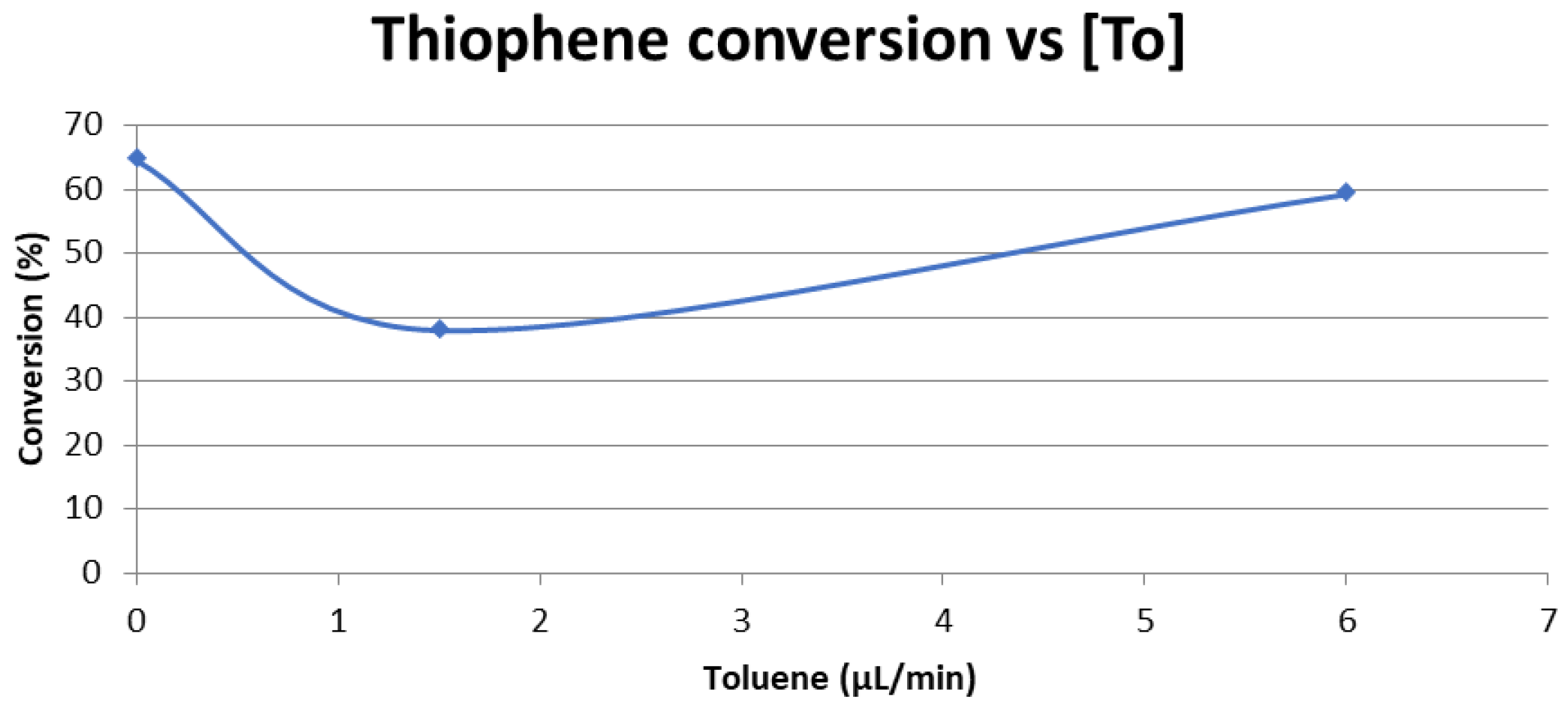
2.1.6. Effect of Aromatic Inorganic Pollutants
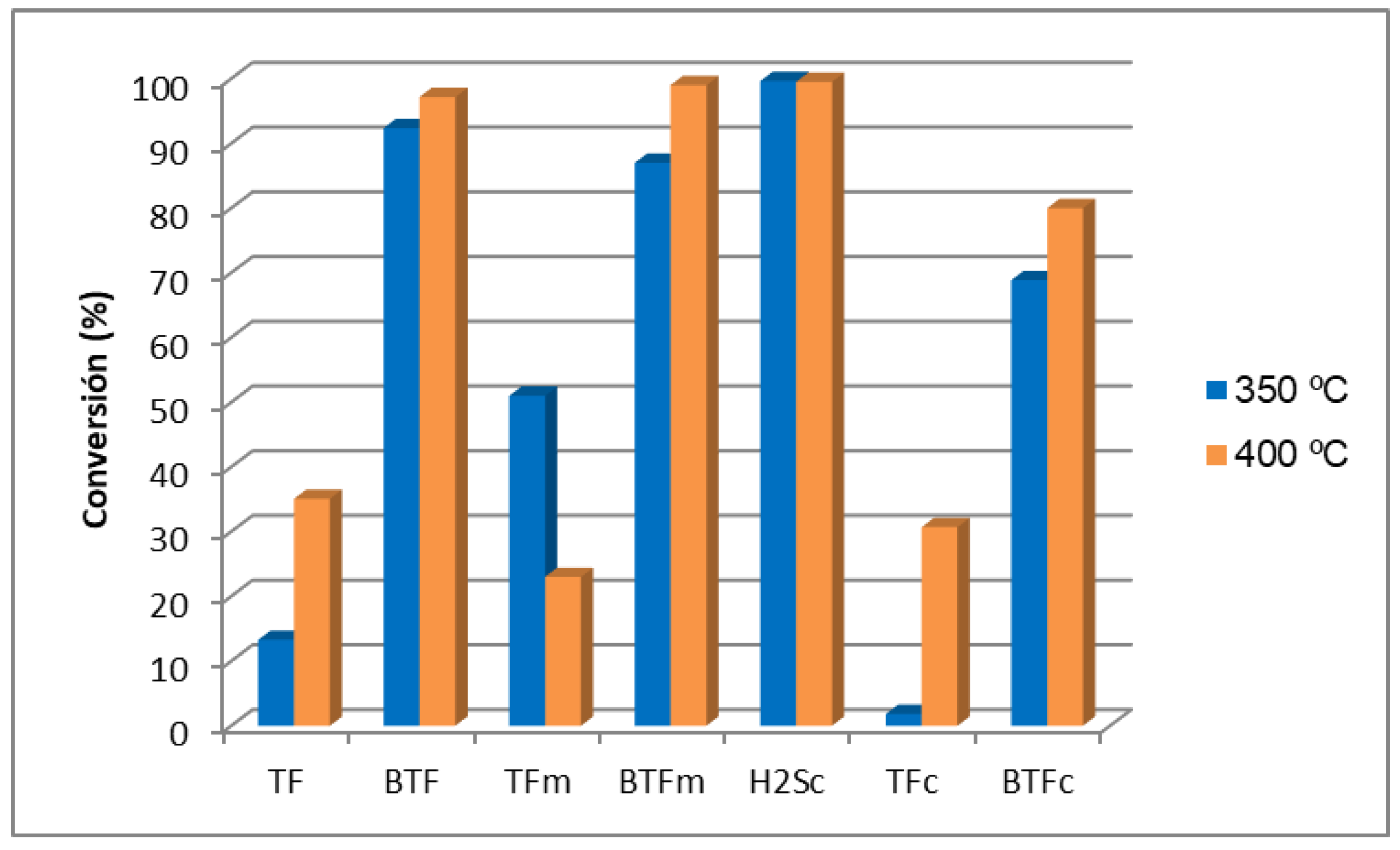
2.2. Removal of Organic Sulfur Species under Synthetic Syngas
3. Materials and Methods
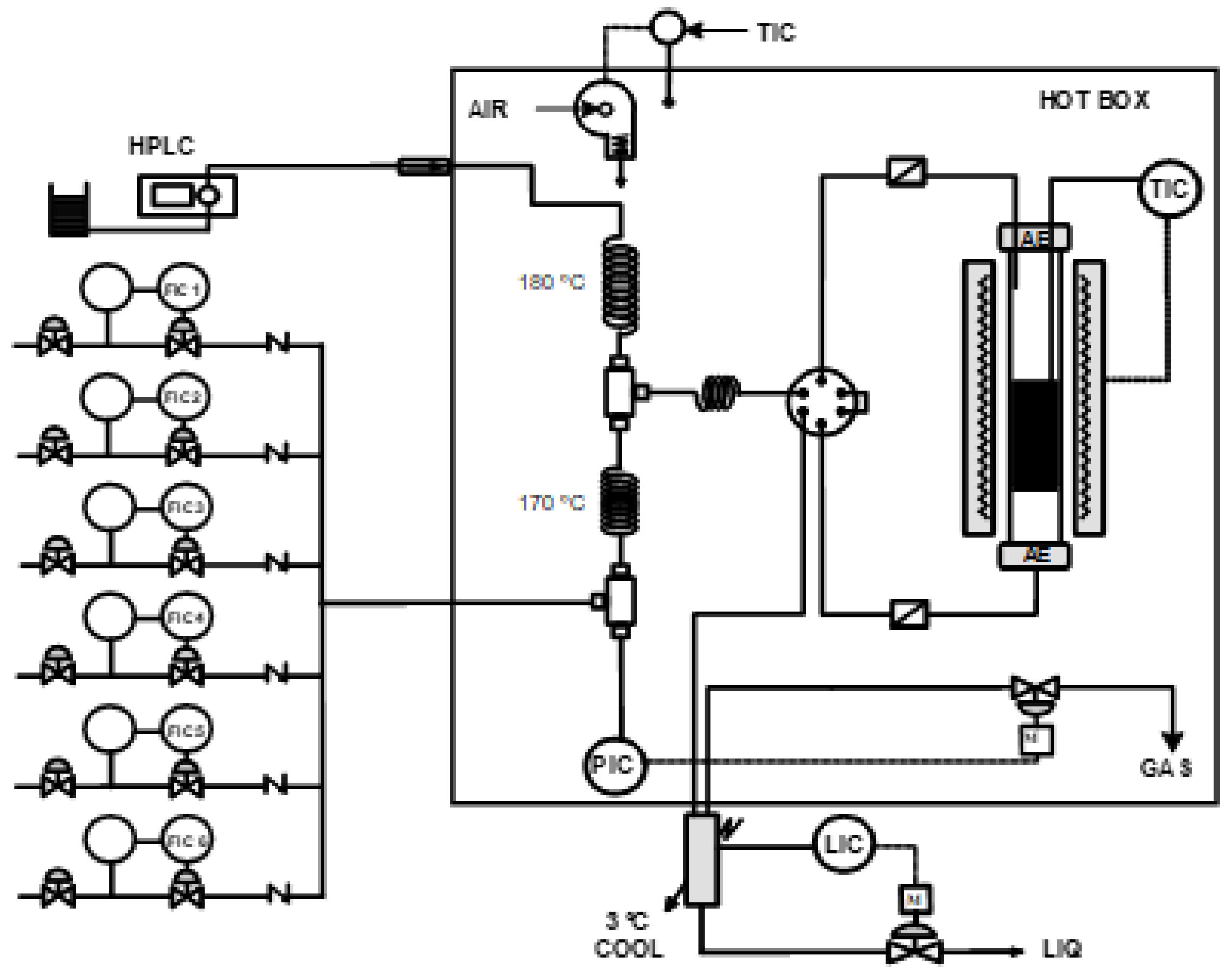
3.1. Experimental Facility
3.2. Sorbent
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jayakumar, M.; Bizuneh Gebeyehu, K.; Deso Abo, L.; Wondimu Tadesse, A.; Vivekanandan, B.; Prabhu Sundramurthy, V.; Bacha, W.; Ashokkumar, V.; Baskar, G. A comprehensive outlook on topical processing methods for biofuel production and its thermal applications: Current advances, sustainability and challenges. Fuel 2023, 349, 128690. [Google Scholar] [CrossRef]
- Cortazar, M.; Santamaria, L.; Lopez, G.; Alvarez, J.; Zhang, L.; Wang, R.; Bi, X.; Olazar, M. A comprehensive review of primary strategies for tar removal in biomass gasification. Energy Convers. Manag. 2023, 276, 116496. [Google Scholar] [CrossRef]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels production by biomass gasification: A review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Materazzi, M.; Lettieri, P.; Mazzei, L.; Taylor, R.; Chapman, C. Reforming of tars and organic sulphur compounds in a plasma-assisted process for waste gasification. Fuel Process. Technol. 2015, 137, 259–268. [Google Scholar] [CrossRef]
- Held, J. Gasification-Status and technology. Rapp. SGC 2012, 240, 1102–7371. [Google Scholar]
- Peters, D.; Van der Leun, K.; Terlouw, W.; Van Tilburg, J.; Berg, T.; Schimmel, M.; Van der Hoorn, I.; Buseman, M.; Maarten Staats, M.; Mark Schenkel, M.; et al. Gas Decarbonisation Pathways 2020–2050: Gas for Climate Guidehouse; Stadsplateau 15: Utrecht, The Netherlands, 2020. [Google Scholar]
- Seiffert, M.; Rönsch, S.; Schmersahl, R.; Zeymer, M.; Majer, S.; Pätz, C.; Kaltschmitt, M.; Rauch, R.; Rehling, B.; Harald Tremmel, H.; et al. Bio-SNG—Demonstration of the Production and Utilization of Synthetic Natural Gas (SNG) from Solid Biofuels; D 1.4 Finla Project Report, TREN/05/FP6EN/S07.56632/019895 6th Framework Programme; DBFZ German Biomass Research Centre: Leipzig, Germany, 2009; pp. 1–46. [Google Scholar]
- Rabou, L.; van Egmond, B.F.; Vreugdenhil, B.J.; van der Drift, A. Measurement of Organic Sulphur and Nitrogen Compounds in Biomass Producer Gas by SPA Sampling; ECN: Petten, The Netherlands, 2014. [Google Scholar]
- Hedenskog, M. Experiences of operating the world’s first industrial scale bio-SNG facility, GoBiGas. In Proceedings of the 2nd International Conference on Renewable Energy Gas Technology, Barcelona, Spain, 7–8 May 2015. [Google Scholar]
- Struis, R.; Schildhauer, T.J.; Czekaj, I.; Janousch, M.; Biollaz, S.M.A.; Ludwig, C. Sulphur poisoning of Ni catalysts in the SNG production from biomass: A TPO/XPS/XAS study. Appl. Catal. A Gen. 2009, 362, 121–128. [Google Scholar] [CrossRef]
- Woolcock, P.J.; Brown, R.C. A review of cleaning technologies for biomass-derived syngas. Biomass Bioenergy 2013, 52, 54–84. [Google Scholar] [CrossRef]
- Aravind, P.V.; De Jong, W. Evaluation of high temperature gas cleaning options for biomass gasification product gas for Solid Oxide Fuel Cells. Prog. Energy Combust. Sci. 2012, 38, 737–764. [Google Scholar] [CrossRef]
- Prabhansu; Karmakar, M.K.; Chandra, P.; Chatterjee, P.K. A review on the fuel gas cleaning technologies in gasification process. J. Environ. Chem. Eng. 2015, 3, 689–702. [Google Scholar] [CrossRef]
- Maroño, M.; Ortiz, I.; Sánchez, J.M.; Alcaraz, L.; Alguacil, F.J.; López, F.A. Effective removal of hydrogen sulfide using Mn-based recovered oxides from recycled batteries. Chem. Eng. J. 2021, 419, 129669. [Google Scholar] [CrossRef]
- Sánchez-Hervás, J.M.; Maroño, M.; Fernández-Martínez, R.; Ortiz, I.; Ortiz, R.; Gómez-Mancebo, M.B. Novel ZnO-NiO-graphene-based sorbents for removal of hydrogen sulfide at intermediate temperature. Fuel 2022, 314, 122724. [Google Scholar] [CrossRef]
- Rhyner, U.; Edinger, P.; Schildhauer, T.J.; Biollaz, S.M.A. Experimental study on high temperature catalytic conversion of tars and organic sulfur compounds. Int. J. Hydrogen Energy 2014, 39, 4926–4937. [Google Scholar] [CrossRef]
- Kienberger, T.; Zuber, C.; Novosel, K.; Baumhakl, C.; Karl, J. Desulfurization and in situ tar reduction within catalytic methanation of biogenous synthesis gas. Fuel 2013, 107, 102–112. [Google Scholar] [CrossRef]
- Zuber, C.; Hochenauer, C.; Kienberger, T. Test of a hydrodesulfurization catalyst in a biomass tar removal process with catalytic steam reforming. Appl. Catal. B Environ. 2014, 156–157, 62–71. [Google Scholar] [CrossRef]
- Zuber, C.; Husmann, M.; Hochenauer, C.; Kienberger, T. Optimization of the desulfurization step in a catalytic gas cleaning process. In Proceedings of the 23rd European Biomass Conference and Exhibition, Wien, Austria, 1–4 June 2015. [Google Scholar]
- Rabou, L.; Aranda, G. 500 Hours Producing bio-SNG from MILENA Gasification Using the ESME System; ECN: Petten, The Netherlands, 2015; pp. 1–49. [Google Scholar]
- Li, L.; Ju, F.; Sun, Y.; Pan, H.; Ling, H. Self-sulfidation adsorbent for reactive adsorption desulfurization. Fuel 2022, 313, 122696. [Google Scholar] [CrossRef]
- Zhang, G.; Xue, P.; Wei, J.; Zhang, Y.; Zhao, L.; Gao, J.; Xu, C. Competitive adsorption mechanism of thiophene with diethyl sulfide in Y zeolite: Displacement and migration. Chem. Eng. J. 2022, 435, 135141. [Google Scholar] [CrossRef]
- Chen, M.; Chen, J.; Liu, Y.; Liu, J.; Li, L.; Yang, B.; Ma, L. Enhanced adsorption of thiophene with the GO-modified bimetallic organic framework Ni-MOF-199. Colloids Surf. A Physicochem. Eng. Asp. 2019, 578, 123553. [Google Scholar] [CrossRef]
- Vafaee, F.; Jahangiri, M.; Salavati-Niasari, M. Adsorptive desulfurization of model fuels using ferrite nickel-silica in continues system: Modelling of breakthrough curves, thermodynamic study and experimental design. Fuel 2022, 327, 124999. [Google Scholar] [CrossRef]
- Bartholomew, C.H.; Farrauto, R.J. Chapter 9: Petroleum Refining and Processing, Hydrotreating. In Fundamentals of Industrial Catalytic Processes, 2nd ed.; Wiley Interscience; John Wiley & Sons Inc. Publisher: Hoboken, NJ, USA, 2006; Volume 2, p. 639. [Google Scholar]
- Sun, H.Y.; Sun, L.P.; Li, F.; Zhang, L. Adsorption of benzothiophene from fuels on modified NaY zeolites. Fuel Process. Technol. 2015, 134, 284–289. [Google Scholar] [CrossRef]
- Wei, F.; Guo, X.; Liao, J.; Bao, W.; Chang, L. Ultra-deep removal of thiophene in coke oven gas over Y zeolite: Effect of acid modification on adsorption desulfurization. Fuel Process. Technol. 2021, 213, 106632. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, X.; Tian, F.; Jia, C.; Chen, Y. Effects of toluene on thiophene adsorption over NaY and Ce(IV)Y zeolites. J. Nat. Gas Chem. 2012, 21, 421–425. [Google Scholar] [CrossRef]
- Edinger, P.; Grimekis, D.; Panopoulos, K.; Karellas, S.; Ludwig, C. Adsorption of thiophene by activated carbon: A global sensitivity analysis. J. Environ. Chem. Eng. 2017, 5, 4173–4184. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sulaiman, K.O.; Al-Hammadi, S.A.; Dafalla, H.; Danmaliki, G.I. Adsorptive desulfurization of thiophene, benzothiophene and dibenzothiophene over activated carbon manganese oxide nanocomposite: With column system evaluation. J. Clean. Prod. 2017, 154, 401–412. [Google Scholar] [CrossRef]
- Malone, W.; Kaden, W.; Kara, A. Exploring thiophene desulfurization: The adsorption of thiophene on transition metal surfaces. Surf. Sci. 2019, 686, 30–38. [Google Scholar] [CrossRef]
- Hernandez, A.D.; Kaisalo, N.; Simell, P.; Scarsella, M. Effect of H2S and thiophene on the steam reforming activity of nickel and rhodium catalysts in a simulated coke oven gas stream. Appl. Catal. B Environ. 2019, 258, 117977. [Google Scholar] [CrossRef]
- Wei, F.; Zhang, X.; Liao, J.; Guo, J.; Bao, W.; Chang, L. Desulfurization mechanism of an excellent Cu/ZnO sorbent for ultra-deep removal of thiophene in simulated coke oven gas. Chem. Eng. J. 2022, 446, 137140. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, Y.; Tian, S.; Chai, Y.; Liu, C. Reactive adsorption of thiophene on Ni/ZnO adsorbent: Effect of ZnO textural structure on the desulfurization activity. J. Nat. Gas Chem. 2010, 19, 327–332. [Google Scholar] [CrossRef]
- Ryzhikov, A.; Bezverkhyy, I.; Bellat, J.P. Reactive adsorption of thiophene on Ni/ZnO: Role of hydrogen pretreatment and nature of the rate determining step. Appl. Catal. B Environ. 2008, 84, 766–772. [Google Scholar] [CrossRef]
- Bezverkhyy, I.; Ryzhikov, A.; Gadacz, G.; Bellat, J.P. Kinetics of thiophene reactive adsorption on Ni/SiO2 and Ni/ZnO. Catal. Today 2008, 130, 199–205. [Google Scholar] [CrossRef]
- Sun, Y.; Li, L.; Ju, F.; Ling, H. Evolution of nickel species in reactive adsorption desulfurization of benzothiophene. Sep. Purif. Technol. 2022, 283, 120204. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.-r.; Zhao, W.; Yang, S.-w.; Hu, B.; Yang, S.-g.; Lu, Q. Mechanical insight into the formation of H2S from thiophene pyrolysis: The influence of H2O. Chemosphere 2021, 279, 130628. [Google Scholar] [CrossRef]
- Mohseni, E.; Rahmani, A.; Hamdi, Z. Poly (4,4′-methylenedianiline)-graphene oxide nanocomposite: Synthesize and application in removal of benzothiophene from model liquid fuel. Environ. Monit. Assess. 2014, 193, 737. [Google Scholar] [CrossRef]
- Liu, W.; Liu, X.; Yang, Y.; Zhang, Y.; Xu, B. Selective removal of benzothiophene and dibenzothiophene from gasoline using double-template molecularly imprinted polymers on the surface of carbon microspheres. Fuel 2014, 117, 184–190. [Google Scholar] [CrossRef]
- Anca-Couce, A.; von Berg, L.; Pongratz, G.; Scharler, R.; Hochenauer, C.; Geusebroek, M.; Kuipers, J.; Vilela, C.M.; Kraia, T.; Panopoulos, K.; et al. Assessment of measurement methods to characterize the producer gas from biomass gasification with steam in a fluidized bed. Biomass Bioenergy 2022, 163, 106527. [Google Scholar] [CrossRef]
- Hydrocarbon Online. S Zorb Sulfur Removal Technology for Gasoline. Available online: http://www.hydrocarbononline.com/doc/s-zorb-sulfur-removal-technology-for-gasoline-0001 (accessed on 12 May 2023).
- Sinopec Tech S ZorbTM Sulfur Removal Technology. Available online: http://www.sinopectech.com/en/index.php?m=Product&a=show&id=35#:~:text=S%20Zorb%20sulfur%20removal%20technology,the%20dominant%20technology%20applied%20in (accessed on 25 May 2023).
- Schmidt, R.; Tsang, A.; Cross, J.; Summers, C.; Kornosky, B. Laboratory simulated slipstream testing of novel sulfur removal processes for gasification application. Fuel Process. Technol. 2008, 89, 589–594. [Google Scholar] [CrossRef]
- Qiu, L.; Zou, K.; Xu, G. Investigation on the sulfur state and phase transformation of spent and regenerated S zorb sorbents using XPS and XRD. Surf. Sci. 2013, 266, 230–234. [Google Scholar] [CrossRef]
- Sánchez, J.M.; Ruiz, E.; Otero, J. Selective removal of hydrogen sulfide from gaseous streams using a zinc-based sorbent. Ind. Eng. Chem. Res. 2005, 44, 241–249. [Google Scholar] [CrossRef]
- Sánchez-Hervás, J.M.; Otero, J.; Ruiz, E. A Study on Sulfidation and Regeneration of Z-Sorb III Sorbent for H2S Removal from Simulated ELCOGAS IGCC Syngas. Chem. Eng. Sci. 2005, 60, 2977–2989. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, Y.; Han, H.; Yang, M.; Wang, L.; Zhang, Y.; Jiang, Z.; Li, C. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: The effect of ZnO particle size on the adsorption performance. Appl. Catal. B Environ. 2012, 119–120, 13–19. [Google Scholar] [CrossRef]
- Lyu, Y.; Sun, Z.; Meng, X.; Wu, Y.; Liu, X.; Hu, Y. Scale-up reactivation of spent S-Zorb adsorbents for gasoline desulfurization. J. Hazard. Mater. 2022, 423, 126903. [Google Scholar] [CrossRef]
- Kong, A.; Wei, Y.; Li, Y. Reactive adsorption desulfurization over a Ni/ZnO adsorbent prepared by homogeneous precipitation. Front. Chem. Sci. Eng. 2013, 7, 170–176. [Google Scholar] [CrossRef]
- Hofbauer, H.; Rauch, R.; Ripfel-Nitsche, K. Report on Gas Cleaning for Synthesis Applications Work Package 2E: Gas Treatment; Thermal Net: University of Technology Institute of Chemical Engineering Viena: Vienna, Austria, 2007; pp. 1–75. [Google Scholar]
- Fan, J.; Wang, G.; Sun, Y.; Xu, C.; Zhou, H.; Zhou, G.; Gao, J. Research on reactive adsorption desulfurization over Ni/ZnO−SiO2−Al2O3 adsorbent in a fixed-fluidized bed reactor. Ind. Eng. Chem. Res. 2010, 49, 8450–8460. [Google Scholar] [CrossRef]
- Vander Laan, J. ConocoPhillips S Zorb Gasoline Sulfur Removal Technology: Unique Chemistry, Proven Performance, and Optimized Design, ConocoPhillips. Available online: https://www.yumpu.com/en/document/view/51243737/1-conocophillips-s-zorb-gasoline-sulfur-removal-technology- (accessed on 22 May 2023).
- Ito, E.; van Veen, J.A.R. On novel processes for removing sulphur from refinery streams. Catal. Today 2006, 116, 446–460. [Google Scholar] [CrossRef]
- Babich, I.V.; Moulijn, J.A. Science and technology of novel processes for deep desulfurization of oil refinery streams: A review. Fuel 2003, 82, 607–631. [Google Scholar] [CrossRef]
- Tawara, K.; Nishimura, T.; Iwanami, H.; Nishimoto, T.; Hasuike, T. New hydrodesulfurization catalyst for petroleum-fed fuel cell vehicles and cogenerations. Ind. Eng. Chem. Res. 2001, 40, 2367–2370. [Google Scholar] [CrossRef]
- Poels, E.K.; van Beek, W.P.; den Hoed, W.; Visser, C. Deactivation of fixed-bed nickel hydrogenation catalysts by sulfur. Fuel 1995, 74, 1800–1805. [Google Scholar] [CrossRef]

| Feedstock | Sulfur (wt% daf) 1 |
|---|---|
| Pine pellets | 0.03 |
| Straw | 0.15 |
| Beer bagasse | 0.26 |
| Cattle manure | 0.95 |
| Municipal Solid Waste | 0.55 |
| Sewage Sludge | 0.40 |
| Test Series | Objective | Operating Conditions 1 |
|---|---|---|
| 1 | Reference case | 350 °C, 3500 h−1, 10 bar, TF (500 ppmv), H2 (20% v/v), N2 makeup |
| 2 | Effect of T | 200, 250, 300, 350, 375, 400, 450 °C, 3500 h−1, 10 bar, TF (500 ppmv), H2 (20% v/v), N2 makeup |
| 3 | Effect of P | 350 °C, 3500 h−1, 0.5, 1, 5, 8, 10 bar, TF (500 ppmv), H2 (20% v/v), N2 makeup |
| 4 | Effect of GHSV | 350 °C, 2000, 2500, 3000, 3500 h−1, 10 bar, TF (500 ppmv), H2 (20% v/v), N2 makeup |
| 5 | Effect of H2 | 350 °C, 3500 h−1, 10 bar, TF (500 ppmv), 10, 15, 20, 30, 40, 50% v/v H2, N2 makeup |
| 6 | Effect of thiophene | 400 °C, 3500 h−1, 10 bar, 500, 1000 ppmv TF, H2 (20% v/v), N2 makeup |
| 7 | Effect of Toluene | 350 °C, 3500 h−1, 10 bar, TF (500 ppmv), 1.5, 3, 4, 6 µL/min TOL, 20% v/v H2, N2 makeup |
| Test Series | Objective | Operating Conditions 1 |
|---|---|---|
| 8 | Effect of CO | 350 °C, 3500 h−1, 10 bar, TF (0.08%), 4 µL/min TOL, 20%, 40% v/v H2, 40% v/v CO (syngas C) |
| 9 | Effect of full syngas | 350 °C, 3500 h−1, 10 bar, TF (500 ppmv), 4 µL/min TOL, syngas A, CO/CO2/CH4/H2 (32.9/11.2/0.7/29.9% v/v), syngas B CO/CO2/CH4/H2 (24.2/8.2/0.5/22% v/v) |
| 10 | BTF removal | 350 °C, 3500 h−1, 10 bar, BTF (1, 3 ppmv), 5850 ppmv TOL, 20%, H2, 40% v/v CO, N2 makeup |
| 11 | TF & BTF combined removal | 350, 400 °C, 3500 h−1, 10 bar, 800 ppv TF, 100 ppmv BTF, 5850 ppmv TOL, 20%, H2, 40% v/v CO, N2 makeup |
| 12 | Effect of H2S | 350, 400 °C, 3500 h−1, 10 bar, 1000 ppv TF, 100 ppmv BTF, 0.36% v/v H2S, 5850 ppmv TOL, 20%, H2, 40% v/v CO, N2 makeup |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Hervás, J.; Ortiz, I.; Martí, V.; Andray, A. Removal of Organic Sulfur Pollutants from Gasification Gases at Intermediate Temperature by Means of a Zinc–Nickel-Oxide Sorbent for Integration in Biofuel Production. Catalysts 2023, 13, 1089. https://doi.org/10.3390/catal13071089
Sánchez-Hervás J, Ortiz I, Martí V, Andray A. Removal of Organic Sulfur Pollutants from Gasification Gases at Intermediate Temperature by Means of a Zinc–Nickel-Oxide Sorbent for Integration in Biofuel Production. Catalysts. 2023; 13(7):1089. https://doi.org/10.3390/catal13071089
Chicago/Turabian StyleSánchez-Hervás, Josemaria, Isabel Ortiz, Veronica Martí, and Alberto Andray. 2023. "Removal of Organic Sulfur Pollutants from Gasification Gases at Intermediate Temperature by Means of a Zinc–Nickel-Oxide Sorbent for Integration in Biofuel Production" Catalysts 13, no. 7: 1089. https://doi.org/10.3390/catal13071089
APA StyleSánchez-Hervás, J., Ortiz, I., Martí, V., & Andray, A. (2023). Removal of Organic Sulfur Pollutants from Gasification Gases at Intermediate Temperature by Means of a Zinc–Nickel-Oxide Sorbent for Integration in Biofuel Production. Catalysts, 13(7), 1089. https://doi.org/10.3390/catal13071089






