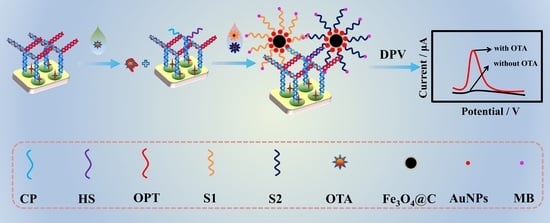
A Novel Ochratoxin A Aptasensor Based on Three-Dimensionally Ordered Macroporous RGO-AuNPs-MoS2 Enhanced Electrocatalysis of Methylene Blue and AuNPs-Fe3O4@C Composite as Signal Probe Carrier
Abstract
1. Introduction
2. Results and Discussion
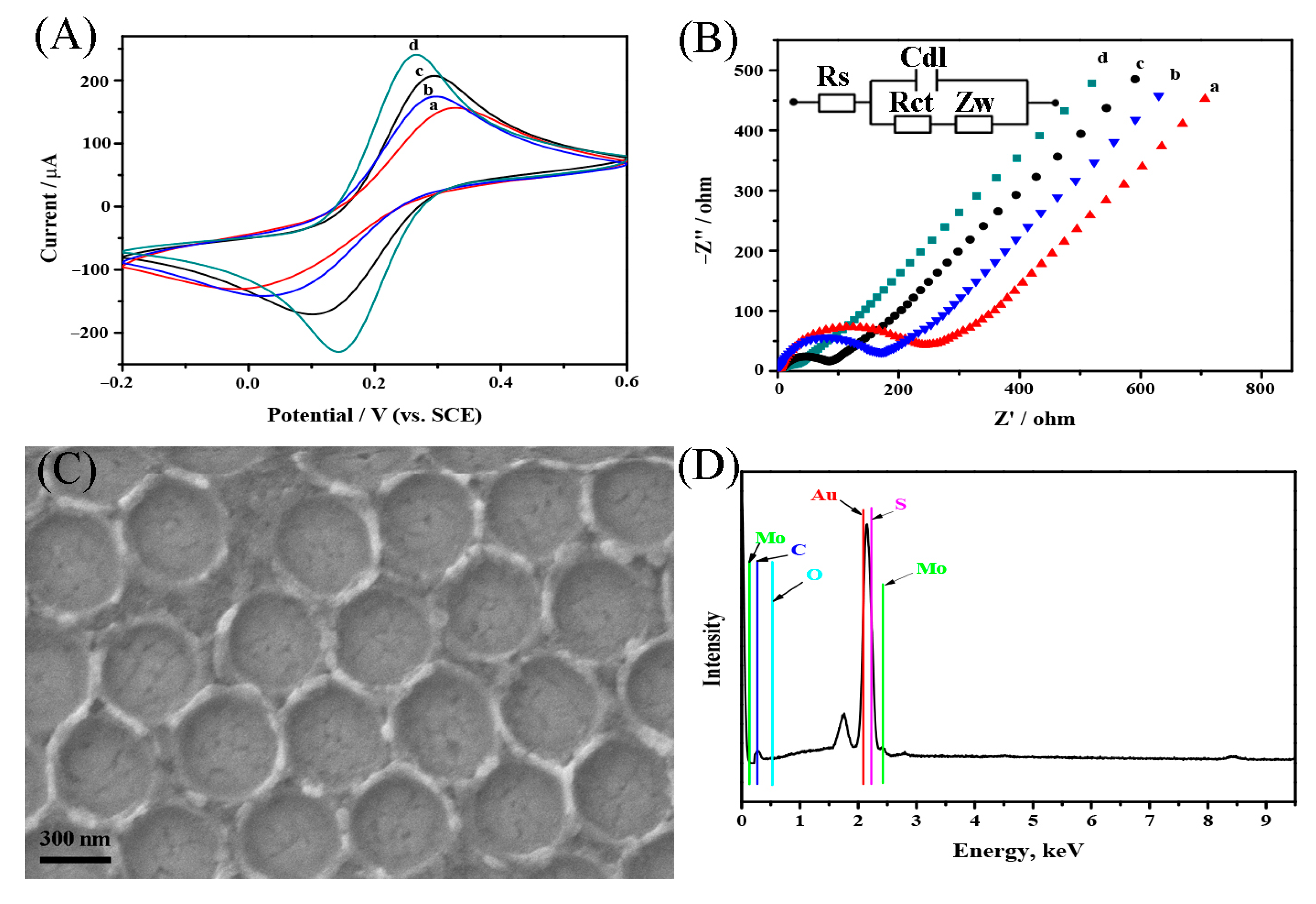
2.1. Characterization of 3DOM RGO-AuNPs-MoS2 Modified Electrode
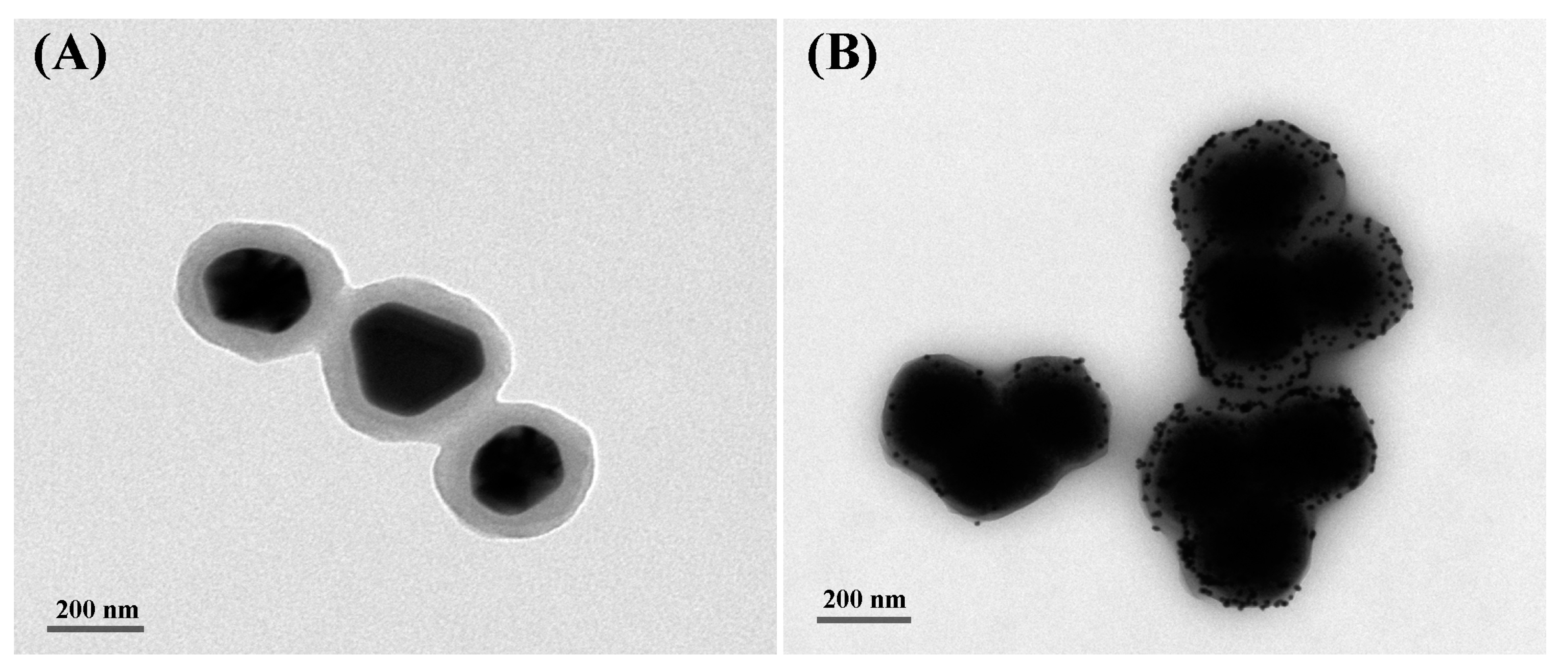
2.2. Characterization of AuNPs-Fe3O4@C
2.3. Electrochemical Characterization of the OTA Aptasensor
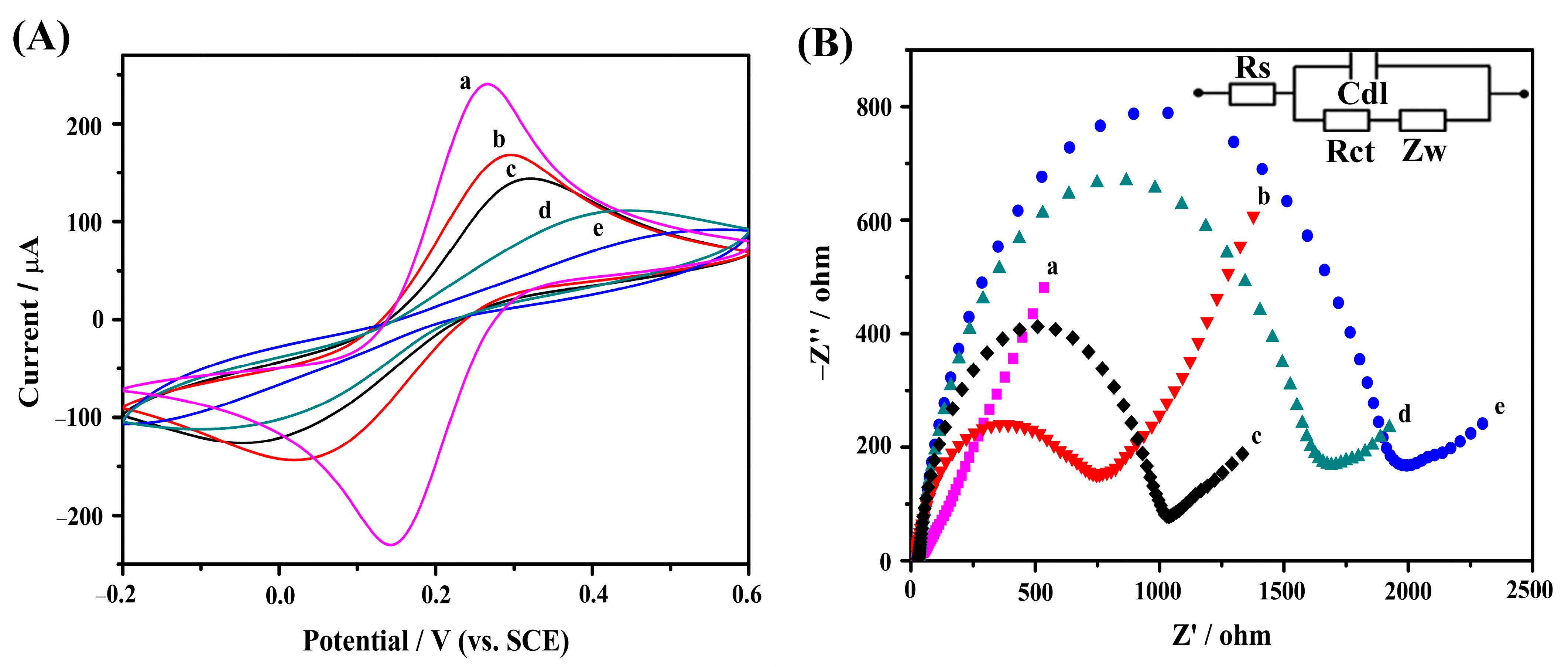
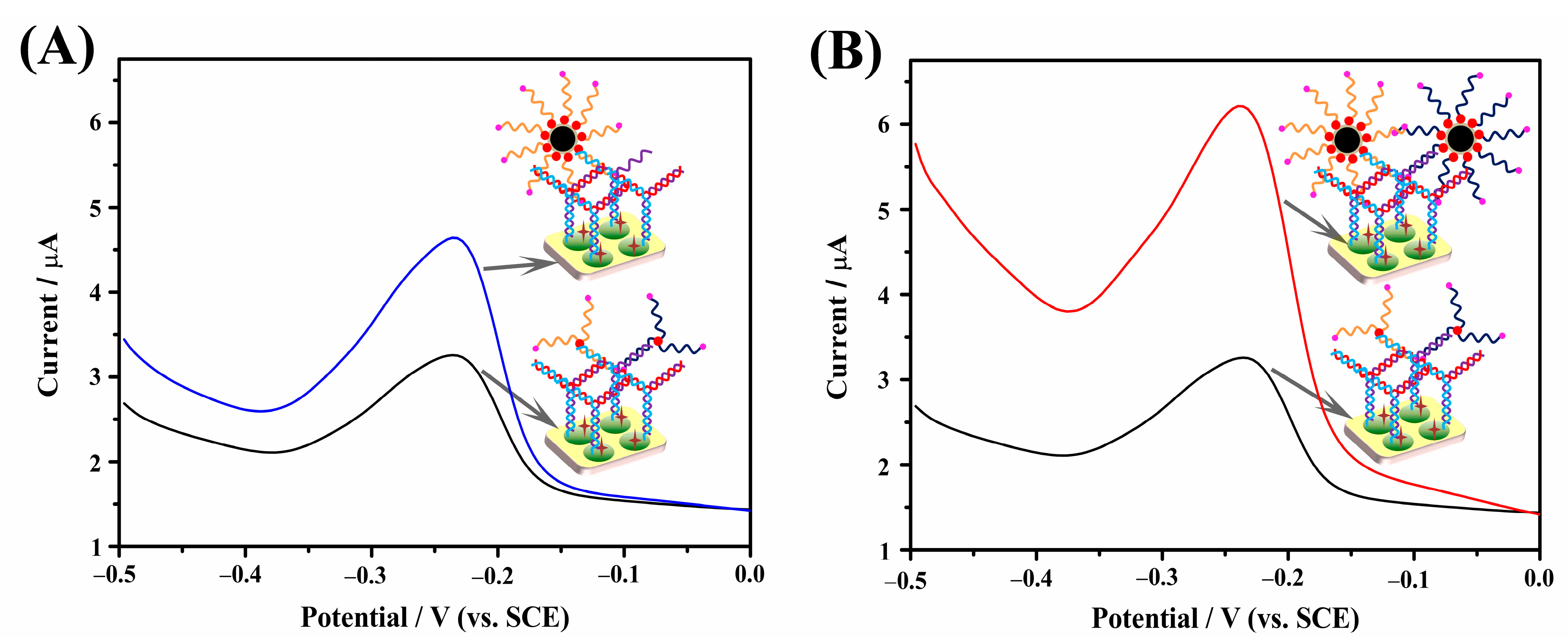
2.4. Signal Amplification Using the Y-Shaped DNA Structure and the Signal Amplification Effect of AuNPs-Fe3O4@C Nanoprobes
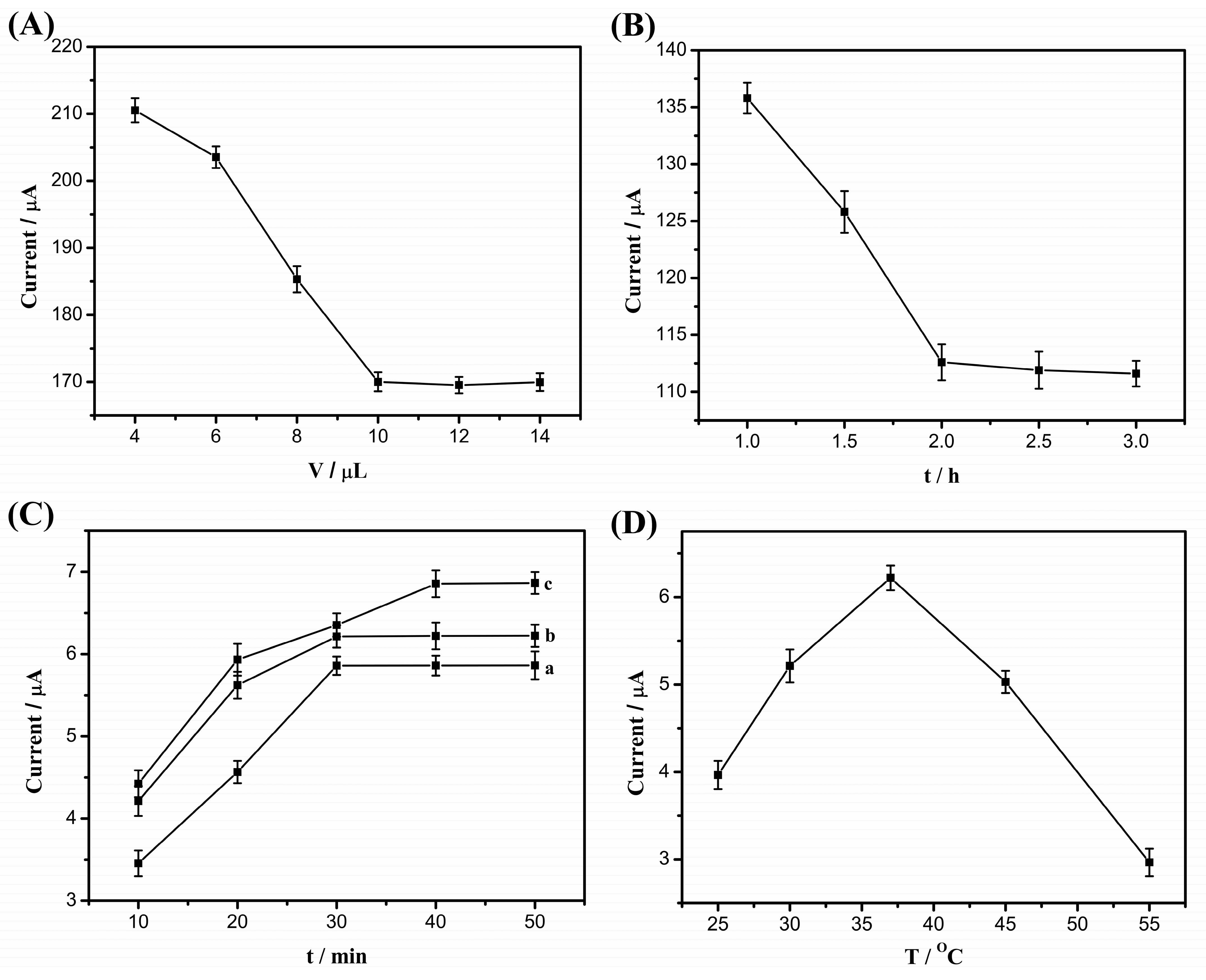
2.5. Optimization of Experimental Conditions
2.5.1. Amount of CP
2.5.2. Hybridization Time between CP and HS
2.5.3. Reaction Time of OPT with OTA
2.5.4. Reaction Temperature of OPT with OTA
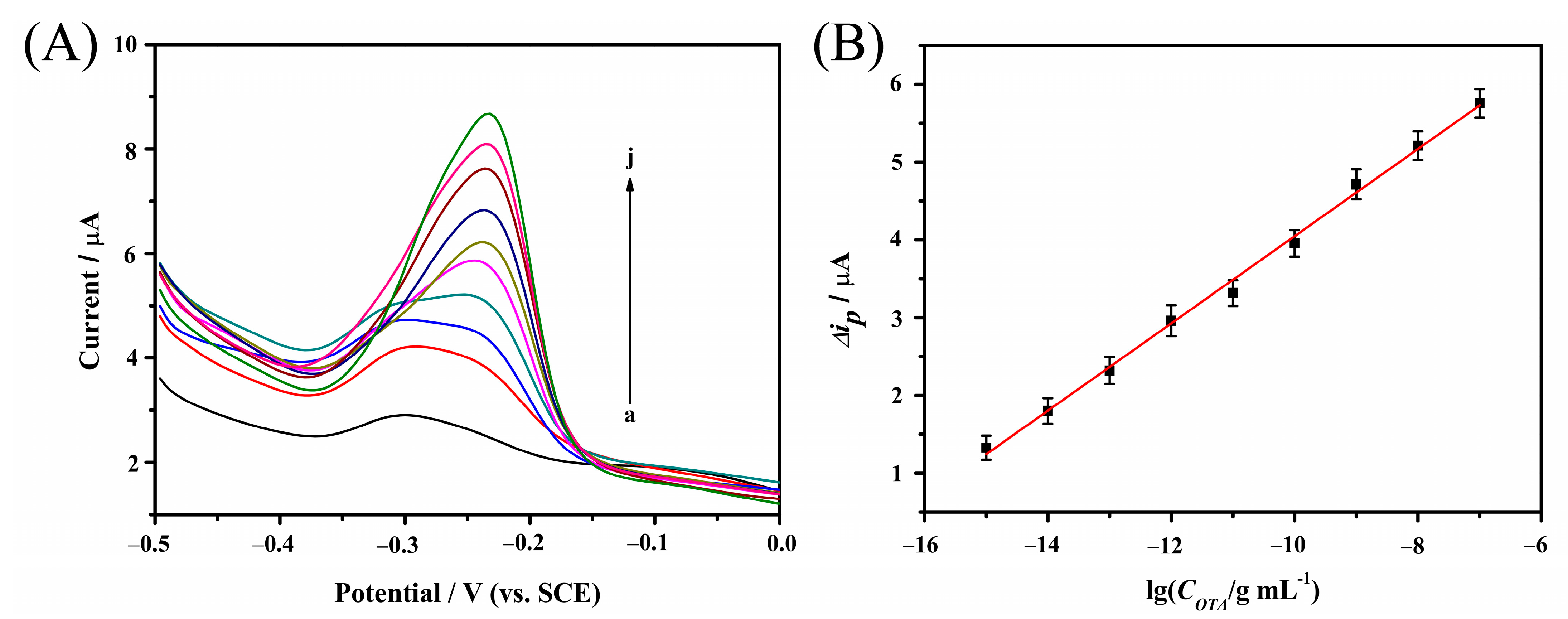
2.6. Amperometric Determination of OTA with Aptasensor
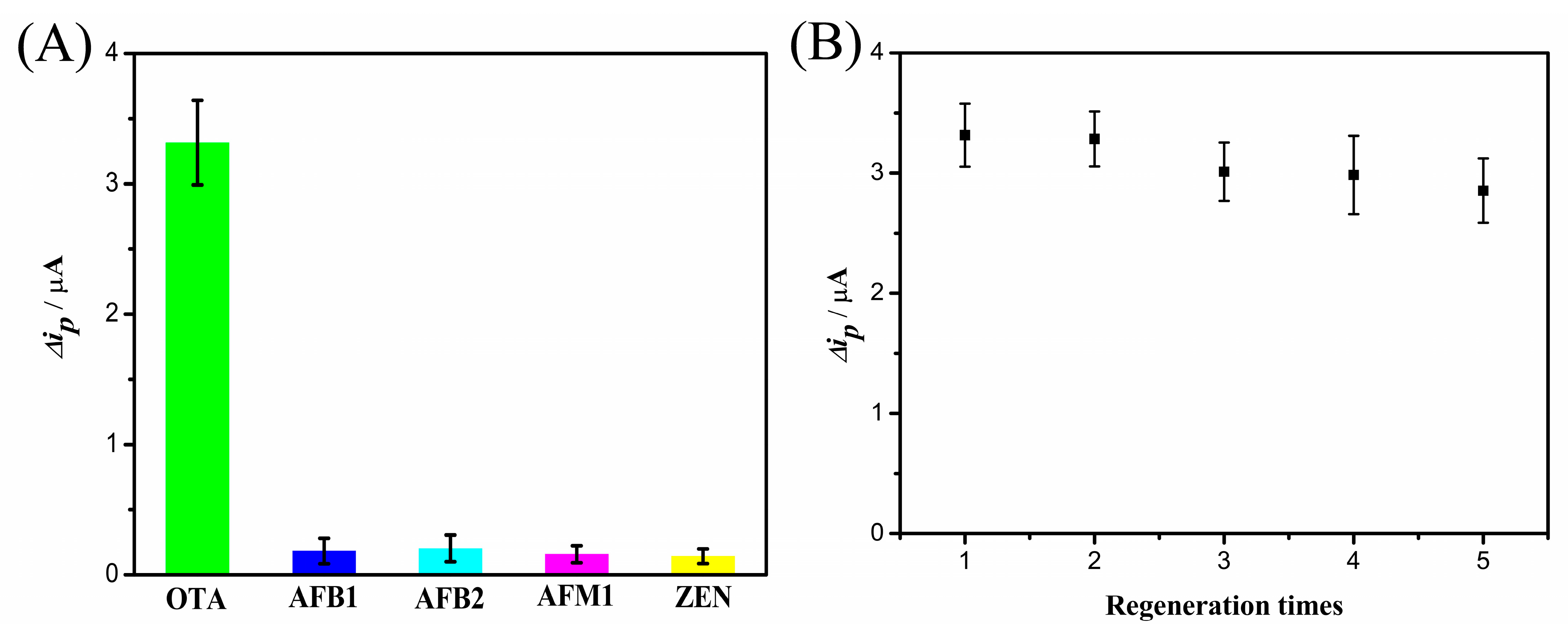
2.7. Specificity, Reproducibility, Regenerability, and Stability of the OTA Aptasensor
2.8. Real Sample Detection
3. Materials and Methods
3.1. Fabrication of the 3DOM RGO-AuNPs-MoS2 Film
3.2. Fabrication of the OTA Aptasensor
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chen, R.P.; Sun, Y.F.; Huo, B.Y.; Zhao, X.D.; Huang, H.; Li, S.; Bai, J.L.; Liang, J.; Gao, Z.X. A Copper Monosulfide-Nanoparticle-Based Fluorescent Probe for the Sensitive and Specific Detection of Ochratoxin A. Talanta 2021, 222, 121678. [Google Scholar] [CrossRef]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary Mycotoxins, Co-exposure, and Carcinogenesis in Humans: Short Review. Mutat. Res.-Rev. Mutat. 2015, 766, 32–41. [Google Scholar] [CrossRef]
- Fromme, H.; Gareis, M.; Völkel, W.; Gottschalk, C. Overall Internal Exposure to Mycotoxins and Their Occurrence in Occupational and Residential Settings-An Overview. Int. J. Hyg. Environ. Health 2016, 219, 143–165. [Google Scholar] [CrossRef]
- Bianco, M.; Sonato, A.; De Girolamo, A.; Pascale, M.; Romanato, F.; Rinaldi, R.; Arima, V. An Aptamer-Based SPR-Polarization Platform for High Sensitive OTA Detection. Sens. Actuators B-Chem. 2017, 241, 314–320. [Google Scholar] [CrossRef]
- Petzinger, E.; Ziegler, K. Ochratoxin A from A Toxicological Perspective. J. Vet. Pharmacol. Ther. 2000, 23, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Pfohl-Leszkowicz, A.; Manderville, R.A. Ochratoxin A: An Overview on Toxicity and Carcinogenicity in Animals and Humans. Mol. Nutr. Food Res. 2007, 51, 61–99. [Google Scholar] [CrossRef] [PubMed]
- Flajs, D.; Domijan, A.M.; Ivić, D.; Cvjetković, B.; Peraica, M. ELISA and HPLC Analysis of Ochratoxin A in Red Wines of Croatia. Food Control 2009, 20, 590–592. [Google Scholar] [CrossRef]
- Campone, L.; Piccinelli, A.L.; Celano, R.; Pagano, I.; Russo, M.; Rastrelli, L. Rapid and Automated on-Line Solid Phase Extraction HPLC–MS/MS with Peak Focusing for the Determination of Ochratoxin A in Wine Samples. Food Chem. 2018, 244, 128–135. [Google Scholar] [CrossRef]
- Frenette, C.; Paugh, R.J.; Tozlovanu, M.; Juzio, M.; Pfohl-Leszkowicz, A.; Manderville, R.A. Structure-Activity Relationships for the Fluorescence of Ochratoxin A: Insight for Detection of Achratoxin A Metabolites. Anal. Chim. Acta 2008, 617, 153–161. [Google Scholar] [CrossRef]
- Zou, X.; Chen, C.; Huang, X.; Chen, X.; Wang, L.; Xiong, Y. Phage-Free Peptide ELISA for Ochratoxin A Detection Based on Biotinylated Mimotope as A Competing Antigen. Talanta 2016, 146, 394–400. [Google Scholar] [CrossRef]
- Qing, Y.; Li, X.; Chen, S.; Zhou, X.; Luo, M.; Xu, X.; Qiu, J. Differential Pulse Voltammetric Ochratoxin A Assay Based on the Use of An Aptamer and Hybridization Chain Reaction. Microchim. Acta 2017, 184, 863–870. [Google Scholar] [CrossRef]
- Li, D.L.; Zhang, X.; Ma, Y.; Deng, Y.; Hu, R.; Yang, Y. Preparation of An OTA Aptasensor Based on A Metal-Organic Framework. Anal. Methods 2018, 10, 3273–3279. [Google Scholar] [CrossRef]
- Sethi, J.; Van Bulck, M.; Suhail, A.; Safarzadeh, M.; Perez-Castillo, A.; Pan, G. A label-free biosensor based on grapheme and reduced grapheme oxide dual-layer for electrochemical determination of beta-amyloid biomarkers. Microchim. Acta 2020, 187, 288. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, M.; Suhail, A.; Sethi, J.; Sattar, A.; Jenkins, D.; Pan, G. A label-free DNA-immunosensor based on aminated rGO electrode for the quantification of DNA methylation. Nanomaterials 2021, 11, 985. [Google Scholar] [CrossRef] [PubMed]
- Bollella, P.; Fusco, G.; Tortolini, C.; Sanzò, G.; Favero, G.; Gorton, L.; Antiochia, R. Beyond Graphene: Electrochemical Sensors and Biosensors for Biomarkers Detection. Biosens. Bioelectron. 2017, 89, 152–166. [Google Scholar] [CrossRef]
- Zarepour, A.; Ahmadi, S.; Rabiee, N.; Zarrabi, A.; Iravani, S. Self-Healing MXene-and Graphene-Based Composites: Properties and Applications. Nano-Micro Lett. 2023, 15, 100. [Google Scholar] [CrossRef]
- Su, S.; Cao, W.; Liu, W.; Lu, Z.; Zhu, D.; Chao, J.; Wang, L. Dual-Mode Electrochemical Analysis of MicroRNA-21 Using Gold Nanoparticle-Decorated MoS2 Nanosheet. Biosens. Bioelectron. 2017, 94, 552–559. [Google Scholar] [CrossRef]
- Loan, P.T.K.; Zhang, W.; Lin, C.T.; Wei, K.H.; Li, L.J.; Chen, C.H. Graphene/MoS2 Heterostructures for Ultrasensitive Detection of DNA Hybridisation. Adv. Mater. 2014, 26, 4838–4844. [Google Scholar] [CrossRef]
- Gan, X.; Zhao, H.; Quan, X. Two-Dimensional MoS2: A Promising Building Block for Biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef]
- Wang, H.; Yao, S.; Liu, Y.; Wei, S.; Su, J.; Hu, G. Molecularly Imprinted Electrochemical Sensor Based on Au Nanoparticles in Carboxylated Multi-Walled Carbon Nanotubes for Sensitive Determination of Olaquindox in Food and Feedstuffs. Biosens. Bioelectron. 2017, 87, 417–421. [Google Scholar] [CrossRef]
- Shuai, H.L.; Huang, K.J.; Zhang, W.J.; Cao, X.; Jia, M.P. Sandwich-Type MicroRNA Biosensor Based on Magnesium Oxide Nanoflower and Graphene Oxide-Gold Nanoparticles Hybrids Coupling with Enzyme Signal Amplification. Sens. Actuat. B-Chem. 2017, 243, 403–411. [Google Scholar] [CrossRef]
- Tian, L.; Qian, K.; Qi, J.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Gold Nanoparticles Superlattices Assembly for Electrochemical Biosensor Detection of MicroRNA-21. Biosens. Bioelectron. 2018, 99, 564–570. [Google Scholar] [CrossRef]
- Baek, S.H.; Roh, J.; Park, C.Y.; Kim, M.W.; Shi, R.; Kailasa, S.K.; Park, T.J. Cu-Nanoflower Decorated Gold Nanoparticles-Graphene Oxide Nanofiber as Electrochemical Biosensor for Glucose Detection. Mat. Sci. Eng. C-Mater. 2020, 107, 110273. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Li, D.; Wei, Q. A Laccase Based Biosensor on AuNPs-MoS2 Modified Glassy Carbon Electrode for Catechol Detection. Colloids Surf. B 2020, 186, 110683. [Google Scholar] [CrossRef]
- Ji, H.; Hu, S.; Jiang, Z.; Shi, S.; Hou, W.; Yang, G. Directly Scalable Preparation of Sandwiched MoS2/Graphene Nanocomposites via Ball-Milling with Excellent Electrochemical Energy Storage Performance. Electrochim. Acta 2019, 299, 143–151. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, Y.; Bao, J.; Huo, D.; Fa, H.; Shen, X.; Hou, C. One-Step Electrodeposition of Au-Pt Bimetallic Nanoparticles on MoS2 Nanoflowers for Hydrogen Peroxide Enzyme-Free Electrochemical Sensor. Electrochim. Acta 2017, 250, 152–158. [Google Scholar] [CrossRef]
- Meyer, S.; Jost, D.; Theodorakopoulos, N.; Peyrard, M.; Lavery, R.; Everaers, R. Temperature dependence of the DNA double helix at the nanoscale: Structure, elasticity, and fluctuations. Biophys. J. 2013, 105, 1904–1914. [Google Scholar] [CrossRef]
- Xu, S.; Dai, B.; Zhao, W.; Jiang, L.; Huang, H. Electrochemical detection of β-lactoglobulin based on a highly selective DNA aptamer and flower-like Au@ BiVO4 microspheres. Anal. Chim. Acta 2020, 1120, 1–10. [Google Scholar] [CrossRef]
- Shao, B.; Ma, X.; Zhao, S.; Lv, Y.; Hun, X.; Wang, H.; Wang, Z. Nanogapped Au (Core)@ Au-Ag (Shell) Structures Coupled with Fe3O4 Magnetic Nanoparticles for The Detection of Ochratoxin A. Anal. Chim. Acta 2018, 1033, 165–172. [Google Scholar] [CrossRef]
- Shen, P.; Li, W.; Ding, Z.; Deng, Y.; Liu, Y.; Zhu, X.; Zheng, T. A Competitive Aptamer Chemiluminescence Assay for Ochratoxin A Using A Single Silica Photonic Crystal Microsphere. Anal. Biochem. 2018, 554, 28–33. [Google Scholar] [CrossRef]
- Zejli, H.; Goud, K.Y.; Marty, J.L. Label Free Aptasensor for Ochratoxin A Detection Using Polythiophene-3-Carboxylic Acid. Talanta 2018, 185, 513–519. [Google Scholar] [CrossRef]
- Liu, R.; Wu, H.; Lv, L.; Kang, X.; Cui, C.; Feng, J.; Guo, Z. Fluorometric Aptamer Based Assay for Ochratoxin A Based on the Use of Exonuclease III. Microchim. Acta 2018, 185, 254. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Zheng, H.; Sun, M.; Guo, Y.; Luo, F.; Guo, L.; Chen, G. Highly Sensitive Colorimetric Aptasensor for Ochratoxin A Detection Based on Enzyme-Encapsulated Liposome. Anal. Chim. Acta 2018, 1002, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wu, S.; Duan, N.; Wang, Z. A Luminescence Resonance Energy Transfer Based Aptasensor for The Mycotoxin Ochratoxin A Using Upconversion Nanoparticles and Gold Nanorods. Microchim. Acta 2016, 183, 1909–1916. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Zhou, J.; Yan, W.; Li, X.; Zhu, J.J. Electrochemical Impedance Immunosensor Based on Three-Dimensionally Ordered Macroporous Gold Film. Anal. Chem. 2008, 80, 2133–2140. [Google Scholar] [CrossRef]
- Xie, W.; Gu, L.; Sun, X.; Liu, M.; Li, S.; Wang, Q.; He, D. Ferrocene Derived Core-Shell Structural Fe3O4@C Nanospheres for Superior Lithium Storage Properties. Electrochim. Acta 2016, 220, 107–113. [Google Scholar] [CrossRef]
- Grabar, K.C.; Freeman, R.G.; Hommer, M.B.; Natan, M.J. Preparation and Characterization of Au Colloid Monolayers. Anal. Chem. 1995, 67, 735–743. [Google Scholar] [CrossRef]
- Tan, Y.; Chu, X.; Shen, G.L.; Yu, R.Q. A Signal-Amplified Electrochemical Immunosensor for Aflatoxin B1 Determination in Rice. Anal. Biochem. 2009, 387, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ye, Z.; Fu, Y.; Xiong, Y.; Li, Y. A Portable Electrochemical Immunosensor for Rapid Detection of Trace Aflatoxin B1 in Rice. Anal. Methods 2016, 8, 548–553. [Google Scholar] [CrossRef]

| Technique | Linear Range | LOD | References |
|---|---|---|---|
| SERS | 0.01–50 ng/mL | 0.004 ng/mL | [29] |
| Chemiluminescence | 0.001–1 ng/mL | 1 pg/mL | [30] |
| EIS | 0.125–2.5 ng/mL | 0.125 ng/mL | [31] |
| Fluorescence | 8–1000 ng/mL | 4.7 ng/mL | [32] |
| Colorimetric | 0.05–2 ng/mL | 0.023 ng/mL | [33] |
| LRET | 0.05–100 ng/mL | 27 pg/mL | [34] |
| SPR | 0.2–40 ng/mL | 5 pg/mL | [4] |
| DPV | 1 × 10−6–100 ng/mL | 0.56 fg/mL | This work |
| Sample | No. | Added (ng/mL) | HPLC-MS (ng/mL) a | Proposed Aptasensor (ng/mL) a | RE (%) |
|---|---|---|---|---|---|
| 1 | 0.8 | 0.783 | 0.796 | +1.7 | |
| Rice | 2 | 10 | 9.907 | 9.632 | −2.8 |
| 3 | 20 | 20.205 | 19.766 | −2.2 | |
| 1 | 0.8 | 0.813 | 0.786 | −3.3 | |
| Wheat powder | 2 | 10 | 9.725 | 9.893 | +1.7 |
| 3 | 20 | 19.797 | 20.282 | +2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, G.; Zhang, X.; Zhang, K.; Chen, X.; Huang, H. A Novel Ochratoxin A Aptasensor Based on Three-Dimensionally Ordered Macroporous RGO-AuNPs-MoS2 Enhanced Electrocatalysis of Methylene Blue and AuNPs-Fe3O4@C Composite as Signal Probe Carrier. Catalysts 2023, 13, 1088. https://doi.org/10.3390/catal13071088
Peng G, Zhang X, Zhang K, Chen X, Huang H. A Novel Ochratoxin A Aptasensor Based on Three-Dimensionally Ordered Macroporous RGO-AuNPs-MoS2 Enhanced Electrocatalysis of Methylene Blue and AuNPs-Fe3O4@C Composite as Signal Probe Carrier. Catalysts. 2023; 13(7):1088. https://doi.org/10.3390/catal13071088
Chicago/Turabian StylePeng, Gang, Xu Zhang, Kaiwen Zhang, Xiaojun Chen, and He Huang. 2023. "A Novel Ochratoxin A Aptasensor Based on Three-Dimensionally Ordered Macroporous RGO-AuNPs-MoS2 Enhanced Electrocatalysis of Methylene Blue and AuNPs-Fe3O4@C Composite as Signal Probe Carrier" Catalysts 13, no. 7: 1088. https://doi.org/10.3390/catal13071088
APA StylePeng, G., Zhang, X., Zhang, K., Chen, X., & Huang, H. (2023). A Novel Ochratoxin A Aptasensor Based on Three-Dimensionally Ordered Macroporous RGO-AuNPs-MoS2 Enhanced Electrocatalysis of Methylene Blue and AuNPs-Fe3O4@C Composite as Signal Probe Carrier. Catalysts, 13(7), 1088. https://doi.org/10.3390/catal13071088