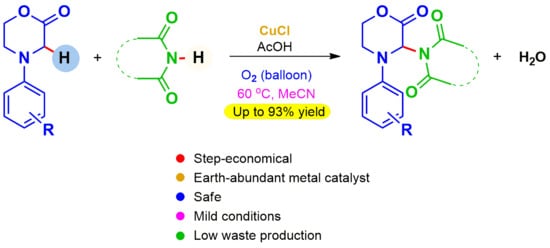
A Mild and Sustainable Procedure for the Functionalization of Morpholin-2-Ones by Oxidative Imidation Reactions
Abstract
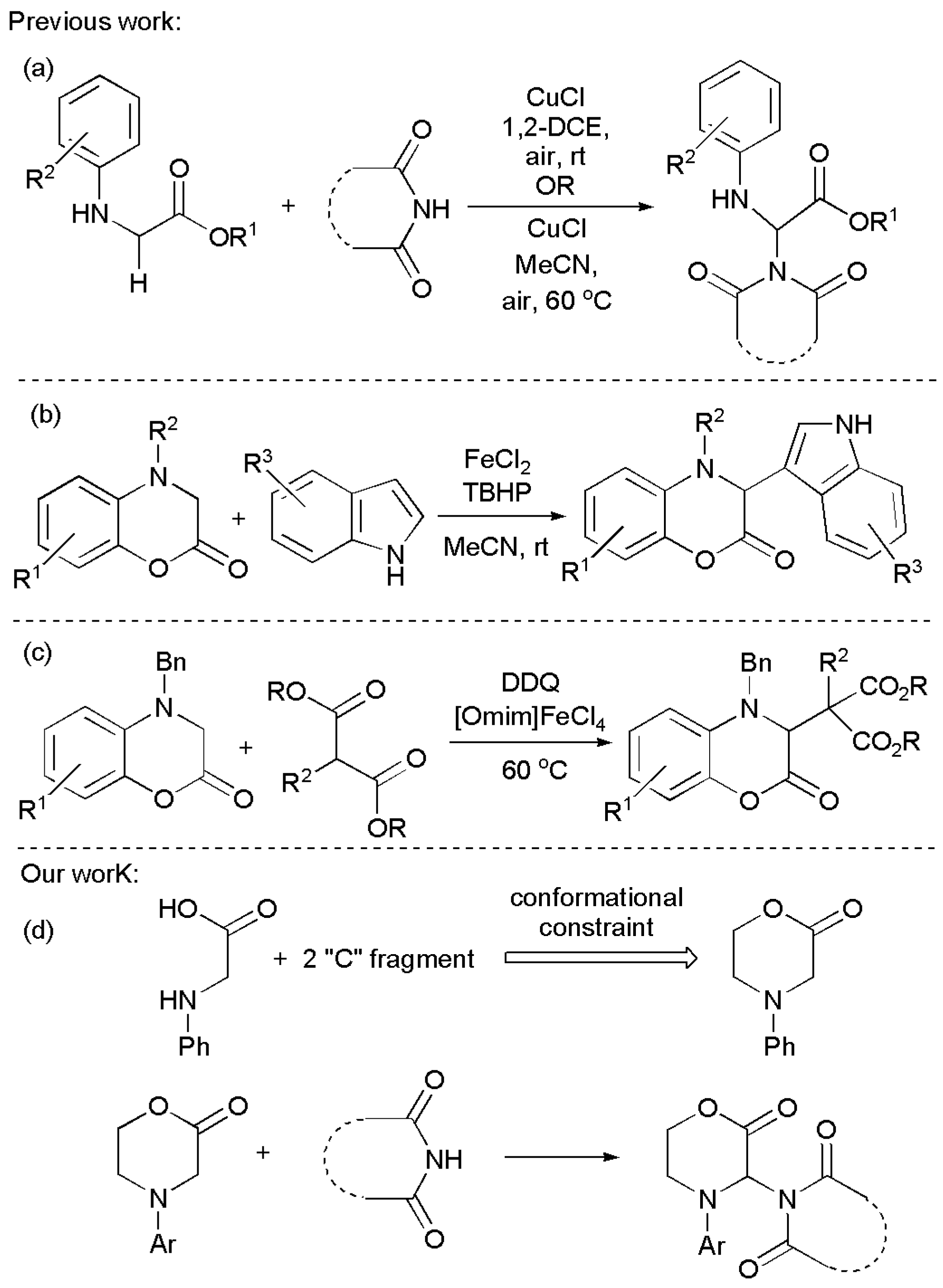
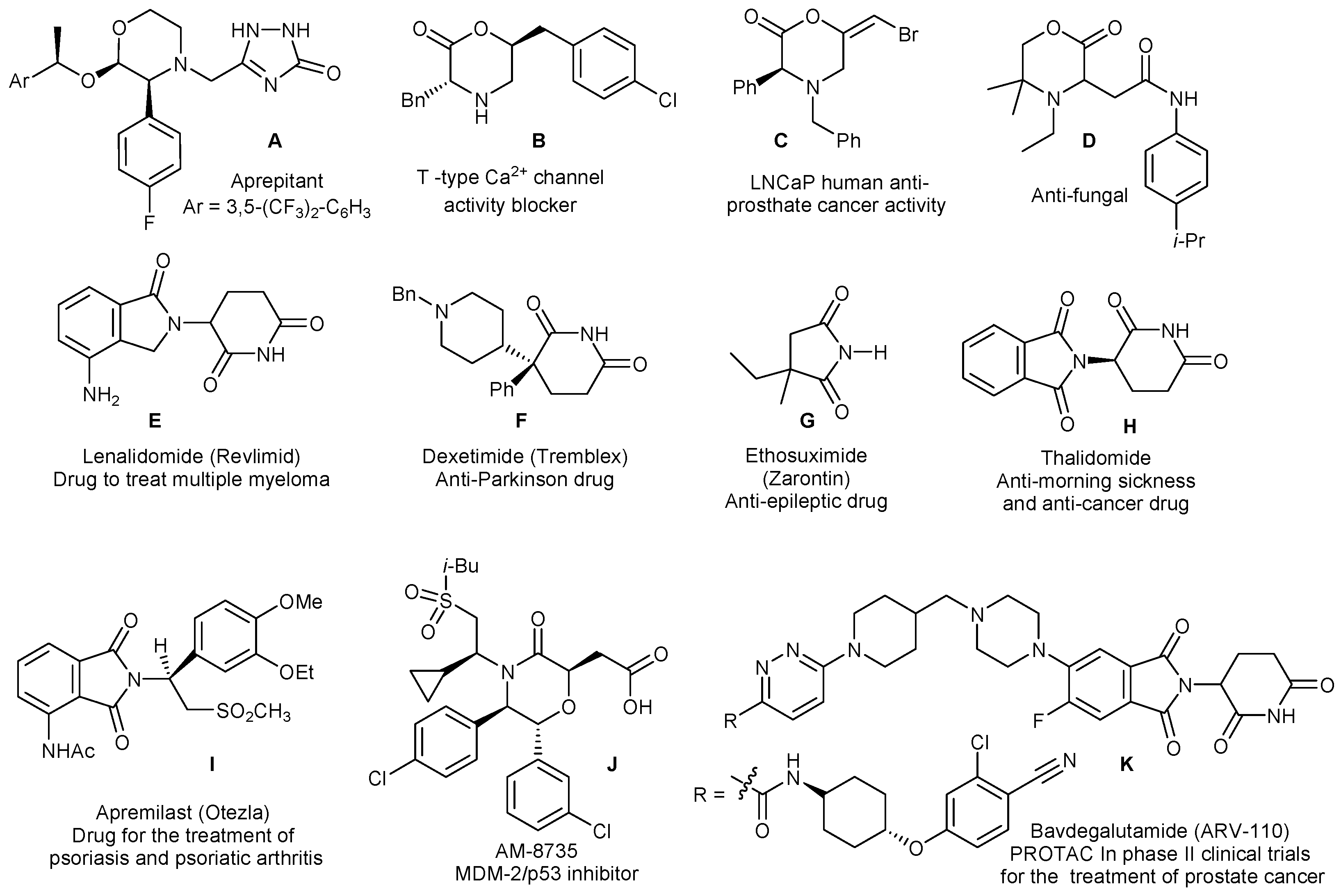
1. Introduction
2. Results
3. Materials and Methods
3.1. General Methods
3.2. General Procedure for the CDC Reaction between Morpholinones and Imides
3.3. Characterization Data of Products and Starting Materials
- 3-(1,3-Dioxoindolin-2-yl)-4-phenylmorpholin-2-one (7a): Prepared from N-phenyl morpholine-2-one and phthalimide according to the general procedure, in a reaction performed at 60 °C. Purified by plate chromatography (silica gel) with dichloromethane/ethyl acetate (8:1) as eluent. Obtained as buff-colored crystals, 57.8 mg, 68% yield, m.p. 166 °C. 1H NMR (CDCl3): δ 3.758 (dt, 1 H, J 2.5, 13.4 Hz, C(5)HH), 4.249 (overlapp ddd, 1 H, J 1.3, 10.8, 13.6 Hz, C(5)HH), 4.62–4.79 (m, 2 H, 2 × H-6), 6.516 (s, 1 H, H-3), 6.911 (t, 1 H, J 7.3 Hz, p-Ph-H), 7.020 (d, 2 H, J 8.5 Hz, 2 × o-Ph-H), 7.279 (overlapp. t, 1 H, J 6.9 Hz, m-Ph-H), 7.282 (overlapp. t, 1 H, J 7.4 Hz, m-Ph-H), 7.734 (dd, 2 H, J 3.1 Hz, 5.5 Hz, H-6′ and H-7′), 7.835 (dd, 2 H, J 3.0, 5.5 Hz, H-5′, H-8′) ppm. 13C NMR (CDCl3): δ 42.27 (CH2, C-5), 61.72 (CH, C-3), 69.32 (CH2, C-6), 115.78 (CH, 2 × o-Ph-H), 121.13 (CH, p-C, Ph), 123.78 (CH, C-5′ and C-8′), 129.41 (CH, 2 × m-Ph-H), 131.55 (Cq, i-C, Ph), 134.42 (CH, C-6′ and C-7′), 144.83 (Cq, C-4′ and C-9′), 164.41 (Cq, CO, C-2), 167.43 (Cq, 2 × Phth-CO) ppm. IR (neat): ṽ 2957, 1742, 1714, 1600, 1500, 1380, 1221, 1186, 1080, 1000, 979, 886, 765, 714, 693, 656, 521 cm−1. Elemental analysis: Calcd for C18H14N2O4·¼H2O: C, 66.15; H, 4.47; N, 8.57. Found C, 66.19; H, 4.12; N, 8.49. HRMS (ESI): Calcd for C18H14N2NaO4: 345.0847. Found: 345.0846. Calcd for C18H14KN2O4: 361.0589. Found: 361.0585.
- 2-(2-Oxo-4-phenylmorpholin-3-yl)-3a,4,7,7a-tetrahydroisoindole-1,3-dione (7b): Prepared from N-phenyl morpholine-2-one and cis-1,2,3,6-tetrahydrophthalimide according to the general procedure, in a reaction performed at 60 °C. Purified by plate chromatography (silica gel) with dichloromethane/ethyl acetate (8:1) as eluent. Obtained as buff-colored crystals, 38.2 mg, 93% yield, m.p. 131–133 °C. 1H NMR (CDCl3): δ 2.129 (dd, 2 H, J 6.6, 15.0 Hz, C(5′)HH and C(8′)HH), 2.363 (d, 2 H, J 13.9 Hz, C(5′)HH and C(8′)HH), 2.94–3.11 (m, 2 H, H-4′ and H-9′), 3.584 (apparent. d, 1 H, 13.0 Hz, C(5)HH), 4.136 (appar. t, 1 H, J 12.7 Hz, C(5)HH), 4.53–4.71 (m, 2 H, 2 × H-6), 5.536 (apparent. s, 2 H, 6′ and 7′), 6.327 (s, 1 H, H-3), 6.880 (d, 2 H, J 7.8 Hz, 2 × o-Ph-H), 6.934 (t, 1 H, J 7.0 Hz, p-Ph-H), 7.258 (t, 2 H, J 7.4 Hz, 2 × m-Ph-H) ppm. 13C NMR (CDCl3): δ 23.15/23.27 (2 × CH2, C-5′ and C-8′), 38.84/39.23 (2 × CH, C-4′ and C-9′), 42.61 (CH2, C-5), 61.88 (CH, C-3), 69.28 (CH2, C-6), 116.46 (CH, 2 × o-Ph-C), 121.28 (CH, p-Ph-C), 126.73/127.06 (2 × CH, C-6 and C-7), 129.19 (CH, 2 × m-Ph-C), 144.70 (Cq, i-Ph-C), 163.99 (Cq, C-2), 179.12/179.19 (2 × Cq, C-1′ and C-3′) ppm. IR (neat): ṽ 3063, 3042, 2971, 2945, 2910, 2853, 1735, 1707, 1600, 1496, 1347, 1274, 1234, 1194, 1165, 1087, 1029, 995, 981, 894, 753, 684 cm−1. Elemental analysis: Calcd for C18H18N2O4·½H2O: C, 64.47; H, 5.71; N, 8.36. Found C, 64.88; H, 5.67; N, 8.49.
- 3-(6-Nitro-1,3-dioxo-indolin-2-yl)-4-phenylmorpholin-2-one (7c): Prepared from N-phenyl morpholine-2-one and 4-nitrophthalimide according to the general procedure, in a reaction performed at 60 °C. Purified by plate chromatography (silica gel) with ethyl acetate/hexane (1:3) as eluent, followed by an elution with dichloromethane/ethyl acetate (8:1). Obtained as pale orange crystals, 12.5 mg, 27% yield, m.p. 192–193 °C. 1H NMR (CDCl3): δ 3.768 (d, 1 H, J 13.4 Hz, C(5)HH), 4.213 (t, 1 H, 11.8 Hz, C(5)HH), 4.65–4.80 (m, 2 H, 6-H), 6.549 (s, H-3), 6.923 (t, 1 H, J 7.1 Hz, p-Ph-H), 6.994 (d, 2 H, 2 × o-Ph-H), 7.23–7.34 (m, 2 H, 2 × m-Ph-H), 8.028 (d, 1 H, J 8.0 Hz, H-8′), 8.597 (d, 1 H, J 8.1 Hz, H-7′), 8.635 (s, 1 H, H-5′) ppm. 13C NMR (CDCl3): δ 42.550 (CH2, C-5), 62.272 (CH, C-3), 69.444 (CH2, C-6), 115.93 (CH, 2 × o-Ph-C), 119.20 (CH, C-5′), 121.58 (CH, p-Ph-C), 125.06 (CH, C-7′), 129.54/129.58 (CH, 2 × m-Ph-C), 132.92 (Cq, C-9′), 135.84 (Cq, C-4′), 144.54 (Cq, i-Ph-C), 151.89 (Cq, C-6′), 163.92 (Cq, C(O)-2), 165.10/165.38 (2 × Cq, C(O)-1′ and C(O)-3′) ppm. IR (neat): ṽ 3100, 2900, 1776, 1722, 1600, 1500, 1338, 1250, 1225, 1200, 1100, 1056, 1013, 962, 932, 856, 744, 716, 687, 641, 524, 500 cm−1. Elemental analysis: Calcd for C18H13N3O6·¼H2O: C, 58.15; H, 3.65; N, 11.30. Found C, 58.01; H, 3.57; N, 11.00.
- 3-(2,5-Dioxopyrrol-1-yl)-4-phenylmorpholin-2-one (7d): Prepared from N-phenyl morpholine-2-one and maleimide according to the general procedure, in a reaction performed at 60 °C. Purified by plate chromatography (silica gel) with ethyl acetate/hexane (1:2) as eluent, followed by an elution with dichloromethane/ethyl acetate (8:1). Obtained as pale yellow crystals, 15.6 mg, 36% yield, m.p. 121 °C. 1H NMR (CDCl3): δ 3.697 (td, 1 H, J 2.6, 13.4 Hz, C(5)HH), 4.104 (overlapp ddd, 1 H, J 3.4, 7.44, 13.7 Hz, C(5)HH), 4.59–4.73 (m, 2 H, 2 × H-6), 6.299 (s, 1 H, H-3), 6.605 (s, 2 H, H-3′ and H-4′), 6.92–6.99 (m, 3 H, o-Ph-H and p-Ph-H), 7.294 (t, 2 H, J 7.8 Hz, 2 × m-Ph-H) ppm. 13C NMR (CDCl3): δ 12.159 (CH2, C-5), 61.651 (CH, C-3), 69.272 (CH2, C-6), 115.92 (2 × CH, o-Ph-C), 121.35 (CH, p-Ph-C), 129.41 (2 × CH, C-3′ and C-4′), 134.41 (2 × CH, m-Ph-C), 144.71 (CH, i-Ph-C), 164.22 (Cq, C-2), 169.71 (2 × Cq, C-2′ and C-5′) ppm. IR (neat): ṽ 3102, 2968, 2909, 2867, 1738, 1704, 1603, 1505, 1462, 1372, 1361, 1341, 1272, 1210, 1145, 1082, 1033, 983, 851, 828, 796, 750, 693, 649, 439 cm−1. Elemental analysis: Calcd for C14H12N2O4·½H2O: C, 59.78; H, 4.66; N, 9.96. Found C, 59.46; H, 4.26; N, 9.39. HRMS (ESI): Calcd for C14H12N2NaO4: 295.0689. Found: 295.08687. Calcd for C14H12KN2O4: 331.0430. Found: 311.0429.
- 3-(2,5-Dioxopyrrolidin-1-yl)-4-phenylmorpholin-2-one (7e): Prepared from N-phenyl morpholine-2-one and succinimide according to the general procedure, in a reaction performed at 60 °C. Purified by plate chromatography (silica gel) with dichloromethane/ethyl acetate (8:1) as eluent. Obtained as buff-colored crystals, 30.6 mg, 83% yield, m.p. 157 °C. 1H NMR (CDCl3): δ 2.663 (s, 4 H, 2 × H-3′ and 2 × H-4′), 3.700 (d, 1 H, J 13.4 Hz, C(5)HH), 4.15 (t, 1 H, J 13.5 Hz, C(5)HH), 4.55–4.71 (m, 2 H, 2 × H-6), 6.340 (s, 1 H, H-3), 6.89–6.98 (m, 3 H, 2 × o-Ph-H + p-Ph-H), 7.290 (t, 1 H, J 7.7 Hz, 2 × m-Ph-H) ppm. 13C NMR (CDCl3): δ 27.98 (2 × CH2, C-3′ and C-4′), 42.55 (CH2, C-5), 61.873 (CH2, C-3), 69.30 (CH2, C-6), 115.59 (2 × CH, o-Ph-C), 121.10 (CH, p-Ph-C), 129.43 (2 × CH, m-Ph-C), 144.64 (CH, i-Ph-C), 164.03 (Cq, C-2), 176.19 (2 × Cq, C-2′ and C-5′) ppm. IR (neat): ṽ 2988, 2937, 2906, 2865, 1741, 1706, 1597, 1496, 1375, 1269, 1208, 1177, 1084, 984, 753, 698 cm−1. Elemental analysis: Calcd for C14H14N2O4·¼H2O: C, 60.31; H, 5.24; N, 10.04. Found C, 60.26; H, 5.22; N, 9.64.
- 3-(3-Ethyl-3-methyl-2,5-dioxopyrrolidin-1-yl)-4-phenylmorpholin-2-one (7f): Prepared N-morpholine-2-one and (R)-ethosuximide according to the general procedure in a reaction performed at 60 °C. Purified by plate chromatography (silica gel) with dichloromethane/ethyl acetate (8:1) as eluent to afford a 1:1 mixture of two inseparable diastereoisomers. Obtained as buff-colored crystals, 29.1 mg, 71% yield, m.p. 78 °C. 1H NMR (CDCl3): δ (mixture of two diastereoisomers) 0.629 and 0.682 (t, 3 H, J 7.4 Hz, CH3-8′), 1.121 (s, 3 H, CH3-6′), 1.36–1.49 (m, 1 H, CHH-7′), 1.52–1.66 (m, 1 H, CHH-7′), 2.319 (2 × overlapp. d, 1 H, CHH-4′), 2.542 (2 × overlapp. d, 1 H, CHH-4′), 3.56–3.66 (m, 1 H, CHH-5), 4.160 (appar. t, 1 H, CHH-5), 4.57–4.71 (m, 2 H, H-6), 6.334 and 6.348 (overlapp. s, 1 H, H-3), 6.87–6.97 (m, 3 H, 2 × o-Ph-H and p-Ph-H), 7.22–7.33 (m, 2 × m-Ph-H) ppm. 13C NMR (CDCl3): δ (mixture of two diastereoisomers) 8.142 and 8.244 (CH3, C-8′), 23.638 and 23.844 (CH3, C-6′), 30.483 and 30.720 (CH2, C-7′), 39.985 and 40.045 (CH2, C-4′), 42.672 and 42.713 (CH2, C-5), 44.166 and 44.336 (Cq, C-3), 61.762 and 61.939 (CH, C-3), 69.234 and 69.293 (CH2, C-6), 116.11 and 116.54 (CH, 2 × o-Ph-C), 121.26 and 121.45 (CH, p-Ph-C), 129.23 (CH, 2 × m-Ph-C), 144.72 and 144.79 (Cq, i-Ph-C), 164.06 (Cq, C(O)-2), 175.12 and 175.22 (Cq, C(O)-2′ and C(O)-5′) ppm. IR (neat): ṽ 3012, 2940, 2900, 2858, 1760, 1712, 1600, 1500, 1383, 1267, 1208, 1146, 1092, 983, 742, 692, 667 cm−1. Elemental analysis: Calcd for C17H20N2O4·½H2O: C, 62.76; H, 6.50; N, 8.61. Found C, 62.84; H, 6.70; N, 8.48.
- 3-(2,6-Dioxopiperidin-1-yl)-4-phenylmorpholin-2-one (7h): Prepared from N-phenyl morpholine-2-one and glutarimide according to the general procedure, but omitting the addition of acetic acid, in a reaction performed at 80 °C. Purified by plate chromatography (silica gel) with hexane/ethyl acetate (2:1) as eluent. Obtained as buff-colored crystals, 16.0 mg, 36% yield, m.p. 165 °C. 1H NMR (CDCl3): δ 1.723 (br s, 2 H, 2 × H-4′), 2.574 (t, J 11.1 Hz, 4 H, 2 × H-3′ and 2 × H-5′), 3.568 (dd, 1 H, J 1.8, 12.7 Hz, C(5)HH), 4.104 (m, 1 H, C(5)HH), 4.50–4.69 (m, 2 H, 2 × H-6), 6.866 (d, 2 H, J 7.5 Hz, 2 × o-Ph-H), 6.923 (t, 1 H, J 7.3 Hz, p-Ph-H), 6.905 (s, 1 H, H-3), 7.261 (t, 2 H, J 7.3. Hz, m-Ph-H) ppm. 13C NMR (CDCl3): δ 16.416 (CH2, C-4′), 32.456 (2 × CH2, C-3′ and C-5′), 43.367 (CH2, C-5), 61.424 (CH2, C-3), 68.907 (CH2, C-6), 116.48 (CH, 2 × o-Ph-C), 120.96 (CH, p-Ph-C), 129.14 (CH, 2 × m-Ph-C), 145.16 (Cq, i-Ph-C), 165.25 (Cq, C(O)-2), 172.23 (Cq, 2 × C(O), C-2′ and C-6′) ppm. IR (neat): ṽ 2967, 2932, 2906, 2862, 1749, 1722, 1671, 1595, 1494, 1368, 1344,1310, 1271, 1247, 1213, 1171, 1134, 1086, 1012, 988, 750, 701, 438 cm−1. Elemental analysis: Calcd for C15H16N2O4·¼H2O: C, 61.68; H, 5.84; N, 9.25. Found C, 61.30; H, 5.76; N, 9.74.
- 3-(1,3-Dioxoindolin-2-yl)-4-(p-tolyl)-morpholin-2-one (7k): Prepared from N-tolylmorpholine-2-one and phthalimide according to the general procedure in a reaction performed at 60 °C. Purified by plate chromatography (silica gel) with dichloromethane/ethyl acetate/hexane (8:1:3) as eluent. Obtained as buff-colored crystals, 33.4 mg, 75% yield. 1H NMR (CDCl3): δ 2.240 (s, 3 H, CH3), 3.669 (d, J 13.3 Hz, C(5)HH), 4.179 (overlapp. ddd, J 7.6, 13.5 Hz, C(5)HH), 4.61–4.79 (m, 2 × H-6), 6.457 (s, 1 H, H-3), 6.924 (d, 2 H, J 8.5 Hz, 2 × o-Ph-H), 7.071 (d, 2 H, J 8.5 Hz, 2 × m-Ph-H), 7.719 (dd, J 3.1, 5.4 Hz, C-6′ and C-7′), 7.824 (dd, J 3.1, 5.5 Hz, H-5′ and H-8′) ppm. 13C NMR (CDCl3): δ 20.402 (CH3), 42.688 (CH2, C-5), 62.113 (CH, C-3), 69.359 (CH2, C-6), 116.46 (CH, 2 × o-Ph-C), 123.76 (CH, C-5′ and C-8′), 129.90 (CH, 2 × m-Ph-C), 130.94 (Cq, p-Ph-C), 131.55 (Cq, C-4′ and C-9′), 134.37 (CH, C-6′ and C-7′), 142.62 (Cq, i-Ph-C), 164.53 (Cq, C(O)-2), 167.44 (Cq, C(O)-1′ and C(O)-3′) ppm. IR (neat): ṽ 3037, 2960, 2922, 2861, 1756, 1712, 1617, 1519, 1470, 1379, 1269, 1204, 1084, 986, 896, 808, 716, 644, 516 cm−1. Elemental analysis: Calcd for C19H16N2O4·¼H2O: C, 63.12; H, 5.24; N, 7.74. Found C, 63.33; H, 4.74; N, 7.37.
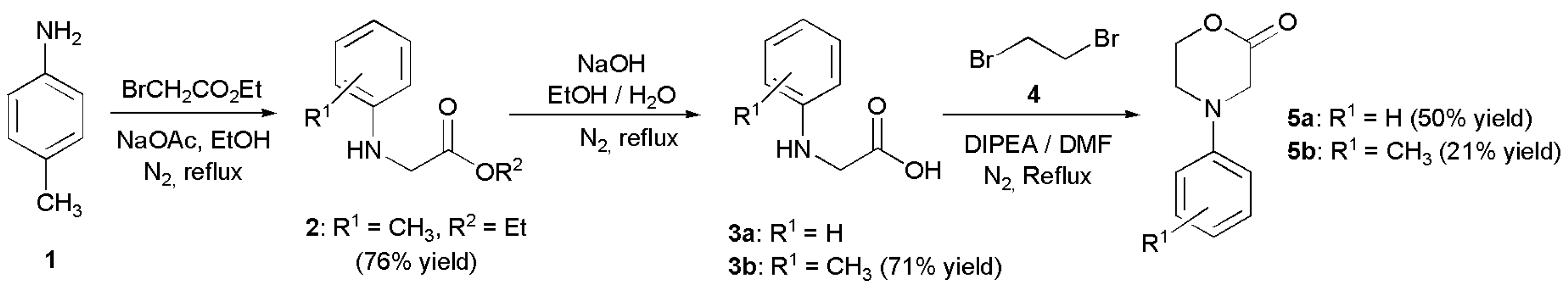
- 4-(p-Tolyl)-morpholin-2-one (5b): Prepared as 5a. Purified by column chromatography (silica gel) with ethyl acetate/hexane (1:2) as eluent. Obtained as buff-coloured crystals, 0.188 mg, 21% yield, m.p. 72 °C. 1H NMR (CDCl3): 2.313 (s, 3 H, CH3), 3.469 (m, 2 × H-5), 4.089 (s, 2 × H-3), 4.575 (m, 2 × H-6),6.76 (d, 2 H, J 7.4 Hz, 2 × o-CH ), 7.15 (d, 2 H, J 7.4 Hz, 2 × m-CH) ppm. 13C NMR (CDCl3): δ 20.355 (CH3), 44.757, (CH2, C-5), 50.868, (CH2, C-3), 67.793, (CH2, C-6), 114, 39 (CH, 2 × o-Ph-C), 129.73 (Cq, p-C), 130.03 (CH, 2 × m-Ph-C), 145.80, (Cq, i-Ph-C), 167.49 (Cq, C(O)-2) ppm. IR (neat): ṽ 3037, 2999, 2960, 2916, 2852, 1718, 1617, 1514, 1462, 1381, 1275, 1234, 1079, 978, 938, 814, 796, 520 cm−1. Elemental analysis: Calcd for C11H13NO2·¼H2O: C, 67.50; H, 6.95; N, 7.16. Found C, 67.12; H, 6.83; N, 7.12.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tian, T.; Li, Z.; Li, C.-J. Cross-dehydrogenative coupling: A sustainable reaction for C–C bond formations. Green Chem. 2021, 23, 6789–6862. [Google Scholar] [CrossRef]
- Faisca Phillips, A.M.; Guedes da Silva, M.d.F.C.; Pombeiro, A.J.L. New trends in enantioselective cross-dehydrogenative coupling. Catalysts 2020, 10, 529. [Google Scholar] [CrossRef]
- Huang, C.Y.; Kang, H.; Li, J.; Li, C.J. En route to intermolecular cross-dehydrogenative coupling Reactions. J. Org. Chem. 2019, 84, 12705–12721. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S. Catalytic enantioselective cross dehydrogenative coupling of sp3 C–H of heterocycles. Org. Biomol. Chem. 2019, 17, 9683–9692. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Pombeiro, A.J.L. Recent developments in transition metal-catalyzed cross-dehydrogenative coupling reactions of ethers and thioethers. ChemCatChem 2018, 10, 3354–3383. [Google Scholar] [CrossRef]
- Varun, B.V.; Dhineshkumar, J.; Bettadapur, K.R.; Siddaraju, Y.; Alagiri, K.; Prabhu, K.R. Recent advancements in dehydrogenative cross coupling reactions for C–C bond formation. Tetrahedron Lett. 2017, 58, 803–824. [Google Scholar] [CrossRef]
- Kozlowski, M.C. Oxidative coupling in complexity building transforms. Acc. Chem. Res. 2017, 50, 638–643. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, J.; Gao, M.; Tang, S.; Li, W.; Shi, R.; Lei, A. Oxidative coupling between two hydrocarbons: An update of recent C-H functionalizations. Chem. Rev. 2015, 115, 12138–12204. [Google Scholar] [CrossRef]
- Yeung, C.S.; Dong, V.M. Catalytic dehydrogenative cross-coupling: Forming carbon-carbon bonds by oxidizing two carbon-hydrogen bonds. Chem. Rev. 2011, 111, 1215–1292. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Pombeiro, A.J.L. The functionalization of amino acids, peptides, and derivatives by cross-dehydrogenative coupling. In Handbook of CH-Functionalization; Maiti, D., Ed.; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar] [CrossRef]
- San Segundo, M.; Correa, A. Cross-dehydrogenative coupling reactions for the functionalization of α-amino acid derivatives and peptides. Synthesis 2018, 50, 2853–2866. [Google Scholar] [CrossRef]
- Noisier, A.F.M.; Brimble, M.A. C–H functionalization in the synthesis of amino acids and peptides. Chem. Rev. 2014, 114, 8775–8806. [Google Scholar] [CrossRef] [PubMed]
- San Segundo, M.; Guerrero, I.; Correa, A. Co-catalyzed C(sp3)–H oxidative coupling of glycine and peptide derivatives. Org. Lett. 2017, 19, 5288–5291. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Bernardes, G.J.L. Advances in chemical protein modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.-Y.; Berti, F.; Adamo, R. Towards the next generation of biomedicines by site-selective conjugation. Chem. Soc. Rev. 2016, 45, 1691–1719. [Google Scholar] [CrossRef]
- Qvit, N.; Rubin, S.J.S.; Urban, T.J.; Mochly-Rosen, D.; Gross, E.C.R. Peptidomimetic therapeutics: Scientific approaches and opportunities. Drug Discov. Today 2017, 22, 454–462. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T. Unusual amino acids in medicinal chemistry. J. Med. Chem. 2016, 59, 10807–10836. [Google Scholar] [CrossRef]
- Elander, R.P. Industrial production of β-lactam antibiotics. Appl. Microbiol. Biotechnol. 2003, 61, 385–392. [Google Scholar] [CrossRef]
- Ballard, A.; Narduolo, S.; Ahmad, H.O.; Keymer, N.I.; Asaad, N.; Cosgrove, D.A.; Buurma, N.J.; Leach, A.G. Racemisation in Chemistry and Biology. Chem. Eur. J. 2020, 26, 3661–3687. [Google Scholar] [CrossRef]
- Shirakawa, S.; Maruoka, K. Recent developments in asymmetric phase-transfer reactions. Angew. Chem. Int. Ed. 2013, 52, 4312–4348. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maruoka, K. Recent development and application of chiral phase-transfer catalysts. Chem. Rev. 2007, 107, 5656–5682. [Google Scholar] [CrossRef]
- Bada, J.L. Racemization of Amino Acids. In Chemistry and Biochemistry of the Amino Acids; Barrett, G.C., Ed.; Springer: Dordrecht, Germany, 1985; pp. 399–414. [Google Scholar]
- King, T.A.; Mandrup Kandemir, J.; Walsh, S.J.; Spring, D.R. Photocatalytic methods for amino acid modification. Chem. Soc. Rev. 2021, 50, 39–57. [Google Scholar] [CrossRef] [PubMed]
- Ohata, J.; Martin, S.C.; Ball, Z.T. Metal-Mediated Functionalization of Natural peptides and proteins: Panning for bioconjugation gold. Angew. Chem. Int. Ed. 2019, 58, 6176–6199. [Google Scholar] [CrossRef] [PubMed]
- de Gruyter, J.N.; Malins, L.R.; Baran, P.S. Residue-specific peptide modification: A chemist’s guide. Biochemistry 2017, 56, 3863–3873. [Google Scholar] [CrossRef] [PubMed]
- Faraggi, T.M.; Rouget-Virbel, C.; Rincón, J.A.; Barberis, M.; Mateos, C.; García-Cerrada, S.; Agejas, J.; de Frutos, O.; MacMillan, D.W.C. Synthesis of enantiopure unnatural amino acids by metallaphotoredox catalysis. Org. Process Res. Dev. 2021, 25, 1966–1973. [Google Scholar] [CrossRef] [PubMed]
- Mani, V.S.; Narayanasamy, R. Conformationally constrained amino acids in peptide design. SSRN 2016. [Google Scholar] [CrossRef]
- Perez, J.J. Designing peptidomimetics. Curr. Top. Med. Chem. 2018, 18, 566–590. [Google Scholar] [CrossRef]
- Lenci, E.; Trabocchi, A. Peptidomimetic toolbox for drug discovery. Chem. Soc. Rev. 2020, 49, 3262–3277. [Google Scholar] [CrossRef]
- Robertson, N.S.; Spring, D.R. Using peptidomimetics and constrained peptides as valuable tools for inhibiting protein–protein interactions. Molecules 2018, 23, 959. [Google Scholar] [CrossRef]
- Morrison, C. Constrained peptides’ time to shine? Nat. Rev. Drug Discov. 2018, 17, 531–533. [Google Scholar] [CrossRef]
- Chowdhury, R. Eosin-Y/Cu(OAc)2-catalyzed aerobic oxidative coupling reactions of glycine esters in the dark. Org. Biomol. Chem. 2022, 20, 5387–5392. [Google Scholar] [CrossRef]
- Daggupati, R.V.; Malapaka, C. Cu(i)-Catalyzed amidation/imidation of N-arylglycine ester derivatives via C–N coupling under mild conditions. Org. Chem. Front. 2018, 5, 788–792. [Google Scholar] [CrossRef]
- Sun, B.; Wang, Y.; Li, D.; Jin, C.; Su, W. A copper/O2-mediated direct sp3 C–H/N–H cross-dehydrogen coupling reaction of acylated amines and N-aryl glycine esters. Org. Biomol. Chem. 2018, 16, 2902–2909. [Google Scholar] [CrossRef]
- Xiao, L.-J.; Zhu, Z.-Q.; Guo, D.; Xie, Z.-B.; Lu, Y.; Le, Z.-G. Copper-catalyzed cross-dehydrogenative-coupling reaction of N-arylglycine esters with imides or amides for synthesis of α-substituted α-amino acid esters. Synlett 2018, 29, 1659–1663. [Google Scholar] [CrossRef]
- Huo, C.; Dong, J.; Su, Y.; Tang, J.; Chen, F. Iron-catalyzed oxidative sp3 carbon-hydrogen bond functionalization of 3,4-dihydro-1,4-benzoxazin-2-ones. Chem. Commun. 2016, 52, 13341–13344. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, A.; Moazami, M.; Abaee, M.S.; Mirzaei, M. Ionic liquid-catalyzed synthesis of (1,4-benzoxazin-3-yl) malonate derivatives via cross-dehydrogenative-coupling reactions. Heterocycl. Commun. 2022, 28, 51–57. [Google Scholar] [CrossRef]
- Ashwood, M.S.; Cottrell, I.F.; Davies, A.J. Stereoselective synthesis of 2-(S)-(3,5-bis(trifluoromethyl)benzyloxy)-3-(S)-phenyl-1,4-oxazine. Tetrahedron Asymmetry 1997, 8, 957–963. [Google Scholar] [CrossRef]
- Trstenjak, U.; Ilaš, J.; Kikelj, D. Advances in the synthesis of morpholin-3-ones and morpholin-2-ones. Synthesis 2012, 44, 3551–3578. [Google Scholar] [CrossRef]
- Nelson, T.D. Synthesis of aprepitant. In Strategies and Tactics in Organic Synthesis; Harmata, M., Ed.; Academic Press: Amsterdam, The Netherlands, 2005; Volume 6, pp. 321–351. [Google Scholar]
- Ku, I.W.; Cho, S.; Doddareddy, M.R.; Jang, M.S.; Keum, G.; Lee, J.H.; Chung, B.Y.; Kim, Y.; Rhim, H.; Kang, S.B. Morpholin-2-one derivatives as novel selective T-type Ca2+ channel blockers. Bioorg. Med. Chem. Lett. 2006, 16, 5244–5248. [Google Scholar] [CrossRef]
- Gonzalez, A.Z.; Eksterowicz, J.; Bartberger, M.D.; Beck, H.P.; Canon, J.; Chen, A.; Chow, D.; Duquette, J.; Fox, B.M.; Fu, J.; et al. Selective and potent morpholinone inhibitors of the MDM2-p53 protein-protein interaction. J. Med. Chem. 2014, 57, 2472–2488. [Google Scholar] [CrossRef]
- Mock, J.N.; Taliaferro, J.P.; Lu, X.; Patel, S.K.; Cummings, B.S.; Long, T.E. Haloenol pyranones and morpholinones as antineoplastic agents of prostate cancer. Bioorg. Med. Chem. Lett. 2012, 22, 4854–4858. [Google Scholar] [CrossRef]
- Bardiot, D.; Thevissen, K.; De Brucker, K.; Peeters, A.; Cos, P.; Taborda, C.P.; McNaughton, M.; Maes, L.; Chaltin, P.; Cammue, B.P.; et al. 2-(2-oxo-morpholin-3-yl)-acetamide derivatives as broad-spectrum antifungal agents. J. Med. Chem. 2015, 58, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Blake, T.R.; Waymouth, R.M. Organocatalytic ring-opening polymerization of morpholinones: New strategies to functionalized polyesters. J. Am. Chem. Soc. 2014, 136, 9252–9255. [Google Scholar] [CrossRef] [PubMed]
- Kashima, C.; Harada, K. Nucleophilic ring-opening reactions of morpholin-2-ones. A resolution of dl-(secondary-alkyl)amines. J. Org. Chem. 1989, 54, 789–792. [Google Scholar] [CrossRef]
- Basu, A.; Kunduru, K.R.; Katzhendler, J.; Domb, A.J. Poly(α-hydroxy acid)s and poly(α-hydroxy acid-co-α-amino acid)s derived from amino acid. Adv. Drug Deliv. Rev. 2016, 107, 82–96. [Google Scholar] [CrossRef]
- Cordeiro, R.A.; Serra, A.; Coelho, J.F.J.; Faneca, H. Poly(β-amino ester)-based gene delivery systems: From discovery to therapeutic applications. J. Control. Release 2019, 310, 155–187. [Google Scholar] [CrossRef]
- Iqbal, S.; Qu, Y.; Dong, Z.; Zhao, J.; Khan, A.R.; Rehman, S.; Zhao, Z. Poly(β-amino esters) based potential drug delivery and targeting polymer; an overview and perspectives (review). Eur. Polym. J. 2020, 141, 110097. [Google Scholar] [CrossRef]
- Zheng, Y.-N.; Zheng, H.; Li, T.; Wei, W.-T. Recent advances in copper-catalyzed C–N bond formation involving N-centered radicals. ChemSusChem 2021, 14, 5340–5358. [Google Scholar] [CrossRef]
- Park, Y.; Kim, Y.; Chang, S. Transition metal-catalyzed C–H amination: Scope, mechanism, and applications. Chem. Rev. 2017, 117, 9247–9301. [Google Scholar] [CrossRef]
- Bariwal, J.; Van der Eycken, E. C–N bond forming cross-coupling reactions: An overview. Chem. Soc. Rev. 2013, 42, 9283–9303. [Google Scholar] [CrossRef]
- Lamberth, C. Synthesis and applications of cyclic imides in agrochemistry. In Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry; Luzzio, F.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 335–352. [Google Scholar] [CrossRef]
- Li, J.J. Imide-containing synthetic drugs. In Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 353–366. [Google Scholar] [CrossRef]
- Luzzio, F.A. Thalidomide and analogues. In Imides: Medicinal, Agricultural, Synthetic Applications and Natural Products Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; pp. 367–429. [Google Scholar] [CrossRef]
- Zerilli, T.; Ocheretyaner, E. Apremilast (Otezla): A new oral treatment for adults with psoriasis and psoriatic arthritis. Pharm. Ther. 2015, 40, 495–500. [Google Scholar]
- In Top 200 Pharmaceuticals by Retail Sales in 2021, compiled and produced by M. H. Qureshi from the Njarðarson group (University of Arizona) as described by McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Ed. 2010, 87, 1348–1349. Available online: https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/Top%20200%20Pharmaceuticals%202021V2.pdf (accessed on 8 June 2023). [CrossRef]
- In Top 200 Small Molecule Pharmaceuticals by Retail Sales in 2021, compiled and produced by M. H. Qureshi from the Njarðarson group (University of Arizona) as described by McGrath, N.A.; Brichacek, M.; Njardarson, J.T. A Graphical Journey of Innovative Organic Architectures That Have Improved Our Lives. J. Chem. Ed. 2010, 87, 1348–1349. Available online: https://njardarson.lab.arizona.edu/sites/njardarson.lab.arizona.edu/files/Top%20200%20Small%20Molecules%202021V3.pdf (accessed on 8 June 2023). [CrossRef]
- Mullard, A. Targeted protein degraders crowd into the clinic. Nat. Rev. Drug Discov. 2021, 20, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Békés, M.; Langley, D.R.; Crews, C.M. PROTAC targeted protein degraders: The past is prologue. Nat. Rev. Drug Discov. 2022, 21, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Crews, C.M. PROTACs: Past, present and future. Chem. Soc. Rev. 2022, 51, 5214–5236. [Google Scholar] [CrossRef] [PubMed]
- Gadd, M.S.; Testa, A.; Lucas, X.; Chan, K.H.; Chen, W.; Lamont, D.J.; Zengerle, M.; Ciulli, A. Structural basis of PROTAC cooperative recognition for selective protein degradation. Nat. Chem. Biol. 2017, 13, 514–521. [Google Scholar] [CrossRef]
- Ishoey, M.; Chorn, S.; Singh, N.; Jaeger, M.G.; Brand, M.; Paulk, J.; Bauer, S.; Erb, M.A.; Parapatics, K.; Müller, A.C.; et al. Translation termination factor GSPT1 is a phenotypically relevant off-target of heterobifunctional phthalimide degraders. ACS Chem. Biol. 2018, 13, 553–560. [Google Scholar] [CrossRef]
- Winter, G.E.; Buckley, D.L.; Paulk, J.; Roberts, J.M.; Souza, A.; Dhe-Paganon, S.; Bradner, J.E. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science 2015, 348, 1376–1381. [Google Scholar] [CrossRef]
- Breugst, M.; Tokuyasu, T.; Mayr, H. Nucleophilic reactivities of imide and amide anions. J. Org. Chem. 2010, 75, 5250–5258. [Google Scholar] [CrossRef]
- Rohlmann, R.; Stopka, T.; Richter, H.; García Mancheño, O. Iron-catalyzed oxidative tandem reactions with TEMPO oxoammonium salts: Synthesis of dihydroquinazolines and quinolines. J. Org. Chem. 2013, 78, 6050–6064. [Google Scholar] [CrossRef]
- Johnston, A.D.; Asmussen, E.; Bowen, R.L. Substitutes for N-phenylglycine in adhesive bonding to dentin. J. Dent. Res. 1989, 68, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Behbehani, H.; Ibrahim, H.M. Synthetic strategy for pyrazolo[1,5-a]pyridine and pyrido[1,2-b]indazole derivatives through AcOH and O2-promoted cross-dehydrogenative coupling reactions between 1,3-dicarbonyl compounds and N-amino-2-iminopyridines. ACS Omega 2019, 4, 15289–15303. [Google Scholar] [CrossRef] [PubMed]
- Forkosh, H.; Vershinin, V.; Reiss, H.; Pappo, D. Stereoselective synthesis of optically pure 2-amino-2′-hydroxy-1,1′-binaphthyls. Org. Lett. 2018, 20, 2459–2463. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Yoshida, K.; Tokuyama, H. Acetic acid promoted metal-free aerobic carbon-carbon bond forming reactions at α-position of tertiary amines. Org. Lett. 2014, 16, 4194–4197. [Google Scholar] [CrossRef]
- Froehr, T.; Sindlinger, C.P.; Kloeckner, U.; Finkbeiner, P.; Nachtsheim, B.J. A metal-free amination of benzoxazoles–The first example of an iodide-catalyzed oxidative amination of heteroarenes. Org. Lett. 2011, 13, 3754–3757. [Google Scholar] [CrossRef]
- Ma, S.; Wu, L.; Liu, M.; Xu, X.; Huang, Y.; Wang, Y. Highly enantioselective aza-Michael addition reactions of 4-nitrophthalimide with α,β-unsaturated ketones. RSC Adv. 2013, 3, 11498–11501. [Google Scholar] [CrossRef]
- Narute, S.; Pappo, D. Iron phosphate catalyzed asymmetric cross-dehydrogenative coupling of 2-naphthols with β-ketoesters. Org. Lett. 2017, 19, 2917–2920. [Google Scholar] [CrossRef]
- Lee, A.; Betori, R.C.; Crane, E.A.; Scheidt, K.A. An enantioselective cross-dehydrogenative coupling catalysis approach to substituted tetrahydropyrans. J. Am. Chem. Soc. 2018, 140, 6212–6216. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization Model List of Essential Medicines: 22nd List 2019; World Health Organization: Geneva, Switzerland, 2019.
- Funes-Ardoiz, I.; Maseras, F. Oxidative coupling mechanisms: Current state of understanding. ACS Catal. 2018, 8, 1161–1172. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic copper-catalyzed organic reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef]
- Wendlandt, A.E.; Suess, A.M.; Stahl, S.S. Copper-catalyzed aerobic oxidative C–H functionalizations: Trends and mechanistic insights. Angew. Chem. Int. Ed. 2011, 50, 11062–11087. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.-J.; Song, L.-J.; Yang, Y.-F.; Zhang, X.; Wiest, O.; Wu, Y.-D. Computational studies on the mechanism of the copper-catalyzed sp3-C–H cross-dehydrogenative coupling reaction. ChemPlusChem 2013, 78, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Young, J.G.; Onyebuagu, W. Synthesis and characterization of di-disubstituted phthalocyanines. J. Org. Chem. 1990, 55, 2155–2159. [Google Scholar] [CrossRef]
- Howell, B.A.; Dangalle, H.; Al-Omari, M. Thermal characteristics of precursors to a difunctional imide monomer. J. Therm. Anal. Calorim. 2011, 106, 129–137. [Google Scholar] [CrossRef]

| Ent. | Catalyst (mol%) | Solvent | Oxidant | [Phth] (M) | Additive | Yield 2 (%) |
|---|---|---|---|---|---|---|
| 1 | Cu(I)Cl | DCE | O2 | 0.1 | None | 23 |
| 2 | Cu(I)Cl | DMSO | air | 0.1 | None | Traces |
| 3 | Cu(I)Cl (10) | MeCN | air | 0.2 | None | 37 |
| 4 | Cu(I)Cl (10) | MeCN | O2 | 0.2 | None | 54 |
| 5 3 | Cu(I)Cl (10) | MeCN | O2 | 0.2 | None | 61 |
| 6 | Cu(I)Cl | MeCN | O2 | 0.1 | None | 70 |
| 7 4 | Cu(I)Cl (10) | MeCN | O2 | 0.1 | None | 70 |
| 8 | Cu(I)Cl | MeCN | O2 | 0.3 | None | 60 |
| 9 | Cu(I)Cl (30) | MeCN | O2 | 0.1 | None | 65 |
| 10 | Cu(II)Cl2 | MeCN | O2 | 0.1 | None | Traces |
| 11 | Cu(I)Br | MeCN | O2 | 0.1 | None | 49 |
| 12 | Fe(II)Cl2 | MeCN | O2 | 0.1 | None | 3 |
| 13 | Cu(II)(OAc)2 | MeCN | O2 | 0.1 | None | 42 |
| 14 5 | Cu(I)Cl (10) | MeCN | DTBP (2 equiv)/N2 | 0.1 | None | 40 |
| 15 5 | Cu(I)Cl (10) | MeCN | DTBP (3 equiv)/N2 | 0.1 | None | 55 |
| 16 | Cu(I)Cl | MeCN | DTBP (1 equiv)/N2 | 0.1 | None | 80 |
| 17 | Cu(I)Cl | MeCN | O2 | 0.1 | Et3N (1.0 equiv) | 0 |
| 18 | Cu(I)Cl | MeCN | O2 | 0.1 | Mol. sieves | Traces |
| 19 | Cu(I)Cl | MeCN | O2 | 0.1 | Pyridine (1.0 equiv) | 31 |
| 20 | Cu(I)Cl | MeCN | O2 | 0.1 | AcOH (1.0 equiv) | 87 |
| 21 | Cu(I)Cl | MeCN | O2 | 0.1 | AcOH (1.5 equiv) | 93 |
| 22 | None | MeCN | air | 0.1 | AcOH (1.5 equiv) | 80 |
| 23 | None | MeCN | N2 | 0.1 | AcOH (1.5 equiv) | 53 |
| 24 | none | MeCN | air | 0.1 | None | 8 |

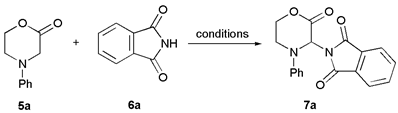
| Entry | Morpholinone | Imide | Product (M) | Yield (%) | |
|---|---|---|---|---|---|
| 1 | 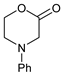 |  | 7a |  | 68 (93) |
| 2 |  |  | 7b |  | 93 2 |
| 3 |  | 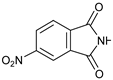 | 7c |  | 27 |
| 4 |  |  | 7d | 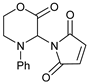 | 36 (85) |
| 5 |  | 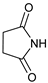 | 7e | 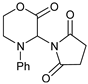 | 83 3 |
| 6 |  |  | 7f |  | 71 (dr = 1:1) |
| 7 |  |  | 7g |  | ND |
| 8 |  | 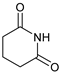 | 7h | 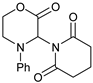 | 36 (75) (at 80 °C) 4 |
| 9 |  | 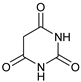 | 7i |  | ND |
| 10 |  |  | 7j | 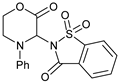 | ND |
| 11 |  | 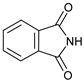 | 7k |  | 75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faisca Phillips, A.M.; Pombeiro, A.J.L. A Mild and Sustainable Procedure for the Functionalization of Morpholin-2-Ones by Oxidative Imidation Reactions. Catalysts 2023, 13, 1072. https://doi.org/10.3390/catal13071072
Faisca Phillips AM, Pombeiro AJL. A Mild and Sustainable Procedure for the Functionalization of Morpholin-2-Ones by Oxidative Imidation Reactions. Catalysts. 2023; 13(7):1072. https://doi.org/10.3390/catal13071072
Chicago/Turabian StyleFaisca Phillips, Ana Maria, and Armando J. L. Pombeiro. 2023. "A Mild and Sustainable Procedure for the Functionalization of Morpholin-2-Ones by Oxidative Imidation Reactions" Catalysts 13, no. 7: 1072. https://doi.org/10.3390/catal13071072
APA StyleFaisca Phillips, A. M., & Pombeiro, A. J. L. (2023). A Mild and Sustainable Procedure for the Functionalization of Morpholin-2-Ones by Oxidative Imidation Reactions. Catalysts, 13(7), 1072. https://doi.org/10.3390/catal13071072