A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol
Abstract
1. Introduction
2. Results and Discussion
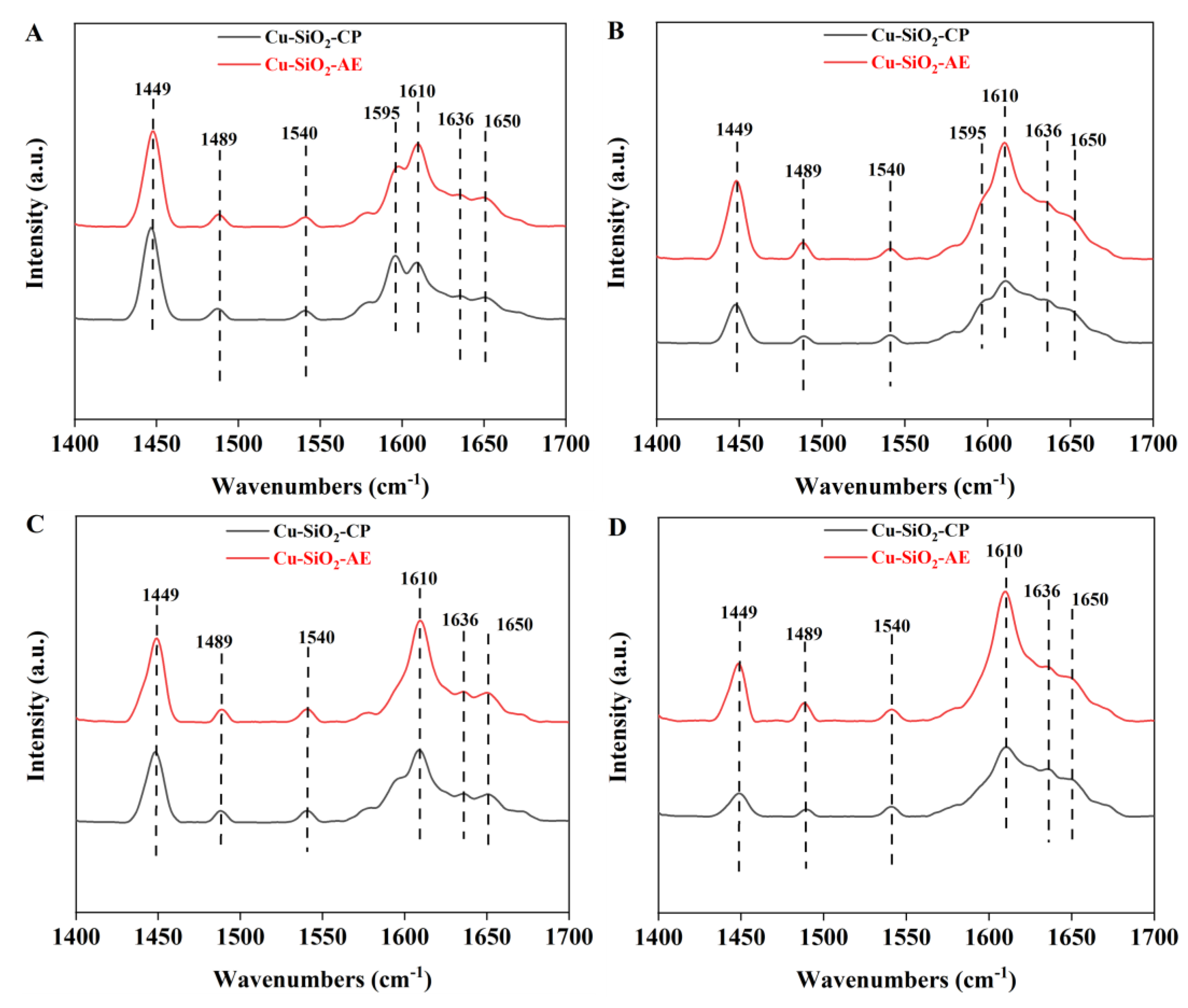
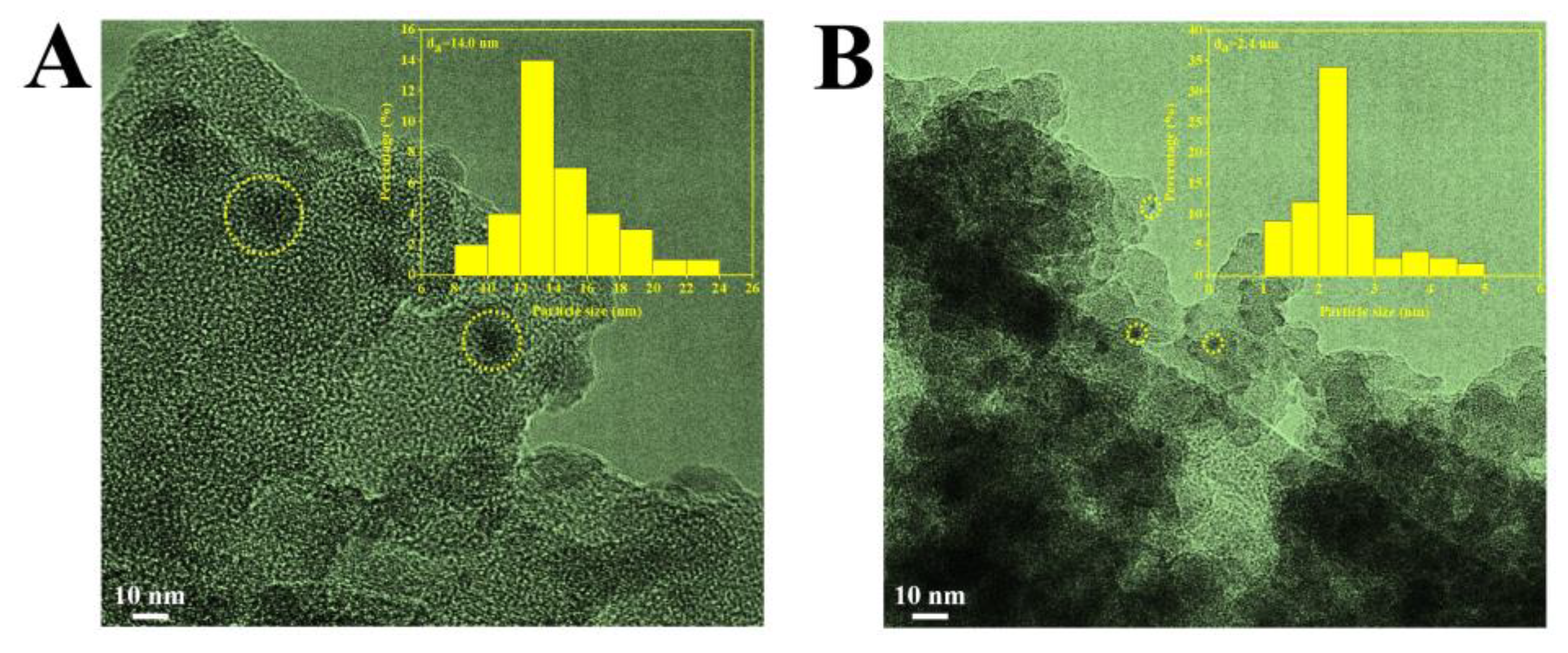
2.1. Characterization of the Catalysts
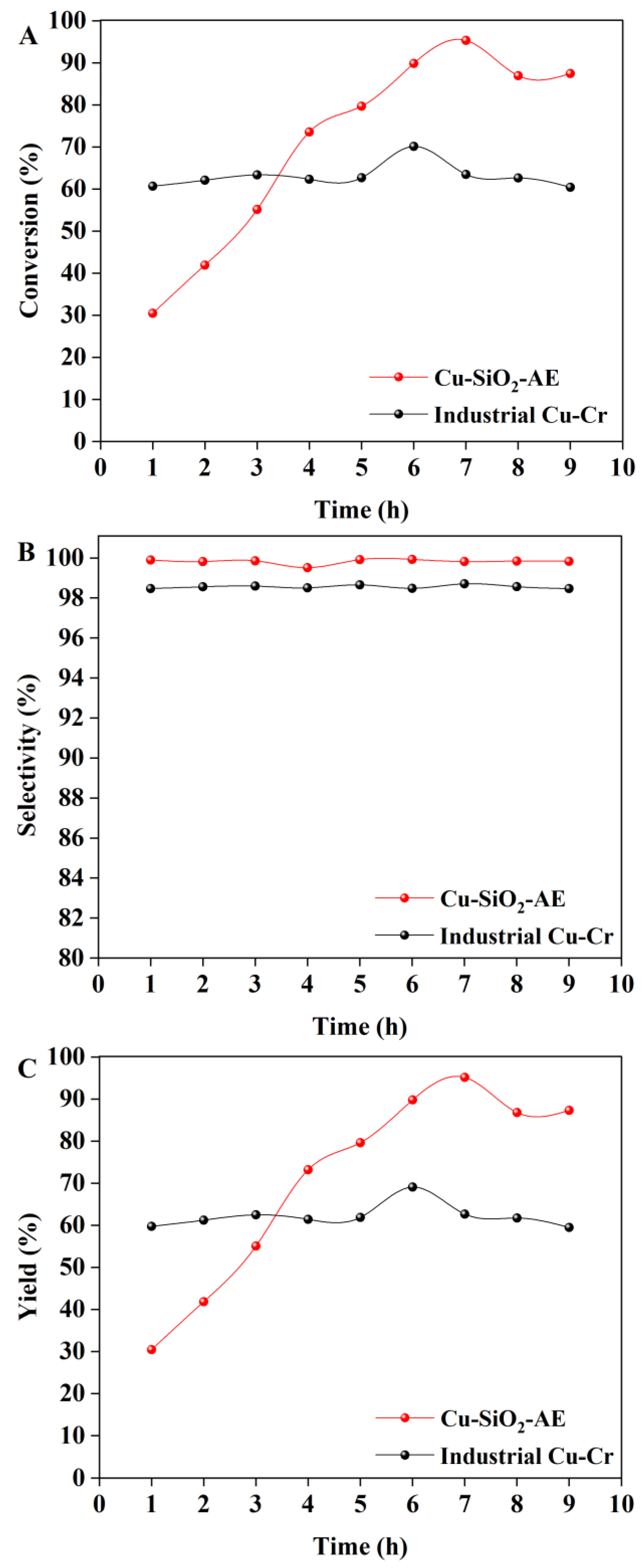
2.2. Catalytic Hydrogenation of Methyl Formate
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Cu-SiO2
3.3. Catalyst Characterization
3.4. Catalytic Reaction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ke, S.; Yang, J.; Wende, X. Intrinsic kinetic research on vapor-phase catalytic coupling of CO and methyl nitrite to DMO. Guangdong Chem. Ind. 2007, 34, 12–14+24. [Google Scholar] [CrossRef]
- Fan, C.; Luo, M.; Xiao, W. Reaction mechanism of methyl nitrite dissociation during co catalytic coupling to dimethyl oxalate: A density functional theory study. Chin. J. Chem. Eng. 2016, 24, 132–139. [Google Scholar] [CrossRef]
- Chuanhao, S. Process optimization of CO coupling production of dimethyl oxalate in the process of ethylene glycol production. Yunnan Chem. Technol. 2017, 44, 36–39. [Google Scholar] [CrossRef]
- Xu, Q.S.; Guo, M.; Xia, L. Temperature gradient analyses of a tubular solid oxide fuel cell fueled by methanol. Trans. Tianjin Univ. 2023, 29, 14–30. [Google Scholar] [CrossRef]
- Qijun, G.; Yuxin, W.; Li, X. Proton-exchange sulfonated poly (ether ether ketone)(SPEEK)/SiOx-S composite membranes in direct methanol fuel cells. Chin. J. Chem. Eng. 2009, 17, 207–213. [Google Scholar] [CrossRef]
- Monti, D.M.; Cant, N.W.; Trimm, D.L. Hydrogenolysis of methyl formate over copper on silica: II. Study of the mechanism using labeled compounds. J. Catal. 1986, 100, 28–38. [Google Scholar] [CrossRef]
- Zhao, H.; Lin, M.; Fang, K. Preparation and evaluation of Cu-Mn/Ca-Zr catalyst for methyl formate synthesis from syngas. Appl. Catal. A: Gen. 2016, 514, 276–283. [Google Scholar] [CrossRef]
- Liu, X.Q.; Wu, Y.T.; Chen, W.K. Concurrent synthesis of methanol and methyl formate catalyzed by copper-based catalysts in a slurry phase. Stud. Surf. Sci. Catal. 1998, 119, 557–560. [Google Scholar] [CrossRef]
- Wang, K.; Chu, W.; He, C. High activity Cu-Cr-Si catalysts for lower temperature liquid phase synthesis of methanol I. Influence of preparation parameters on catalysts activities. Petrochem. Technol. 2001, 30, 686–688. [Google Scholar] [CrossRef]
- Yi, L.; Wei, C.; Yanghong, Y. Study of the effect of additives on Cu-Cr-Si catalyst for methanol synthesis from the hydrogenolysis of methyl formate. Chem. Peking 2003, 6, 663–666. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, S. Advances in copper-based catalyst for the methanol synthesis from CO2 hydrogenation. Chem. Indus. Engineer Pro. 2016, 35, 159–166. [Google Scholar] [CrossRef]
- Haiguang, G.; Wenfeng, H.; Huazhang, L. Study on copper-based catalysts for methanol synthesis. J. Zhejiang Univ. Technol. 2003, 31, 508–511. [Google Scholar] [CrossRef]
- Zu, Y.; Guo, Z.; Zheng, J. Investigation of Cu (I)-Y zeolites with different Cu/Al ratios towards the ultra-deep adsorption desulfurization: Discrimination and role of the specific adsorption active sites. Chem. Eng. J. 2020, 380, 122319. [Google Scholar] [CrossRef]
- Suzuki, N.; Asami, H.; Nakamura, T. Immobilization of a C2-symmetric ansa-zirconocene complex on silica surfaces using a Si-Cl Anchor: Catalysts for isospecific propene polymerization. Chem. Lett. 1999, 28, 341–342. [Google Scholar] [CrossRef]
- Zhang, H.; Tan, H.R.; Jaenicke, S. Highly efficient and robust Cu catalyst for non-oxidative dehydrogenation of ethanol to acetaldehyde and hydrogen. J. Catal. 2020, 389, 19–28. [Google Scholar] [CrossRef]
- Wu, L.; Li, B.; Zhao, C. Direct synthesis of hydrogen and dimethoxylmethane from methanol on copper/silica catalysts with optimal Cu+/Cu0 sites. ChemCatChem 2018, 10, 1140–1147. [Google Scholar] [CrossRef]
- Jinyong, L.; Ming, M.; Yuqing, Z. Characterization on the fine structures of the copper species in the highly dispersed CuO/CeO2-Al2O3 catalysts. Chin. J. Inorg. Chem. 2006, 22, 861–866. [Google Scholar] [CrossRef]
- Weng, Z.; Wu, Y.; Wang, M. Active sites of copper-complex catalytic materials for electrochemical carbon dioxide reduction. Nat. Commun. 2018, 9, 415. [Google Scholar] [CrossRef]
- Li, G. Catalytic property and reactional mechanism of Cu-ZnO-ZrO2/CFG for reaction of methanol dehydrogenation to methyl formate. J. Fushun Petrochem. Inst. 1995, 1, 1–6. [Google Scholar]
- Kannapu, H.P.R.; Neeli, C.K.P.; Rao, K.S.R. Unusual effect of cobalt on Cu-MgO catalyst for the synthesis of γ-butyrolactone and aniline via coupling reaction. Catal. Sci. Technol. 2016, 6, 5494–5503. [Google Scholar] [CrossRef]
- Yu, J.; Suocai, R.; Liu, B. Advances in deactivation and solutions of Cu-based methanol catalysts. Nat. Gas Chem. Ind. 2019, 44, 118–122. [Google Scholar] [CrossRef]
- Cho, J.M.; Lee, S.R.; Sun, J.; Tsubaki, N.; Jang, E.J.; Bae, J.W. Highly Ordered Mesoporous Fe2O3-ZrO2 Bimetal Oxides for an Enhanced CO Hydrogenation Activity to Hydrocarbons with Their Structural Stability. ACS Catal. 2017, 7, 5955–5964. [Google Scholar] [CrossRef]
- Yuanzhi, L.; Ergan, C. Inverstigation on Co-Fe-Cu/SiO2 Catalyst by Unsingtemperature Programmed Reduction. J. Jingzhou Teach. Coll. 1998, 21, 60–62. [Google Scholar]
- Zhang, C.; Peiyi, L.; Zhibiao, S. Preparation of Cu/ZnO/MCM-41 catalyst with double-solvent impregnation method and catalytic performance in methanol synthesis by CO2 hydrogenation. J. Shanghai Univ. Nat. Sci. 2019, 25, 109–119. [Google Scholar] [CrossRef]
- Ding, D.; Wang, Q.; Jin, F. Influences of the ammonia evaporation pressure on the structure of Cu-SiO2 catalysts and catalytic performances for dimethyl oxalate hydrogenation to ethylene glycol. Chin. J. Appl. Chem. 2016, 33, 466. [Google Scholar] [CrossRef]
- Siqi, B.; Renjiang, L.; Lidi, G. Synthesis and electrochemical study of Sn-Ni core-shell nanoparticles modified carbon one-dimensional nanostructures. Chem. Res. Appl. 2022, 34, 2920–2926. [Google Scholar] [CrossRef]
- Cui, A.L.; Bai, Y.; Yu, H.Y. Electrocatalytic “Volcano-Type” effect of Nano-TiO2 (A)/(R) phase content in Pt/TiO2-CNx catalyst. J. Electrochem. 2022, 28, 2110021. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, X.; Mao, H. Photoluminescence and XPS investigations of Cu2+-doped ZnS quantum dots capped with polyvinylpyrrolidone. Phys. Status Solidi B 2009, 246, 2333–2336. [Google Scholar] [CrossRef]
- Hongfei, Y.; Yu, Z.; Guixian, L. Effect of carrier surface hydroxyl group on performance of Cu/SiO2 catalyst for DMO hydrogenation. Chem. Ind. Eng. Prog. 2022, 41, 6338–6349. [Google Scholar] [CrossRef]
- Bian, Z.; Zhong, W.; Yu, Y. Cu-SiO2 derived from copper phyllosilicate for low-temperature water-gas shift reaction: Role of Cu+ sites. Int. J. Hydrog. Energy 2020, 45, 27078–27088. [Google Scholar] [CrossRef]
- Yang, R.; Liu, Y.; Zhong, B. H2 and CO desorption properties of raney Cu-Nd catalysts and correlation with their catalytic activity. Petrochem. Technol. 1999, 28, 219–222. [Google Scholar] [CrossRef]
- Haiyan, L.; Hongbin, Z.; Guodong, L. Study of Promoted Cu based catalysts for hydrogenolysis of methyl formate to methanol. J. Xiamen Univ. Nat. Sci. 1997, 36, 381–387. [Google Scholar]
- Jitao, L.; Qiangu, Y.; Weide, Z. Synthesis methanol under low temperature and low pressure by two step process. Sci. Technol. Chem. Ind. 2000, 8, 17–19. [Google Scholar] [CrossRef]
- Chen, Y.Z.; Chung, B.Z.; Hsieh, C.R. An effective catalyst, Cu-B2O3/SiO2, for hydrogenolysis of methyl formate and one-step synthesis of methanol in slurry-phase with potassium methoxide. Catal. Lett. 1996, 41, 213–220. [Google Scholar] [CrossRef]
- Parry, E.P. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar] [CrossRef]
- Huang, F. Research for control the acidity of RFCC catalyst. Chem. Eng. Oil Gas 2001, 6, 287–289. [Google Scholar]
- Lu, S.; Fu, H.; Wang, L. Infrared spectroscopic study on the surface Bronsted and Lewis acidity of fluoridated hydrotreating catalysts F·N·W/Al2O3·SiO2. J. Mol. Catal. 1993, 7, 471–474. [Google Scholar] [CrossRef]
- Wang, J.; Shan, J.; Tian, Y. Catalytic cracking of n-heptane over Fe modified HZSM-5 nanosheet to produce light olefins. Fuel 2021, 306, 121725. [Google Scholar] [CrossRef]
- Kamiguchi, S.; Seki, Y.; Satake, A. Catalytic cracking of methyl tert-butyl ether to isobutene over Brønsted and Lewis acid sites on solid-state molybdenum sulfide clusters with an octahedral metal framework. J. Clust. Sci. 2015, 26, 653–660. [Google Scholar] [CrossRef]
- Yang, J.; Wu, X.; Cheng, Y. Novel solid acid with both Brønsted and Lewis acid sites for biodiesel synthesis. Kinet. Catal. 2013, 54, 703–708. [Google Scholar] [CrossRef]
- Jiang, W.; Gao, X.; Dong, L. Aerobic oxidative desulfurization via magnetic mesoporous silica-supported tungsten oxide catalysts. Pet. Sci. 2020, 17, 1422–1431. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Wang, Y. High performance of supported Cu-based catalysts modulated via phosphamide coordination in acetylene hydrochlorination. Appl. Catal. A Gen. 2020, 591, 117408. [Google Scholar] [CrossRef]
- Elgayyar, T.; Atwi, R.; Tuel, A. Contributions and limitations of IR spectroscopy of CO adsorption to the characterization of bimetallic and nanoalloy catalysts. Catal. Today 2021, 373, 59–68. [Google Scholar] [CrossRef]
- Niu, Y.; Li, F.; Yang, K. Preparation and characterization of sulfated TiO2 with rhodium modification used in esterification reaction and decomposition of methyl orange. Chin. J. Chem. Eng. 2016, 24, 767–774. [Google Scholar] [CrossRef]
- Moayedi, H.; Kazemian, S. Zeta potentials of suspended humus in multivalent cationic saline solution and its effect on electro-osomosis behavior. J. Dispers. Sci. Technol. 2013, 34, 283–294. [Google Scholar] [CrossRef]
- Song, M.; Zhang, B.; Zhai, Z.; Liu, S.; Wang, L.; Liu, G. Highly dispersed Pt stabilized by ZnOx-Si on self-pillared zeolite nanosheets for propane dehydrogenation. Ind. Eng. Chem. Res. 2023, 62, 3853–3861. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, X.; Wei, S.; Yang, H.; Zhang, F.; Wang, P.; Xie, M.; Ma, J. Cu (I) immobilized on functionalized SBA-15: A recyclable catalyst for the synthesis of 1,3-diynes using terminal alkynes without base. Catal. Commun. 2013, 39, 24–29. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Zhou, H.; Chu, S.; Liu, L.; Feng, Z.; Qin, X.; Qi, J.; Hou, J.; Li, H.; et al. Rivet of cobalt in siliceous zeolite for catalytic ethane dehydrogenation. Chem 2023, 9, 637–649. [Google Scholar] [CrossRef]
- Wang, L.; Cui, Y.; Meng, X.; Gates, B.C.; Xiao., F.; Guan, E.; Wang, Y. Silica accelerates the selective hydrogenation of CO2 to methanol on cobalt catalysts. Nat. Commun. 2020, 11, 1033. [Google Scholar] [CrossRef]
- Tehrani, S.; Irani, M.; Tavasoli, A. Studies on accelerated deactivation of ruthenium-promoted alumina-supported alkalized cobalt Fischer-Tropsch synthesis catalyst. J. Nat. Gas Chem. 2011, 20, 65–71. [Google Scholar] [CrossRef]
- Xie, Y.; Li, B.; Weng, W.Z. Mechanistic aspects of formation of sintering-resistant palladium nanoparticles over SiO2 prepared using Pd(acac)2 as precursor. Appl. Catal. A Gen. 2015, 504, 179–186. [Google Scholar] [CrossRef]
- Li, D.; Xu, F.; Tang, X.; Dai, S.; Pu, T.; Liu, X.; Tian, P.; Xuan, F.; Xu, Z.; Wachs, I.E.; et al. Induced activation of the commercial Cu/ZnO/Al2O3 catalyst for the steam reforming of methanol. Nat. Catal. 2022, 5, 99–108. [Google Scholar] [CrossRef]
- Li, H.; Fang, W.; Wang, L.; Liu, Y.; Liu, L.; Sun, T.; Liao, C.; Zhu, Y.; Wang, L.; Xiao, F. Physical regulation of copper catalyst with a hydrophobic promoter for enhancing CO2 hydrogenation to methanol. Innovation 2023, 4, 100445. [Google Scholar] [CrossRef]
- Shi, W.; Gao, T.; Zhang, L. Tailoring the surface structures of iron oxide nanorods to support Au nanoparticles for CO oxidation. Chin. J. Catal. 2019, 40, 1884–1894. [Google Scholar] [CrossRef]
- Xiao, P.; Zhao, Y.; Wang, T. Polymeric carbon nitride/mesoporous silica composites as catalyst support for Au and Pt nanoparticles. Chem. Eur. J. 2014, 20, 2872–2878. [Google Scholar] [CrossRef]
- Ewing, C.S.; Veser, G.; McCarthy, J.J. Effect of support preparation and nanoparticle size on catalyst-support interactions between Pt and amorphous silica. J. Phys. Chem. C 2015, 119, 19934–19940. [Google Scholar] [CrossRef]
- Lueking, A.D.; Yang, R.T. Hydrogen spillover to enhance hydrogen storage-study of the effect of carbon physicochemical properties. Appl. Catal. A Gen. 2004, 265, 259–268. [Google Scholar] [CrossRef]
- Kong, X.; Xiao, J.; Chen, A. Enhanced catalytic denitrification performance of ruthenium-based catalysts by hydrogen spillover from a palladium promoter. J. Colloid Interface Sci. 2022, 608, 2973–2984. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.; Ryoo, R. Hydrogen spillover in nonreducible oxides: Mechanism and catalytic utilization. Nano Res. 2022, 15, 10357–10365. [Google Scholar] [CrossRef]
- Lennon, D.; Kennedy, D.R.; Webb, G. Deactivation and selectivity: The effect of hydrogen concentration in propyne hydrogenation over a silica-supported palladium catalyst//studies in surface science and catalysis. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 1999; Volume 126, pp. 341–348. [Google Scholar] [CrossRef]
- Behrens, M. Chemical hydrogen storage by methanol: Challenges for the catalytic methanol synthesis from CO2. Recycl. Catal. 2015, 2, 78–86. [Google Scholar] [CrossRef]


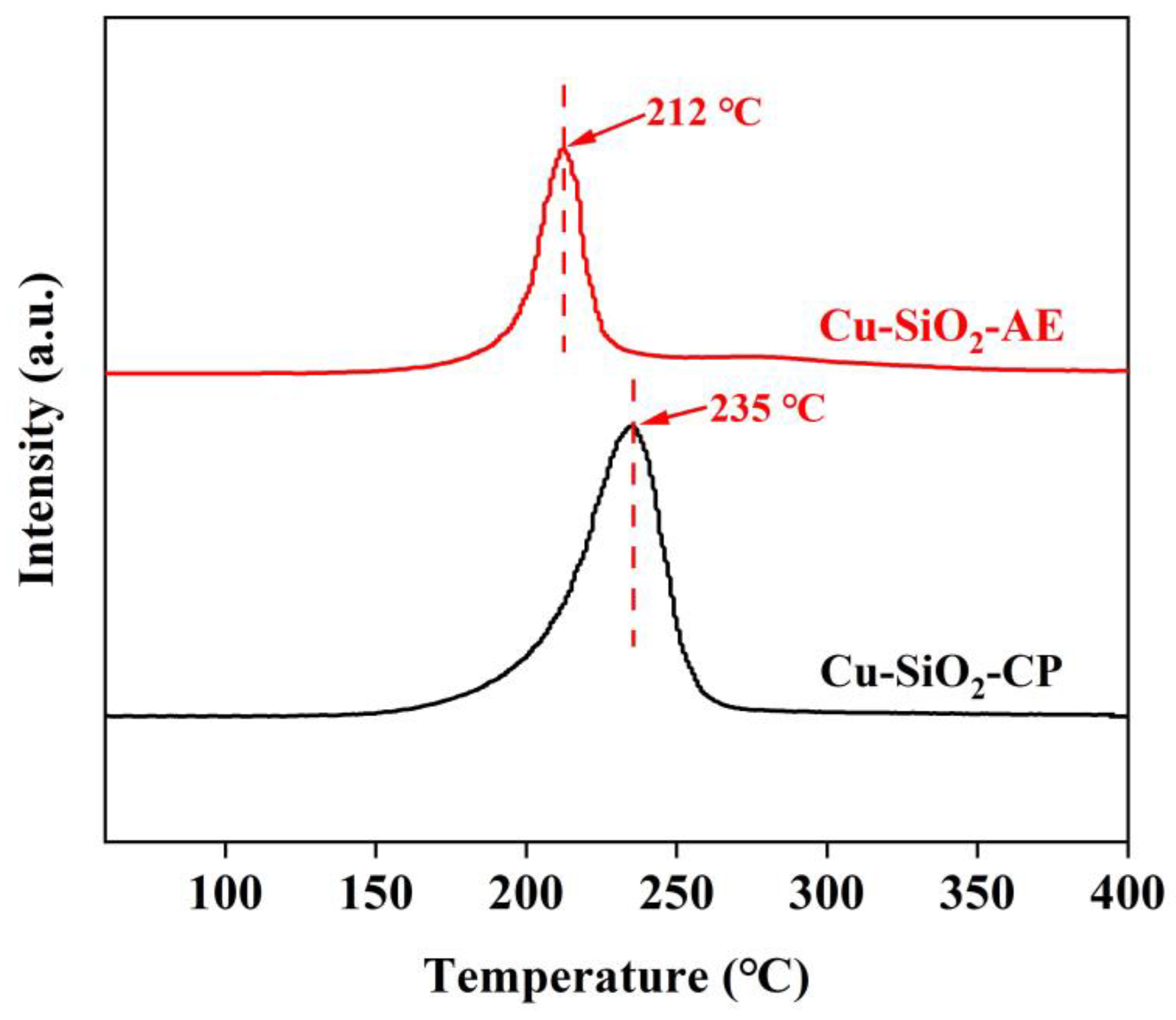
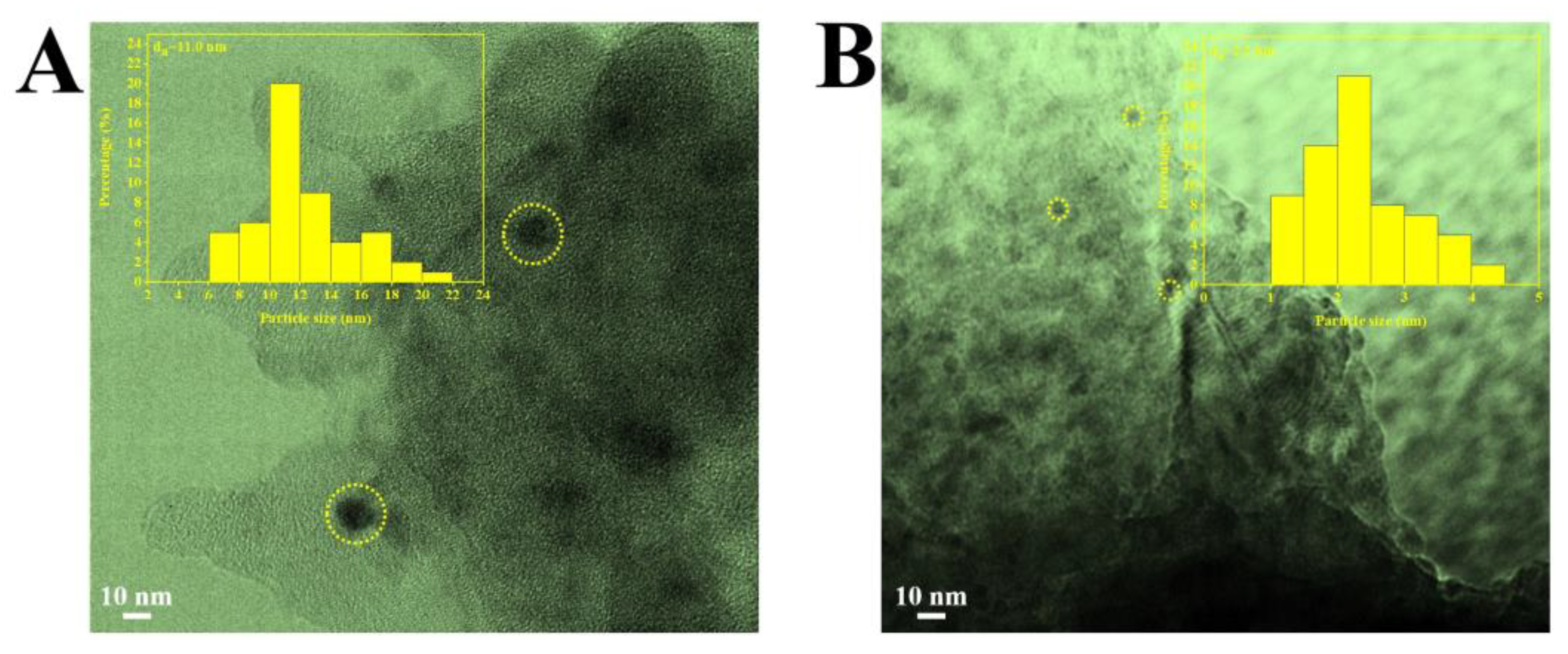

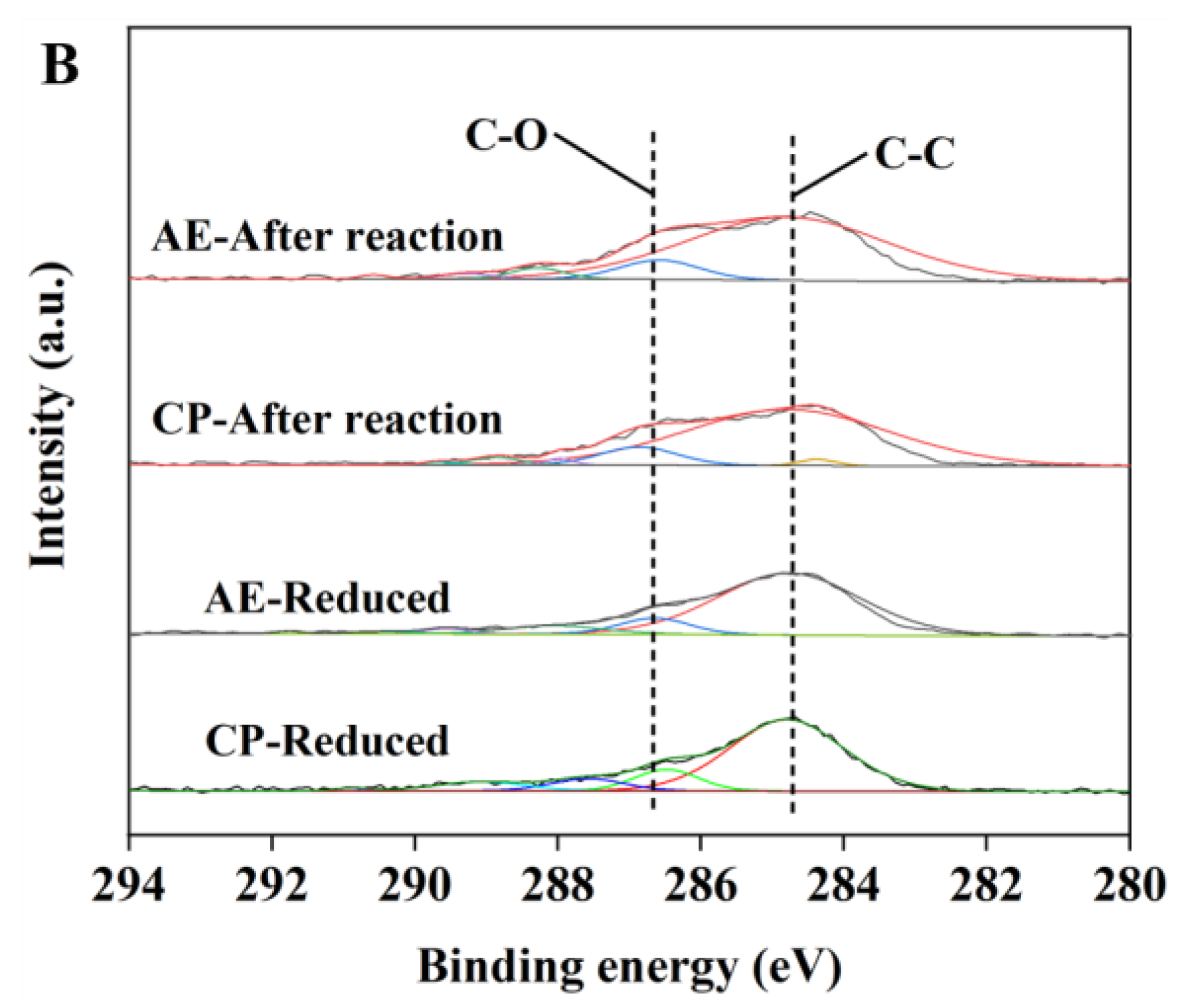


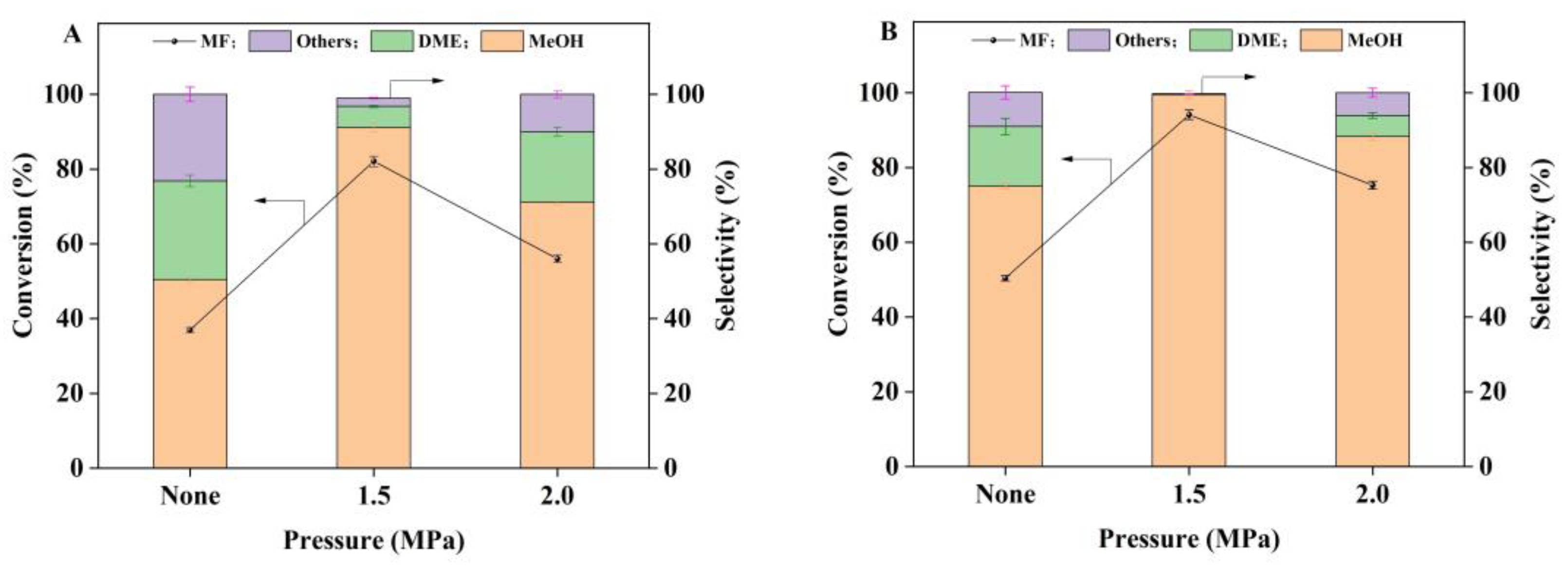




| Samples | a SBET (m2/g) | a V (cm3/g) | a d (nm) | b D (%) | b SCu (m2/g) | b dCu (nm) |
|---|---|---|---|---|---|---|
| Cu-SiO2-CP | 208.3 | 0.8 | 18.4 | 50.0 | 339.0 | 2.0 |
| Cu-SiO2-AE | 350.8 | 0.7 | 18.4 | 109.6 | 742.9 | 0.9 |
| Catalyst | MF Conversion/% | Product Selectivity/% | ||
|---|---|---|---|---|
| MeOH | DME a | Others b | ||
| Cu-SiO2-CP | 83.9 | 92.1 | 5.7 | 2.2 |
| Cu-SiO2-AE | 95.3 | 99.8 | 0.1 | 0.1 |
| Catalyst | Cu Loading (wt%) | H2/MF (v/v) | Temp. (°C) | P (MPa) | LHSV (h−1) | MF Conv. (%) | MeOH Sel. (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Cu-Cr | 23 | 4/1 | 140 | 0.1 | 1800 | 73.1 | 98.0 | [32] |
| Cu-Cr-Mn | 23 | 4/1 | 140 | 0.1 | 1800 | 76.15 | 99.55 | [32] |
| Cu-Mn-SiO2 | 19.2 | 4/1 | 180 | 0.5 | 10 | 84.4 | 94.4 | [33] |
| Cu-SiO2 | 19.2 | 4/1 | 180 | 0.5 | 10 | 51.5 | 90.4 | [33] |
| Cu-B2O3/SiO2 | - | 3/1 | 150 | 2.8 | - | 39 | 99.8 | [34] |
| Cu-SiO2-AE | 15 | 4/1 | 140 | 1.5 | 2.4 | 95.3 | 99.8 | This study |
| Cu-SiO2-CP | 15 | 4/1 | 140 | 1.5 | 2.4 | 83.9 | 92.1 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Liu, G.; Liu, Q.; Zhang, Y.; Ding, F.; Wang, K. A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol. Catalysts 2023, 13, 1038. https://doi.org/10.3390/catal13071038
Wu J, Liu G, Liu Q, Zhang Y, Ding F, Wang K. A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol. Catalysts. 2023; 13(7):1038. https://doi.org/10.3390/catal13071038
Chicago/Turabian StyleWu, Jincheng, Guoguo Liu, Qin Liu, Yajing Zhang, Fu Ding, and Kangjun Wang. 2023. "A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol" Catalysts 13, no. 7: 1038. https://doi.org/10.3390/catal13071038
APA StyleWu, J., Liu, G., Liu, Q., Zhang, Y., Ding, F., & Wang, K. (2023). A Cu-SiO2 Catalyst for Highly Efficient Hydrogenation of Methyl Formate to Methanol. Catalysts, 13(7), 1038. https://doi.org/10.3390/catal13071038







