Abstract
The abundant C1 source CO2 can be utilized to produce value-added chemicals through hydrogenation technology. A bifunctional catalyst consisting of reducible metal oxide Cr2O3 and acidic zeolite ZSM-5 was designed for the direct conversion of CO2 + H2 into valuable aromatics, especially para-xylene (PX), via the methanol-mediated pathway. The twin structure of ZSM-5 (ZSM-5T), with sinusoidal channels that are predominantly exposed to the external surface, enhances the possibility of the transformation of methanol into PX due to the favorable diffusion dynamic of PX in the sinusoidal channels. Via the bifunctional catalyst Cr2O3&ZSM-5T, a PX selectivity of 28.7% and PX space-time yield (STY) of 2.5 gCH2 h−1 kgcat−1 are achieved at a CO2 conversion rate of 16.5%. Furthermore, we rationally modify the ZSM-5T zeolite via Cu species doping and amorphous SiO2 shell coating (Cu-ZSM-5T@SiO2). After combining with the Cr2O3 catalytic component, the CO2 conversion (18.4%) and PX selectivity (33.8%) are increased to some extent, which systematically increases the STY of PX to 3.0 gCH2 h−1 kgcat−1. The physicochemical property of the acidic zeolite and the corresponding structure-function relationship in enhancing the PX productivity are discovered. Our work provides a novel catalyst design idea to boost PX synthesis performance from CO2 hydrogenation.
1. Introduction
As a major contributor to the greenhouse effect, the capture and resource utilization of CO2 can not only alleviate the trend of global warming but also provide a feasible technical route for the synthesis of high-value-added chemicals [1,2,3]. CO2 hydrogenation is currently the most efficient means of CO2 conversion into valuable chemicals (i.e., methanol, light olefins, aromatics, etc.) due to its high efficiency and industrial applicability [4,5,6]. Among all of these products, aromatics—especially para-xylene (PX), as the most valuable aromatic hydrocarbons—are basic building blocks in the polymer industries [7]. Compared to the traditional fossil fuel-based PX production pathway, the direct transformation of CO2 + H2 into PX holds great potential to alleviate the pressures from the depletion of fossil fuel reserves and environmental issues [8,9]. It is of strategic significance to develop synthetic routes towards aromatic compounds, especially PX, based on the CO2 hydrogenation process.
Until now, two main pathways have been proposed to realize direct PX synthesis from CO2 + H2, including the modified Fischer–Tropsch synthesis (FTS) pathway and the methanol-mediated pathway (Scheme 1) [10,11]. During the modified FTS process, the multifunctional catalyst composed of an Fe-based catalytic component (Fe-based oxide and carbide) and acidic zeolite could cascade the reverse water-gas shift reaction (CO2 + H2 → CO + H2O), FTS, aromatization process, etc. into a one-pass for PX synthesis [12,13,14,15,16]. For the methanol-mediated pathway, the combination of the methanol synthesis and the classical methanol-to-aromatics (MTA) process via a bifunctional catalyst involved in a reducible metal oxide (e.g., ZnZrOx, ZnAlOx, ZnCrOx, etc.) and acidic zeolite guarantees the direct conversion of CO2 + H2 into PX [17,18,19,20,21,22]. It is obvious that no matter which route is chosen, the physicochemical property of the acidic zeolite plays a vital role in altering the selectivity of aromatics, especially the most valuable PX.
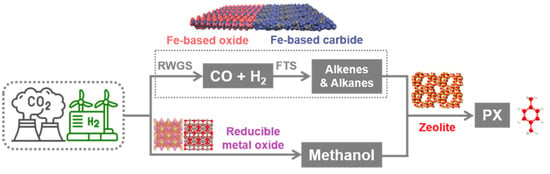
Scheme 1.
The modified FTS and the methanol-mediated pathways for PX synthesis from CO2 hydrogenation.
ZSM-5 zeolite with an MFI topology and a three-dimensional 10-membered ring channel system features two distinct types of channels (Scheme 2a): the two-dimensional sinusoidal channel (0.51 nm × 0.55 nm) opening on 100 plane and the straight channel (0.53 nm × 0.56 nm) opening on 010 plane [23,24]. Ideally, the channel structure of ZSM-5 is accommodative for shape-selective PX synthesis due to the close dynamic diameter of PX (ca. 0.58 nm) with the channel structure of ZSM-5. The formation of isomers ortho-xylene (OX) and meta-xylene (MX) as well as the heavy aromatics (C9+) with larger molecular dynamic diameters (>0.68 nm) can be well suppressed by the narrow channels of ZSM-5 (Scheme 2b) [25]. However, the undesirable isomerization and alkylation reactions on the external acid site or at the pore mouth of ZSM-5, without shape-selective function, always lower the PX selectivity in the ZSM-5-catalyzed aromatics synthesis reactions, such as toluene alkylation or MTA (Scheme 2c) [26]. At the same time, owing to the thermodynamic equilibrium limitation and the change in the non-rigid skeleton structure under high reaction temperatures, the molecules with a close dynamic diameter to PX, such as OX and MX, can also diffuse out of the ZSM-5 channels as final products, which is another cause of lowered PX selectivity. For instance, the PX selectivity in X is usually lower than 24% [27]. To tackle above obstacles, much attention has been paid to rationally tailoring the acidic and architectural properties of the ZSM-5. On the one hand, the external acid sites of ZSM-5 can be erased by coating an amorphous SiO2 layer or epitaxially growing a non-acidic zeolite membrane on the surface of ZSM-5 crystals. This external acid site erasing strategy has been widely employed to boost the PX selectivity of several ZSM-5-catalyzed reactions, such as toluene alkylation, MTA, syngas conversion, and CO2 hydrogenation [28,29,30,31,32]. However, it should be noted that the adhesion of amorphous SiO2 and non-acidic zeolite onto the external surface of ZSM-5 will inevitably decrease the reactivity due to the loss of acid site. On the other hand, considering that the shape-selective capability of the sinusoidal channels with smaller pore size, predominantly opening on the 100 crystal plane of ZSM-5, is stronger than that of the straight channels with larger pore size, primarily opening on the 010 crystal plane, the precise control of the priority exposure of 100 crystal plane, i.e., increasing the opening of sinusoidal channels, has been proven to be feasible in optimizing the PX selectivity by tailoring the diffusion behaviors of PX and the isomers in the elongated channels. Recently, Ma et al. synthesized a twin-structured ZSM-5 for toluene alkylation with methanol, in which the intergrowth morphology with 100-plane-dominated twin crystal covering the body crystal maximizes the exposure of sinusoidal channels, therefore optimizing the shape-selective capability of ZSM-5 for PX synthesis (˃99% PX selectivity) [33]. Furthermore, this twin-structured ZSM-5 possessed a decreasing acid site density gradient from the core to the surface. The surface with poor acidic sites could prohibit the undesirable side reactions that occurred on the external surface or in the pore mouth of ZSM-5 due to the decreased accessibility of reactive centers, which is another key factor in boosting PX selectivity. Tsubaki et al. explored the possibility of this twin-structured ZSM-5 in tandem catalysis of CO2 + H2 into PX via the methanol-mediated pathway. Interestingly, after combining this twin-structured ZSM-5 zeolite with ZnCr2O4, which can convert CO2 into methanol efficiently, high PX/X and PX/aromatics ratios of 97.3% and 63.9% were achieved, respectively [34]. Sun et al. presented a series of novel HZSM-5 nanocrystal clusters with chain-like morphology and chain length. With the combination of highly active ZnZrOx oxides, the composite catalyst achieved excellent performance of CO2 hydrogenation to aromatics. They found that a proper extension of the b-axis length and passivation of external surface acid sites of HZSM-5 could greatly enhance the selectivity of PX [35]. Tsubaki et al. tailored the physicochemical properties of the parent H-ZSM-5 by metal ion doping and subsequent SiO2 coating to study the effect of the zeolite component on the aromatics synthesis performance from syngas. The strong CO adsorption capacity and the lower C-C coupling barrier derived from Ga doping guaranteed the superior aromatics yield. The blocking of the external acid sites of H-ZSM-5 by SiO2 deposition is beneficial to suppress the undesirable side reactions, therefore boosting light aromatics synthesis [36]. Even though great progress has been made in the rational design of the ZSM-5 zeolitic component to enhance the PX selectivity of various ZSM-5-catalyzed aromatics synthesis reactions, there is still a lack of innovative works on optimizing PX synthesis performance by systematically considering most of the essential factors that affect the shape-selective property of ZSM-5.
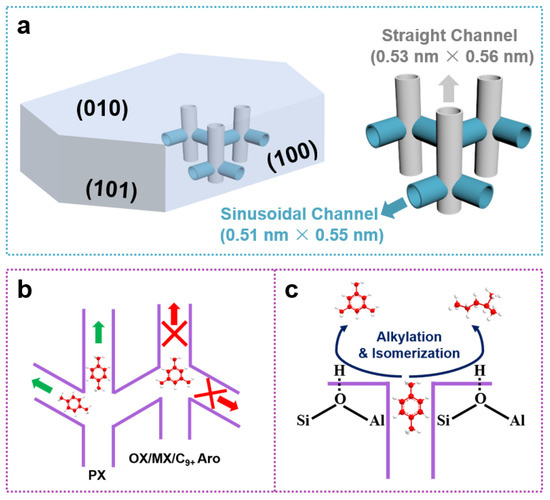
Scheme 2.
(a) The topology structure of ZSM-5 (the gray arrow represents the straight channel, and the blue arrow represents the sinusoidal channel), (b) shape-selective synthesis of ZSM-5 (the green arrows mean that the products could pass the channels easily, while the red arrows mean it is difficult to pass), and (c) side reactions that occurred on the external surface or at the pore mouth of ZSM-5.
In this work, we focus on enhancing the PX selectivity of the methanol-mediated tandem CO2 hydrogenation process by simultaneously tailoring the architectural and acidic properties of the ZSM-5 component in the bifunctional catalyst. The twin-structured ZSM-5 zeolite is employed as an initial research subject. To further increase its shape-selective capability, an amorphous SiO2 shell is coated onto the external surface to erase the undesirable acid sites. More importantly, to eliminate the negative effect brought about by SiO2 deposition, i.e., the loss of catalytic reactivity stemming from the decrease in the amount of acid sites, Cu ions, which are widely used to enrich the acid site density and tailor the acidic property of zeolite, are exchanged into the zeolite framework to strengthen the driving force in the tandem process. Benefiting from this comprehensive regulation strategy, the PX selectivity from CO2 hydrogenation could clearly be improved. We hope that our work will provide new insights into the rational design of ZSM-5 zeolite with excellent shape-selective ability.
2. Results
2.1. Physicochemical Property of the Twin-Structured Zeolite
The twin-structured ZSM-5 zeolite (denoted as H-ZSM-5T) was prepared by the traditional hydrothermal method. Cu ions were exchanged into the zeolite framework to enrich the acid site density and tailor the acidic property of H-ZSM-5T. It should be noted that in our previous work, the transition metals, such as Zn, Ga, La, Mg, etc., were doped into the zeolitic framework to investigate their effects on tailoring the catalytic performance of H-ZSM-5 zeolite in the methanol-mediated conversion of CO2 + H2 or syngas into aromatics. However, the effect of Cu ions has been rarely clarified. Finally, a non-acidic and amorphous SiO2 shell was coated onto the external surface of Cu-ZSM-5T to further suppress the undesirable alkylation and isomerization reactions that usually occurred on the external surface. The final catalyst was denoted as Cu-ZSM-5T@SiO2.
Multiple techniques were employed to examine the physicochemical properties of the obtained zeolitic catalysts. The X-ray fluorescence (XRF) spectra verified that the Si/Al ratio in H-ZSM-5T is ca. 70. The X-ray diffraction (XRD) patterns of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2 are depicted in Figure 1. All of these three zeolites exhibited typical characteristics of MFI zeolite structure, with notable peaks at 7°, 8°, 23°, and 24°, which were attributed to the (101), (200), (332), and (303) crystal planes of MFI-type zeolite (PDF#44-0003), confirming the successful synthesis of H-ZSM-5 zeolite. The following Cu ion exchange and SiO2 coating steps had no influence on the crystal structure of the samples. No diffraction peaks of Cu species were detected in the XRD patterns, confirming that the ion exchange strategy guarantees the homogeneous dispersion of them in the zeolitic framework.
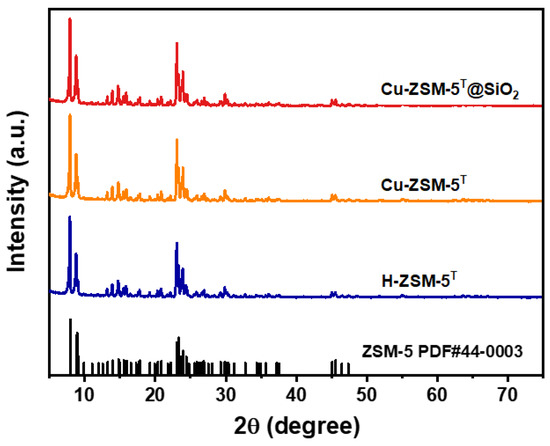
Figure 1.
XRD patterns of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2.
Scanning electron microscopy (SEM) images (Figure 2a) revealed that the synthesized H-ZSM-5T had a grain size of approximately 8 μm along the c-axis, a relatively smooth surface, and an obvious intergrowth structure. Specially, in the case of a single crystal, the body crystal was mostly covered by an intergrowth twin crystal (Figure 2b). The texture property of this twin-structured zeolite has been clarified in detail by Ma et al. On the basis of their high-resolution microscopy, selected-area electron diffraction (SAED), and laser confocal fluorescence results, it is clearly demonstrated that the two 010 planes (up and down) of each main crystal with straight channel openings was mostly covered by two independent twin crystals, and the 100 planes with sinusoidal channel openings were well maintained (Figure 2c and Scheme 2). Owing to the fact that the two independent twin crystals mainly exposed the 100 planes with sinusoidal channels opening along the a-axis, the final twin-structured H-ZSM-5T maximized the opening of sinusoidal channels, accounting for 73% of the total openings on the external surface, which is a great deal higher than that of the classical coffin-shape H-ZSM-5 (ca. 43%). This unique characteristic of sinusoidal channel maximization exposure will enhance the shape-selective capability of the H-ZSM-5 zeolite. The morphology of Cu-ZSM-5T obtained by ion exchange treatment was nearly identical to that of H-ZSM-5T (Figure 2d). However, the SiO2-coated Cu-ZSM-5T@SiO2 catalyst was significantly different from the other two samples, as evidenced by the aggregation between zeolitic crystals (Figure 2e,f). The amorphous SiO2 shell enwrapped several Cu-ZSM-5T crystals to fabricate the core-shell structured capsule catalyst, in which the undesirable external acid sites could be eliminated to some extent. The SEM energy dispersive spectroscopy (EDS) mapping images, as shown in Figure 3, indicated that the Si, Al, and Cu elements are uniformly distributed throughout the synthesized Cu-ZSM-5T@SiO2 capsule catalyst. In particular, the strong Si signals confirmed the successful and complete encapsulation of the twin-structured zeolites by the non-acidic SiO2 shell. Moreover, no regional enrichment of Cu species was detected in the EDS mapping images due to the homogeneous distribution in the zeolite framework, which is in line with the XRD results. Only 1.7 wt% Cu was doped into Cu-ZSM-5T as characterized by XRF, and this value could decrease after SiO2 deposition. As we know, Cu nanoparticles are ideal catalytic components of hydrogenation reaction. During the MTA process catalyzed by acidic zeolite, the existence of Cu nanoparticles could facilitate the hydrogenation of the key reaction intermediates (e.g., unsaturated alkenes or cyclo-alkenes), which is contrary to the dehydrogenation reaction mechanism for aromatics synthesis. Herein, the homogeneously dispersed Cu species (even single atoms) in Cu-ZSM-5T@SiO2 will mainly play their roles in tailoring the acidic property of the zeolite rather than delivering the hydrogenation functions due to the low Cu content and even dispersion in zeolite skeleton. One may also wonder whether the Cu species in acidic zeolite catalyze CO2 + H2 into methanol because Cu-based catalysts are ideal components for methanol synthesis from syngas or CO2 + H2. However, the low Cu content and the homogeneous dispersion in the zeolite skeleton, as confirmed by the XRD, XRF, and SEM-EDS results, make them difficult to use for methanol synthesis. We tested the CO2 hydrogenation performance of the sole Cu-ZSM-5T catalytic component under 350 °C and 3 MPa, and Cu-ZSM-5T exhibited no CO2 hydrogenation activity, which again verifies our viewpoint.
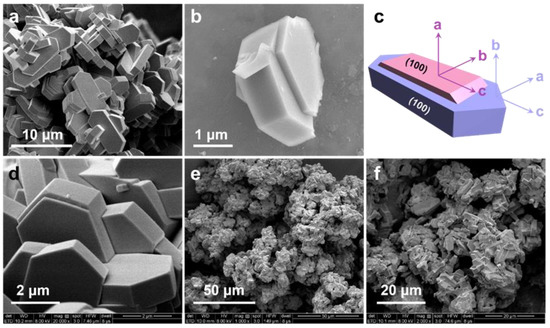
Figure 2.
(a,b) SEM images of H-ZSM-5T, (c) I schematic illustration of the twin-structured zeolite (The a-axis, b-axis, and c-axis are perpendicular to the 100, 010, and 101 crystal planes, respectively), SEM image of Cu-ZSM-5T, (d) and Cu-ZSM-5T@SiO2 (e,f).
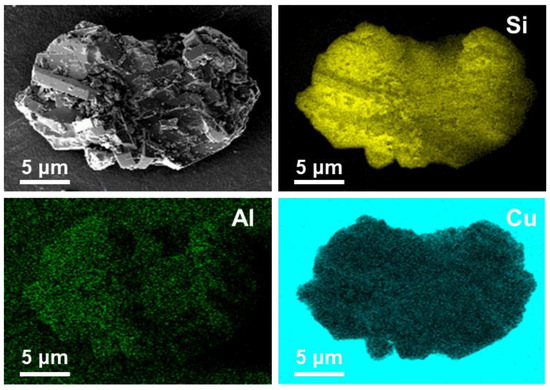
Figure 3.
SEM EDS mapping images of Si, Al, and Cu elements in the Cu-ZSM-5T@SiO2 capsule catalyst.
N2 adsorption and desorption tests were performed on the aforementioned three catalysts to investigate the impacts of Cu doping and SiO2 coating on the specific surface area and pore structure of the zeolites. As demonstrated in Figure 4a, H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2 all displayed type-I(b) N2 adsorption and desorption isotherms. The rapid N2 absorption at low relative pressures indicated that these three zeolitic components consist primarily of micropores. The hysteresis loop at P/Po ~ 0.1 can be ascribed to pore filling into supermicropores or small mesopores [37]. The pore size distribution obtained using the DFT model provided additional evidence of the microporous property of the zeolites (Figure 4b). Additionally, as indicated in Table 1, the specific surface area of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2 declined gradually. This suggests that the introduction of Cu ions occupied part of the pore of the parent H-ZSM-5T zeolite, leading to a decrease in the specific surface area of the Cu-ZSM-5T from 376 m2 g−1 to 372 m2 g−1. Furthermore, subsequent deposition of SiO2 shell on the exterior of the zeolite obstructed the pore mouth of the microporous surface, resulting in further reduction in the specific surface area of Cu-ZSM-5T@SiO2 to 363 m2 g−1. The BET surface area of the three zeolites was indeed very similar, but the microporous and mesoporous specific surface area exhibited a clear variation pattern. Compared to H-ZSM-5T, the microporous specific surface area of Cu-ZSM-5T decreased from 144 m2 g−1 to 129 m2 g−1, indicating that Cu ions occupied part of the micropores of the parent H-ZSM-5T zeolite. Furthermore, subsequent deposition of SiO2 shell on the exterior of the zeolite obstructed the pore mouth of the microporous surface, and the microporous-specific surface area of Cu-ZSM-@SiO2 reduced to 78 m2 g−1. The increase in mesoporous-specific surface area of Cu-ZSM-@SiO2 was attributed to the mesoporous property of SiO2.

Figure 4.
(a) Nitrogen adsorption-desorption isotherms of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2, and (b) pore size distribution calculated by DFT model.

Table 1.
Texture property of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2.
The acid properties of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2 were evaluated using NH3 temperature-programmed desorption experiments. As shown in Figure 5, two distinctive NH3 desorption peaks appeared in all three catalysts, including the low-temperature peak that corresponded to the weak acid sites and the high-temperature peak that corresponded to the medium/strong acid sites. Compared to H-ZSM-5T, the NH3 desorption peak of Cu-ZSM-5T shifted to higher temperatures, indicating the enhanced acid strength due to the formation of Lewis acid sites after Cu ion doping and the synergistic effect between the Brønsted and Lewis acid sites. Meanwhile, the NH3 desorption peak of Cu-ZSM-5T@SiO2 slightly shifted to the low-temperature region relative to Cu-ZSM-5T due to SiO2 deposition on the external surface of the zeolite, leading to the shielding of some acidic sites and the slight weakening of the adsorption capacity for NH3 molecules. The reduction in the undesirable acid sites can suppress undesirable isomerization and alkylation reactions, thereby boosting the PX selectivity [35]. Nonetheless, the acid strength of Cu-ZSM-5T@SiO2 remained greater than that of H-ZSM-5T. Benefiting from the enhanced acid strength after Cu ion doping, the consumption of the reaction intermediates by acid zeolites could be facilitated, therefore continuously and efficiently cleaning up the catalytic interface for intermediate synthesis and shifting the tandem process forward [36].
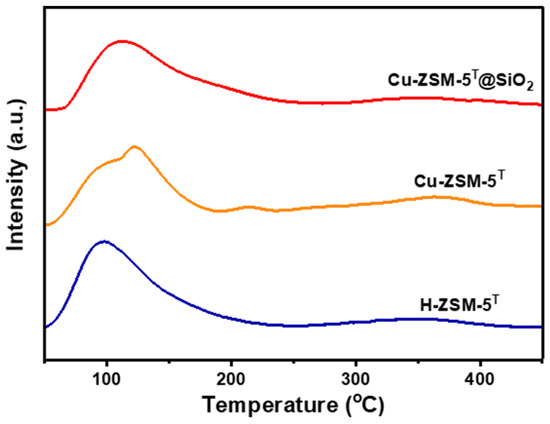
Figure 5.
NH3-TPD profiles of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2.
To analyze the strength of the basic sites of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2, the CO2-TPD experiments were conducted. As Figure 6 shows, all three zeolites displayed one desorption peak in the range of 100~200 °C, which implied that they contained predominantly weak basic sites. As we know, the Brønsted (Si-OH-Al) and Lewis acid (derived from Cu species) in zeolite are the key active sites for methanol conversion into aromatics via dehydration, dehydrogenation, and aromatization steps, and the basic site is deemed to be harmful for aromatics synthesis.
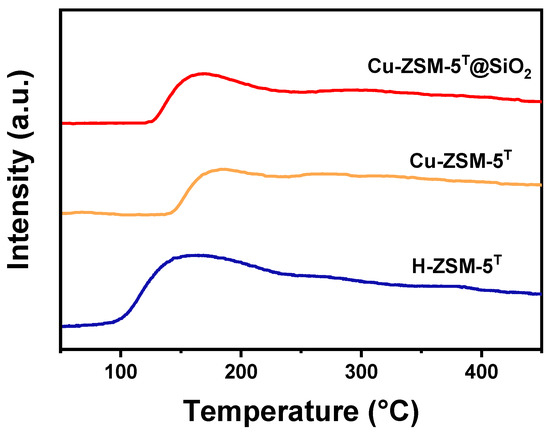
Figure 6.
CO2-TPD profiles of H-ZSM-5T, Cu-ZSM-5T, and Cu-ZSM-5T@SiO2.
The Cr2O3-based catalyst was prepared by subsequently carbonizing and oxidizing the Cr-based metal-organic frameworks’ (MOFs’) precursors (denoted as Cr2O3/C). The MOFs-derived Cr2O3/C catalyst exhibited excellent methanol selectivity of 98.1% (without CO) at a CO2 conversion of 16.8%. The detailed physicochemical properties of the Cr2O3/C catalyst have been investigated in our previous work with the aid of multiple characterization techniques [38]. In this work, we mainly paid attention to improving the PX synthesis performance of the acidic zeolite. The bifunctional catalyst was prepared by physically grinding the methanol synthesis Cr2O3/C catalyst and the acidic zeolite component. With Cr2O3/C&Cu-ZSM-5T as an example, the nanoscale morphology of the bifunctional catalyst was observed by SEM. As shown in Figure 7, the Cr2O3/C nanoparticles with loose and spherical morphology distributed on the external surface of Cu-ZSM-5T homogeneously. The nanoscale size of Cr2O3/C is favorable to enhance the proximity between different catalytic components by increasing the contact area. It should be noted that for tandem catalysis of CO2 + H2 into aromatics, the close proximity between different catalytic components is essential. On the one hand, the close proximity is beneficial for transporting the reaction intermediates from reducible metal oxide to the acidic zeolite for aromatics synthesis via the tandem process. On the other hand, the aromatics synthesis process follows the dehydrogenation mechanism. The continuous extraction of H* species from the zeolite component is the prerequisite for the success of aromatics synthesis. The reducible metal oxide as the H* species acceptor and consumer plays a key role during the dehydrogenation process by extracting and consuming the H* species, and the close proximity between the reducible metal oxide and acidic zeolite could facilitate this dehydrogenation process, therefore boosting the aromatics synthesis process.
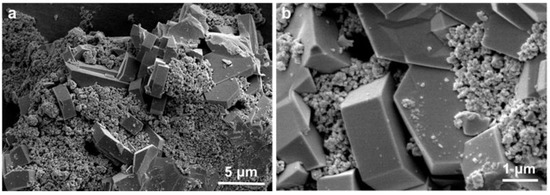
Figure 7.
(a,b) SEM images of Cr2O3&H-ZSM-5T.
2.2. The Catalytic Performance of PX Synthesis from CO2 Hydrogenation
To investigate the effect of different catalysts on PX selectivity, a CO2 hydrogenation reaction of Cr2O3/C&H-ZSM-5T, Cr2O3/C&Cu-ZSM-5T, and Cr2O3/C&Cu-ZSM-5T@SiO2 was conducted under 350 °C and 3 Mpa (Figure 8, Table 2 and Table 3). Cr2O3/C&H-ZSM-5T exhibited an aromatics selectivity of 78.7% at a CO2 conversion of 17.2%, with PX selectivity reaching 28.7%. The PX selectivity obtained from the bare H-ZSM-5T zeolite significantly outperformed that of the classical coffin-shape H-ZSM-5, which can be attributed to the unique twin structure with dominant sinusoidal channel openings. After Cu ion doping and CO2 conversion, the aromatics selectivity of Cr2O3/C&Cu-ZSM-5T increased to 19.2% and 80.4%, respectively, while the PX selectivity decreased slightly (27.4%). This catalytic performance variation trend confirmed that Cu ion doping is favorable to increase the CO2 transformation efficiency and the total aromatics selectivity due to the enhanced acid strength and aromatization capability of Cu-ZSM-5T. Even though Cu ion doping slightly increased the space-time yield (STY) of PX, owing to the enhanced CO2 conversion, the PX selectivity from Cr2O3/C&Cu-ZSM-5T was decreased due to the reduced shape-selective capability of Cu-ZSM-5T. There is no doubt that the Cu ions in the zeolite framework could act as Lewis acid sites, as well as enhance the acid strength by cooperating with the originally existing Brønsted acid, but the ion exchange strategy could also introduce undesirable acid sites on the external surface or pore mouth of microporous channels of Cu-ZSM-5T, therefore decreasing its shape-selective property.
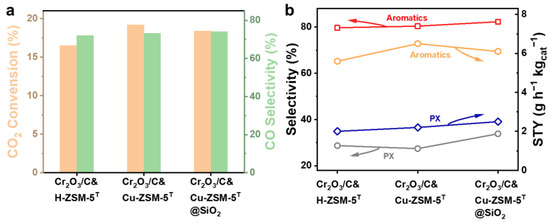
Figure 8.
Catalytic performance of the bifunctional catalysts composed of Cr2O3/C and H-ZSM-5T or Cu-ZSM-5T or Cu-ZSM-5T@SiO2: (a) CO2 conversion and CO selectivity, (b) space-time yield of (STY) and selectivity of PX and aromatics. Reaction conditions: 350 °C, 3 Mpa, (24.3% CO2, 71.8% H2, and 3.9% Ar), GHSV = 1200 mL gcat−1 h−1, time on stream (TOS) = 8 h, metal oxide/zeolite mass ratio = 1, and catalyst of 1 g.

Table 2.
Catalytic performance of Cr2O3/C&H-ZSM-5T, Cr2O3/C&Cu-ZSM-5T, and Cr2O3/C&Cu-ZSM-5T@SiO2 for CO2 hydrogenation into aromatics a.

Table 3.
Products distribution in aromatics.
Therefore, our attention turned to erasing the undesirable external acid sites by coating a non-acidic SiO2 shell onto the external surface of Cu-ZSM-5T by chemical liquid deposition. Benefiting from the cooperative interplay between the enhanced acid strength via Cu ion doping and the erasing of the undesirable acid sites via SiO2 shell coating, the selectivity of total aromatics and PX were increased to 82.3% and 33.8%, respectively, via the bifunctional catalyst of Cr2O3/C&Cu-ZSM-5T@SiO2. Even though the CO2 conversion slightly decreased to 18.4%, the STY of PX from Cr2O3/C&Cu-ZSM-5T@SiO2 (3.0 gCH2 h−1 kgcat−1) was superior to that from the counterparts Cr2O3/C&H-ZSM-5T (2.5 gCH2 h−1 kgcat−1) and Cr2O3/C&Cu-ZSM-5T (2.6 gCH2 h−1 kgcat−1).
3. Materials and Methods
3.1. Materials and Chemicals
The chromium nitrate nonahydrate [Cr(NO3)3·9H2O], terephthalic acid (PTA), concentrated nitric acid, sodium aluminate (NaAlO2), tetrapropylammonium bromide (TPABr), n-butylamine, silica sol, copper nitrate trihydrate [Cu(NO3)2·3H2O], ethanol, n-hexane, and tetraethyl orthosilicate (TEOS) that purchased from Aladdin Biochemical Technology Co., Ltd (Shanghai, China) were of analytical grade and utilized without further purification. The deionized water used for catalyst preparation had a resistivity of 18.2 megohms and was obtained through an ultrapure water system (Milli-Q, Merck, Germany).
3.2. Catalyst Preparation
3.2.1. Synthesis of Cr2O3/C Catalyst
Scheme 3 shows the preparation process of MOFs-derived, Cr-based catalysts. Cr-MOFs were synthesized via a hydrothermal method. Typically, Cr(NO3)3·9H2O of 10.0 mmol (4.00 g) and PTA of 10.0 mmol (1.66 g) were dissolved into the deionized water of 48 mL. Subsequently, concentrated nitric acid of 680 μL was added dropwise under stirring, and the mixture was stirred for 30 min to ensure complete phase dispersion. The resulting solution was transferred into an 80 mL Teflon-lined autoclave and kept at 220 °C for 8 h. Green crystalline products obtained after centrifugation underwent washing three times with deionized water and ethanol, followed by drying overnight at 60 °C in an oven. These products were then pyrolyzed at 500 °C for 2 h in a tube furnace under an N2 atmosphere with a heating rate of 5 °C min−1 to obtain the black powders. Finally, calcination of the aforementioned powders was undertaken at 500 °C for 2 h in a muffle furnace under air atmosphere with a heating rate of 5 °C min−1 yielded the Cr-based catalyst, denoted as Cr2O3/C.
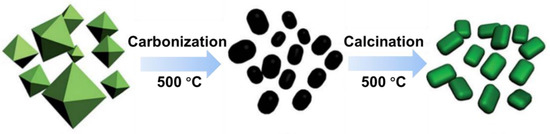
Scheme 3.
Preparation process of MOFs-derived Cr-based catalysts.
3.2.2. Synthesis of H-ZSM-5T
An H-ZSM-5T zeolite with twin-structure was synthesized via a hydrothermal method. NaAlO2 (0.080 g), TPABr (1.834 g), and n-butylamine (3.916 g) were dissolved into 91.5 mL of deionized water by stirring for 15 min. Subsequently, silica sol of 22.53 g was slowly added dropwise under stirring for 30 min. The resulting solution was divided evenly into two 80 mL Teflon-lined autoclaves, fixed on the rotary support of a rotary blast drying oven, and kept at a temperature of 180 °C for 48 h with rotating speed of 1.5 rpm. The obtained emulsion was centrifugally washed three times by deionized water, followed by drying overnight at 60 °C to acquire the H-ZSM-5T precursor. Calcination of the precursor was undertaken at 550 °C for 6 h in a muffle furnace with a heating rate of 5 °C min−1 to remove the organic template in H-ZSM-5T.
3.2.3. Synthesis of Cu-ZSM-5T
The Cu-H-ZSM-5T zeolite was synthesized via an ion exchange method. Initially, Cu(NO3)2·3H2O (6.5 mmol, 1.57 g) was dissolved into 91.5 mL of deionized water, followed by the addition of H-ZSM-5T of 1.3 g under stirring. The resulting mixture was moved to an 80 °C oil bath and stirred for 15 h for ion exchange. After ion exchange, the product was centrifugally washed three times by deionized water and dried overnight at 60 °C in an oven. The dried product was then calcined at 500 °C for 3 h in a muffle furnace to yield the Cu-ZSM-5T zeolite.
3.2.4. Synthesis of Cu-ZSM-5T@SiO2
The Cu-ZSM-5T@SiO2 capsule catalyst was obtained by employing TEOS to coat the external surface of Cu-ZSM-5T via a chemical liquid deposition method. Initially, Cu-ZSM-5T of 1 g was dispersed into n-hexane of 8 mL via ultrasonication for 15 min to prevent agglomeration. Next, TEOS of 2.25 g was slowly added dropwise to the solution under stirring for 4 h. The resulting mixture was further transferred to an 80 °C oil bath until complete evaporation of n-hexane. Finally, the coated molecular sieve was calcined at 500 °C for 2 h in a muffle furnace with a heating rate of 5 °C min−1 to yield the Cu-ZSM-5T@SiO2 capsule catalyst. Scheme 4 shows the preparation process of Cu-ZSM-5T and Cu-ZSM-5T@SiO2.
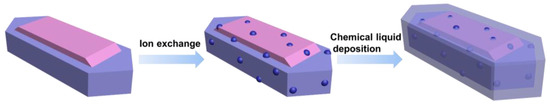
Scheme 4.
Preparation process of Cu-ZSM-5T and Cu-ZSM-5T@SiO2.
3.3. Catalyst Characterization
X-ray diffraction (XRD) analysis of the obtained catalysts was conducted using Cu Kα radiation and a Rigaku RINT 2400 X-ray diffractometer (Tokyo, Japan). The morphology of the catalysts was observed via scanning electron microscopy (SEM) on a Zeiss Genimi 300 instrument (Oberkochen, Germany) equipped with an energy dispersive spectroscopy (EDS) system using an Oxford Inca Energy X-max 50 X-ray EDS detector. Furthermore, the specific surface area and pore size distribution of the samples were determined by a Micromeritics 3Flex 2MP instrument (Norcross, GA, USA). The surface area was determined by the Brunauer–Emmett–Teller (BET) method using a relative pressure (P/Po) in the range of 0.05–0.3. The pore size distribution was calculated by the non-local density functional theory (NLDFT) method. NH3 temperature-programmed desorption (NH3-TPD) and CO2 temperature-programmed desorption (CO2-TPD) experiments were carried out on a MicrotracBEL BELCATII-T-SP instrument (Osaka, Japan). Prior to the experiment, the catalyst was pretreated at 150 °C for 1 h to eliminate the H2O present in the microporous zeolite. Subsequently, the sample was saturated with NH3 or CO2 at 50 °C and the physisorbed NH3 or CO2 was removed using a He flow. Furthermore, the desorption of NH3 or CO2 was recorded under an He flow with a heating rate of 10 °C min−1. X-ray fluorescence (XRF) spectroscopy was employed to evaluate the Si/Al ratio in H-ZSM-5T and the Cu content in Cu-ZSM-5T.
3.4. Catalytic Activity Evaluation
Scheme 5 shows the CO2 hydrogenation reactor for investigating the activities of the catalysts. The catalytic performance of the catalysts was measured in a fixed-bed reactor (ZXBLUE Co., Ltd., Beijing, China) with an internal diameter of 8 mm. Prior to the reaction, the catalyst was reduced by pure H2 at 400 °C for 2 h. After cooling down to the room temperature, the reactant gas (24.3% CO2, 71.8% H2, and 3.9% Ar) was fed into the reactor until the pressure reached 3 MPa. At the same time, the temperature of the reactor was increased to 350 °C. The exhaust gas was analyzed by an online gas chromatograph (Fuli 9790II, Fuli Analytical Instruments Co., Ltd., Zhejiang, China) equipped with two detectors (thermal conductivity detector (TCD) and flame ionization detector (FID)). The TCD was connected to the TDX-01 (60–80 mesh, 3 mm × 2 m) and Porapak-Q (60–80 mesh, 3 mm × 1 m) packed columns for the analysis of Ar, CO, CH4, and CO2, and the FID was connected to the HP-PLOT/Q capillary column (0.53 mm × 30 m) to detect gas-phase hydrocarbons. An off-line gas chromatograph (Fuli 9790II, Fuli Analytical Instruments Co., Ltd., Zhejiang, China) equipped with an FID and two separated columns (DB-1 and DB-WAX) was employed to further identify the xylene isomers in the collected liquid products. In order to suppress the possible condensation during the reaction process, the pipeline from the catalyst bed to the gas chromatograph was heated at 190 °C. This type of catalytic performance test instrument is highly efficient due to the advantage of quick and in-time product analysis.
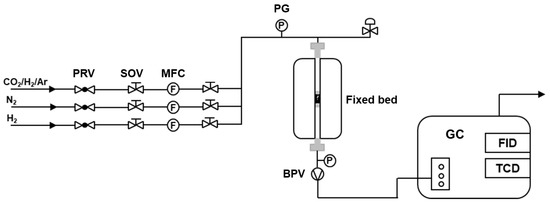
Scheme 5.
Schematic of CO2 hydrogenation reactor.
CO2 conversion and product selectivity were calculated by the following equations.
(1) CO2 conversion was calculated according to:
where and represent moles of the CO2 at the inlet and outlet, respectively.
(2) Product selectivity was calculated as the percentage of CO2 converted into a given product and according to:
where and represent the mole percentage and carbon number of product i.
(3) CO selectivity was calculated according to:
Typically, the experimental data after the reaction of 8 h were used for discussion.
4. Conclusions
In conclusion, an efficient bifunctional catalyst composed of an MOFs-derived Cr2O3/C and Cu ion doped H-ZSM-5T capsule catalyst has been developed for the direct conversion of CO2 + H2 into valuable aromatics, especially PX, via the methanol-mediated tandem process. We proposed a comprehensive strategy to boost the PX selectivity, in which the texture and acid properties of the acidic zeolite were systematically considered. The twin-structured H-ZSM-5T with dominant sinusoidal channel openings was beneficial for increasing the PX selectivity owing to its enhanced shape-selective capability and controlled PX diffusion dynamic. Furthermore, Cu ion doping and SiO2 coating were subsequently employed to enhance the acid strength and erase the undesirable external acid sites, respectively. Benefiting from the multiple zeolite modification steps, the PX selectivity and STY were increased to 33.8% and 3.0 gCH2 h−1 kgcat−1, respectively, via the bifunctional catalyst Cr2O3/C&Cu-ZSM-5T@SiO2. With the aid of catalyst characterization, it is concluded that the dominant sinusoidal channel openings and external acid site suppression guaranteed the increased PX selectivity. At the same time, the enhanced acid strength stems from Cu ion doping and could increase the CO2 conversion by facilitating the consumption of key reaction intermediates and strengthening the driving force in the tandem process. We anticipate that the comprehensive strategy proposed in this work could guide the rational design of bifunctional catalysts for the oriented conversion of CO2 + H2 or syngas into the targeted product.
Author Contributions
Conceptualization, S.L. and Y.W.; methodology, S.L. and R.H.; validation, S.L., W.W. and Y.G.; formal analysis, S.L., R.H., W.W. and Q.L.; investigation, S.L. and R.H.; resources, Y.W. and M.W.; writing—original draft preparation, S.L.; writing—review and editing, Y.W. and M.W.; visualization, S.L., R.H., W.W. and Y.G.; supervision, Y.W. and M.W.; project administration, Y.W. and M.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (22108310), the Shandong Province Key Research Program (Major Scientific and Technological Innovation Project, 2020CXGC010402), the Science and Technology Innovation Project of the Shandong Energy Group Co., Ltd. (SNKJ2021BJ04, SNKJ2023A03), the CNPC Innovation Foundation (2020D-5007-0407), and the Foundation of State Key Laboratory of High-Efficiency Utilization of Coal and Green Chemical Engineering (2022-K-75).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ding, M.; Flaig, R.W.; Jiang, H.-L.; Yaghi, O.M. Carbon capture and conversion using metal–organic frameworks and MOF-based materials. Chem. Soc. Rev. 2019, 48, 2783–2828. [Google Scholar] [CrossRef]
- Feldman, D.R.; Collins, W.D.; Gero, P.J.; Torn, M.S.; Mlawer, E.J.; Shippert, T.R. Observational determination of surface radiative forcing by CO2 from 2000 to 2010. Nature 2015, 519, 339–343. [Google Scholar] [CrossRef]
- Keith, D.W. Why capture CO2 from the atmosphere? Science 2009, 325, 1654–1655. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.; Zhou, C.; Subramanian, V.; Zhang, Q.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Wang, W.-H.; Himeda, Y.; Muckerman, J.T.; Manbeck, G.F.; Fujita, E. CO2 hydrogenation to formate and methanol as an alternative to photo- and electrochemical CO2 reduction. Chem. Rev. 2015, 115, 12936–12973. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Han, Y.; Ge, Q.; Sun, J. Towards the development of the emerging process of CO2 heterogenous hydrogenation into high-value unsaturated heavy hydrocarbons. Chem. Soc. Rev. 2021, 50, 10764–10805. [Google Scholar] [CrossRef]
- Lyons, T.W.; Guironnet, D.; Findlater, M.; Brookhart, M. Synthesis of p-Xylene from ethylene. J. Am. Chem. Soc. 2012, 134, 15708–15711. [Google Scholar] [CrossRef]
- Tsubaki, N.; Wang, Y.; Yang, G.; He, Y. Rational design of novel reaction pathways and tailor-made catalysts for value-added chemicals synthesis from CO2 hydrogenation. Bull. Chem. Soc. Jpn. 2023, 96, 291–302. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.; Wu, M.; Tsubaki, N.J.E. Thermocatalytic hydrogenation of CO2 into aromatics by tailor-made catalysts: Recent advancements and perspectives. EcoMat. 2021, 3, e12080. [Google Scholar] [CrossRef]
- Tian, G.; Zhang, C.; Wei, F. COx conversion to aromatics: A mini-review of nanoscale performance. Nanoscale Horiz. 2022, 7, 1478–1487. [Google Scholar] [CrossRef]
- Wang, D.; Xie, Z.H.; Porosoff, M.; Chen, J.G. Recent advances in carbon dioxide hydrogenation to produce olefins and aromatics. Chem 2021, 7, 2277–2311. [Google Scholar] [CrossRef]
- Song, G.; Li, M.; Yan, P.; Nawaz, M.A.; Liu, D. High conversion to aromatics via CO2-FT over a CO-reduced Cu-Fe2O3 Catalyst integrated with HZSM-5. ACS Catal. 2020, 10, 11268–11279. [Google Scholar] [CrossRef]
- Wang, Y.; Kazumi, S.; Gao, W.; Gao, X.; Li, H.; Guo, X.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Direct conversion of CO2 to aromatics with high yield via a modified Fischer-Tropsch synthesis pathway. Appl. Catal. B Environ. 2020, 269, 118792. [Google Scholar] [CrossRef]
- Cui, X.; Gao, P.; Li, S.; Yang, C.; Liu, Z.; Wang, H.; Zhong, L.; Sun, Y. Selective production of aromatics directly from carbon dioxide hydrogenation. ACS Catal. 2019, 9, 3866–3876. [Google Scholar] [CrossRef]
- Xu, Y.; Shi, C.; Liu, B.; Wang, T.; Zheng, J.; Li, W.; Liu, D.; Liu, X. Technology. Selective production of aromatics from CO2. Catal. Sci. Technol. 2019, 9, 593–610. [Google Scholar] [CrossRef]
- Wei, J.; Yao, R.; Ge, Q.; Xu, D.; Fang, C.; Zhang, J.; Xu, H.; Sun, J. Precisely regulating Bronsted acid sites to promote the synthesis of light aromatics via CO2 hydrogenation. Appl. Catal. B. Environ. 2021, 283, 119648. [Google Scholar] [CrossRef]
- Wang, Y.; Tan, L.; Tan, M.; Zhang, P.; Fang, Y.; Yoneyama, Y.; Yang, G.; Tsubaki, N. Rationally designing bifunctional catalysts as an efficient strategy to boost CO2 hydrogenation producing value-added aromatics. ACS Catal. 2019, 9, 895–901. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Chen, S.; Wang, X.; Zhou, Z.; Wu, Y.; Zhang, T.; Yang, G.; Han, Y.; Tan, Y. Hydrogenation of CO2 into aromatics over a ZnCrOx–zeolite composite catalyst. Chem. Commun. 2019, 55, 973–976. [Google Scholar] [CrossRef]
- Zhou, C.; Shi, J.; Zhou, W.; Cheng, K.; Zhang, Q.; Kang, J.; Wang, Y. Highly active ZnO-ZrO2 aerogels integrated with H-ZSM-5 for aromatics synthesis from carbon dioxide. ACS Catal. 2020, 10, 302–310. [Google Scholar] [CrossRef]
- Ni, Y.; Chen, Z.; Fu, Y.; Liu, Y.; Zhu, W.; Liu, Z. Selective conversion of CO2 and H2 into aromatics. Nat. Commun. 2018, 9, 3457. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Kazumi, S.; Li, H.; Yang, G.; Tsubaki, N. Direct and oriented conversion of CO2 into value-added aromatics. Chem. Eur. J. 2019, 25, 5149–5153. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qu, Y.; Wang, J.; Liu, H.; Li, M.; Miao, S.; Li, C. Highly selective conversion of carbon dioxide to aromatics over tandem catalysts. Joule 2019, 3, 570–583. [Google Scholar] [CrossRef]
- Liu, Y.; Qiang, W.; Ji, T.; Zhang, M.; Li, M.; Lu, J.; Lu, Y. Uniform hierarchical MFI nanosheets prepared via anisotropic etching for solution-based sub–100-nm-thick oriented MFI layer fabrication. Sci. Adv. 2020, 6, eaay5993. [Google Scholar] [CrossRef]
- Dai, W.; Kouvatas, C.; Tai, W.; Wu, G.; Guan, N.; Li, L.; Valtchev, V. Platelike MFI crystals with controlled crystal faces aspect ratio. J. Am. Chem. Soc. 2021, 143, 1993–2004. [Google Scholar] [CrossRef]
- Chen, N.Y.; Kaeding, W.W.; Dwyer, F.G. Para-directed aromatic reactions over shape-selective molecular sieve zeolite catalysts. J. Am. Chem. Soc. 1979, 101, 6783–6784. [Google Scholar] [CrossRef]
- Cheng, Y.-T.; Wang, Z.; Gilbert, C.J.; Fan, W.; Huber, G.W. Production of p-Xylene from biomass by catalytic fast pyrolysis using ZSM-5 catalysts with reduced pore openings. Angew. Chem. 2012, 124, 11259–11262. [Google Scholar] [CrossRef]
- Vu, D.V.; Miyamoto, M.; Nishiyama, N.; Egashira, Y.; Ueyama, K. Selective formation of para-xylene over H-ZSM-5 coated with polycrystalline silicalite crystals. J. Catal. 2006, 243, 389–394. [Google Scholar] [CrossRef]
- Miyake, K.; Hirota, Y.; Ono, K.; Uchida, Y.; Tanaka, S.; Nishiyama, N. Direct and selective conversion of methanol to para-xylene over Zn ion doped ZSM-5/silicalite-1 core-shell zeolite catalyst. J. Catal. 2016, 342, 63–66. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, W.; Kong, C.; Wei, F. Increasing para-Xylene selectivity in making aromatics from methanol with a surface-modified Zn/P/ZSM-5 catalyst. ACS Catal. 2015, 5, 2982–2988. [Google Scholar] [CrossRef]
- Lu, P.; Fei, Z.; Li, L.; Feng, X.; Ji, W.; Ding, W.; Chen, Y.; Yang, W.; Xie, Z. Effects of controlled SiO2 deposition and phosphorus and nickel doping on surface acidity and diffusivity of medium and small sized HZSM-5 for para-selective alkylation of toluene by methanol. Appl. Catal. A Gen. 2013, 453, 302–309. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, L.; Yang, G.; Tsubaki, N. One-pass selective conversion of syngas to para-xylene. Chem. Sci. 2017, 8, 7941–7946. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Wu, Q.; Wang, C.; Guo, X.; He, Y.; Zhang, P.; Yang, G.; Liu, G.; Wu, J.; et al. Capsule-like zeolite catalyst fabricated by solvent-free strategy for para-Xylene formation from CO2 hydrogenation. Appl. Catal. B Environ. 2022, 303, 120906. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Huang, X.; Zhu, Y.; Li, G.; Gu, Q.; Chen, J.; Ma, L.; Li, X.; He, Q.; et al. Maximizing sinusoidal channels of HZSM-5 for high shape-selectivity to p-xylene. Nat. Commun. 2019, 10, 4348. [Google Scholar] [CrossRef]
- Gao, W.; Guo, L.; Cui, Y.; Yang, G.; He, Y.; Zeng, C.; Taguchi, A.; Abe, T.; Ma, Q.; Yoneyama, Y.; et al. Selective conversion of CO2 into para-Xylene over a ZnCr2O4-ZSM-5 catalyst. ChemSusChem 2020, 13, 6541–6545. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, C.; Gao, P.; Zhou, S.; Li, S.; Wang, H.; Sun, Y. ZnZrOx integrated with chain-like nanocrystal HZSM-5 as efficient catalysts for aromatics synthesis from CO2 hydrogenation. Appl. Catal. B Environ. 2021, 286, 119929. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, W.; Wang, K.; Gao, X.; Zhang, B.; Zhao, H.; Ma, Q.; Zhang, P.; Yang, G.; Wu, M.; et al. Boosting the synthesis of value-added aromatics directly from syngas via a Cr2O3 and Ga doped zeolite capsule catalyst. Chem. Sci. 2021, 12, 7786–7792. [Google Scholar] [CrossRef]
- Yang, Z.X.; Xia, Y.D.; Mokaya, R. Zeolite ZSM-5 with unique supermicropores synthesized using mesoporous carbon as a template. Adv. Mater. 2004, 16, 727–732. [Google Scholar] [CrossRef]
- Wang, W.; He, R.; Wang, Y.; Li, M.; Liu, J.; Liang, J.; Yasuda, S.; Liu, Q.; Wu, M.; Tsubaki, N. Boosting methanol-mediated CO2 hydrogenation into aromatics by synergistically tailoring oxygen vacancy and acid site properties of multifunctional catalyst. Chem. A Eur. J. 2023, e202301135. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).