Biosynthesis of Furfurylamines in Batch and Continuous Flow by Immobilized Amine Transaminases
Abstract
1. Introduction
2. Results and Discussion
2.1. Activity of Soluble ATAs towards HMF and DFF
2.2. Immobilization of ATAs
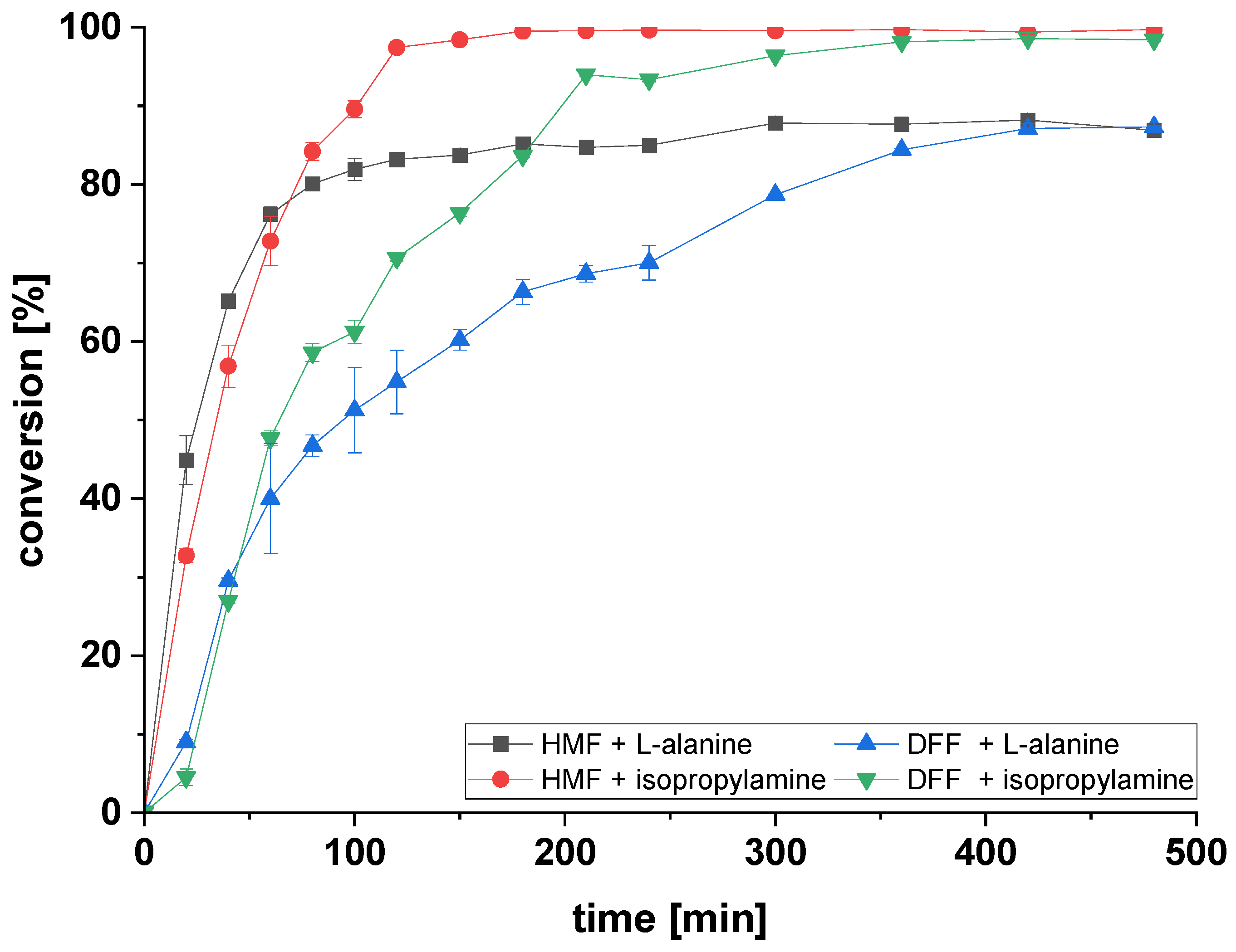
2.3. Amination of HMF and DFF Using Immobilized ATA-Spo in Batch
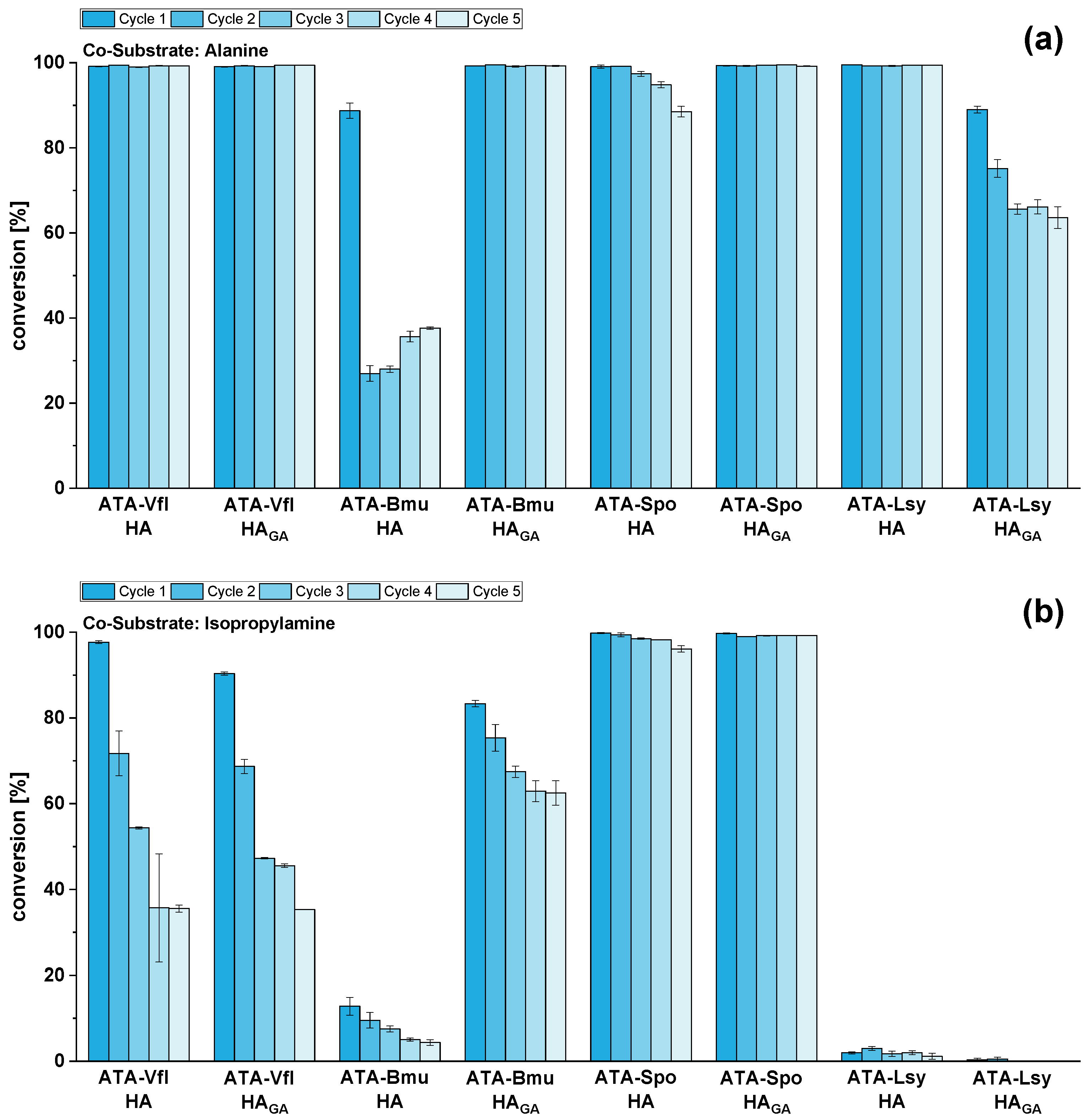
2.4. Reusability of Immobilized ATAs for Amination of HMF Using Alanine or Isopropylamine
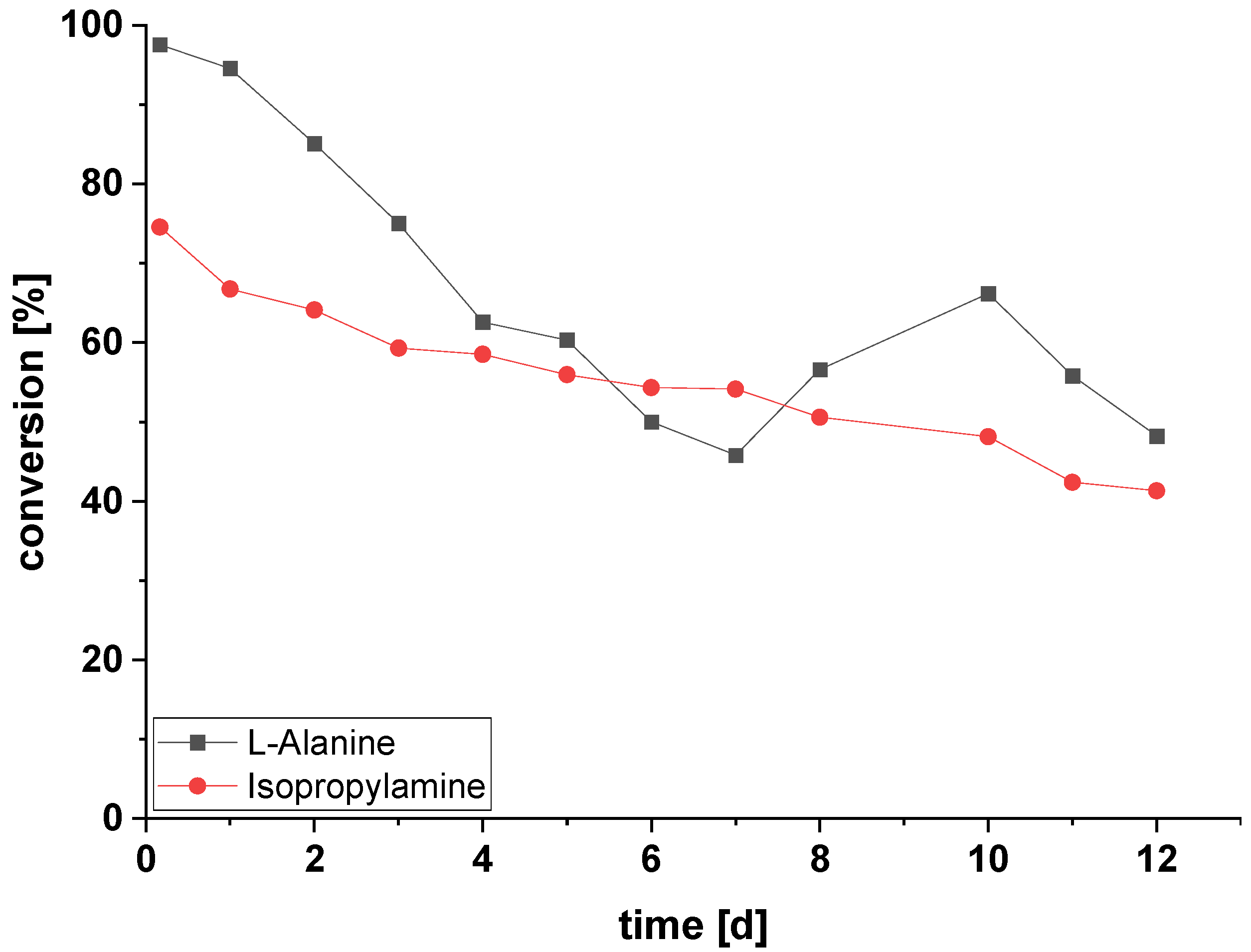
2.5. Amination of HMF in Continuous Flow Using Immobilized ATA-Spo with Alanine or Isopropylamine
3. Materials and Methods
3.1. General Information
3.2. Preparation of Glutaraldehyde-Functionalized Amine Beads
3.3. Cloning of the Aldehyde-tagged ATA-Spo
3.4. Expression and Purification of Enzymes
3.5. Conversion of the Aldehyde Tag
3.6. Immobilization of ATAs on the Solid Supports
3.7. Activity Assay of Soluble and Immobilized ATAs
3.8. Batch Reusability Study
3.9. Continuous Flow Catalysis
3.10. HPLC Analysis
3.11. Determination of Protein Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef] [PubMed]
- Hayes, K.S. Industrial Processes for Manufacturing Amines. Appl. Catal. A Gen. 2001, 221, 187–195. [Google Scholar] [CrossRef]
- Ragno, D.; Brandolese, A.; Di Carmine, G.; Buoso, S.; Belletti, G.; Leonardi, C.; Bortolini, O.; Bertoldo, M.; Massi, A. Exploring Oxidative NHC-Catalysis as Organocatalytic Polymerization Strategy towards Polyamide Oligomers. Chem. A Eur. J. 2021, 27, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Dangerfield, E.M.; Plunkett, C.H.; Win-Mason, A.L.; Stocker, B.L.; Timmer, M.S.M. Protecting-Group-Free Synthesis of Amines: Synthesis of Primary Amines from Aldehydes via Reductive Amination. J. Org. Chem. 2010, 75, 5470–5477. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; ISBN 9780333227794. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass Volume I.; DOE/GO-102004-1992; National Renewable Energy Lab: Golden, CO, USA, 2004. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; He, M.; Ren, H.; Zhou, S.; Lai, D.; Wang, Z.; Jiang, L. Semi-Rational Directed Evolution of Monoamine Oxidase for Kinetic Resolution of Rac-Mexiletine. Appl. Biochem. Biotechnol. 2015, 176, 2267–2278. [Google Scholar] [CrossRef] [PubMed]
- Eve, T.S.C.; Wells, A.; Turner, N.J. Enantioselective Oxidation of O-Methyl-N-Hydroxylamines Using Monoamine Oxidase N as Catalyst. Chem. Commun. 2007, 1530–1531. [Google Scholar] [CrossRef] [PubMed]
- Mitsukura, K.; Suzuki, M.; Tada, K.; Yoshida, T.; Nagasawa, T. Asymmetric Synthesis of Chiral Cyclic Amine from Cyclic Imine by Bacterial Whole-Cell Catalyst of Enantioselective Imine Reductase. Org. Biomol. Chem. 2010, 8, 4533–4535. [Google Scholar] [CrossRef]
- Wetzl, D.; Gand, M.; Ross, A.; Müller, H.; Matzel, P.; Hanlon, S.P.; Müller, M.; Wirz, B.; Höhne, M.; Iding, H. Asymmetric Reductive Amination of Ketones Catalyzed by Imine Reductases. ChemCatChem 2016, 8, 2023–2026. [Google Scholar] [CrossRef]
- Poulhès, F.; Vanthuyne, N.; Bertrand, M.P.; Gastaldi, S.; Gil, G. Chemoenzymatic Dynamic Kinetic Resolution of Primary Amines Catalyzed by CAL-B at 38–40 °C. J. Org. Chem. 2011, 76, 7281–7286. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Y.; Zhang, D.; Feng, Y.; Li, J. Efficient Kinetic Resolution of Amino Acids Catalyzed by Lipase AS ‘Amano’ via Cleavage of an Amide Bond. Tetrahedron Asymmetry 2012, 23, 1338–1342. [Google Scholar] [CrossRef]
- Oliveira, E.F.; Cerqueira, N.M.F.S.A.; Fernandes, P.A.; Ramos, M.J. Mechanism of Formation of the Internal Aldimine in Pyridoxal 5′-Phosphate-Dependent Enzymes. J. Am. Chem. Soc. 2011, 133, 15496–15505. [Google Scholar] [CrossRef] [PubMed]
- Cassimjee, K.E.; Manta, B.; Himo, F. A Quantum Chemical Study of the ω-Transaminase Reaction Mechanism. Org. Biomol. Chem. 2015, 13, 8453–8464. [Google Scholar] [CrossRef] [PubMed]
- Slabu, I.; Galman, J.L.; Lloyd, R.C.; Turner, N.J. Discovery, Engineering, and Synthetic Application of Transaminase Biocatalysts. ACS Catal. 2017, 7, 8263–8284. [Google Scholar] [CrossRef]
- Steffen-Munsberg, F.; Vickers, C.; Kohls, H.; Land, H.; Mallin, H.; Nobili, A.; Skalden, L.; van den Bergh, T.; Joosten, H.-J.; Berglund, P.; et al. Bioinformatic Analysis of a PLP-Dependent Enzyme Superfamily Suitable for Biocatalytic Applications. Biotechnol. Adv. 2015, 33, 566–604. [Google Scholar] [CrossRef] [PubMed]
- Dunbabin, A.; Subrizi, F.; Ward, J.M.; Sheppard, T.D.; Hailes, H.C. Furfurylamines from Biomass: Transaminase Catalysed Upgrading of Furfurals. Green Chem. 2017, 19, 397–404. [Google Scholar] [CrossRef]
- Chatterjee, M.; Ishizaka, T.; Kawanami, H. Reductive Amination of Furfural to Furfurylamine Using Aqueous Ammonia Solution and Molecular Hydrogen: An Environmentally Friendly Approach. Green Chem. 2016, 18, 487–496. [Google Scholar] [CrossRef]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel Routes in Transformation of Lignocellulosic Biomass to Furan Platform Chemicals: From Pretreatment to Enzyme Catalysis. Catalysts 2020, 10, 743. [Google Scholar] [CrossRef]
- van Putten, R.-J.; van der Waal, J.C.; de Jong, E.; Rasrendra, C.B.; Heeres, H.J.; de Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Yuan, H.; Li, J.P.; Su, F.; Yan, Z.; Kusema, B.T.; Streiff, S.; Huang, Y.; Pera-Titus, M.; Shi, F. Reductive Amination of Furanic Aldehydes in Aqueous Solution over Versatile NiyAlOx Catalysts. ACS Omega 2019, 4, 2510–2516. [Google Scholar] [CrossRef]
- Dijkman, W.P.; Groothuis, D.E.; Fraaije, M.W. Enzyme-Catalyzed Oxidation of 5-Hydroxymethylfurfural to Furan-2,5-Dicarboxylic Acid. Angew. Chemie Int. Ed. 2014, 53, 6515–6518. [Google Scholar] [CrossRef]
- Hu, L.; Lin, L.; Wu, Z.; Zhou, S.; Liu, S. Recent Advances in Catalytic Transformation of Biomass-Derived 5-Hydroxymethylfurfural into the Innovative Fuels and Chemicals. Renew. Sustain. Energy Rev. 2017, 74, 230–257. [Google Scholar] [CrossRef]
- Petri, A.; Masia, G.; Piccolo, O. Biocatalytic Conversion of 5-Hydroxymethylfurfural: Synthesis of 2,5-Bis(Hydroxymethyl)Furan and 5-(Hydroxymethyl)Furfurylamine. Catal. Commun. 2018, 114, 15–18. [Google Scholar] [CrossRef]
- Qin, Y.Z.; Li, Y.M.; Zong, M.H.; Wu, H.; Li, N. Enzyme-Catalyzed Selective Oxidation of 5-Hydroxymethylfurfural (HMF) and Separation of HMF and 2,5-Diformylfuran Using Deep Eutectic Solvents. Green Chem. 2015, 17, 3718–3722. [Google Scholar] [CrossRef]
- Ponto, L.L.; Schoenwald, R.D. Furosemide (Frusemide). A Pharmacokinetic/Pharmacodynamic Review (Part I). Clin. Pharmacokinet. 1990, 18, 381–408. [Google Scholar] [CrossRef]
- Öǧütlü, A.; Genç, H.; Dursun, T.; Zengin, M.; Karabay, O. A New Antiseptic-Furfurylamine Biguanidine Derivative Synthesis and Its Effect on Multi-Drug Resistant Acinetobacter Baumannii Strains. Acta Med. Mediterr. 2014, 30, 133–136. [Google Scholar]
- Aggarwal, N.; Kumar, R.; Dureja, P.; Rawat, D.S. Schiff Bases as Potential Fungicides and Nitrification Inhibitors. J. Agric. Food Chem. 2009, 57, 8520–8525. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and Derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2007; ISBN 9783527306732. [Google Scholar]
- Arena, E.; Ballistreri, G.; Tomaselli, F.; Fallico, B. Survey of 1,2-Dicarbonyl Compounds in Commercial Honey of Different Floral Origin. J. Food Sci. 2011, 76, C1203-10. [Google Scholar] [CrossRef]
- Kirit, A.B.; Eerdogdu, F.; Ozdemir, Y. Accumulation of 5-Hydroxymethyl-2-Furfural During Toasting of White Bread Slices. J. Food Process Eng. 2013, 36, 241–246. [Google Scholar] [CrossRef]
- Murkovic, M.; Pichler, N. Analysis of 5-Hydroxymethylfurfual in Coffee, Dried Fruits and Urine. Mol. Nutr. Food Res. 2006, 50, 842–846. [Google Scholar] [CrossRef]
- Rosatella, A.A.; Simeonov, S.P.; Frade, R.F.M.; Afonso, C.A.M. 5-Hydroxymethylfurfural (HMF) as a Building Block Platform: Biological Properties, Synthesis and Synthetic Applications. Green Chem. 2011, 13, 754–793. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Suryadharma, M.S.; Pham, T.P.T.; Mahmood, R.; Balasubramanian, R. Heterogeneous Catalyst-Assisted Thermochemical Conversion of Food Waste Biomass into 5-Hydroxymethylfurfural. Bioresour. Technol. 2015, 178, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.K.M.; Tsang, D.C.W.; Yip, A.C.K.; Chen, S.S.; Wang, L.; Ok, Y.S.; Poon, C.S. Catalytic Valorization of Starch-Rich Food Waste into Hydroxymethylfurfural (HMF): Controlling Relative Kinetics for High Productivity. Bioresour. Technol. 2017, 237, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Danielli, C.; van Langen, L.; Boes, D.; Asaro, F.; Anselmi, S.; Provenza, F.; Renzi, M.; Gardossi, L. 2{,}5-Furandicarboxaldehyde as a Bio-Based Crosslinking Agent Replacing Glutaraldehyde for Covalent Enzyme Immobilization. RSC Adv. 2022, 12, 35676–35684. [Google Scholar] [CrossRef] [PubMed]
- Laugel, C.; Estrine, B.; Le Bras, J.; Hoffmann, N.; Marinkovic, S.; Muzart, J. NaBr/DMSO-Induced Synthesis of 2,5-Diformylfuran from Fructose or 5-(Hydroxymethyl)Furfural. ChemCatChem 2014, 6, 1195–1198. [Google Scholar] [CrossRef]
- Kumar, A.; Armstrong, D.; Peters, G.; Nagala, M.; Shirran, S. Direct Synthesis of Polyureas from the Dehydrogenative Coupling of Diamines and Methanol. Chem. Commun. 2021, 57, 6153–6156. [Google Scholar] [CrossRef]
- Sen, C.P.; Chong, N.X.; Wai Tam, E.K.; Seayad, A.M.; Seayad, J.; Jana, S. Biobased Nonisocyanate Polyurethanes as Recyclable and Intrinsic Self-Healing Coating with Triple Healing Sites. ACS Macro Lett. 2021, 10, 635–641. [Google Scholar] [CrossRef]
- Gao, R.; Li, Q.; Di, J.; Li, Q.; He, Y.C.; Ma, C. Improved 5-Hydroxymethyl-2-Furfurylamine Production from D-Fructose-Derived 5-Hydroxymethylfurfural by a Robust Double Mutant Aspergillus Terreus ω-Transaminase Biocatalyst in a Betaine-Formic Acid Medium. Ind. Crops Prod. 2023, 193, 116199. [Google Scholar] [CrossRef]
- Wang, Z.; Chai, H.; Ren, J.; Tao, Y.; Li, Q.; Ma, C.; Ai, Y.; He, Y. Biocatalytic Valorization of Biobased 5-Hydroxymethylfurfural to 5-Hydroxymethyl-2-Furfurylamine in a Three-Constituent Deep Eutectic Solvent–Water System. ACS Sustain. Chem. Eng. 2022, 10, 8452–8463. [Google Scholar] [CrossRef]
- Wei, Z.; Cheng, Y.; Zhou, K.; Zeng, Y.; Yao, E.; Li, Q.; Liu, Y.; Sun, Y. One-Step Reductive Amination of 5-Hydroxymethylfurfural into 2,5-Bis(Aminomethyl)Furan over Raney Ni. ChemSusChem 2021, 14, 2308–2312. [Google Scholar] [CrossRef]
- Chen, W.; Sun, Y.; Du, J.; Si, Z.; Tang, X.; Zeng, X.; Lin, L.; Liu, S.; Lei, T. Preparation of 5-(Aminomethyl)-2-Furanmethanol by Direct Reductive Amination of 5-Hydroxymethylfurfural with Aqueous Ammonia over the Ni/SBA-15 Catalyst. J. Chem. Technol. Biotechnol. 2018, 93, 3028–3034. [Google Scholar] [CrossRef]
- Börner, T.; Rämisch, S.; Reddem, E.R.; Bartsch, S.; Vogel, A.; Thunnissen, A.M.W.H.; Adlercreutz, P.; Grey, C. Explaining Operational Instability of Amine Transaminases: Substrate-Induced Inactivation Mechanism and Influence of Quaternary Structure on Enzyme-Cofactor Intermediate Stability. ACS Catal. 2017, 7, 1259–1269. [Google Scholar] [CrossRef]
- Börner, T.; Rämisch, S.; Bartsch, S.; Vogel, A.; Adlercreutz, P.; Grey, C. Three in One: Temperature, Solvent and Catalytic Stability by Engineering the Cofactor-Binding Element of Amine Transaminase. ChemBioChem 2017, 18, 1482–1486. [Google Scholar] [CrossRef] [PubMed]
- Roura Padrosa, D.; Alaux, R.; Smith, P.; Dreveny, I.; López-Gallego, F.; Paradisi, F. Enhancing PLP-Binding Capacity of Class-III ω-Transaminase by Single Residue Substitution. Front. Bioeng. Biotechnol. 2019, 7, 282. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.D.; Grogan, G.; Bommarius, A.; Yun, H. Recent Advances in ω-Transaminase-Mediated Biocatalysis for the Enantioselective Synthesis of Chiral Amines. Catalysts 2018, 8, 254. [Google Scholar] [CrossRef]
- Land, H.; Campillo-Brocal, J.C.; Svedendahl Humble, M.; Berglund, P. B-Factor Guided Proline Substitutions in Chromobacterium Violaceum Amine Transaminase: Evaluation of the Proline Rule as a Method for Enzyme Stabilization. ChemBioChem 2019, 20, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Merz, L.M.; van Langen, L.M.; Berglund, P. The Role of Buffer, Pyridoxal 5′-Phosphate and Light on the Stability of the Silicibacter Pomeroyi Transaminase. ChemCatChem 2022, 15, e202201174. [Google Scholar] [CrossRef]
- Gerlach, T.; Nugroho, D.L.; Rother, D. The Effect of Visible Light on the Catalytic Activity of PLP-Dependent Enzymes. ChemCatChem 2021, 13, 2398–2406. [Google Scholar] [CrossRef]
- Truppo, M.D.; Strotman, H.; Hughes, G. Development of an Immobilized Transaminase Capable of Operating in Organic Solvent. ChemCatChem 2012, 4, 1071–1074. [Google Scholar] [CrossRef]
- Kaličanin, N.; Kovačević, G.; Spasojević, M.; Prodanović, O.; Jovanović-Šanta, S.; Škorić, D.; Opsenica, D.; Prodanović, R. Immobilization of ArRMut11 Omega-Transaminase for Increased Operational Stability and Reusability in the Synthesis of 3α-Amino-5α-Androstan-17β-Ol. Process Biochem. 2022, 121, 674–680. [Google Scholar] [CrossRef]
- Yi, S.-S.; Lee, C.; Kim, J.; Kyung, D.; Kim, B.-G.; Lee, Y.-S. Covalent Immobilization of ω-Transaminase from Vibrio Fluvialis JS17 on Chitosan Beads. Process Biochem. 2007, 42, 895–898. [Google Scholar] [CrossRef]
- Singh, R.K.; Tiwari, M.K.; Singh, R.; Lee, J.-K. From Protein Engineering to Immobilization: Promising Strategies for the Upgrade of Industrial Enzymes. Int. J. Mol. Sci. 2013, 14, 1232–1277. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Immobilized Enzymes. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Elsevier, B.V.: Amsterdam, Netherlands, 2011; Volume 2, ISBN 9780080885049. [Google Scholar]
- Van Den Biggelaar, L.; Soumillion, P.; Debecker, D.P. Enantioselective Transamination in Continuous Flow Mode with Transaminase Immobilized in a Macrocellular Silica Monolith. Catalysts 2017, 7, 54. [Google Scholar] [CrossRef]
- Heinks, T.; Montua, N.; Teune, M.; Liedtke, J.; Höhne, M.; Bornscheuer, U.T.; von Mollard, G.F. Comparison of Four Immobilization Methods for Different Transaminases. Catalysts 2023, 13, 300. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying Enzyme Activity and Selectivity by Immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef]
- Liese, A.; Hilterhaus, L. Evaluation of Immobilized Enzymes for Industrial Applications. Chem. Soc. Rev. 2013, 42, 6236–6249. [Google Scholar] [CrossRef]
- Mallin, H.; Höhne, M.; Bornscheuer, U.T. Immobilization of (R)- and (S)-Amine Transaminases on Chitosan Support and Their Application for Amine Synthesis Using Isopropylamine as Donor. J. Biotechnol. 2014, 191, 32–37. [Google Scholar] [CrossRef]
- Neto, W.; Schürmann, M.; Panella, L.; Vogel, A.; Woodley, J.M. Immobilisation of ω-Transaminase for Industrial Application: Screening and Characterisation of Commercial Ready to Use Enzyme Carriers. J. Mol. Catal. B Enzym. 2015, 117, 54–61. [Google Scholar] [CrossRef]
- Velasco-Lozano, S.; Jackson, E.; Ripoll, M.; López-Gallego, F.; Betancor, L. Stabilization of ω-Transaminase from Pseudomonas Fluorescens by Immobilization Techniques. Int. J. Biol. Macromol. 2020, 164, 4318–4328. [Google Scholar] [CrossRef]
- Deepankumar, K.; Nadarajan, S.P.; Mathew, S.; Lee, S.-G.; Yoo, T.H.; Hong, E.Y.; Kim, B.-G.; Yun, H. Engineering Transaminase for Stability Enhancement and Site-Specific Immobilization through Multiple Noncanonical Amino Acids Incorporation. ChemCatChem 2015, 7, 417–421. [Google Scholar] [CrossRef]
- Khanam, W.; Dubey, N.C. Recent Advances in Immobilized ω-Transaminase for Chiral Amine Synthesis. Mater. Today Chem. 2022, 24, 100922. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in Bio-Catalysts Design: A Useful Crosslinker and a Versatile Tool in Enzyme Immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Abaházi, E.; Sátorhelyi, P.; Erdélyi, B.; Vértessy, B.G.; Land, H.; Paizs, C.; Berglund, P.; Poppe, L. Covalently Immobilized Trp60Cys Mutant of ω-Transaminase from Chromobacterium Violaceum for Kinetic Resolution of Racemic Amines in Batch and Continuous-Flow Modes. Biochem. Eng. J. 2018, 132, 270–278. [Google Scholar] [CrossRef]
- Mallin, H.; Menyes, U.; Vorhaben, T.; Höhne, M.; Bornscheuer, U.T. Immobilization of Two (R)-Amine Transaminases on an Optimized Chitosan Support for the Enzymatic Synthesis of Optically Pure Amines. ChemCatChem 2013, 5, 588–593. [Google Scholar] [CrossRef]
- de Souza, S.P.; Junior, I.I.; Silva, G.M.A.; Miranda, L.S.M.; Santiago, M.F.; Leung-Yuk Lam, F.; Dawood, A.; Bornscheuer, U.T.; de Souza, R.O.M.A. Cellulose as an Efficient Matrix for Lipase and Transaminase Immobilization. RSC Adv. 2016, 6, 6665–6671. [Google Scholar] [CrossRef]
- Jia, H.; Huang, F.; Gao, Z.; Zhong, C.; Zhou, H.; Jiang, M.; Wei, P. Immobilization of ω-Transaminase by Magnetic PVA-Fe3O4 Nanoparticles. Biotechnol. Reports 2016, 10, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Cui, W.; Du, K.; Gao, Q.; Du, M.; Ji, P.; Feng, W. Immobilization of R-ω-Transaminase on MnO2 Nanorods for Catalyzing the Conversion of (R)-1-Phenylethylamine. J. Biotechnol. 2017, 245, 14–20. [Google Scholar] [CrossRef]
- Engelmark Cassimjee, K.; Kadow, M.; Wikmark, Y.; Svedendahl Humble, M.; Rothstein, M.L.; Rothstein, D.M.; Bäckvall, J.-E. A General Protein Purification and Immobilization Method on Controlled Porosity Glass: Biocatalytic Applications. Chem. Commun. 2014, 50, 9134–9137. [Google Scholar] [CrossRef]
- Böhmer, W.; Knaus, T.; Volkov, A.; Slot, T.K.; Shiju, N.R.; Engelmark Cassimjee, K.; Mutti, F.G. Highly Efficient Production of Chiral Amines in Batch and Continuous Flow by Immobilized ω-Transaminases on Controlled Porosity Glass Metal-Ion Affinity Carrier. J. Biotechnol. 2019, 291, 52–60. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Bertella, S.; de Bueren, J.; Luterbacher, J.S.; Paradisi, F. Dual Valorization of Lignin as a Versatile and Renewable Matrix for Enzyme Immobilization and (Flow) Bioprocess Engineering. ChemSusChem 2021, 14, 3198–3207. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous Flow Biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- De Santis, P.; Meyer, L.E.; Kara, S. The Rise of Continuous Flow Biocatalysis-Fundamentals, Very Recent Developments and Future Perspectives. React. Chem. Eng. 2020, 5, 2155–2184. [Google Scholar] [CrossRef]
- Molnár, Z.; Farkas, E.; Lakó, Á.; Erdélyi, B.; Kroutil, W.; Vértessy, B.G.; Paizs, C.; Poppe, L. Immobilized Whole-Cell Transaminase Biocatalysts for Continuous-Flow Kinetic Resolution of Amines. Catalysts 2019, 9, 438. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Contente, M.L.; Velasco-Lozano, S.; Paradisi, F.; López-Gallego, F. Self-Sufficient Flow-Biocatalysis by Coimmobilization of Pyridoxal 5′-Phosphate and ω-Transaminases onto Porous Carriers. ACS Sustain. Chem. Eng. 2018, 6, 13151–13159. [Google Scholar] [CrossRef]
- Semproli, R.; Vaccaro, G.; Ferrandi, E.E.; Vanoni, M.; Bavaro, T.; Marrubini, G.; Annunziata, F.; Conti, P.; Speranza, G.; Monti, D.; et al. Use of Immobilized Amine Transaminase from Vibrio Fluvialis under Flow Conditions for the Synthesis of (S)-1-(5-Fluoropyrimidin-2-Yl)-Ethanamine. ChemCatChem 2020, 12, 1359–1367. [Google Scholar] [CrossRef]
- Gruber, P.; Carvalho, F.; Marques, M.P.C.; O’Sullivan, B.; Subrizi, F.; Dobrijevic, D.; Ward, J.; Hailes, H.C.; Fernandes, P.; Wohlgemuth, R.; et al. Enzymatic Synthesis of Chiral Amino-Alcohols by Coupling Transketolase and Transaminase-Catalyzed Reactions in a Cascading Continuous-Flow Microreactor System. Biotechnol. Bioeng. 2018, 115, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.H.; Kroutil, W.; Jamison, T.F. Continuous Flow Synthesis of Chiral Amines in Organic Solvents: Immobilization of E. Coli Cells Containing Both ω-Transaminase and PLP. Org. Lett. 2014, 16, 6092–6095. [Google Scholar] [CrossRef] [PubMed]
- Kohrt, J.T.; Dorff, P.H.; Burns, M.; Lee, C.; O’Neil, S.V.; Maguire, R.J.; Kumar, R.; Wagenaar, M.; Price, L.; Lall, M.S. Application of Flow and Biocatalytic Transaminase Technology for the Synthesis of a 1-Oxa-8-Azaspiro [4.5]Decan-3-Amine. Org. Process Res. Dev. 2022, 26, 616–623. [Google Scholar] [CrossRef]
- Planchestainer, M.; Contente, M.L.; Cassidy, J.; Molinari, F.; Tamborini, L.; Paradisi, F. Continuous Flow Biocatalysis: Production and in-Line Purification of Amines by Immobilised Transaminase from Halomonas Elongata. Green Chem. 2017, 19, 372–375. [Google Scholar] [CrossRef]
- Böhmer, W.; Volkov, A.; Engelmark Cassimjee, K.; Mutti, F.G. Continuous Flow Bioamination of Ketones in Organic Solvents at Controlled Water Activity Using Immobilized ω-Transaminases. Adv. Synth. Catal. 2020, 362, 1858–1867. [Google Scholar] [CrossRef]
- Contente, M.L.; Paradisi, F. Transaminase-Catalyzed Continuous Synthesis of Biogenic Aldehydes. ChemBioChem 2019, 20, 2830–2833. [Google Scholar] [CrossRef]
- Heckmann, C.M.; Dominguez, B.; Paradisi, F. Enantio-Complementary Continuous-Flow Synthesis of 2-Aminobutane Using Covalently Immobilized Transaminases. ACS Sustain. Chem. Eng. 2021, 9, 4122–4129. [Google Scholar] [CrossRef]
- Janson, N.; Heinks, T.; Beuel, T.; Alam, S.; Höhne, M.; Bornscheuer, U.T.; Fischer von Mollard, G.; Sewald, N. Efficient Site-Selective Immobilization of Aldehyde-Tagged Peptides and Proteins by Knoevenagel Ligation. ChemCatChem 2022, 14, e202101485. [Google Scholar] [CrossRef]
- Peng, J.; Alam, S.; Radhakrishnan, K.; Mariappan, M.; Rudolph, M.G.; May, C.; Dierks, T.; von Figura, K.; Schmidt, B. Eukaryotic Formylglycine-Generating Enzyme Catalyses a Monooxygenase Type of Reaction. FEBS J. 2015, 282, 3262–3274. [Google Scholar] [CrossRef] [PubMed]
- Dierks, T.; Lecca, M.R.; Schlotterhose, P.; Schmidt, B.; von Figura, K. Sequence Determinants Directing Conversion of Cysteine to Formylglycine in Eukaryotic Sulfatases. EMBO J. 1999, 18, 2084–2091. [Google Scholar] [CrossRef] [PubMed]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein Immobilization Strategies for Protein Biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal Chemistry: Fishing for Selectivity in a Sea of Functionality. Angew. Chemie Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Kelefiotis-Stratidakis, P.; Tyrikos-Ergas, T.; Pavlidis, I.V. The Challenge of Using Isopropylamine as an Amine Donor in Transaminase Catalysed Reactions. Org. Biomol. Chem. 2019, 17, 1634–1642. [Google Scholar] [CrossRef]
- Dawood, A.W.H.; Weiß, M.S.; Schulz, C.; Pavlidis, I.V.; Iding, H.; de Souza, R.O.M.A.; Bornscheuer, U.T. Isopropylamine as Amine Donor in Transaminase-Catalyzed Reactions: Better Acceptance through Reaction and Enzyme Engineering. ChemCatChem 2018, 10, 3943–3949. [Google Scholar] [CrossRef]
- Shin, J.S.; Kim, B.G. Asymmetric Synthesis of Chiral Amines with Omega-Transaminase. Biotechnol. Bioeng. 1999, 65, 206–211. [Google Scholar] [CrossRef]
- Shin, J.-S.; Kim, B.-G. Kinetic Modeling of ω-Transamination for Enzymatic Kinetic Resolution of α-Methylbenzylamine. Biotechnol. Bioeng. 1998, 60, 534–540. [Google Scholar] [CrossRef]
- Höhne, M.; Kühl, S.; Robins, K.; Bornscheuer, U.T. Efficient Asymmetric Synthesis of Chiral Amines by Combining Transaminase and Pyruvate Decarboxylase. ChemBioChem 2008, 9, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-H.; Kyung, D.; Joo, K.; Lee, J.; Kim, B.-G. Necessary and Sufficient Conditions for the Asymmetric Synthesis of Chiral Amines Using ω-Aminotransferases. Biotechnol. Bioeng. 2011, 108, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Tufvesson, P.; Bach, C.; Woodley, J.M. A Model to Assess the Feasibility of Shifting Reaction Equilibrium by Acetone Removal in the Transamination of Ketones Using 2-Propylamine. Biotechnol. Bioeng. 2014, 111, 309–319. [Google Scholar] [CrossRef]
- Han, S.-W.; Park, E.-S.; Dong, J.-Y.; Shin, J.-S. Mechanism-Guided Engineering of ω-Transaminase to Accelerate Reductive Amination of Ketones. Adv. Synth. Catal. 2015, 357, 1732–1740. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Contente, M.L.; Roura Padrosa, D.; Paradisi, F. Flow Biocatalysis 101: Design, Development and Applications. React. Chem. Eng. 2021, 6, 599–611. [Google Scholar] [CrossRef]
- Romero-Fernández, M.; Paradisi, F. Protein Immobilization Technology for Flow Biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 1–8. [Google Scholar] [CrossRef]
- Kollipara, M.; Matzel, P.; Sowa, M.; Brott, S.; Bornscheuer, U.; Höhne, M. Characterization of Proteins from the 3N5M Family Reveals an Operationally Stable Amine Transaminase. Appl. Microbiol. Biotechnol. 2022, 106, 5563–5574. [Google Scholar] [CrossRef]
- Meng, Q.; Capra, N.; Palacio, C.M.; Lanfranchi, E.; Otzen, M.; van Schie, L.Z.; Rozeboom, H.J.; Thunnissen, A.-M.W.H.; Wijma, H.J.; Janssen, D.B. Robust ω-Transaminases by Computational Stabilization of the Subunit Interface. ACS Catal. 2020, 10, 2915–2928. [Google Scholar] [CrossRef]
- Iwasaki, A.; Matsumoto, K.; Hasegawa, J.; Yasohara, Y. A Novel Transaminase, (R)-Amine:Pyruvate Aminotransferase, from Arthrobacter Sp. KNK168 (FERM BP-5228): Purification, Characterization, and Gene Cloning. Appl. Microbiol. Biotechnol. 2012, 93, 1563–1573. [Google Scholar] [CrossRef]
- Park, E.-S.; Dong, J.-Y.; Shin, J.-S. ω-Transaminase-Catalyzed Asymmetric Synthesis of Unnatural Amino Acids Using Isopropylamine as an Amino Donor. Org. Biomol. Chem. 2013, 11, 6929–6933. [Google Scholar] [CrossRef]
- Dawood, A.W.H.; de Souza, R.O.M.A.; Bornscheuer, U.T. Asymmetric Synthesis of Chiral Halogenated Amines Using Amine Transaminases. ChemCatChem 2018, 10, 951–955. [Google Scholar] [CrossRef]
- Shin, J.-S.; Kim, B.-G. Exploring the Active Site of Amine:Pyruvate Aminotransferase on the Basis of the Substrate Structure−Reactivity Relationship: How the Enzyme Controls Substrate Specificity and Stereoselectivity. J. Org. Chem. 2002, 67, 2848–2853. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-S.; Yun, H.; Jang, J.-W.; Park, I.; Kim, B.-G. Purification, Characterization, and Molecular Cloning of a Novel Amine:Pyruvate Transaminase from Vibrio Fluvialis JS17. Appl. Microbiol. Biotechnol. 2003, 61, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, Y.; Wang, Z.; Zhang, K.; Wang, H.; Wei, D. Efficient Synthesis of (S)-1-Boc-3-Aminopiperidine in a Continuous Flow System Using ω-Transaminase-Immobilized Amino-Ethylenediamine-Modified Epoxide Supports. Org. Process Res. Dev. 2022, 26, 1351–1359. [Google Scholar] [CrossRef]
- Schätzle, S.; Höhne, M.; Redestad, E.; Robins, K.; Bornscheuer, U.T. Rapid and Sensitive Kinetic Assay for Characterization of ω-Transaminases. Anal. Chem. 2009, 81, 8244–8248. [Google Scholar] [CrossRef] [PubMed]
- Heinks, T.; Paulus, J.; Koopmeiners, S.; Beuel, T.; Sewald, N.; Höhne, M.; Bornscheuer, U.T.; Fischer von Mollard, G. Recombinant L-Amino Acid Oxidase with Broad Substrate Spectrum for Co-Substrate Recycling in (S)-Selective Transaminase-Catalyzed Kinetic Resolutions. ChemBioChem 2022, 23, e202200329. [Google Scholar] [CrossRef]

| Transaminase | Substrate | Specific Activity at pH 8.0 [mU/mg Enzyme] | Specific Activity at pH 9.0 [mU/mg Enzyme] |
|---|---|---|---|
| ATA-Vfl | HMF | 105.3 ± 0.3 | 126.1 ± 0.5 |
| DFF | 113.1 ± 3.1 | 96.1 ± 0.7 | |
| ATA-Bmu | HMF | 117.0 ± 1.0 | 138.4 ± 4.2 |
| DFF | 63.3 ± 1.7 | 69.1 ± 0.3 | |
| ATA-Spo | HMF | 48.4 ± 0.1 | 57.7 ± 0.5 |
| DFF | 39.1 ± 0.8 | 47.8 ± 1.5 | |
| ATA-Lsy | HMF | 30.8 ± 0.7 | 46.1 ± 3.0 |
| DFF | 27.3 ± 0.4 | 34.8 ± 2.4 |
| Transaminase | Specific Activity of Soluble Enzyme [a] [U/mg Enzyme] | Bead Type | Specific Activity of Immobilized Enzyme [b] [U/g Bead] | Binding Efficiency [c] [%] | Activity Recovery [d] [%] |
|---|---|---|---|---|---|
| ATA-Vfl | 4.2 | HA | 48.6 | 74.5 | 10.6 |
| HAGA | 56.3 | 61.9 | 9.7 | ||
| ATA-Bmu | 2.0 | HA | 26.6 | 97.6 | 18.2 |
| HAGA | 51.8 | 71.1 | 17.7 | ||
| ATA-Spo | 0.8 | HA | 35.8 | 92.5 | 14.2 |
| HAGA | 57.6 | 93.8 | 12.4 | ||
| ATA-Lsy | 1.0 | HA | 52.3 | 75.3 | 14.4 |
| HAGA | 33.6 | 96.2 | 16.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinks, T.; Merz, L.M.; Liedtke, J.; Höhne, M.; van Langen, L.M.; Bornscheuer, U.T.; Fischer von Mollard, G.; Berglund, P. Biosynthesis of Furfurylamines in Batch and Continuous Flow by Immobilized Amine Transaminases. Catalysts 2023, 13, 875. https://doi.org/10.3390/catal13050875
Heinks T, Merz LM, Liedtke J, Höhne M, van Langen LM, Bornscheuer UT, Fischer von Mollard G, Berglund P. Biosynthesis of Furfurylamines in Batch and Continuous Flow by Immobilized Amine Transaminases. Catalysts. 2023; 13(5):875. https://doi.org/10.3390/catal13050875
Chicago/Turabian StyleHeinks, Tobias, Luisa M. Merz, Jan Liedtke, Matthias Höhne, Luuk M. van Langen, Uwe T. Bornscheuer, Gabriele Fischer von Mollard, and Per Berglund. 2023. "Biosynthesis of Furfurylamines in Batch and Continuous Flow by Immobilized Amine Transaminases" Catalysts 13, no. 5: 875. https://doi.org/10.3390/catal13050875
APA StyleHeinks, T., Merz, L. M., Liedtke, J., Höhne, M., van Langen, L. M., Bornscheuer, U. T., Fischer von Mollard, G., & Berglund, P. (2023). Biosynthesis of Furfurylamines in Batch and Continuous Flow by Immobilized Amine Transaminases. Catalysts, 13(5), 875. https://doi.org/10.3390/catal13050875








