Improving the Kinetics of H2-Fueled Biological Methanation with Quinone-Based Redox Mediators
Abstract
1. Introduction
2. Results and Discussion
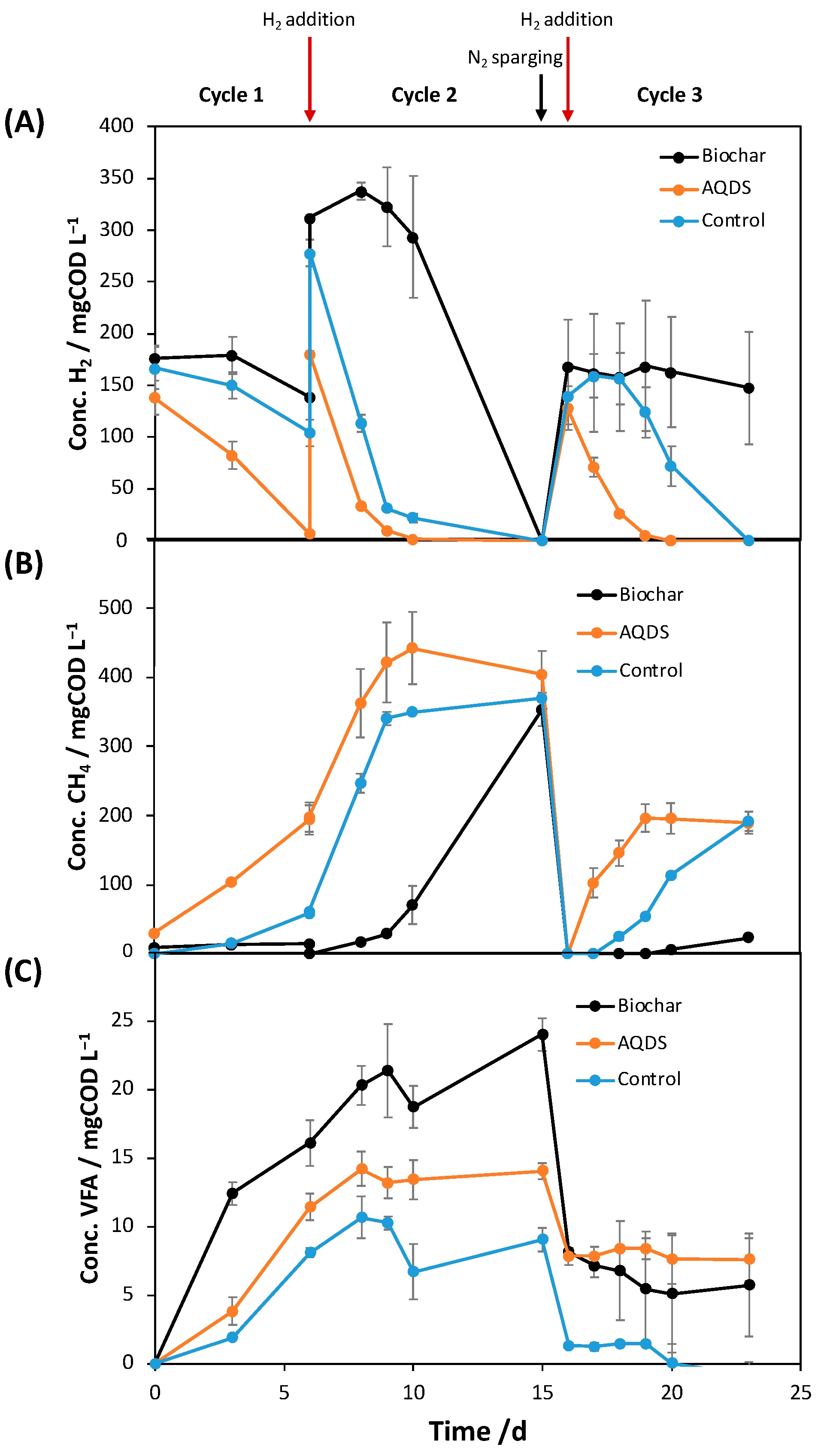
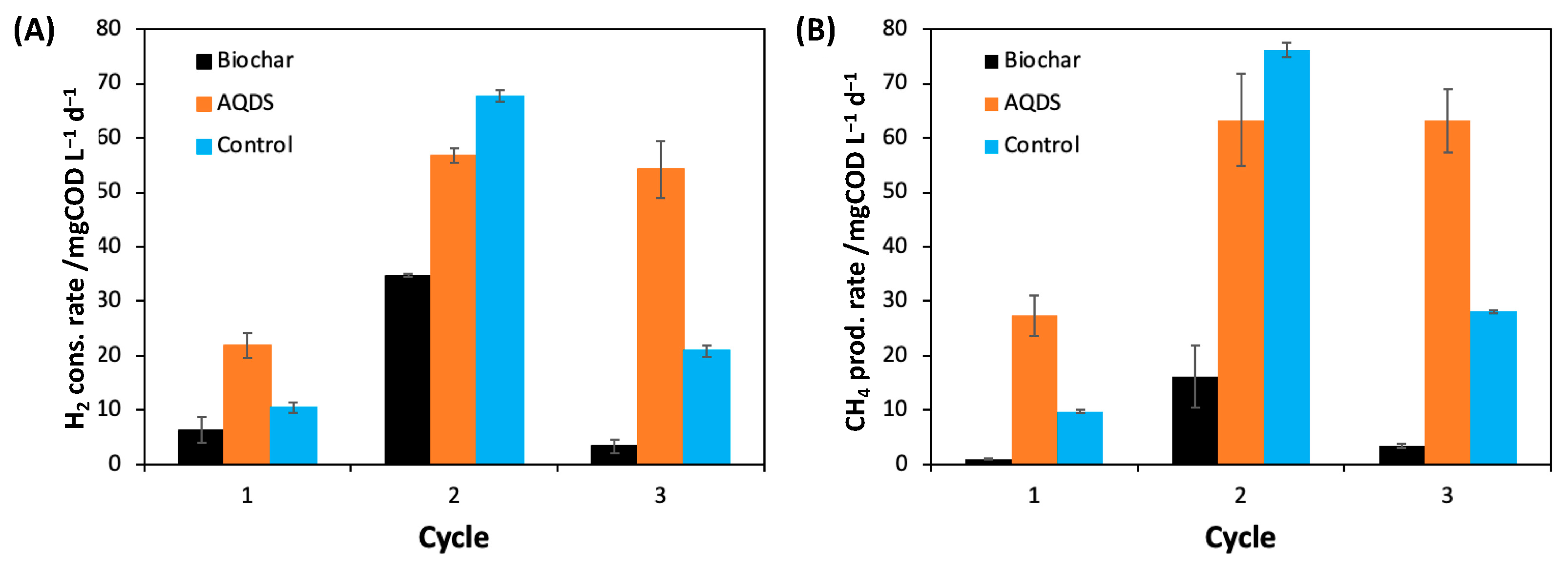
2.1. Impact of Quinone-Based Mediators on H2 Consumption and CH4 Production
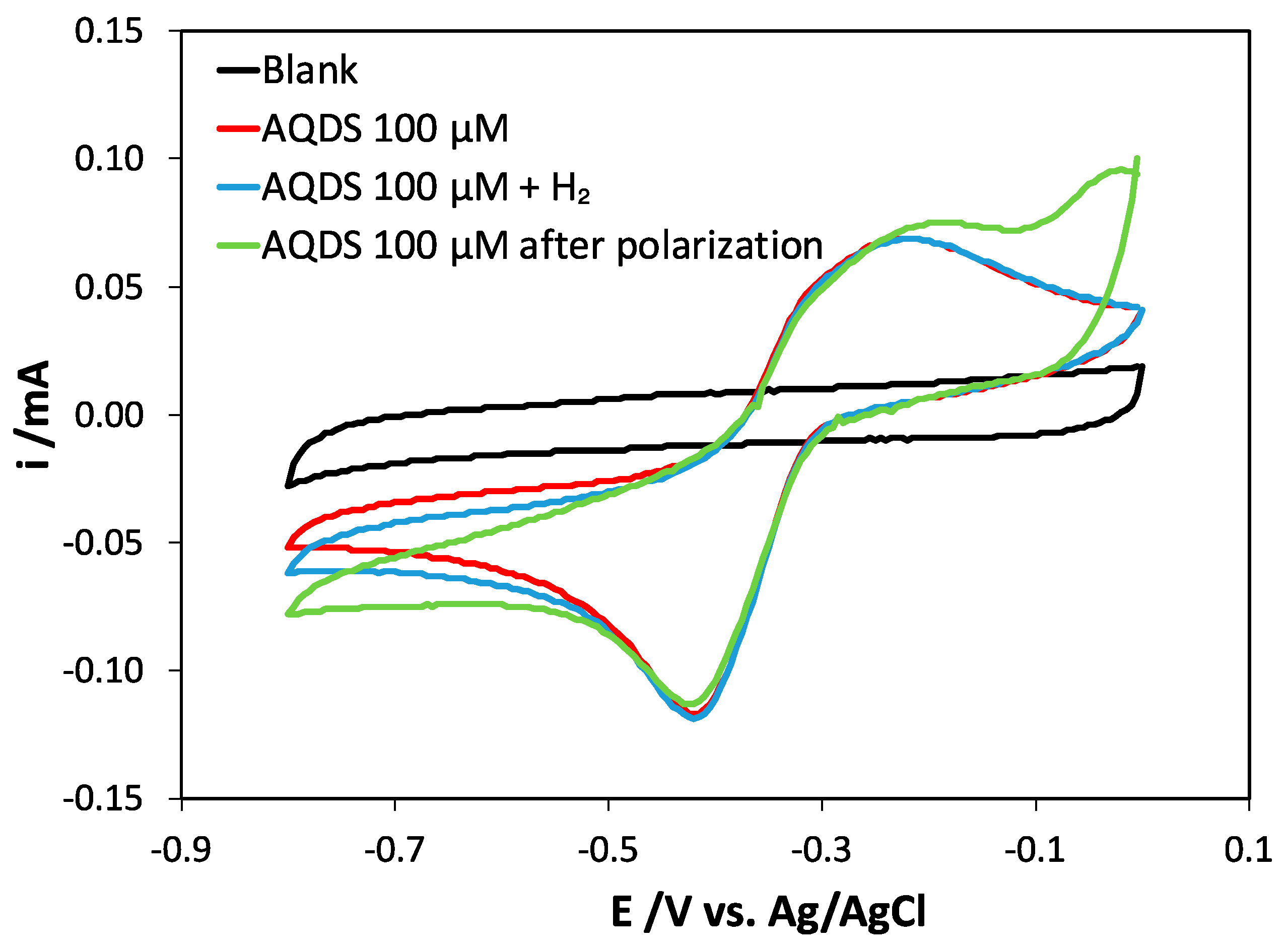
2.2. Deciphering the Mechanisms of AQDS-Enhanced Methanogenesis
3. Materials and Methods
3.1. Chemicals
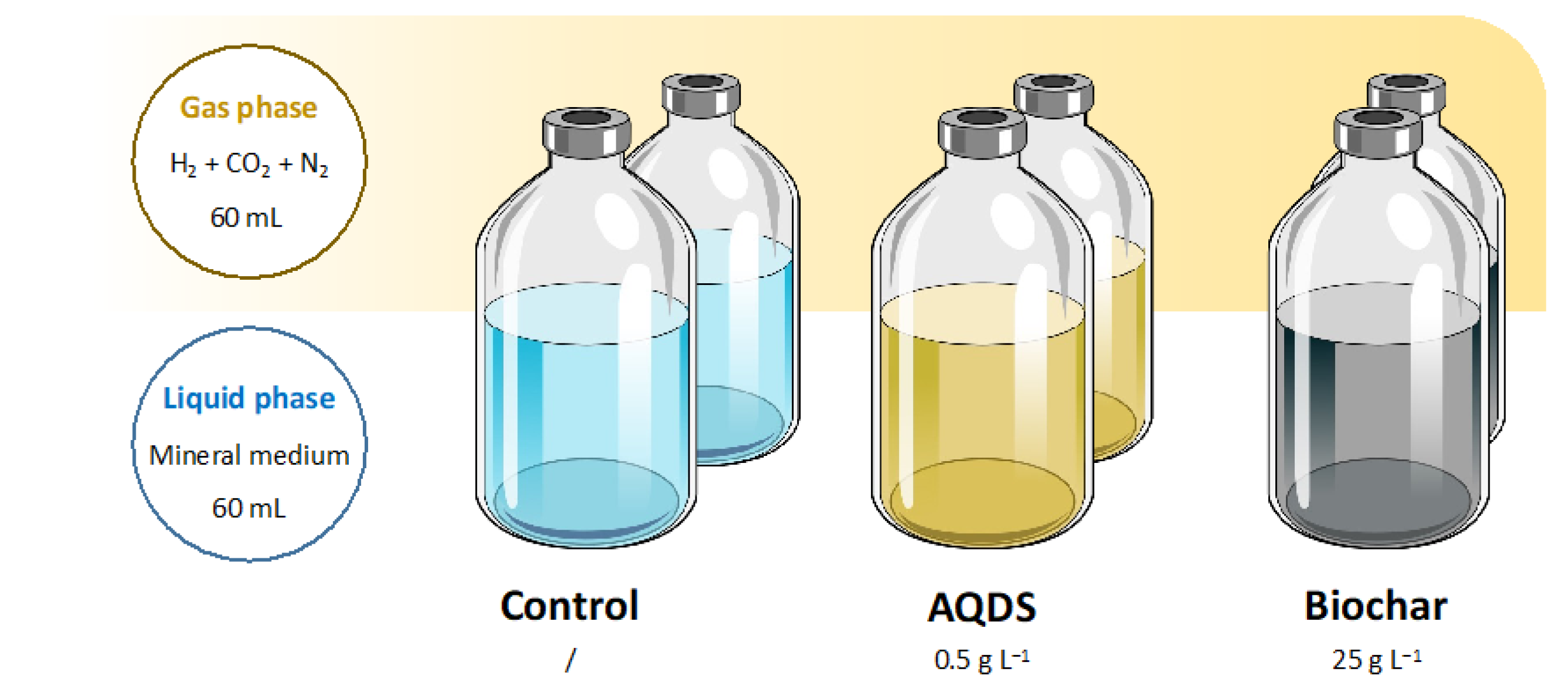
3.2. Microcosms Setup
3.3. Analytical Methods
3.4. Cyclic Voltammograms
3.5. Calculations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Raven, J.A.; Beardall, J. Influence of Global Environmental Change on Plankton. J. Plankton Res. 2021, 43, 779–800. [Google Scholar] [CrossRef]
- Ghiat, I.; Al-Ansari, T. A Review of Carbon Capture and Utilisation as a CO2 abatement Opportunity within the EWF Nexus. J. CO2 Util. 2021, 45, 101432. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Kamkeng, A.D.N.; Wang, M.; Hu, J.; Du, W.; Qian, F. Transformation Technologies for CO2 Utilisation: Current Status, Challenges and Future Prospects. Chem. Eng. J. 2021, 409, 128138. [Google Scholar] [CrossRef]
- UNFCCC. What Is the Paris Agreement? UNFCCC: Rio de Janeiro, Brazil; New York, NY, USA, 2020. [Google Scholar]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon Capture and Storage (CCS): The Way Forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Spiess, S.; Sasiain Conde, A.; Kucera, J.; Novak, D.; Thallner, S.; Kieberger, N.; Guebitz, G.M.; Haberbauer, M. Bioelectrochemical Methanation by Utilization of Steel Mill Off-Gas in a Two-Chamber Microbial Electrolysis Cell. Front. Bioeng. Biotechnol. 2022, 10, 972653. [Google Scholar] [CrossRef] [PubMed]
- Sposob, M.; Wahid, R.; Fischer, K. Ex-Situ Biological CO2 Methanation Using Trickle Bed Reactor: Review and Recent Advances. Rev. Environ. Sci. Biotechnol. 2021, 20, 1087–1102. [Google Scholar] [CrossRef]
- Barbaresi, A.; Morini, M.; Gambarotta, A. Review on the Status of the Research on Power-to-Gas Experimental Activities. Energies 2022, 15, 5942. [Google Scholar] [CrossRef]
- Lewandowska-Bernat, A.; Desideri, U. Opportunities of Power-to-Gas Technology in Different Energy Systems Architectures. Appl. Energy 2018, 228, 57–67. [Google Scholar] [CrossRef]
- Dong, Z.; Ding, Y.; Chen, F.; Zhu, X.; Wang, H.; Cheng, M.; Liao, Q. Enhanced Carbon Dioxide Biomethanation with Hydrogen Using Anaerobic Granular Sludge and Metal–Organic Frameworks: Microbial Community Response and Energy Metabolism Analysis. Bioresour. Technol. 2022, 362, 127822. [Google Scholar] [CrossRef]
- Vogt, C.; Monai, M.; Kramer, G.J.; Weckhuysen, B.M. The Renaissance of the Sabatier Reaction and Its Applications on Earth and in Space. Nat. Catal. 2019, 2, 188–197. [Google Scholar] [CrossRef]
- Lee, W.J.; Li, C.; Prajitno, H.; Yoo, J.; Patel, J.; Yang, Y.; Lim, S. Recent Trend in Thermal Catalytic Low Temperature CO2 Methanation: A Critical Review. Catal. Today 2021, 368, 2–19. [Google Scholar] [CrossRef]
- Thema, M.; Bauer, F.; Sterner, M. Power-to-Gas: Electrolysis and Methanation Status Review. Renew. Sustain. Energy Rev. 2019, 112, 775–787. [Google Scholar] [CrossRef]
- Jiang, B.; Hu, X.; Söderlind, U.; Göransson, K.; Zhang, W.; Yu, C. Identification of the Biomethanation Pathways during Biological CO2 Fixation with Exogenous H2 Addition. Fuel Process. Technol. 2022, 238, 107478. [Google Scholar] [CrossRef]
- Geppert, F.; Liu, D.; van Eerten-Jansen, M.; Weidner, E.; Buisman, C.; ter Heijne, A. Bioelectrochemical Power-to-Gas: State of the Art and Future Perspectives. Trends Biotechnol. 2016, 34, 879–894. [Google Scholar] [CrossRef] [PubMed]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on Methanation—From Fundamentals to Current Projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Diender, M.; Uhl, P.S.; Bitter, J.H.; Stams, A.J.M.; Sousa, D.Z. High Rate Biomethanation of Carbon Monoxide-Rich Gases via a Thermophilic Synthetic Coculture. ACS Sustain. Chem. Eng. 2018, 6, 2169–2176. [Google Scholar] [CrossRef]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological Biogas Upgrading Capacity of a Hydrogenotrophic Community in a Trickle-Bed Reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Pauss, A.; Andre, G.; Perrier, M.; Guiot, S.R. Liquid-to-Gas Mass Transfer in Anaerobic Processes: Inevitable Transfer Limitations of Methane and Hydrogen in the Biomethanation Process. Appl. Environ. Microbiol. 1990, 56, 1636–1644. [Google Scholar] [CrossRef]
- Alfaro, N.; Fdz-Polanco, M.; Fdz-Polanco, F.; Díaz, I. Evaluation of Process Performance, Energy Consumption and Microbiota Characterization in a Ceramic Membrane Bioreactor for Ex-Situ Biomethanation of H2 and CO2. Bioresour. Technol. 2018, 258, 142–150. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; McDaniel Koch, A.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A Technological and Economic Review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Wegener Kofoed, M.V.; Jensen, M.B.; Mørck Ottosen, L.D. Biological Upgrading of Biogas through CO2 Conversion to CH4. In Emerging Technologies and Biological Systems for Biogas Upgrading; Aryal, N., Ottosen, L.D.M., Kofoed, M.V.W., Pant, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 321–362. ISBN 978-0-12-822808-1. [Google Scholar]
- Xu, J.; Zhuang, L.; Yang, G.; Yuan, Y.; Zhou, S. Extracellular Quinones Affecting Methane Production and Methanogenic Community in Paddy Soil. Microb. Ecol. 2013, 66, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Xie, J.; Wang, Y.; Xu, L.; Zong, Y.; Pang, W.; Xie, L. Effect of Anthraquinone-2,6-Disulfonate (AQDS) on Anaerobic Digestion under Ammonia Stress: Triggering Mediated Interspecies Electron Transfer (MIET). Sci. Total Environ. 2022, 828, 154158. [Google Scholar] [CrossRef] [PubMed]
- Beckmann, S.; Welte, C.; Li, X.; Oo, Y.M.; Kroeninger, L.; Heo, Y.; Zhang, M.; Ribeiro, D.; Lee, M.; Bhadbhade, M.; et al. Novel Phenazine Crystals Enable Direct Electron Transfer to Methanogens in Anaerobic Digestion by Redox Potential Modulation. Energy Environ. Sci. 2016, 9, 644–655. [Google Scholar] [CrossRef]
- Zhuang, L.; Ma, J.; Tang, J.; Tang, Z.; Zhou, S. Cysteine-Accelerated Methanogenic Propionate Degradation in Paddy Soil Enrichment. Microb. Ecol. 2017, 73, 916–924. [Google Scholar] [CrossRef]
- Hernandez, M.E.; Newman, D.K. Extracellular Electron Transfer. Cell. Mol. Life Sci. 2001, 58, 1562–1571. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Mellage, A.; Cirpka, O.A.; Sun, T.; Angenent, L.T.; Haderlein, S.B.; Kappler, A. AQDS and Redox-Active NOM Enables Microbial Fe(III)-Mineral Reduction at Cm-Scales. Environ. Sci. Technol. 2020, 54, 4131–4139. [Google Scholar] [CrossRef]
- Chen, M.; Tong, H.; Liu, C.; Chen, D.; Li, F.; Qiao, J. A Humic Substance Analogue AQDS Stimulates Geobacter Sp. Abundance and Enhances Pentachlorophenol Transformation in a Paddy Soil. Chemosphere 2016, 160, 141–148. [Google Scholar] [CrossRef]
- An, W.; Wu, C.; Xue, S.; Liu, Z.; Liu, M.; Li, W. Effects of Biochar/AQDS on As(III)-Adsorbed Ferrihydrite Reduction and Arsenic (As) and Iron (Fe) Transformation: Abiotic and Biological Conditions. Chemosphere 2022, 291, 133126. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Y.; Xie, H.; Cao, W.; Zhang, Y. Varied Promotion Effects and Mechanisms of Biochar on Anaerobic Digestion (AD) under Distinct Food-to-Microorganism (F/M) Ratios and Biochar Dosages. Waste Manag. 2023, 155, 118–128. [Google Scholar] [CrossRef]
- Patel, M.R.; Rathore, N.; Panwar, N.L. Influences of Biochar in Biomethanation and CO2 Mitigation Potential. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Shen, Y.; Linville, J.L.; Urgun-Demirtas, M.; Schoene, R.P.; Snyder, S.W. Producing Pipeline-Quality Biomethane via Anaerobic Digestion of Sludge Amended with Corn Stover Biochar with in-Situ CO2 Removal. Appl. Energy 2015, 158, 300–309. [Google Scholar] [CrossRef]
- Saif, I.; Thakur, N.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; Usman, M.; et al. Biochar Assisted Anaerobic Digestion for Biomethane Production: Microbial Symbiosis and Electron Transfer. J. Environ. Chem. Eng. 2022, 10, 107960. [Google Scholar] [CrossRef]
- Palanisamy, G.; Jung, H.-Y.; Sadhasivam, T.; Kurkuri, M.D.; Kim, S.C.; Roh, S.-H. A Comprehensive Review on Microbial Fuel Cell Technologies: Processes, Utilization, and Advanced Developments in Electrodes and Membranes. J. Clean. Prod. 2019, 221, 598–621. [Google Scholar] [CrossRef]
- Guo, S.; Li, Y.; Wang, Y.; Wang, L.; Sun, Y.; Liu, L. Recent Advances in Biochar-Based Adsorbents for CO2 Capture. Carbon Capture Sci. Technol. 2022, 4, 100059. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, X.; Xu, Y.; Xiang, W.; Wang, R.; Ding, F.; Hong, P.; Gao, B. Straw and Wood Based Biochar for CO2 Capture: Adsorption Performance and Governing Mechanisms. Sep. Purif. Technol. 2022, 287, 120592. [Google Scholar] [CrossRef]
- Dantas, J.M.; Ferreira, M.R.; Catarino, T.; Kokhan, O.; Pokkuluri, P.R.; Salgueiro, C.A. Molecular Interactions between Geobacter Sulfurreducens Triheme Cytochromes and the Redox Active Analogue for Humic Substances. Biochim. Biophys. Acta-Bioenerg. 2018, 1859, 619–630. [Google Scholar] [CrossRef]
- Viggi, C.C.; Tucci, M.; Resitano, M.; Matturro, B.; Crognale, S.; Feigl, V.; Molnár, M.; Rossetti, S.; Aulenta, F. Passive Electrobioremediation Approaches for Enhancing Hydrocarbons Biodegradation in Contaminated Soils. Sci. Total Environ. 2022, 845, 157325. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Aulenta, F.; Canosa, A.; Reale, P.; Rossetti, S.; Panero, S.; Majone, M. Microbial Reductive Dechlorination of Trichloroethene to Ethene with Electrodes Serving as Electron Donors without the External Addition of Redox Mediators. Biotechnol. Bioeng. 2009, 103, 85–91. [Google Scholar] [CrossRef]


| Consumed H2/mgCOD L−1 | Produced CH4+ VFAs/mgCOD L−1 | Recovery/% | |
|---|---|---|---|
| Biochar | 513 ± 36 | 516 ± 2 | 101 |
| AQDS | 610 ± 53 | 717± 68 | 117 |
| Control | 639 ± 14 | 725 ± 16 | 113 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucci, M.; Colantoni, S.; Cruz Viggi, C.; Aulenta, F. Improving the Kinetics of H2-Fueled Biological Methanation with Quinone-Based Redox Mediators. Catalysts 2023, 13, 859. https://doi.org/10.3390/catal13050859
Tucci M, Colantoni S, Cruz Viggi C, Aulenta F. Improving the Kinetics of H2-Fueled Biological Methanation with Quinone-Based Redox Mediators. Catalysts. 2023; 13(5):859. https://doi.org/10.3390/catal13050859
Chicago/Turabian StyleTucci, Matteo, Simone Colantoni, Carolina Cruz Viggi, and Federico Aulenta. 2023. "Improving the Kinetics of H2-Fueled Biological Methanation with Quinone-Based Redox Mediators" Catalysts 13, no. 5: 859. https://doi.org/10.3390/catal13050859
APA StyleTucci, M., Colantoni, S., Cruz Viggi, C., & Aulenta, F. (2023). Improving the Kinetics of H2-Fueled Biological Methanation with Quinone-Based Redox Mediators. Catalysts, 13(5), 859. https://doi.org/10.3390/catal13050859







