A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation
Abstract
1. Introduction
2. Results
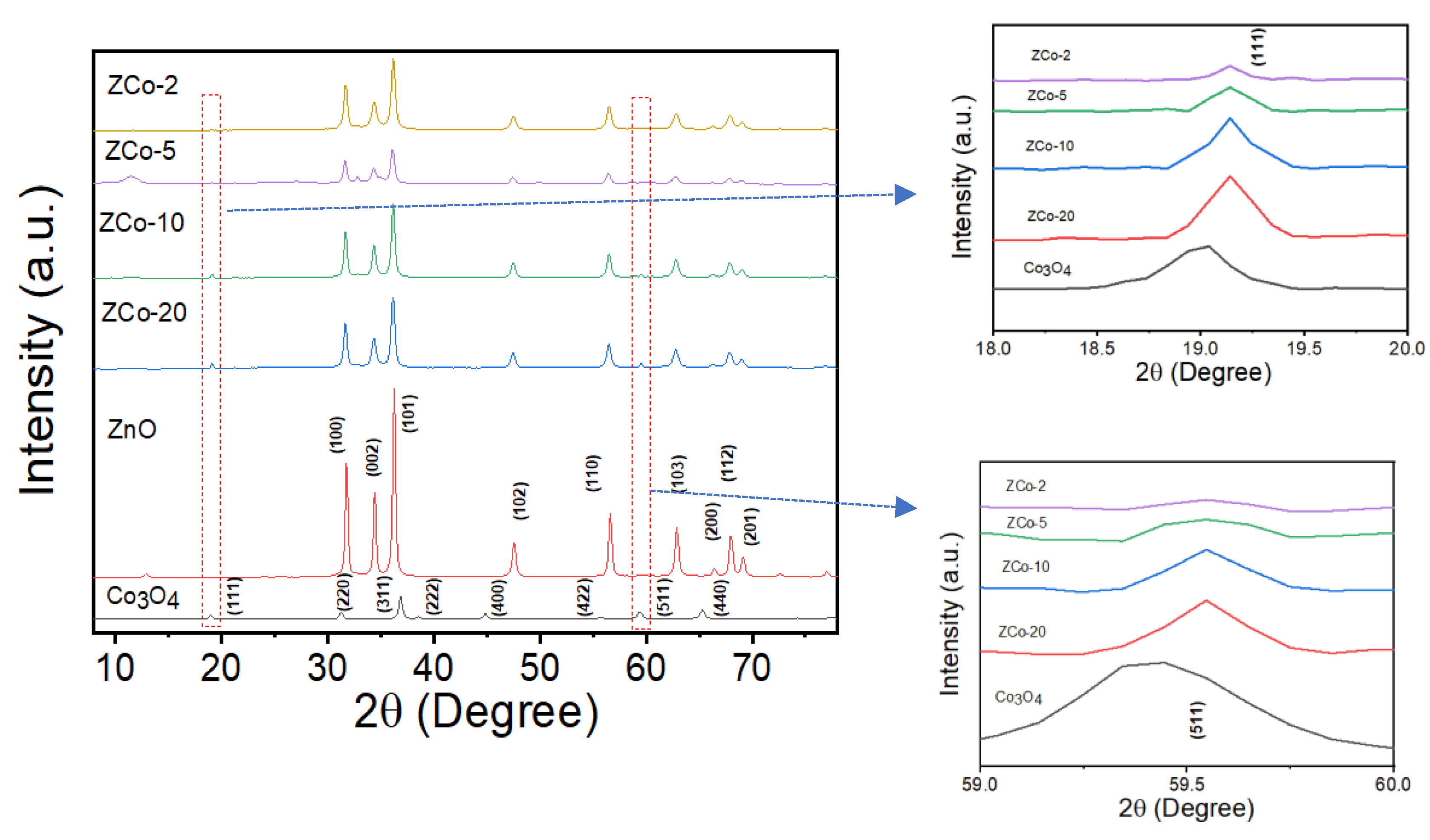
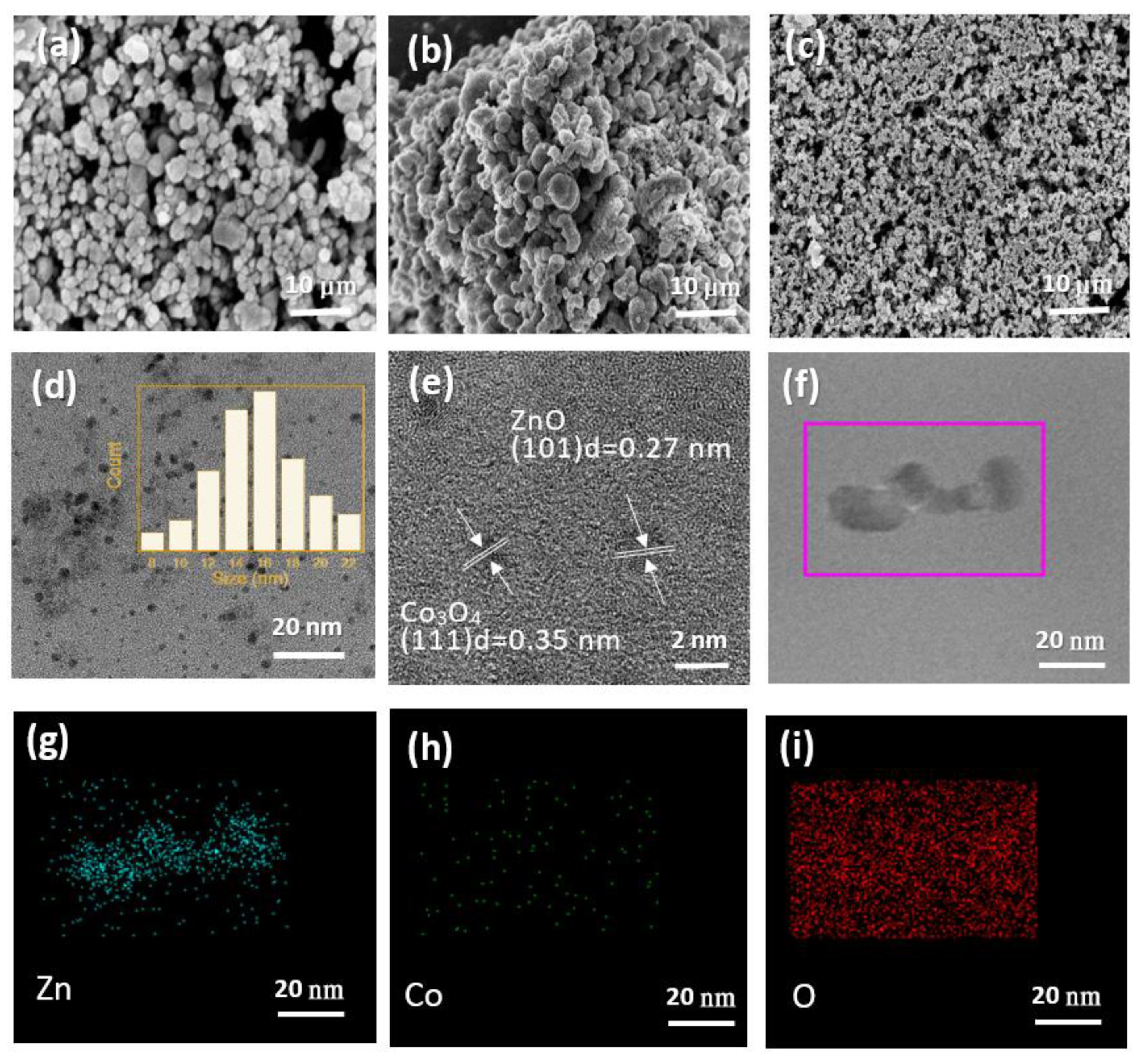
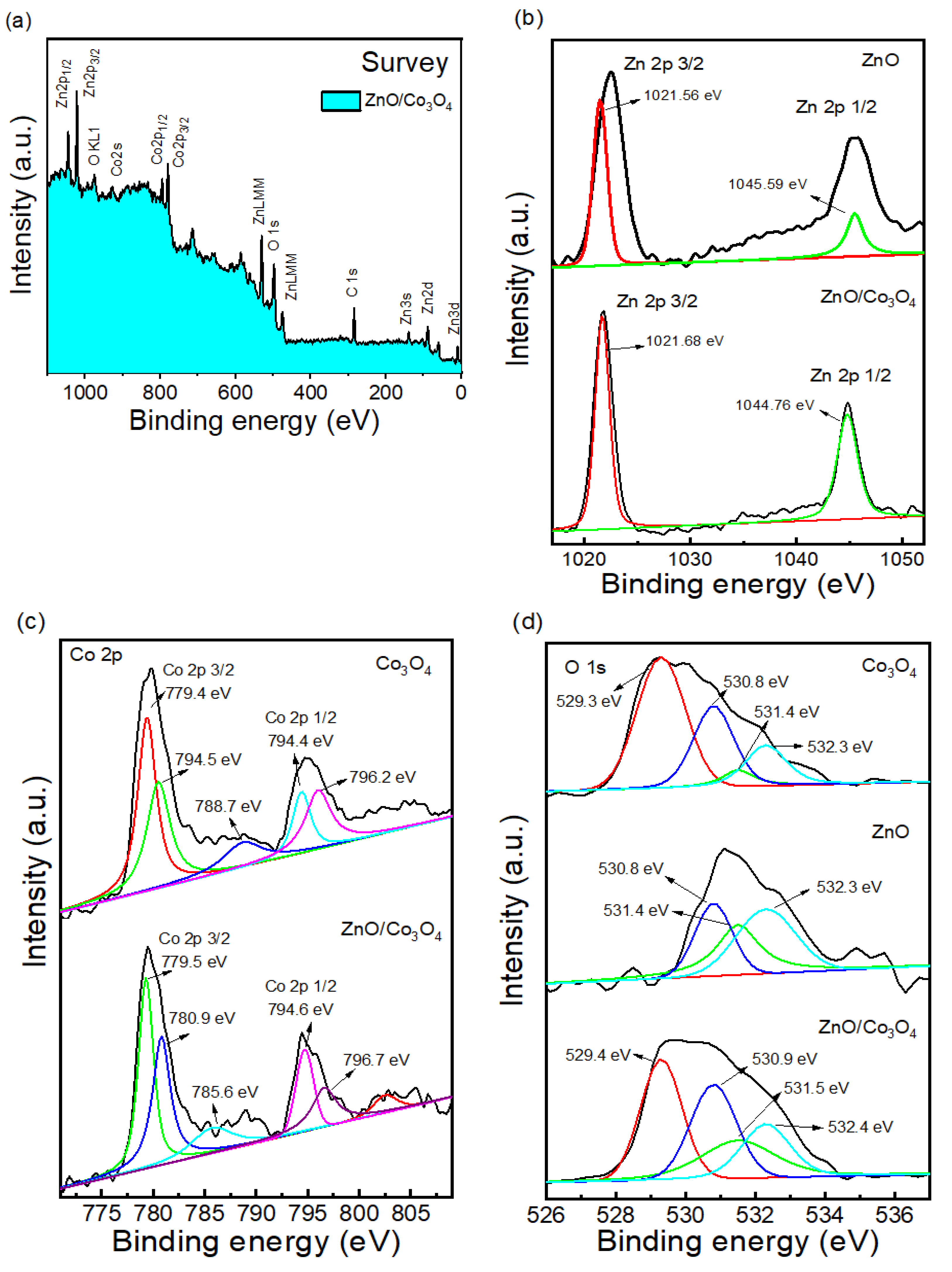
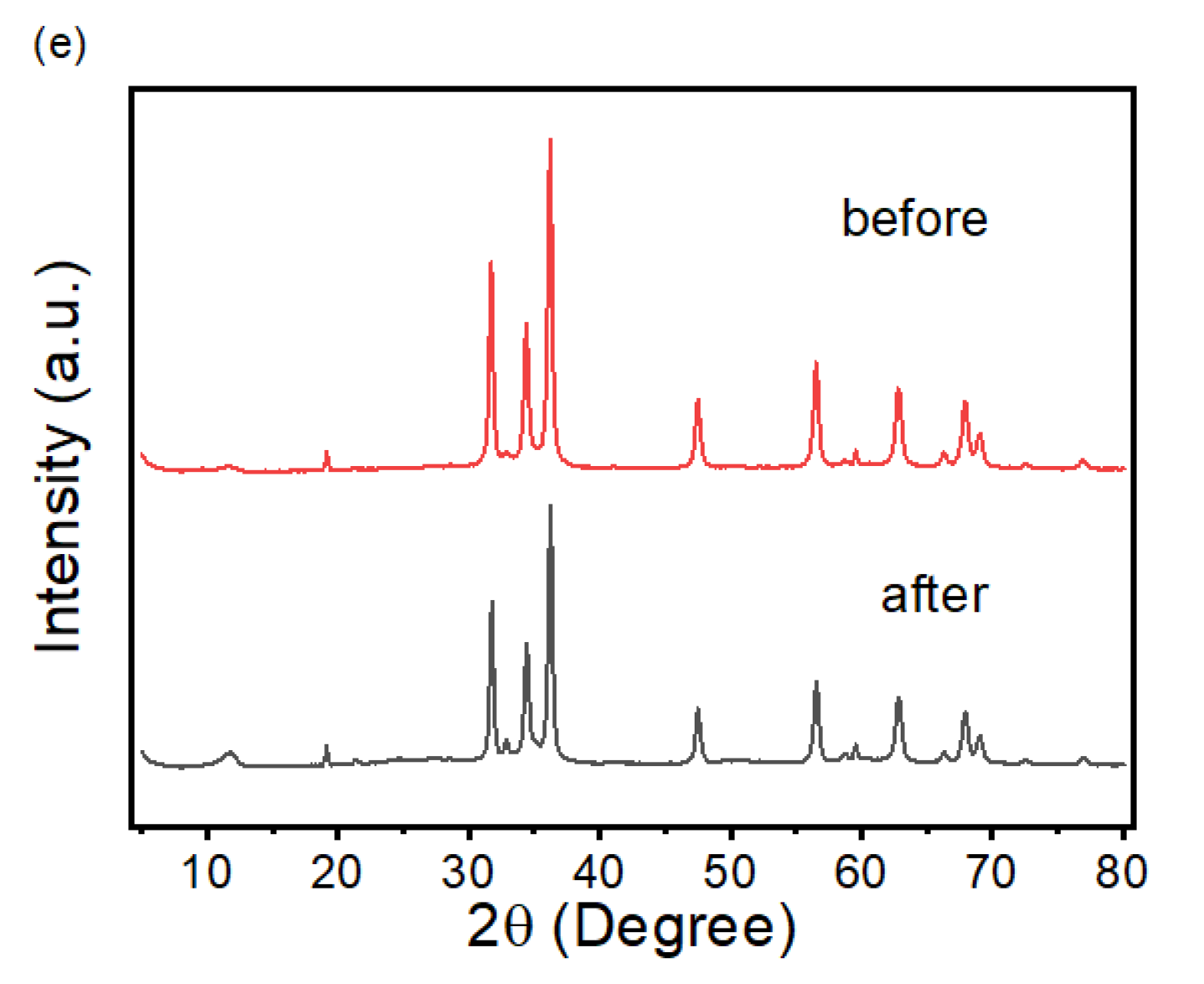
2.1. Microstructure Characterization
2.2. Photoelectrochemical Behavior
2.3. Hydrogen Production Behavior
3. Materials and Methods
3.1. Preparation of Materials
3.2. Synthesis
3.3. Characterization
3.4. Photocatalytic Hydrogen Evolution Tests
3.5. Photoelectrochemical Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, G.; Thummavichai, K.; Lv, X.; Chen, W.; Lin, T.; Tan, S.; Zeng, M.; Chen, Y.; Wang, N.; Zhu, Y. Defect-Rich Heterogeneous MoS2/rGO/NiS Nanocomposite for Efficient pH-Universal Hydrogen Evolution. Nanomaterials 2021, 11, 662. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2020, 44, 4110. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602. [Google Scholar] [CrossRef]
- Song, F.; Li, W.; Sun, Y. Metal–Organic Frameworks and Their Derivatives for Photocatalytic Water Splitting. Inorganics 2017, 5, 40. [Google Scholar] [CrossRef]
- Goldsmith, J.I.; Hudson, W.R.; Lowry, M.S.; Anderson, T.H.; Bernhard, S. Discovery and high-throughput screening of heteroleptic iridium complexes for photoinduced hydrogen production. J. Am. Chem. Soc. 2005, 127, 7502. [Google Scholar] [CrossRef]
- Mehtab, A.; Alshehri, S.M.; Ahmad, T. Photocatalytic and photoelectrocatalytic water splitting by porous g-C3N4 nanosheets for hydrogen generation. ACS Appl. Nano Mater. 2022, 5, 12656. [Google Scholar] [CrossRef]
- Zhao, W.; Chen, Z.; Yang, X.; Qian, X.; Liu, C.; Zhou, D.; Sun, T.; Zhang, M.; Wei, G.; Dissanayake, P.D.; et al. Recent advances in photocatalytic hydrogen evolution with high-performance catalysts without precious metals. Renew. Sustain. Energy Rev. 2020, 132, 110040. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-Functionalized Titanium Based Metal-Organic Framework for Photocatalytic Hydrogen Production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Ren, X.; Luo, S.; Huang, H.; Chen, R.; Shao, S.; Liu, D.; Gao, J.; Gui, J.; et al. Building rapid charge transfer channel via engineering Ni coordinated flexible polymer for efficient solar hydrogen evolution. Chem. Eng. J. 2023, 456, 141032. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Zhao, Y.; Yang, T.; Liu, X.; Xie, J.; Li, G.; Zhu, D.; Tan, H.; Su, Z. Photosensitizers based on Ir(III) complexes for highly efficient photocatalytic hydrogen generation. Dyes Pigm. 2019, 170, 107547. [Google Scholar] [CrossRef]
- Yang, Y.; Niu, S.; Han, D.; Liu, T.; Wang, G.; Li, Y. Progress in Developing Metal Oxide Nanomaterials for Photoelectrochemical Water Splitting. Adv. Energy Mater. 2017, 7, 1700555. [Google Scholar] [CrossRef]
- Ishchenko, O.; Rogé, V.; Lamblin, G.; Lenoble, D.; Fechete, I. TiO2, ZnO, and SnO2 -based metal oxides for photocatalytic applications: Principles and development. C. R. Chim. 2021, 24, 103. [Google Scholar]
- Singh, R.; Dutta, S. A review on H2 production through photocatalytic reactions using TiO2/TiO2 -assisted catalysts. Fuel 2018, 220, 607. [Google Scholar] [CrossRef]
- Shaikh, Z.A.; Laghari, A.A.; Litvishko, O.; Litvishko, V.; Kalmykova, T.; Meynkhard, A. Liquid-Phase Deposition Synthesis of ZIF-67-Derived Synthesis of Co3O4@TiO2 Composite for Efficient Electrochemical Water Splitting. Metals 2021, 11, 420. [Google Scholar] [CrossRef]
- Yao, X.; Ho, P.-Y.; Yiu, S.-C.; Suramitr, S.; Li, W.-B.; Ho, C.-L.; Hannongbua, S. Development of new thiocyanate-free Ruthenium(II) dyes bearing isoquinoline chromophores for hydrogen production via water splitting. Dyes Pigm. 2022, 205, 110508. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Ababtain, A.S.; Alanazi, H.S.; Al-Nafaei, W.S.; Hasan, I. Synthesis of Zn3V2O8/rGO Nanocomposite for Photocatalytic Hydrogen Production. Inorganics 2023, 11, 93. [Google Scholar] [CrossRef]
- Tien, T.M.; Chung, Y.J.; Huang, C.T.; Chen, E.L. WSSe Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methylene Blue Removal under Visible-Light Irradiation. Materials 2022, 15, 5616. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, T.; Tian, W.; Zhang, Y.; Shan, P.; Chen, Y.; Wei, W.; Yuan, H.; Cui, H. Mechanism for Hydrogen Evolution from Water Splitting Based on a MoS2/WSe2 Heterojunction Photocatalyst: A First-Principle Study. RSC Adv. 2020, 10, 41127. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.J.; Liao, J.D.; Chen, L.J. Polyvinylbutyral-assisted synthesis and characterization of mesoporous silica nanofibers by electrospinning route. J. Compos. Mater. 2012, 46, 227. [Google Scholar] [CrossRef]
- Chen, L.J.; Chen, C.W.; Dong, C.D. Direct Z-Scheme Heterostructures Based on MoSSe Quantum Dots for Visible Light-Driven Photocatalytic Tetracycline Degradation. ACS Appl. Nano Mater. 2021, 4, 1038. [Google Scholar] [CrossRef]
- Tien, T.M.; Chen, E.L. S-Scheme System of MoS2/Co3O4 Nanocomposites for Enhanced Photocatalytic Hydrogen Evolution and Methyl Violet Dye Removal under Visible Light Irradiation. Coatings 2023, 13, 80. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J. Quaternary Semiconductor Derived and formation mechanism by non-vacuum Route from Solvothermal Nanostructures for High-Performance application. Mater. Lett. 2013, 91, 372. [Google Scholar] [CrossRef]
- Chen, L.J. Synthesis and optical properties of lead-free cesium germanium halide perovskite quantum rods. RSC Adv. 2018, 18, 18396. [Google Scholar] [CrossRef] [PubMed]
- Sanakousar, F.M.; Vidyasagar, C.C.; Jimenez-Perez, V.M.; Prakash, K. Recent progress on visible-light-driven metal and nonmetal doped ZnO nanostructures for photocatalytic degradation of organic pollutants. Mater. Sci. Semicond. 2022, 140, 106390. [Google Scholar] [CrossRef]
- Biswal, H.J.; Srivastava, T.; Vundavilli, P.R.; Gupta, A. Facile fabrication of hydrophobic ZnO nanostructured nickel microtubes through pulse electrodeposition as promising photocatalyst for wastewater remediation. J. Manuf. Process. 2022, 75, 538. [Google Scholar] [CrossRef]
- Le Pivert, M.; Martin, N.; Leprince-Wang, Y. Hydrothermally Grown ZnO Nanostructures for Water Purification via Photocatalysis. Crystals 2022, 12, 308. [Google Scholar] [CrossRef]
- Wu, T.; Huang, J.; Cheng, G.; Pang, Y. Enhanced photocatalytic hydrogen evolution based on ternary noblemetal-free Co3O4/CdS/g-C3N4 composite. Mater. Lett. 2021, 292, 129274. [Google Scholar]
- Tien, T.M.; Chen, C.H.; Huang, C.T.; Chen, E.L. Photocatalytic Degradation of Methyl Orange Dyes Using Green Synthesized MoS2/Co3O4 Nanohybrids. Catalysts 2022, 12, 1474. [Google Scholar] [CrossRef]
- Chen, L.J. Tunable photoluminescence emission from Cadmium Tellurium nanorods with ethylenediamine template-assistance at a low temperature. Mater. Lett. 2013, 101, 83. [Google Scholar] [CrossRef]
- Tien, T.M.; Chung, Y.J.; Huang, C.T.; Chen, E.L. Fabrication of WS2/WSe2 Z-Scheme Nano-Heterostructure for Efficient Photocatalytic Hydrogen Production and Removal of Congo Red under Visible Light. Catalysts 2022, 12, 852. [Google Scholar] [CrossRef]
- Kavan, L. Conduction band engineering in semiconducting oxides (TiO2, SnO2): Applications in perovskite photovoltaics and beyond. Catal. Today 2019, 328, 50. [Google Scholar] [CrossRef]
- Krobthong, S.; Rungsawang, T.; Wongrerkdee, S. Comparison of ZnO Nanoparticles Prepared by Precipitation and Combustion for UV and Sunlight-Driven Photocatalytic Degradation of Methylene Blue. Toxics 2023, 11, 266. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Zhang, X.-K.; Fan, J.-Q.; Zhan, X.-Q.; Xie, L.; Shi, D.; Jiang, T.; Tsai, F.-C. MOF-Templated Synthesis of Co3O4@TiO2 Hollow Dodecahedrons for High-Storage-Density Lithium-Ion Batteries. ACS Omega 2019, 4, 13241. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.J.; Lee, C.R.; Chuang, Y.J.; Wu, Z.H.; Chen, C. Synthesis and Optical Properties of Lead-Free Cesium Tin Halide Perovskite Quantum Rods with High-Performance Solar Cell Application. J. Phys. Chem. Lett. 2016, 7, 5028. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Feng, Y.; Jeon, Y.; Guan, B.; Lou, X.W.D.; Paik, U. Formation of Ni-Co-MoS2 nanoboxes with enhanced electrocatalytic activity for hydrogen evolution. Adv. Mater. 2016, 28, 9006. [Google Scholar] [CrossRef]
- Ahmad, I.; Shukrullah, S.; Naz, M.Y.; Bhatt, H.N.; Ahmad, M.; Ahmed, E.; Ullah, S.; Hussien, M. Recent progress in rare earth oxides and carbonaceous materials modified ZnO heterogeneous photocatalysts for environmental and energy applications. J. Environ. Chem. Eng. 2022, 10, 107762. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J.; Chen, C. Surface passivation assisted lasing emission in the quantum dots doped cholesteric liquid crystal resonating cavity with polymer template. RSC Adv. 2014, 4, 18600. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, P.; Li, M.; Li, Y.; Zhang, X.; Chen, J.; Fang, X.; Liu, Y.; Yuan, X.; Dai, X.; et al. Interfacial synergy between dispersed Ru sub-nanoclusters and porous NiFe layered double hydroxide on accelerated overall water splitting by intermediate modulation. Nanoscale 2020, 12, 9669. [Google Scholar] [CrossRef]
- Chen, D.; Lin, Z.; Sartin, M.M.; Huang, T.X.; Liu, J.; Zhang, Q.; Han, L.; Li, J.F.; Tian, Z.Q.; Zhan, D. Photosynergetic electrochemical synthesis of graphene oxide. J. Am. Chem. Soc. 2020, 142, 6516. [Google Scholar] [CrossRef]
- Altaa, S.H.A.; Alshamsi, H.A.H.; Al-Hayder, L.S.J. Synthesis and characterization of rGO/Co3O4 composite as nanoadsorbent for Rhodamine 6G dye removal. Desalin. Water Treat. 2018, 114, 320. [Google Scholar] [CrossRef]
- Shathy, R.A.; Fahim, S.A.; Sarker, M.; Quddus, M.S.; Moniruzzaman, M.; Masum, S.M.; Molla, M.A.I. Natural sunlight driven photocatalytic removal of toxic textile dyes in water using B-doped ZnO/TiO2 nanocomposites. Catalysts 2022, 12, 308. [Google Scholar] [CrossRef]
- Chen, L.J.; Chuang, Y.J. Directly electrospinning growth of single crystal Cu2ZnSnS4 nanowires film for high performance thin film solar cell. J. Power Sources 2013, 241, 259. [Google Scholar] [CrossRef]
- Qureshi, M.; Takanabe, K. Insights on Measuring and Reporting Heterogeneous Photocatalysis: Efficiency Definitions and Setup Examples. Chem. Mater. 2017, 29, 158. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tien, T.-M.; Chen, E.L. A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts 2023, 13, 852. https://doi.org/10.3390/catal13050852
Tien T-M, Chen EL. A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts. 2023; 13(5):852. https://doi.org/10.3390/catal13050852
Chicago/Turabian StyleTien, Tsung-Mo, and Edward L. Chen. 2023. "A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation" Catalysts 13, no. 5: 852. https://doi.org/10.3390/catal13050852
APA StyleTien, T.-M., & Chen, E. L. (2023). A Novel ZnO/Co3O4 Nanoparticle for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Catalysts, 13(5), 852. https://doi.org/10.3390/catal13050852






