Triton-X-100 as an Organic Catalyst for One-Pot Synthesis of Arylmethyl-H-phosphinic Acids from Red Phosphorus and Arylmethyl Halides in the KOH/H2O/Toluene Multiphase Superbase System
Abstract
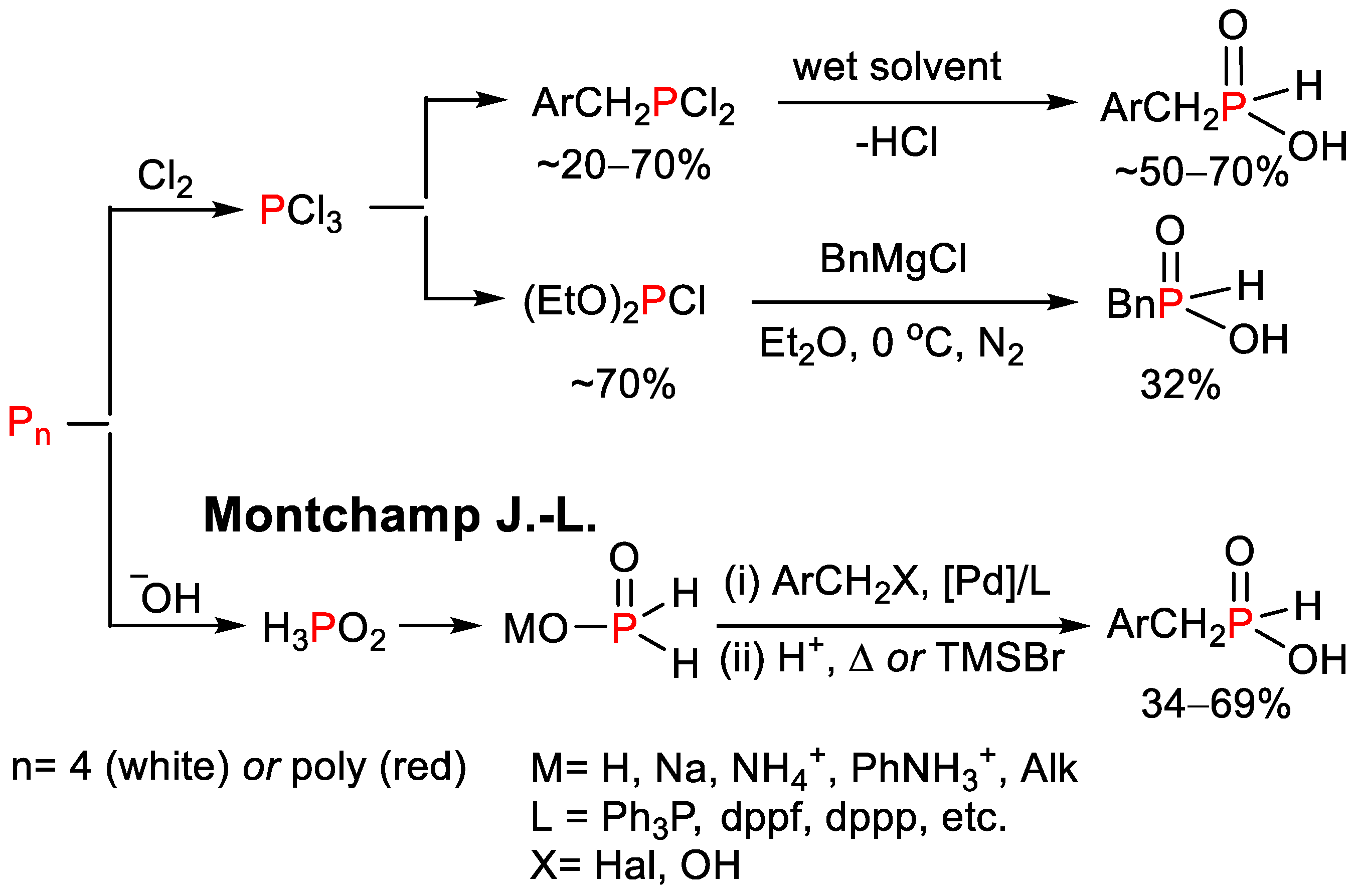
1. Introduction
2. Results
2.1. Optimization of the Reaction Conditions
2.2. Study on Substrate Scope
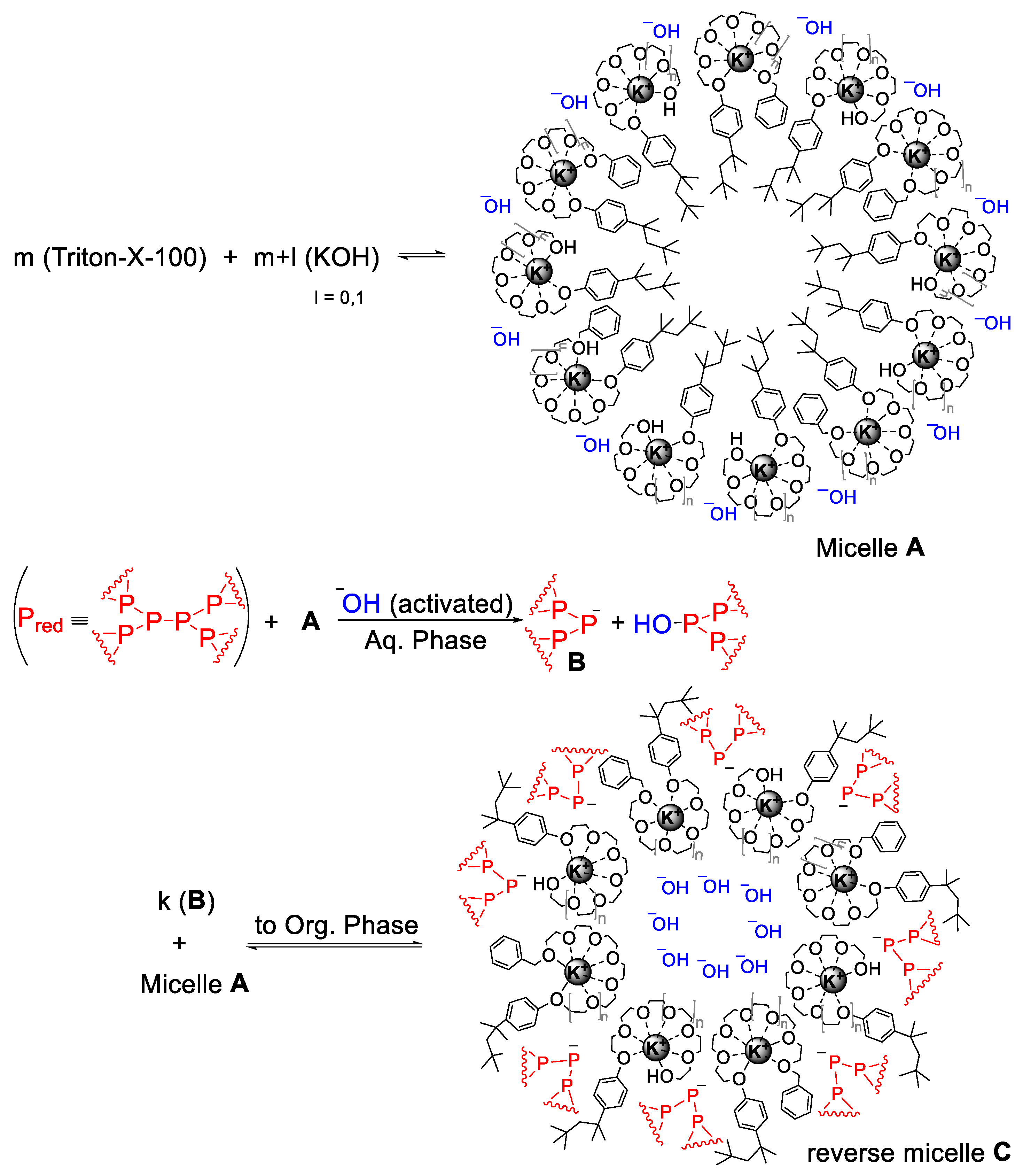
3. Discussion
4. Materials and Methods
4.1. General Considerations
4.2. General Procedure for the Synthesis of H-Phosphinic Acids 2
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdou, M.M.; O’Neill, P.M.; Amigues, E.; Matziari, M. Phosphinic acids: Current status and potential for drug discovery. Drug Discov. Today 2019, 24, 916–929. [Google Scholar] [CrossRef]
- Makki, M.S.T.; Abdel-Rahman, R.M.; Alharbi, A.S. Synthetic Approach for Novel Fluorine Substituted α-Aminophosphonic Acids Containing 1,2,4-Triazin-5-One Moiety as Antioxidant Agents. Int. J. Org. Chem. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Chu, L.; Luo, X.; Zhu, T.; Cao, Y.; Zhang, L.; Deng, Z.; Gao, J. Harnessing phosphonate antibiotics argolaphos biosynthesis enables a synthetic biology-based green synthesis of glyphosate. Nat. Commun. 2022, 13, 1736. [Google Scholar] [CrossRef]
- Nifant’ev, I.E.; Minyaev, M.E.; Tavtorkin, A.N.; Vinogradov, A.A.; Ivchenko, P.V. Branched alkylphosphinic and disubstituted phosphinic and phosphonic acids: Effective synthesis based on alpha-olefin dimers and applications in lanthanide extraction and separation. RSC Adv. 2017, 7, 24122–24128. [Google Scholar] [CrossRef]
- Agarwal, V.; Safarzadeh, M.S. Solvent extraction and molecular modeling studies of Dy(III) using acidic extractants. J. Mol. Liq. 2020, 304, 112452. [Google Scholar] [CrossRef]
- Safiulina, A.M.; Ivanets, D.V.; Kudryavtsev, E.M.; Baulin, D.V.; Baulin, V.E.; Tsivadze, A.Y. Liquid- and Solid-Phase Extraction of Uranium(VI), Thorium(IV), and Rare Earth Elements(III) from Nitric Acid Solutions Using Acid-Type Phosphoryl-Containing Podands. Russ. J. Inorg. Chem. 2019, 64, 536–542. [Google Scholar] [CrossRef]
- Zhang, R.; Khan, S.; Azimi, G. Microstructured silicon substrates impregnated with bis(2,4,4-trimethylpentyl) phosphinic acid for selective scandium recovery. Appl. Surf. Sci. 2023, 622, 156852. [Google Scholar] [CrossRef]
- Han, Y.; Chen, J.; Deng, Y.; Liu, T.; Li, H. A leaching, solvent extraction, stripping, precipitation and calcination process for the recovery of MoO3 and NiO from spent hydrofining catalysts. Hydrometallurgy 2023, 218, 106046. [Google Scholar] [CrossRef]
- Sait, N.; Aliouane, N.; Toukal, L.; Hammache, H.; Al-Noaimi, M.; Helesbeux, J.J.; Duval, O. Synthesis of ethylene bis [(2-hydroxy-5,1,3-phenylene) bis methylene] tetraphosphonic acid and their anticorrosive effect on carbon steel in 3%NaCl solution. J. Mol. Liq. 2021, 326, 115316. [Google Scholar] [CrossRef]
- Francos, J.; Elorriaga, D.; Crochet, P.; Cadierno, V. The chemistry of Group 8 metal complexes with phosphinous acids and related P OH ligands. Coordin. Chem. Rev. 2019, 387, 199–234. [Google Scholar] [CrossRef]
- Schneider, F.; Osterod, F.; Bauer, H.; Sicken, M. Mixtures of bis-phosphinic acids and alkylphosphinic acids as additives for polymer formulations for control of thermal stability and thermal expansion coefficient. U.S. Patent 20,180,030,355, 1 February 2018. [Google Scholar]
- Dhaene, E.; Coppenolle, S.; Deblock, L.; De Buysser, K.; De Roo, J. Binding Affinity of Monoalkyl Phosphinic Acid Ligands toward Nanocrystal Surfaces. Chem. Mater. 2023, 35, 558–569. [Google Scholar] [CrossRef]
- Petit, C.; Fécourt, F.; Montchamp, J.-L. Synthesis of Disubstituted Phosphinates via Palladium-Catalyzed Hydrophosphinylation of H-Phosphinic Acids. Adv. Synth. Catal. 2011, 353, 1883–1888. [Google Scholar] [CrossRef]
- Berger, O.; Petit, C.; Deal, E.L.; Montchamp, J.L. Phosphorus-Carbon Bond Formation: Palladium-Catalyzed Cross-Coupling of H-Phosphinates and Other P(O)H-Containing Compounds. Adv. Synth. Catal. 2013, 355, 1361–1373. [Google Scholar] [CrossRef]
- Li, Y.; Jin, X.; Liu, P.; Zhang, H.; Yu, X.; Liu, Y.; Liu, B.; Yang, W. Copper-Catalyzed Dynamic Kinetic C−P Cross-Coupling/Cyclization for the Concise Asymmetric Synthesis of Six-, Seven- and Eight-Membered P-Stereogenic Phosphorus Heterocycles. Angew. Chem. Int. Ed. 2022, 61, E202117093. [Google Scholar] [CrossRef]
- Montchamp, J.-L. Challenges and solutions in phosphinate chemistry. Pure Appl. Chem. 2019, 91, 113–120. [Google Scholar] [CrossRef]
- Winters, K.R.; Ricke, C.; Montchamp, J.L. Synthesis of Adamantyl H-Phosphinate Esters. Eur. J. Org. Chem. 2021, 2022, e202101130. [Google Scholar] [CrossRef]
- Troev, K.D. Reactivity of P–H Group of H-Phosphinic Acid and Its Derivatives. In Reactivity of P–H Group of Phosphorus Based Compounds; Academic Press: Cambridge, MA, USA, 2018; pp. 245–290. [Google Scholar] [CrossRef]
- Chen, T.; Han, L.-B. Optically Active H-Phosphinates and Their Stereospecific Transformations into Optically Active P-Stereogenic Organophosphoryl Compounds. Synlett 2015, 26, 1153–1163. [Google Scholar] [CrossRef]
- Greco, M.N.; Hawkins, M.J.; Powell, E.T.; Almond, H.R.; de Garavilla, L.; Hall, J.; Minor, L.K.; Wang, Y.; Corcoran, T.W.; Di Cera, E.; et al. Discovery of Potent, Selective, Orally Active, Nonpeptide Inhibitors of Human Mast Cell Chymase. J. Med. Chem. 2007, 50, 1727–1730. [Google Scholar] [CrossRef]
- Rudovský, J.; Kotek, J.; Hermann, P.; Lukeš, I.; Mainero, V.; Aime, S. Synthesis of a bifunctional monophosphinic acid DOTA analogue ligand and its lanthanide(iii) complexes. A gadolinium(iii) complex endowed with an optimal water exchange rate for MRI applications. Org. Biomol. Chem. 2005, 3, 112–117. [Google Scholar] [CrossRef]
- Froestl, W.; Mickel, S.J.; von Sprecher, G.; Diel, P.J.; Hall, R.G.; Maier, L.; Strub, D.; Melillo, V.; Baumann, P.A. Phosphinic Acid Analogs of GABA. 2. Selective, Orally Active GABAB Antagonists. J. Med. Chem. 1995, 38, 3313–3331. [Google Scholar] [CrossRef]
- Wolińska, E.; Hałdys, K.; Góra, J.; Olszewski, T.K.; Boduszek, B.; Latajka, R. Phosphonic and Phosphinic Acid Derivatives as Novel Tyrosinase Inhibitors: Kinetic Studies and Molecular Docking. Chem. Biodivers. 2019, 16, e1900167. [Google Scholar] [CrossRef] [PubMed]
- Duro, M.V.V.; Mustafa, D.; Kashemirov, B.A.; McKenna, C.E. Phosphorus in Chemical Biology and Medicinal Chemistry. In Organophosphorus Chemistry: From Molecules to Applications; Iaroshenko, V., Ed.; Wiley-VCH: Weinheim, Germany, 2019; Chapter 10; pp. 499–544. [Google Scholar] [CrossRef]
- Jackson, P.F.; Tays, K.L.; Maclin, K.M.; Ko, Y.-S.; Li, W.; Vitharana, D.; Tsukamoto, T.; Stoermer, D.; Lu, X.-C.M.; Wozniak, K.; et al. Design and Pharmacological Activity of Phosphinic Acid Based NAALADase Inhibitors. J. Med. Chem. 2001, 44, 4170–4175. [Google Scholar] [CrossRef] [PubMed]
- Slusher, B.S.; Jackson, P.F.; Tays, K.L.; Maclin, K.M. Methods of Cancer Treatment Using Naaladase Inhibitors. U.S. Patent 6,011,021, 4 January 2000. [Google Scholar]
- Virieux, D.; Volle, J.-N.; Bakalara, N.; Pirat, J.-L. Synthesis and Biological Applications of Phosphinates and Derivatives. In Phosphorus Chemistry I. Asymmetric Synthesis and Bioactive Compounds. Topics in Current Chemistry; Montchamp, J.-L., Ed.; Springer: Cham, Switzerland, 2015; Volume 360, pp. 39–114. [Google Scholar] [CrossRef]
- Raguin, O.; Fournié-Zaluski, M.-C.; Romieu, A.; Pèlegrin, A.; Chatelet, F.; Pélaprat, D.; Barbet, J.; Roques, B.P.; Gruaz-Guyon, A. A Labeled Neutral Endopeptidase Inhibitor as a Potential Tool for Tumor Diagnosis and Prognosis. Angew. Chem. Int. Ed. 2005, 44, 4058–4061. [Google Scholar] [CrossRef] [PubMed]
- Schwier, C.E.; Chapman, R.D.; Ayotte, R.C. Linear Amorphous Polyamides with Excess Amine Endgroups and Their Production. U.S. Patent 5,245,005, 14 September 1993. [Google Scholar]
- Balavoine, F.; Compere, D.; Llorens-Cortes, C.; Marc, Y. Aminopeptidase a Inhibitors and Pharmaceutical Compositions Comprising the Same. WO Patent 2020/084131, 30 April 2020. [Google Scholar]
- Sachais, B.; Rux, J. Screening Methods for Identifying Small Molecule Antagonists of Platelet Factor-4 (PF4) Containing Ultra Large Complexes and Uses Thereof for Treating Medical Conditions Such as Heparin-Induced Thrombocytopenia and Related Diseases. WO Patent 2,013,142,328, 26 September 2013. [Google Scholar]
- Corbridge, D.E.C. Phosphorus. Chemistry, Biochemistry and Technology, 6th ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Pietrusiewicz, K.M.; Stankevic, M. Product Class 8: Alkylphosphonous Acids and Derivatives. In Science of Synthesis; Mathey, F., Trost, B.M., Eds.; Thieme: Leipzig, Germany, 2009; Volume 42.8.6, p. 251. [Google Scholar] [CrossRef]
- Jackson, P.F.; Slusher, B.S. Prodrugs of NAALADase Inhibitors. U.S. Patent 6,384,022, 7 May 2002. [Google Scholar]
- Bravo-Altamirano, K.; Montchamp, J.-L. Phosphinic Acid, Alkyl Esters. Encyclopedia of Reagents for Organic Synthesis (e-EROS); Wiley: Hoboken, NJ, USA, 2007. [Google Scholar] [CrossRef]
- Coudray, L.; Montchamp, J.L. Green, palladium-catalyzed synthesis of benzylic H-phosphinates from hypophosphorous acid and benzylic alcohols. Eur. J. Org. Chem. 2008, 2008, 4101–4103. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa-Thomas, I.; Ribiere, P.; Adcock, A.C.; Montchamp, J.L. Direct monoalkylation of alkyl phosphinates to access H-phosphinic acid esters. Synthesis 2006, 2006, 325–331. [Google Scholar] [CrossRef]
- Montchamp, J.L.; Dumond, Y.R. Synthesis of monosubstituted phosphinic acids: Palladium-catalyzed cross-coupling reactions of anilinium hypophosphite. J. Am. Chem. Soc. 2001, 123, 510–511. [Google Scholar] [CrossRef]
- Fu, X.; Loh, W.-T.; Zhang, Y.; Chen, T.; Ma, T.; Liu, H.; Wang, J.; Tan, C.-H. Chiral Guanidinium Salt Catalyzed Enantioselective Phospha-Mannich Reactions. Angew. Chem. Int. Ed. 2009, 48, 7387–7390. [Google Scholar] [CrossRef]
- Kalek, M.; Stawinski, J. Efficient synthesis of mono- and diarylphosphinic acids: A microwave-assisted palladium-catalyzed cross-coupling of aryl halides with phosphinate. Tetrahedron 2009, 65, 10406–10412. [Google Scholar] [CrossRef]
- Montchamp, J.-L. Recent advances in phosphorus–carbon bond formation: Synthesis of H-phosphinic acid derivatives from hypophosphorous compounds. J. Organomet. Chem. 2005, 690, 2388–2406. [Google Scholar] [CrossRef]
- Albouy, D.; Etemad-Moghadam, G.; Koenig, M. Phosphorylating Power of Red Phosphorus towards Aldehydes in Basic and in Acidic Media. Eur. J. Org. Chem. 1999, 1999, 861–868. [Google Scholar] [CrossRef]
- Dragulescu-Andrasi, A.; Miller, L.Z.; Chen, B.H.; McQuade, D.T.; Shatruk, M. Facile Conversion of Red Phosphorus into Soluble Polyphosphide Anions by Reaction with Potassium Ethoxide. Angew. Chem. Int. Ed. 2016, 55, 3904–3908. [Google Scholar] [CrossRef]
- Caporali, M.; Serrano-Ruiz, M.; Peruzzini, M. Benign Chlorine-Free Approaches to Organophosphorus Compounds. In Chemistry Beyond Chlorine, Part II; Tundo, P., He, L.N., Lokteva, E., Mota, C., Eds.; Springer: Cham, Switzerland, 2016; pp. 97–136. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Trofimov, B.A. Organophosphorus chemistry based on elemental phosphorus: Advances and horizons. Russ. Chem. Rev. 2020, 89, 225–249. [Google Scholar] [CrossRef]
- Jo, M.; Dragulescu-Andrasi, A.; Miller, L.Z.; Pak, C.; Shatruk, M. Nucleophilic Activation of Red Phosphorus for Controlled Synthesis of Polyphosphides. Inorg. Chem. 2020, 59, 5483–5489. [Google Scholar] [CrossRef]
- Olmstead, W.N.; Margolin, Z.; Bordwell, F.G. Acidities of Water and Simple Alcohols in Dimethylsulfoxide Solution. J. Org. Chem. 1980, 45, 3295–3299. [Google Scholar] [CrossRef]
- Malysheva, S.F.; Kuimov, V.A.; Belogorlova, N.A.; Albanov, A.I.; Gusarova, N.K.; Trofimov, B.A. Superbase-Assisted Selective Synthesis of Triarylphosphines from Aryl Halides and Red Phosphorus: Three Consecutive Different SNAr Reactions in One Pot. Eur. J. Org. Chem. 2019, 2019, 6240–6245. [Google Scholar] [CrossRef]
- Malysheva, S.F.; Kuimov, V.A.; Trofimov, A.B.; Belogorlova, N.A.; Litvintsev, Y.I.; Belogolova, A.M.; Gusarova, N.K.; Trofimov, B.A. 2-Halopyridines in the triple reaction in the Pn/KOH/DMSO system to form tri(2-pyridyl)phosphine: Experimental and quantum-chemical dissimilarities. Mendeleev Commun. 2018, 28, 472–474. [Google Scholar] [CrossRef]
- Malysheva, S.F.; Belogorlova, N.A.; Kuimov, V.A.; Litvintsev, Y.I.; Sterkhova, I.V.; Albanov, A.I.; Gusarova, N.K.; Trofimov, B.A. PCl3- and organometallic-free synthesis of tris(2-picolyl)phosphine oxide from elemental phosphorus and 2-(chloromethyl)pyridine hydrochloride. Tetrahedron Lett. 2018, 59, 723–726. [Google Scholar] [CrossRef]
- Trofimov, B.A.; Artem’ev, A.V.; Gusarova, N.K.; Sutyrina, A.O.; Malysheva, S.F.; Oparina, L.A. Hydrophosphorylation of vinyl sulfides with elemental phosphorus in the KOH/DMSO(H2O) system: Synthesis of 2-alkyl(aryl) thioethylphosphinic acids. J. Sulfur Chem. 2018, 39, 112–118. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Sutyrina, A.O.; Matveeva, E.A.; Sterkhova, I.V.; Smirnov, V.I.; Trofimov, B.A. One-Pot Chlorine-Free Synthesis of Chiral Organophosphorus Compounds from Elemental Phosphorus and α-Methylstyrene Dimer. Dokl. Chem. 2018, 478, 5–8. [Google Scholar] [CrossRef]
- Gusarova, N.K.; Sutyrina, A.O.; Kuimov, V.A.; Malysheva, S.F.; Belogorlova, N.A.; Volkov, P.A.; Trofimov, B.A. Single-stage synthesis of alkyl-H-phosphinic acids from elemental phosphorus and alkyl bromides. Mendeleev Commun. 2019, 29, 328–330. [Google Scholar] [CrossRef]
- Kuimov, V.A.; Malysheva, S.F.; Belogorlova, N.A.; Albanov, A.I.; Gusarova, N.K.; Trofimov, B.A. Synthesis of Long-Chain n-Alkylphosphonic Acids by Phosphonylation of Alkyl Bromides with Red Phosphorus and Superbase under Micellar/Phase Transfer Catalysis. Eur. J. Org. Chem. 2021, 2021, 1596–1602. [Google Scholar] [CrossRef]
- Kuimov, V.A.; Malysheva, S.F.; Belogorlova, N.A.; Gusarova, N.K.; Trofimov, B.A. Chemoselective synthesis of long-chain alkyl-H-phosphinic acids via one-pot alkylation/oxidation of red phosphorus with alkyl-PEGs as recyclable micellar catalysts. Org. Biomol. Chem. 2021, 19, 10587–10595. [Google Scholar] [CrossRef] [PubMed]
- Brewer, S.E.; Vickery, T.P.; Bachert, D.C.; Brands, K.M.J.; Emerson, K.M.; Goodyear, A.; Kumke, K.J.; Lam, T.; Scott, J.P. Thermal Hazard Evaluation of 4-Methoxybenzyl Chloride (PMB-Cl). Org. Process Res. Dev. 2005, 9, 1009–1012. [Google Scholar] [CrossRef]
- Denegri, B.; Matić, M.; Vaško, M. Mechanism of solvolysis of substituted benzyl chlorides in aqueous ethanol. Tetrahedron 2022, 103, 132548. [Google Scholar] [CrossRef]
- Levshina, K.V.; Sergievskaya, S.I. Preparation of N,N-bis(chloroethyl) alkaryl amines. Zh. Obshch. Khim. 1954, 24, 905–909. [Google Scholar]
- Karmanova, I.B.; Vol'kenshtein, Y.B.; Belen'kii, L.I. Alkyl m-chloromethylphenyl ketones. U.S.S.R. Patent SU585150, 25 December 1977. [Google Scholar]
- 2-Chloromethylthiophene. Organic Syntheses 1949, 29, 31. Available online: https://orgsyn.org/Content/pdfs/procedures/CV3P0197.pdf (accessed on 7 April 2023).
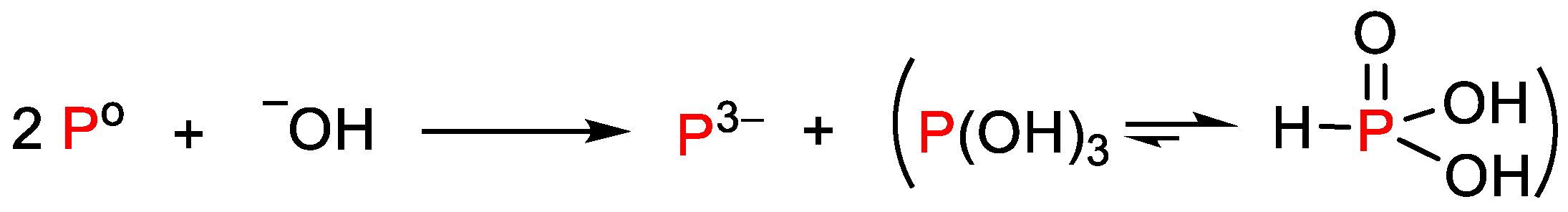


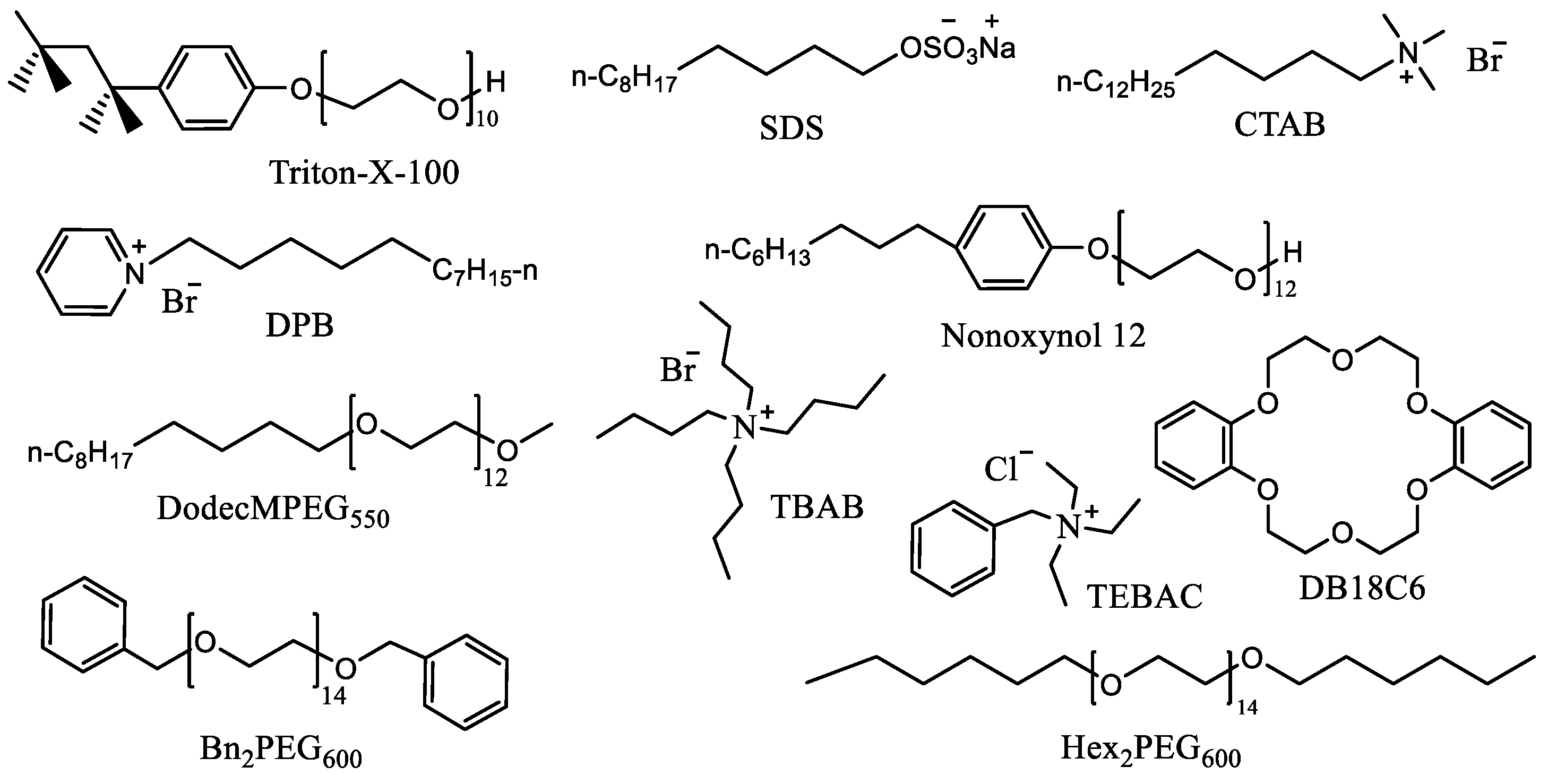
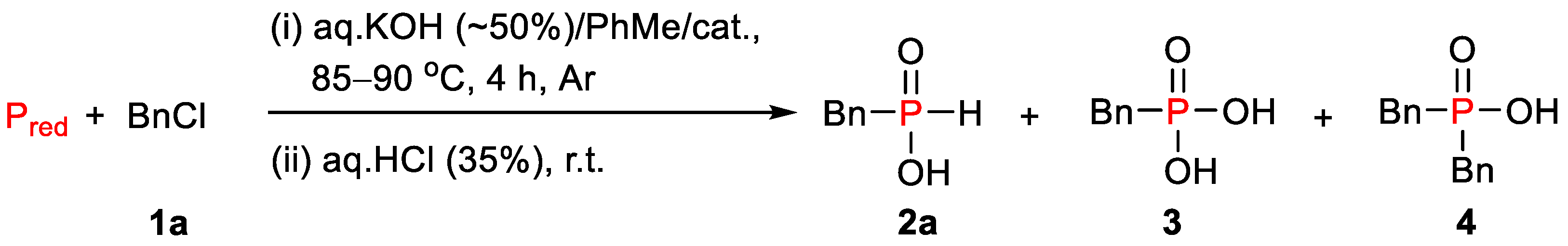



| Entry | Catalyst | Conversion of Pred, % | Content of Acids in the Crude Product | ||
|---|---|---|---|---|---|
| 2a | 3 | 4 | |||
| 1 | Bu3N | 99 | 0 | 0 | 0 |
| 2 | TBAB | 40 | 7 | 1 | 11 |
| 3 2 | TEBAC | 59 | 4 | 1 | 5 |
| 4 | [Ph4P]Br | 98 | 0 | 0 | 0 |
| 5 | DPB | 97 | 0 | 0 | 0 |
| 6 | CTAB | 70 | 31 | 2 | 6 |
| 7 3 | Stearate Na | 77 | 0 | 0 | 2 |
| 8 | SDS | 89 | 0 | 0 | 0 |
| 9 | DB18C6 | 74 | 10 | 0 | 6 |
| 10 | PEG1000 | 70 | 15 | 2 | 2 |
| 11 | Hex2PEG600 | 73 | 11 | 2 | 3 |
| 12 | DodecMPEG550 | 96 | 27 | trace | 0 |
| 13 | Bn2PEG600 | 87 | 11 | trace | 0 |
| 14 | Nonoxynol-12 | 97 | 16 | 0 | 3 |
| 15 | Triton-X-100 | 73 | 50 | 0 | 4 |
| Entry | Catalyst (mol%) | Temp., °C | Feeding Time of BnCl | Reaction Time | Concentration of KOH, % | Conversion of Pred, % | 31P NMR Yield of Acids | ||
|---|---|---|---|---|---|---|---|---|---|
| 2a | 3 | 4 | |||||||
| 1 | CTAB (5) | 85–90 | 2 h | 6 | 50 | 99 | 31 | 2 | 6 |
| 2 | CTAB (5) | 90–95 | 1.25 h | 6 | 50 | 98 | 16 | 0 | 0 |
| 3 | CTAB (5) | 90–95 | 0.7 h | 1 | 50 | 47 | 8 | 3 | 9 |
| 4 | CTAB (5) | 90–95 | 1.5 h | 3 | 50 | 48 | 32 | 0 | 0 |
| 5 | CTAB (5) | 95–97 | 1 h | 3 | 50 | 61 | 34 | 0 | 0 |
| 6 | Triton-X-100 (10) | 80 | 1 min | 3 | 55 | 76 | 40 | 0 | 0 |
| 7 | Triton-X-100 (5) | 85–90 | 2 h | 4.5 | 55 | 71 | 41 | 2 | 4 |
| 8 | Triton-X-100 (2.5) | 80–85 | 2 h | 5 | 55 | 83 | 3 | 1 | 2 |
| 9 | Triton-X-100 (1) | 80–85 | 2 h | 5 | 50 | 92 | 2 | 1 | 3 |
| 10 | Triton-X-100 (5) | 85–90 | 1.5 h | 4 | 50 | 73 | 50 | 0 | 4 |
| 11 | Triton-X-100 (2.5) | 85–90 | 2 h | 6 | 55 | 92 | 63 | 0 | 2 |
| 12 | Triton-X-100 (2.5) | 95–97 | 1 min | 3 | 55 | 97 | 65 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuimov, V.A.; Malysheva, S.F.; Belogorlova, N.A.; Fattakhov, R.I.; Albanov, A.I.; Trofimov, B.A. Triton-X-100 as an Organic Catalyst for One-Pot Synthesis of Arylmethyl-H-phosphinic Acids from Red Phosphorus and Arylmethyl Halides in the KOH/H2O/Toluene Multiphase Superbase System. Catalysts 2023, 13, 720. https://doi.org/10.3390/catal13040720
Kuimov VA, Malysheva SF, Belogorlova NA, Fattakhov RI, Albanov AI, Trofimov BA. Triton-X-100 as an Organic Catalyst for One-Pot Synthesis of Arylmethyl-H-phosphinic Acids from Red Phosphorus and Arylmethyl Halides in the KOH/H2O/Toluene Multiphase Superbase System. Catalysts. 2023; 13(4):720. https://doi.org/10.3390/catal13040720
Chicago/Turabian StyleKuimov, Vladimir A., Svetlana F. Malysheva, Natalia A. Belogorlova, Ruslan I. Fattakhov, Alexander I. Albanov, and Boris A. Trofimov. 2023. "Triton-X-100 as an Organic Catalyst for One-Pot Synthesis of Arylmethyl-H-phosphinic Acids from Red Phosphorus and Arylmethyl Halides in the KOH/H2O/Toluene Multiphase Superbase System" Catalysts 13, no. 4: 720. https://doi.org/10.3390/catal13040720
APA StyleKuimov, V. A., Malysheva, S. F., Belogorlova, N. A., Fattakhov, R. I., Albanov, A. I., & Trofimov, B. A. (2023). Triton-X-100 as an Organic Catalyst for One-Pot Synthesis of Arylmethyl-H-phosphinic Acids from Red Phosphorus and Arylmethyl Halides in the KOH/H2O/Toluene Multiphase Superbase System. Catalysts, 13(4), 720. https://doi.org/10.3390/catal13040720






