Molecular Cluster Complex of High-Valence Chromium Selenide Carbonyl as Effective Electrocatalyst for Water Oxidation
Abstract
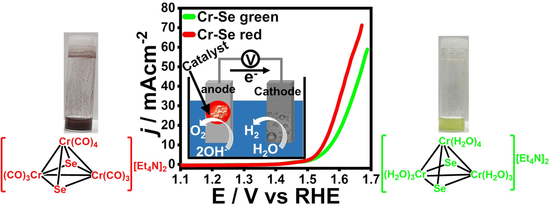
1. Introduction
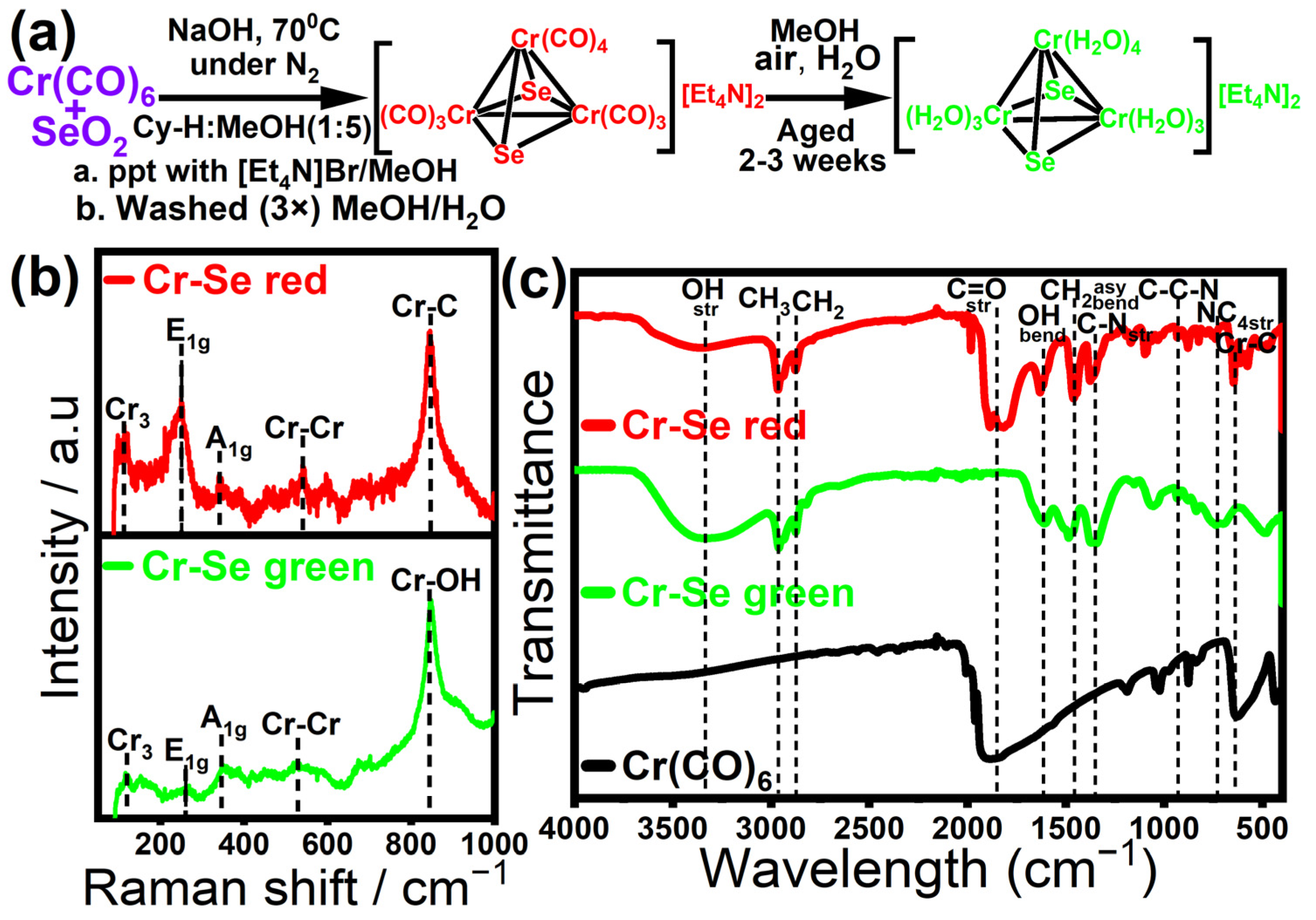
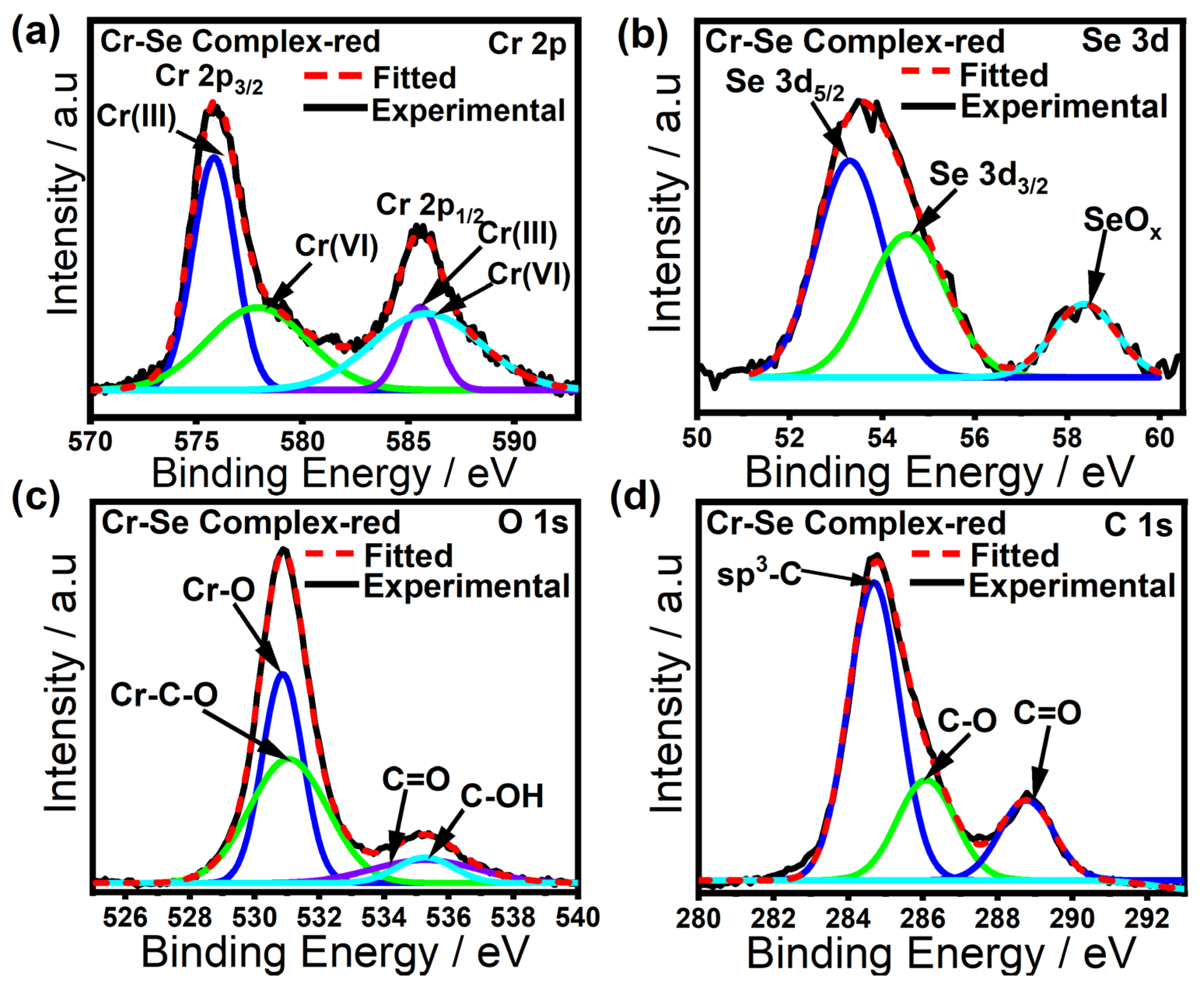
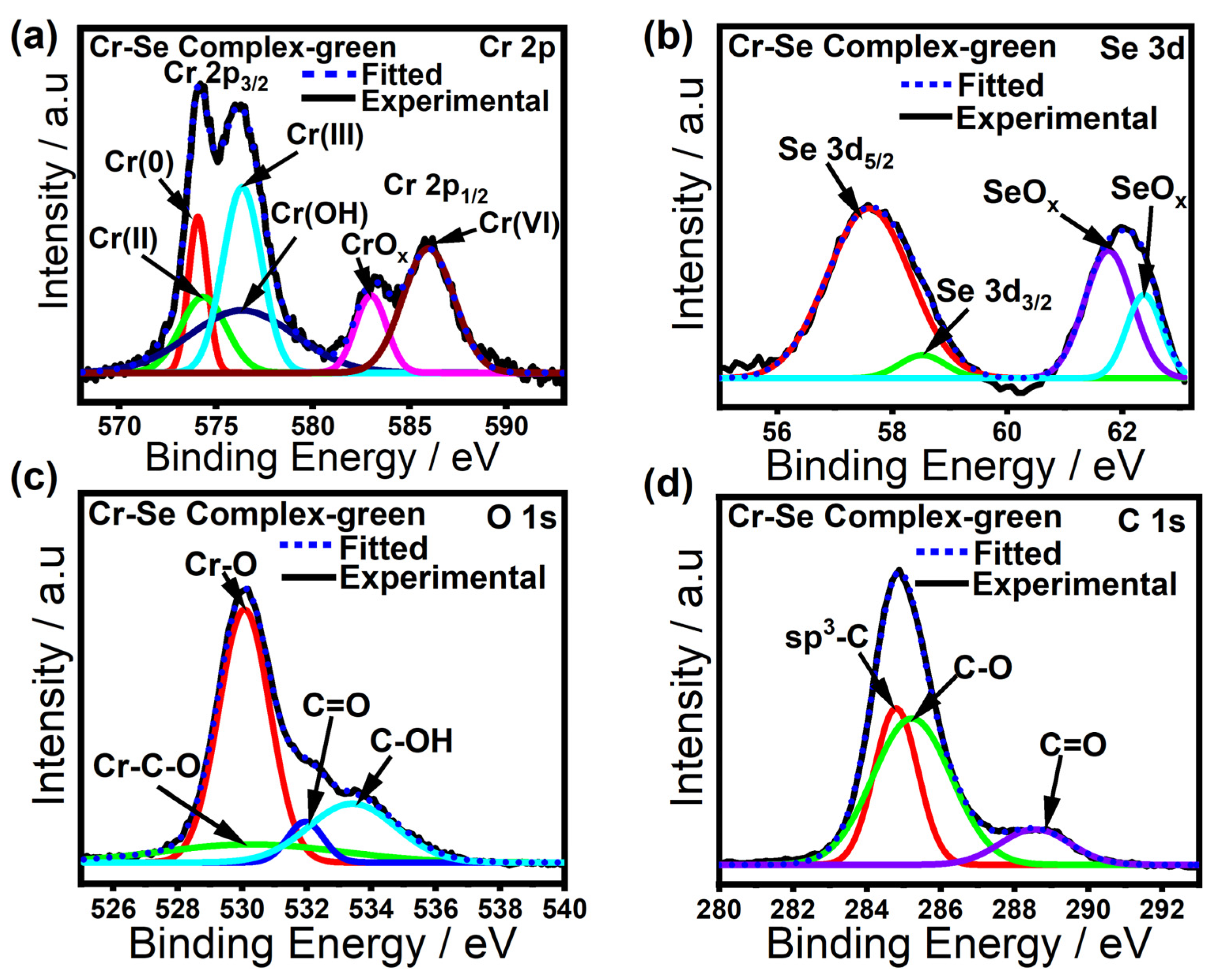
2. Results
3. Materials and Methods
3.1. Preparation of Cr-Se Red Complex
3.2. Preparation of Cr-Se Green Complex
3.3. Electrode Preparation
3.4. Characterization Methods
3.5. Chronoamperometry Stability Study
3.6. Tafel Slope
3.7. Electrochemical Impedance Spectroscopy (EIS)
3.8. Turnover Frequency (TOF)
3.9. Mass Activity
3.10. Electrochemically Active Surface Area (ECSA) and Roughness Factor (RF)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khatun, S.; Roy, P. Cobalt Chromium Vanadium Layered Triple Hydroxides as an Efficient Oxygen Electrocatalyst for Alkaline Seawater Splitting. Chem. Commun. 2022, 58, 1104–1107. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.B.; Akbar, K.; Jeon, J.H.; Jerng, S.K.; Truong, L.; Kim, K.; Yi, Y.; Chun, S.H. Iridium on Vertical Graphene as an All-Round Catalyst for Robust Water Splitting Reactions. J. Mater. Chem. A Mater. 2019, 7, 20590–20596. [Google Scholar] [CrossRef]
- Lee, Y.; Suntivich, J.; May, K.J.; Perry, E.E.; Shao-Horn, Y. Synthesis and Activities of Rutile IrO2 and RuO2 Nanoparticles for Oxygen Evolution in Acid and Alkaline Solutions. J. Phys. Chem. Lett. 2012, 3, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Antolini, E. Iridium as Catalyst and Cocatalyst for Oxygen Evolution/Reduction in Acidic Polymer Electrolyte Membrane Electrolyzers and Fuel Cells. ACS Catal. 2014, 4, 1426–1440. [Google Scholar] [CrossRef]
- Hong, W.T.; Risch, M.; Stoerzinger, K.A.; Grimaud, A.; Suntivich, J.; Shao-Horn, Y. Toward the Rational Design of Non-Precious Transition Metal Oxides for Oxygen Electrocatalysis. Energy Environ. Sci. 2015, 8, 1404–1427. [Google Scholar] [CrossRef]
- Masud, J.; Ioannou, P.C.; Levesanos, N.; Kyritsis, P.; Nath, M. A Molecular Ni-Complex Containing Tetrahedral Nickel Selenide Core as Highly Efficient Electrocatalyst for Water Oxidation. ChemSusChem 2016, 9, 3128–3132. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, I.M.; Masud, J.; Ioannou, P.-C.; Ferentinos, E.; Kyritsis, P.; Nath, M. A Molecular Tetrahedral Cobalt–Seleno-Based Complex as an Efficient Electrocatalyst for Water Splitting. Molecules 2021, 26, 945. [Google Scholar] [CrossRef]
- Geer, A.M.; Musgrave, C.; Webber, C.; Nielsen, R.J.; McKeown, B.A.; Liu, C.; Schleker, P.P.M.; Jakes, P.; Jia, X.; Dickie, D.A.; et al. Electrocatalytic Water Oxidation by a Trinuclear Copper(II) Complex. ACS Catal. 2021, 11, 7223–7240. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Allakhverdiev, S.I. Manganese Compounds as Water Oxidizing Catalysts for Hydrogen Production via Water Splitting: From Manganese Complexes to Nano-Sized Manganese Oxides. Int. J. Hydrogen Energy 2012, 37, 8753–8764. [Google Scholar] [CrossRef]
- Schmid, G. Clusters and Colloids. From Theory to Applications; VCH Verlagsgesellschaft: Weinheim, Germany, 1994; ISBN 0195069005. [Google Scholar]
- Darensbourg, D.J.; Zalewski, D.J.; Sanchez, K.M.; Delord, T. Cluster Synthesis via Aggregation: Synthesis and Solution and Solid-State Characterization of Sulfur-Capped Group 6 Metal Carbonyl Clusters. Inorg. Chem. 1988, 27, 821–829. [Google Scholar] [CrossRef]
- Hoefler, M.; Tebbe, K.-F.; Veit, H.; Weiler, N.E. Synthesis and Structure of [PPN]2[M2-CO)3(CO)9Cr3(M4-S)Cr(CO)5]: A Chromium Cluster. J. Am. Chem. Soc. 1983, 3, 6338–6339. [Google Scholar] [CrossRef]
- Hessen, B.; Siegrist, T.; Palstra, T.; Tanzler, S.M.; Steigerwald, M.L. Cr6Te8(PEt3)6 and a Molecule-Based Synthesis of Cr3Te4. Inorg. Chem. 1993, 32, 5165. [Google Scholar] [CrossRef]
- Tsuge, K.; Imoto, H.; Saito, T. Syntheses, Structures, and Molecular-Orbital Calculations of Chromium Chevrel-Type Cluster Complexes [Cr6E8(PR3)6] (E=S, PR3=PEt3, PMe3; E=Se, PR3=PEt3, PMe3, PMe2Ph). Bull. Chem. Soc. Jpn. 1996, 69, 627–636. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Zhang, Z.; Yuan, N.; Wang, X. The Long-Sought Seventeen-Electron Radical [(C6Me6)Cr(CO)3]+: Isolation, Crystal Structure and Substitution Reaction. Chem. Commun. 2015, 51, 8410–8413. [Google Scholar] [CrossRef]
- Bügel, P.; Krummenacher, I.; Weigend, F.; Eichhöfer, A. Experimental and Theoretical Evidence for Low-Lying Excited States in [Cr6E8(PEt3)6] (E = S, Se, Te) Cluster Molecules. Dalton Trans. 2022, 51, 14568–14580. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Zalewskl, D.J. Synthesis and Characterization of Sulfur-Capped Trlnuclear Group 6B Metal Clusters. Organometallics 1984, 3, 1598–1600. [Google Scholar] [CrossRef]
- Hsu, M.H.; Miu, C.Y.; Lin, Y.C.; Shieh, M. Selenium-Capped Trimolybdenum and Tritungsten Carbonyl Clusters [Se2M3(CO)10]2− (M = Mo, W). J. Organomet. Chem. 2006, 691, 966–974. [Google Scholar] [CrossRef]
- Shieh, M.; Ho, L.F.; Jang, L.F.; Ueng, C.H.; Peng, S.M.; Liu, Y.H. First Selenium-Capped Carbonyltrichromium Complex [Se2Cr3(CO)10]2−: A Novel Cr3 Ring Cluster. Chem. Commun. 2001, 3, 1014–1015. [Google Scholar] [CrossRef]
- Shieh, M.; Lin, C.N.; Miu, C.Y.; Hsu, M.H.; Pan, Y.W.; Ho, L.F. Chromium-Manganese Selenide Carbonyl Complexes: Paramagnetic Clusters and Relevance to C = O Activation of Acetone. Inorg. Chem. 2010, 49, 8056–8066. [Google Scholar] [CrossRef]
- Shieh, M.; Chung, R.L.; Yu, C.H.; Hsu, M.H.; Ho, C.H.; Peng, S.M.; Liu, Y.H. The Unusual Paramagnetic Mixed-Metal Carbonyl Chalcogenide Clusters: [E2Cr2Fe(CO)10]2− (E = Te, Se). Inorg. Chem. 2003, 42, 5477–5479. [Google Scholar] [CrossRef]
- Yeh, H.H.; Hsu, M.C.; Li, Y.H.; Hsu, Y.N.; Shr, F.Y.; Shieh, M. Ternary Antimony–Chalcogen Iron Carbonyl Complexes and Their Derivatives: Syntheses, Structures, Reactivities, and Low-Energy-Gap Characteristics. J. Organomet. Chem. 2021, 937, 121717. [Google Scholar] [CrossRef]
- Jin, D.; Kang, J.; Prabhakaran, S.; Lee, Y.; Kim, M.H.; Kim, D.H.; Lee, C. Chromium-Rich CrxIr1−xO2 Wire-in-Tube Alloys for Boosted Water Oxidation with Long Standing Electrocatalytic Activity. J. Mater. Chem. A Mater. 2022, 10, 13803–13813. [Google Scholar] [CrossRef]
- Kong, S.; Lu, M.; Yan, S.; Zou, Z. High-Valence Chromium Accelerated Interface Electron Transfer for Water Oxidation. Dalton Trans. 2022, 51, 16890–16897. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; McCrory, C.C.L. Effect of Chromium Doping on Electrochemical Water Oxidation Activity by Co3−xCrxO4 Spinel Catalysts. ACS Catal. 2017, 7, 443–451. [Google Scholar] [CrossRef]
- Du, X.; Su, H.; Zhang, X. Cr Doped-Co9S8 Nanoarrays as High-Efficiency Electrocatalysts for Water Splitting. J. Alloys Compd. 2020, 824, 153965. [Google Scholar] [CrossRef]
- Moltved, K.A.; Kepp, K.P. The Chemical Bond between Transition Metals and Oxygen: Electronegativity, d-Orbital Effects, and Oxophilicity as Descriptors of Metal-Oxygen Interactions. J. Phys. Chem. C 2019, 123, 18432–18444. [Google Scholar] [CrossRef]
- Shufler, L.; Sternberg, H.W.; Friedel, R.A. Infrared Spectrum and Structure of Chromium Hexacarbonyl, Cr(CO)6. J. Am. Chem. Soc. 1956, 78, 2687–2688. [Google Scholar] [CrossRef]
- Thalassinos, G.; Stacey, A.; Dontschuk, N.; Murdoch, B.J.; Mayes, E.; Girard, H.A.; Abdullahi, I.M.; Thomsen, L.; Tadich, A.; Arnault, J.-C.; et al. Fluorescence and Physico-Chemical Properties of Hydrogenated Detonation Nanodiamonds. C J. Carbon Res. 2020, 6, 7. [Google Scholar] [CrossRef]
- He, T.; Zhou, T.; Wan, Y.; Tan, T. A Simple Strategy Based on Deep Eutectic Solvent for Determination of Aflatoxins in Rice Samples. Food Anal. Methods 2020, 13, 542–550. [Google Scholar] [CrossRef]
- Amirthaganesan, G.; Kandhaswamy, M.A.; Srinivasan, V. Thermal, Phase Transition and FTIR Spectroscopic Studies in Tetraethylammonium Trichlorocadmiate Crystals. Cryst. Res. Technol. 2005, 40, 680–683. [Google Scholar] [CrossRef]
- Swesi, A.T.; Masud, J.; Nath, M. Nickel Selenide as a High-Efficiency Catalyst for Oxygen Evolution Reaction. Energy Environ. Sci. 2016, 9, 1771–1782. [Google Scholar] [CrossRef]
- Liu, B.; Han, W.; Li, X.; Li, L.; Tang, H.; Lu, C.; Li, Y.; Li, X. Quasi Metal Organic Framework with Highly Concentrated Cr2O3 Molecular Clusters as the Efficient Catalyst for Dehydrofluorination of 1,1,1,3,3-Pentafluoropropane. Appl. Catal. B 2019, 257, 117939. [Google Scholar] [CrossRef]
- Ghosh, T.; Maayan, G. Efficient Homogeneous Electrocatalytic Water Oxidation by a Manganese Cluster with an Overpotential of Only 74 MV. Angew. Chem. Int. Ed. 2019, 58, 2785–2790. [Google Scholar] [CrossRef]
- Stern, C.M.; Jegede, T.O.; Hulse, V.A.; Elgrishi, N. Electrochemical Reduction of Cr(vi) in Water: Lessons Learned from Fundamental Studies and Applications. Chem. Soc. Rev. 2021, 50, 1642–1667. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.N.; Fonkeng, B.; Barr-David, G.; Farrell, R.P.; Judd, R.J.; Lay, P.A.; Sangster, D.F. Redox Potentials of Chromium(V)/(IV),-(V)/(III), and -(IV)/(III) Complexes with 2-Ethyl-2-Hydroxybutanoato(2-/1-) Ligands. J. Am. Chem. Soc. 1996, 118, 7139–7144. [Google Scholar] [CrossRef]
- Guziejewski, D.; Stojanov, L.; Gulaboski, R.; Mirceski, V. Reversible and Quasireversible Electron Transfer under Conditions of Differential Square-Wave Voltammetry. J. Phys. Chem. C 2022, 126, 5584–5591. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.; Chen, X.; Zhao, C. High Valence Chromium Regulated Cobalt-Iron-Hydroxide for Enhanced Water Oxidation. J. Power Sources 2018, 402, 381–387. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, B.; Zhao, G.; Wang, J.; Liu, D.; Chen, Y.; Xia, L.; Gao, M.; Liu, Y.; Sun, W.; et al. Atomically Dispersed Chromium Coordinated with Hydroxyl Clusters Enabling Efficient Hydrogen Oxidation on Ruthenium. Nat. Commun. 2022, 13, 5894. [Google Scholar] [CrossRef]
- Gomes, A.S.O.; Yaghini, N.; Martinelli, A.; Ahlberg, E. A Micro-Raman Spectroscopic Study of Cr(OH)3 and Cr2O3 Nanoparticles Obtained by the Hydrothermal Method. J. Raman Spectrosc. 2017, 48, 1256–1263. [Google Scholar] [CrossRef]
- Huang, Y.M.; Tsai, H.R.; Lai, S.H.; Lee, S.J.; Chen, I.C.; Huang, C.L.; Peng, S.M.; Wang, W.Z. Bonding between Chromium Atoms in Metal-String Complexes from Raman Spectra and Surface-Enhanced Raman Scattering: Vibrational Frequency of the Chromium Quadruple Bond. J. Phys. Chem. C 2011, 115, 13919–13926. [Google Scholar] [CrossRef]
- Abdullahi, I.M.; Langenderfer, M.; Shenderova, O.; Nunn, N.; Torelli, M.D.; Johnson, C.E.; Mochalin, V.N. Explosive Fragmentation of Luminescent Diamond Particles. Carbon N. Y. 2020, 164, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.A.; Al-Hammadi, S.A.; Abdullahi, I.M.; Mustaqeem, M. Synthesis of Molybdenum Cobalt Nanocatalysts Supported on Carbon for Hydrodesulfurization of Liquid Fuels. J. Mol. Liq. 2018, 272, 715–721. [Google Scholar] [CrossRef]
- Beltz, J.; Pfaff, A.; Abdullahi, I.M.; Cristea, A.; Mochalin, V.N.; Ercal, N. Effect of Nanodiamond Surface Chemistry on Adsorption and Release of Tiopronin. Diam. Relat. Mater. 2019, 100, 107590. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, J.; Lian, K. Alkaline Quaternary Ammonium Hydroxides and Their Polymer Electrolytes for Electrochemical Capacitors. RSC Adv. 2014, 4, 21332–21339. [Google Scholar] [CrossRef]
- Bo, X.; Li, Y.; Chen, X.; Zhao, C. Operando Raman Spectroscopy Reveals Cr-Induced-Phase Reconstruction of NiFe and CoFe Oxyhydroxides for Enhanced Electrocatalytic Water Oxidation. Chem. Mater. 2020, 32, 4303–4311. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Beom Lee, K.; Choi, G.J.; Park, I.K.; Inn Sohn, J. Porous Hollow Nanorod Structured Chromium-Substituted Inverse Spinel Compound: An Efficient Oxygen Evolution Reaction Catalyst. J. Ind. Eng. Chem. 2021, 101, 178–185. [Google Scholar] [CrossRef]
- Shifa, T.A.; Mazzaro, R.; Morandi, V.; Vomiero, A. Controllable Synthesis of 2D Nonlayered Cr2S3 Nanosheets and Their Electrocatalytic Activity Toward Oxygen Evolution Reaction. Front. Chem. Eng. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Shamsipur, M.; Taherpour, A.; Sharghi, H.; Lippolis, V.; Pashabadi, A. A Low-Overpotential Nature-Inspired Molecular Chromium Water Oxidation Catalyst. Electrochim. Acta 2018, 265, 316–325. [Google Scholar] [CrossRef]
- Craig, M.J.; García-Melchor, M. High-Throughput Screening and Rational Design to Drive Discovery in Molecular Water Oxidation Catalysis. Cell Rep. Phys. Sci. 2021, 2. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, M.; Yang, H.; Lin, L.; Jiang, H.; Ge, S.; Li, H.; Sun, G.; Ma, S.; Yang, X. 3D Porous Amorphous γ-CrOOH on Ni Foam as Bifunctional Electrocatalyst for Overall Water Splitting. Inorg. Chem. 2019, 58, 4014–4018. [Google Scholar] [CrossRef]
- Kumar, U.N.; Malek, A.; Rao, G.R.; Thomas, T. Chromium Oxynitride (CrON) Nanoparticles: An Unexplored Electrocatalyst for Oxygen Evolution Reaction. Electrocatalysis 2022, 13, 62–71. [Google Scholar] [CrossRef]
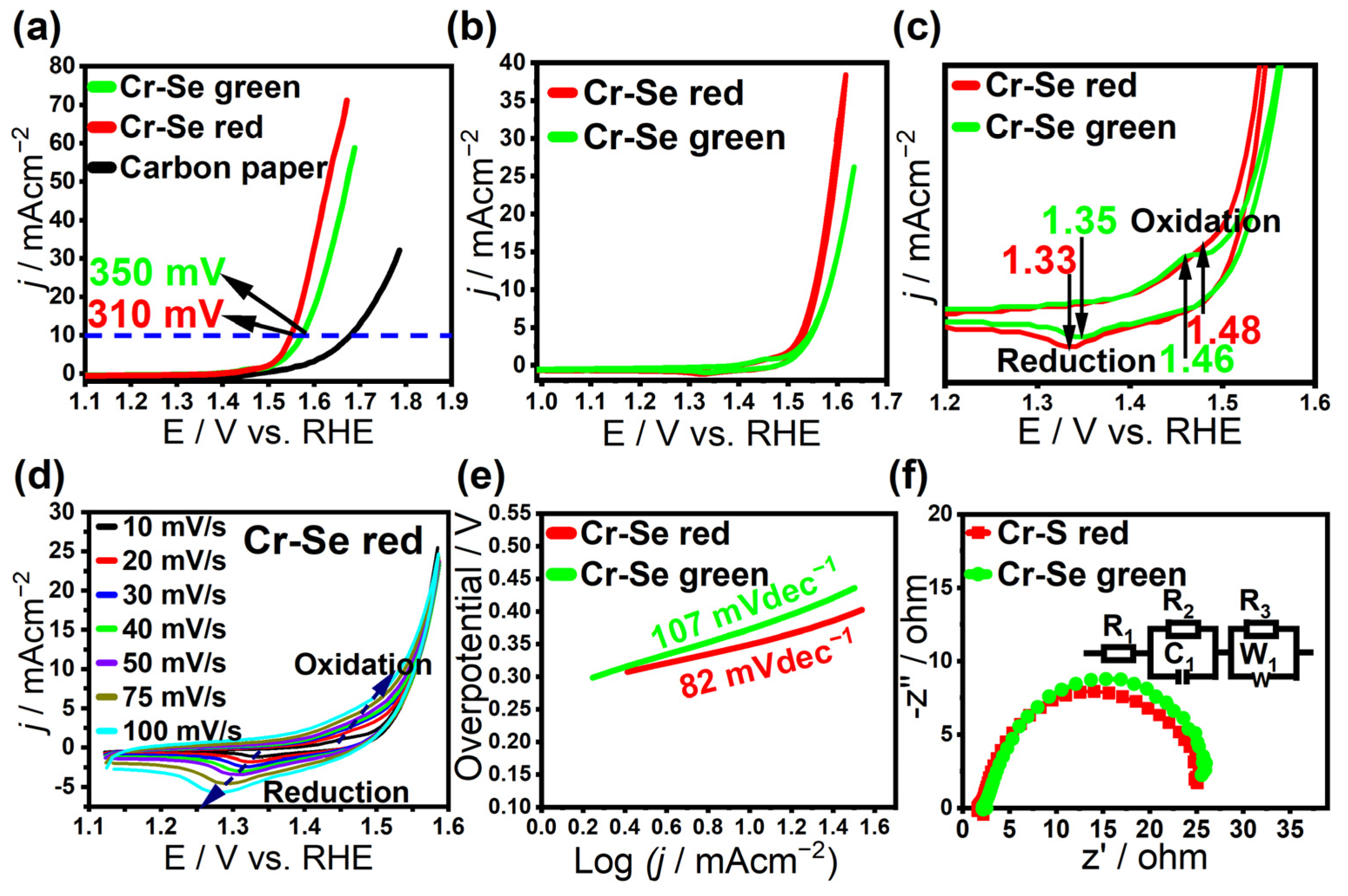
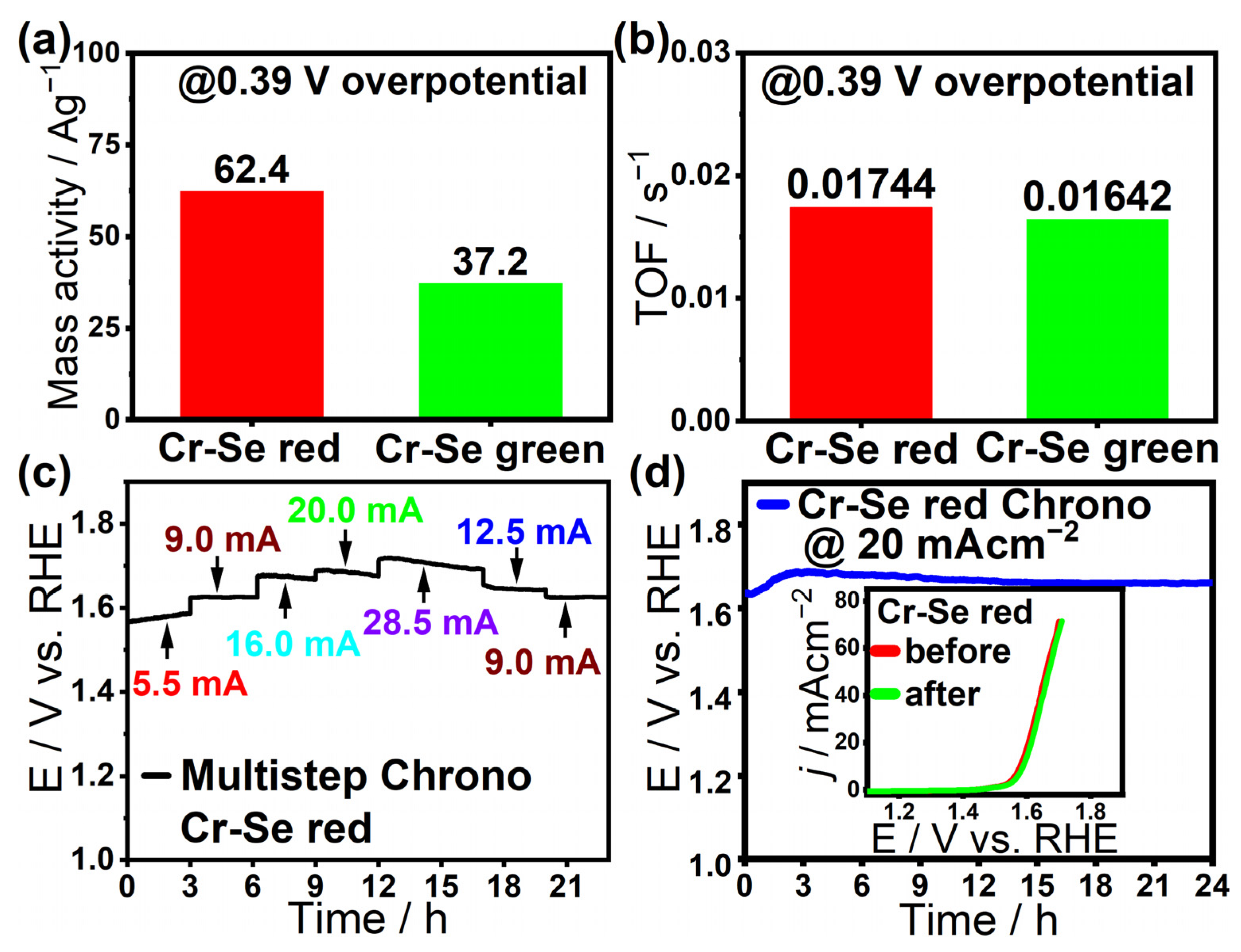
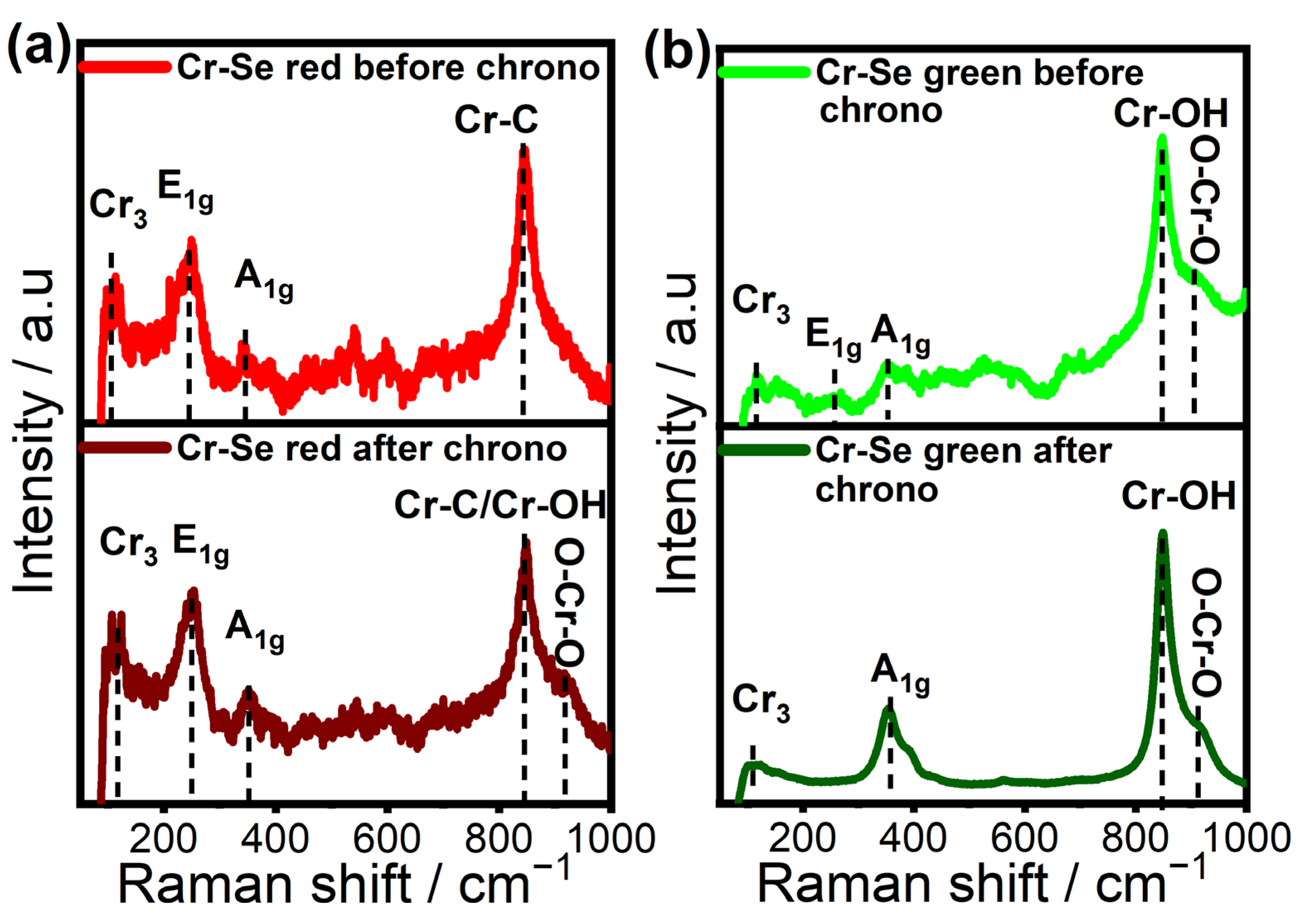
| Material | Cr-Se Red | Cr-Se Green |
|---|---|---|
| Onset potential (V) vs. RHE | 1.47 | 1.58 |
| Overpotential @ 10 mA cm−2 (mV) | 310 | 350 |
| TOF (s−1) @ 0.39 V | 0.0174 | 0.0164 |
| Mass activity (Ag−1) @ 0.39 V | 62.4 | 37.2 |
| Tafel slope (mV dec−1) | 82 | 107 |
| ECSA (cm−2) | 289 | 139 |
| RF | 1023 | 492 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullahi, I.M.; Nath, M. Molecular Cluster Complex of High-Valence Chromium Selenide Carbonyl as Effective Electrocatalyst for Water Oxidation. Catalysts 2023, 13, 721. https://doi.org/10.3390/catal13040721
Abdullahi IM, Nath M. Molecular Cluster Complex of High-Valence Chromium Selenide Carbonyl as Effective Electrocatalyst for Water Oxidation. Catalysts. 2023; 13(4):721. https://doi.org/10.3390/catal13040721
Chicago/Turabian StyleAbdullahi, Ibrahim Munkaila, and Manashi Nath. 2023. "Molecular Cluster Complex of High-Valence Chromium Selenide Carbonyl as Effective Electrocatalyst for Water Oxidation" Catalysts 13, no. 4: 721. https://doi.org/10.3390/catal13040721
APA StyleAbdullahi, I. M., & Nath, M. (2023). Molecular Cluster Complex of High-Valence Chromium Selenide Carbonyl as Effective Electrocatalyst for Water Oxidation. Catalysts, 13(4), 721. https://doi.org/10.3390/catal13040721