Four-Component Synthesis of 9H-Pyrimido[4,5-b]indoles Using Ammonium Iodide as the Nitrogen Source
Abstract
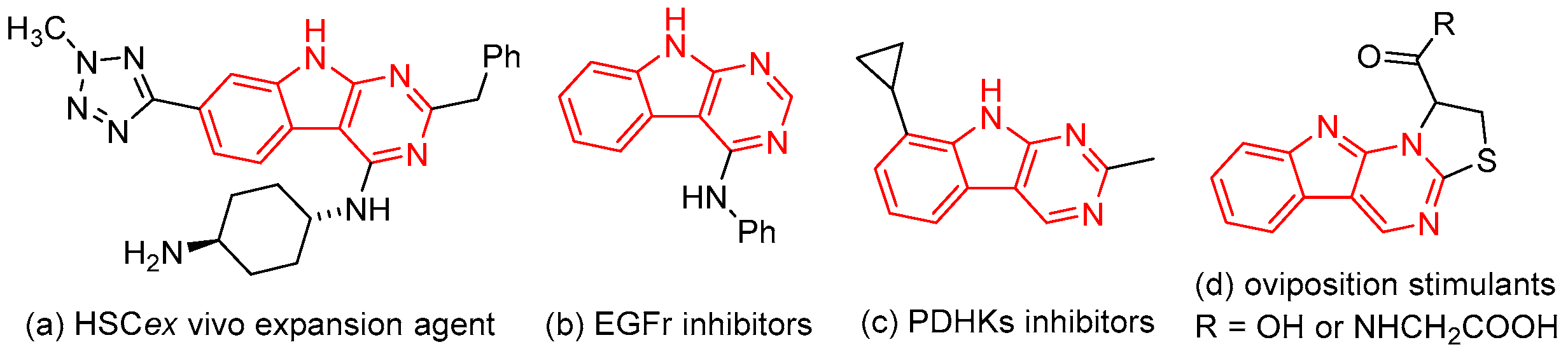
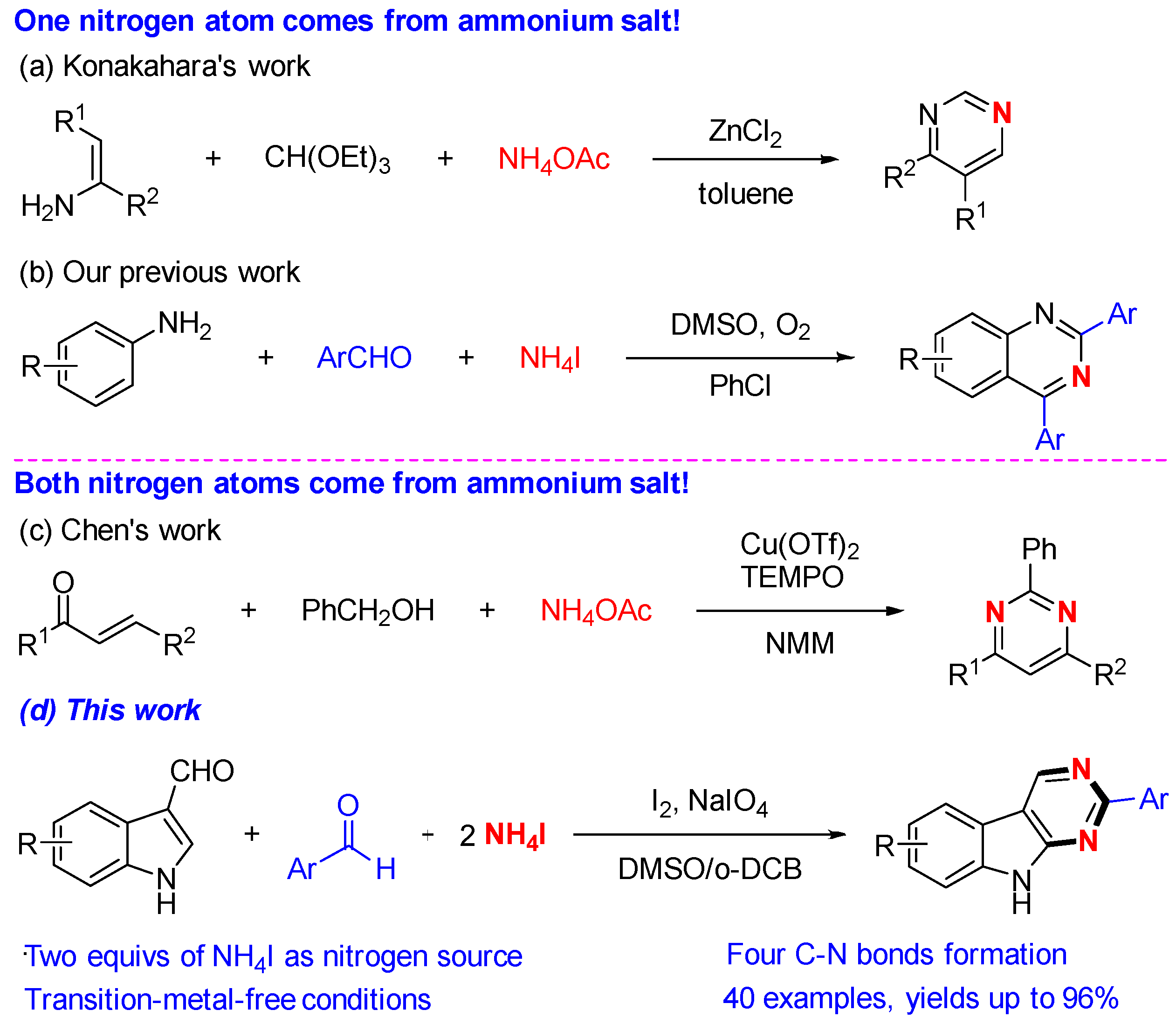
1. Introduction
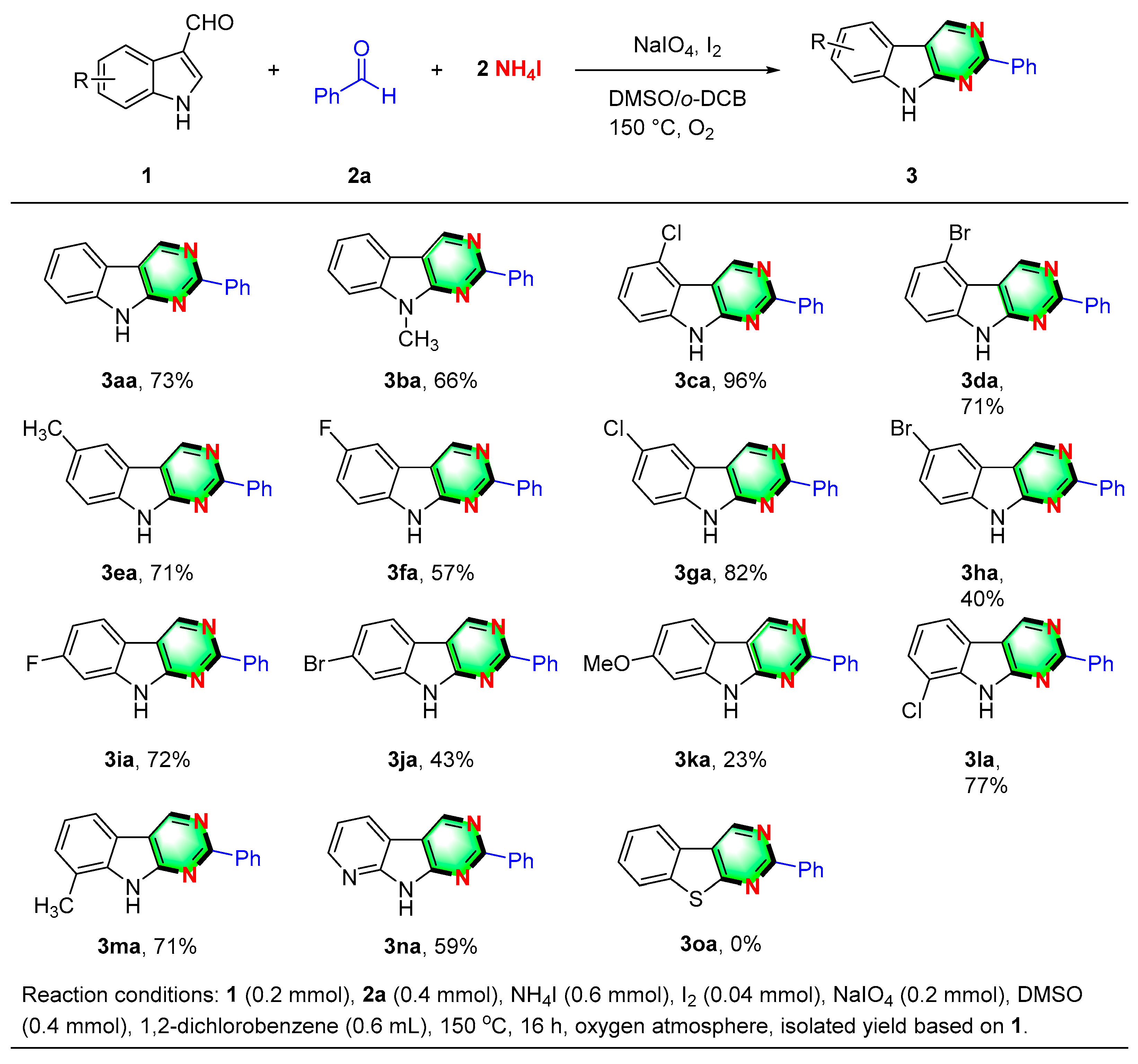
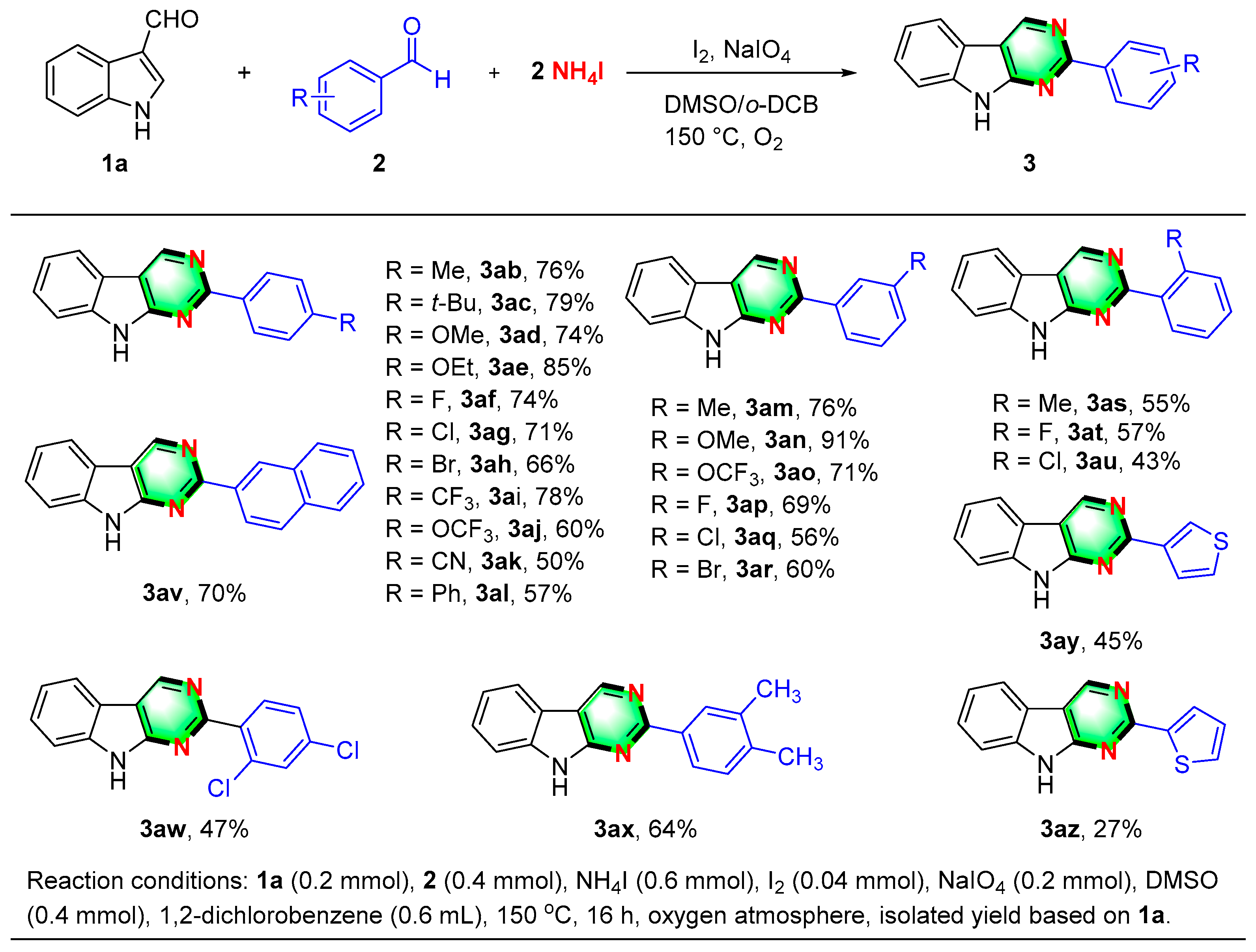
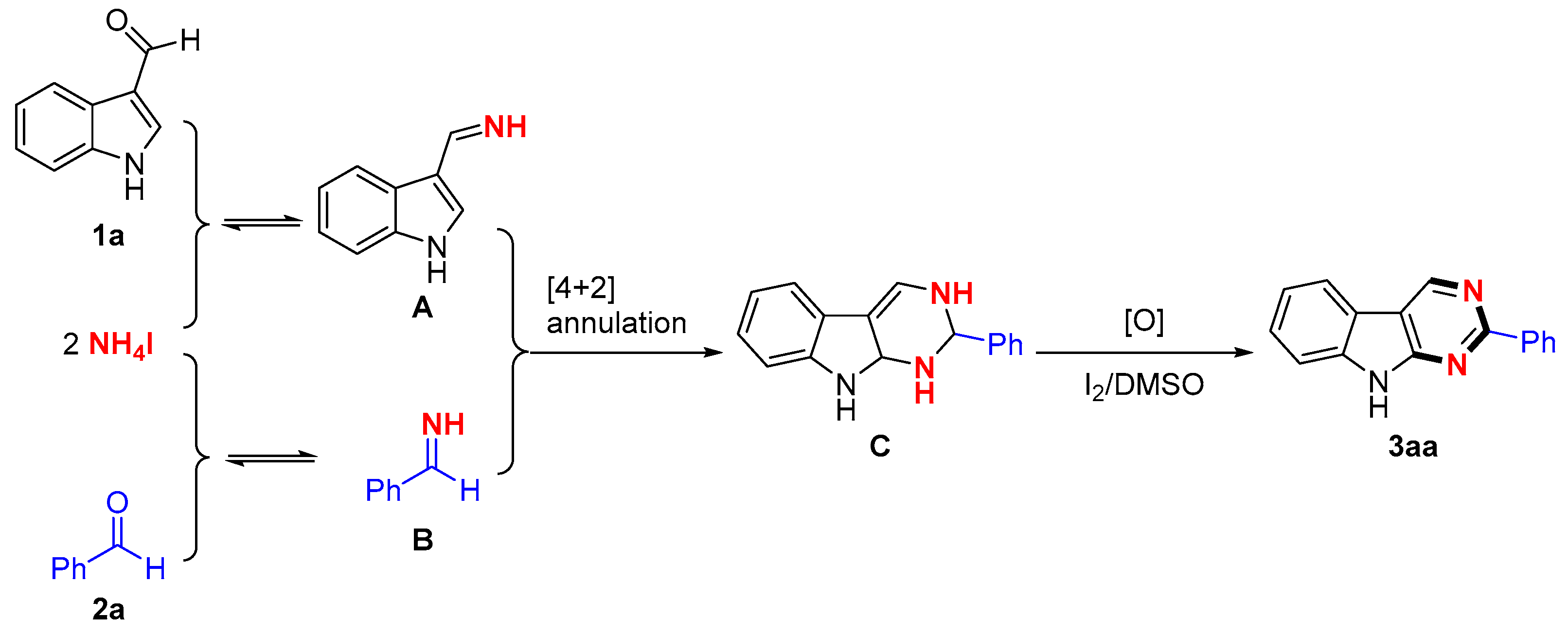
2. Results
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. General Procedure for the Synthesis of 2-Phenyl-9H-pyrimido[4,5-b]indole Derivatives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Okasha, R.M.; Albalawi, F.F.; Afifi, T.H.; Fouda, A.M.; Al-Dies, A.-A.M.; El-Agrody, A.M. Structural Characterization and Antimicrobial Activities of 7H-Benzo[h]chromeno[2,3-d]pyrimidine and 14H-Benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c] pyrimidine Derivatives. Molecules 2016, 21, 1450. [Google Scholar] [CrossRef] [PubMed]
- De Coen, L.M.; Heugebaert, T.S.A.; Garcia, D.; Stevens, C.V. Synthetic entries to and biological activity of pyrrolopyrimidines. Chem. Rev. 2016, 116, 80–139. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, R.; Siddiqi, A.A.; Dwivedi, J. Synthesis of pyrimidino derivatives: Potent antimicrobial candidates. Pharm. Chem. J. 2017, 50, 678–683. [Google Scholar] [CrossRef]
- Guillena, G.; Ramon, D.J.; Yus, M. Hydrogen autotransfer in the N-alkylation of amines and related compounds using alcohols and amines as electrophiles. Chem. Rev. 2010, 110, 1611–1641. [Google Scholar] [CrossRef]
- Walker, S.R.; Carter, E.J.; Huff, B.C.; Morris, J.C. Variolins and related alkaloids. Chem. Rev. 2009, 109, 3080–3098. [Google Scholar] [CrossRef]
- Parker, W.B. Enzymology of purine and pyrimidine antimetabolites used in the treatment of cancer. Chem. Rev. 2009, 109, 2880–2893. [Google Scholar] [CrossRef]
- Feng, Y.; Xie, X.Y.; Yang, Y.Q.; Sun, Y.T.; Ma, W.H.; Zhou, P.J.; Li, Z.Y.; Liu, H.Q.; Wang, Y.F.; Huang, Y.S. Synthesis and evaluation of pyrimidoindole analogs in umbilical cord blood ex vivo expansion. Eur. J. Med. Chem. 2019, 174, 181–197. [Google Scholar] [CrossRef]
- Akaki, T.; Bessho, Y.; Ito, T.; Fujioka, S.; Ubukata, M.; Mori, G.; Yamanaka, K.; Orita, T.; Doi, S.; Iwanaga, T.; et al. Fragment-based lead discovery to identify novel inhibitors that target the ATP binding site of pyruvate dehydrogenase kinases. Bioorg. Med. Chem. 2021, 44, 116283. [Google Scholar] [CrossRef]
- Hollis Showalter, H.D.; Bridges, A.J.; Zhou, H.; Sercel, A.D.; McMichael, A.; Fry, D.W. Tyrosine Kinase Inhibitors. 16. 6,5,6-Tricyclic Benzothieno[3,2-d]pyrimidines and Pyrimido[5,4-b]- and -[4,5-b]indoles as Potent Inhibitors of the Epidermal Growth Factor Receptor Tyrosine Kinase. J. Med. Chem. 1999, 42, 5464–5474. [Google Scholar] [CrossRef]
- Eschke, D.; Brand, A.; Scheibler, P.; Hess, S.; Eger, K.; Allgaier, C.; Nieber, K. Effect of an adenosine A1 receptor agonist and a novel pyrimidoindole on membrane properties and neurotransmitter release in rat cortical and hippocampal neurons, Neurochemistry International. Neurochem. Int. 2001, 38, 391–398. [Google Scholar] [CrossRef]
- Hurter, J.; Ramp, T.; Patrian, B.; Städler, E.; Roessingh, P.; Baur, R.; De Jong, R.; Nielsen, J.K.; Winkler, T.; Richter, W.J.; et al. Oviposition stimulants for the cabbage root fly: Isolation from cabbage leaves. Phytochemistry 1999, 51, 377–382. [Google Scholar] [CrossRef]
- Portonov, Y.N.; Bulaga, S.N.; Zabrodnyaya, V.G.; Smirnov, L.D. Synthesis of 9H-pyrimido[4,5-b]indoles from 2-amino-3-cyanoindoles. Chem. Heterocycl. Compd. 1991, 27, 325–327. [Google Scholar] [CrossRef]
- Li, B.; Guo, S.; Zhang, J.; Zhang, X.; Fan, X. Selective Access to 3-Cyano-1H-indoles, 9H-Pyrimido[4,5-b]indoles, or 9H-Pyrido[2,3-b]indoles through Copper-Catalyzed One-Pot Multicomponent Cascade Reactions. J. Org. Chem. 2015, 80, 5444–5456. [Google Scholar] [CrossRef] [PubMed]
- Adib, M.; Mohammadi, B.; Bijanzadeh, H.R. A Novel and Simple Synthesis of 9H-Pyrimido[4,5-b]indoles under Microwave Irradiation and Solvent-Free Conditions. Synlett 2008, 2008, 177–180. [Google Scholar] [CrossRef]
- Jafari, B.; Safarov, S.; Khalikova, M.; Ehlers, P.; Langer, P. Synthesis of 1,3,4-Thiadiazolo[2′,3′:2,3]imidazo[4,5-b]indoles. Synlett 2019, 30, 1791–1794. [Google Scholar] [CrossRef]
- Hyatt, J.A.; Swenton, J.S. A facile synthesis of 9H-pyrimido [4,5-b]indole via photolysis of 4-azido-5-phenylpyrimidine. J. Heterocycl. Chem. 1972, 9, 409–410. [Google Scholar] [CrossRef]
- Xu, G.; Zheng, L.; Wang, S.; Wang, Q.; Bai, X. Regiospecific Syntheses of 3-Aza-α-carbolines via Inverse Electron-Demand Diels-Alder Reactions of 2-Aminoindoles with 1,3,5-Triazines. Synlett 2009, 2009, 3206–3210. [Google Scholar] [CrossRef]
- Gao, W.; Wang, D.; Li, Y. A simple and facile synthesis of tricyclic-fused pyrimido[4,5-b]indol-2-amines. Mol. Divers. 2017, 21, 831–840. [Google Scholar] [CrossRef]
- Qu, Z.; Wang, P.; Chen, X.; Deng, G.-J.; Huang, H. Visible-light-driven Cadogan reaction. Chin. Chem. Lett. 2021, 32, 2582–2586. [Google Scholar] [CrossRef]
- Borovik, V.P.; Gatilov, Y.V.; Shkurko, O.P. Reaction of 3-(R-methylidene)-2-ethoxylindolenines with N,N′-binucleophiles. Russ. J. Org. Chem. 2016, 52, 228–234. [Google Scholar] [CrossRef]
- Burange, A.S.; Gopinath, C.S. Catalytic applications of hydrotalcite and related materials in multi -component reactions: Concepts, challenges and future scope. Sustain. Chem. Pharm. 2021, 22, 100458. [Google Scholar] [CrossRef]
- Vachan, B.S.; Karuppasamy, M.; Vinoth, P.; Vivek Kumar, S.; Perumal, S.; Sridharan, V.; Menéndez, J.C. Proline and its Derivatives as Organocatalysts for Multi- Component Reactions in Aqueous Media: Synergic Pathways to the Green Synthesis of Heterocycles. Adv. Synth. Catal. 2020, 362, 87. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Zhang, J.-W.; Xiang, S.-H.; Guo, Z.; Tan, B. Stereoselective Construction of Complex Spirooxindoles via Bisthiourea Catalyzed Three-Component Reactions. Chin. J. Chem. 2018, 36, 1182–1186. [Google Scholar] [CrossRef]
- Ren, C.; Wang, Z.; Wang, T.; Guo, J.; Dai, Y.; Yuan, H.; Tan, Y. Ultralong Organic Phosphorescence Modulation of Aromatic Carbonyls and Multi-Component Systems. Chin. J. Chem. 2022, 40, 1987–2000. [Google Scholar] [CrossRef]
- Khan, I.; Zaib, S.; Ibrar, A. New frontiers in the transition-metal-free synthesis of heterocycles from alkynoates: An overview and current status. Org. Chem. Front. 2020, 7, 3734–3791. [Google Scholar] [CrossRef]
- Guo, H.; Tian, L.; Liu, Y.; Wan, J.-P. DMSO as a C1 Source for [2 + 2 + 1] Pyrazole Ring Construction via Metal-Free Annulation with Enaminones and Hydrazines. Org. Lett. 2022, 24, 228–233. [Google Scholar] [CrossRef]
- Liu, H.; Cai, Z.-J.; Ji, S.-J. Access to 3-alkylselenindoles by multicomponent reaction of indoles, selenium powder and unactivated alkyl halides under transition-metal-free conditions. Chin. Chem. Lett. 2022, 33, 4303–4305. [Google Scholar] [CrossRef]
- Xu, Z.; Fu, L.; Fang, X.; Huang, B.; Zhou, L.; Wan, J.-P. Tunable Trifunctionalization of Tertiary Enaminones for the Regioselective and Metal-Free Synthesis of Discrete and Proximal Phosphoryl Nitriles. Org. Lett. 2021, 23, 5049–5053. [Google Scholar] [CrossRef]
- Han, C.; Nie, L.; Han, X.; Zhang, Y.; Sun, K.; Shi, L.; Cui, G.; Meng, W. One-Pot Three-Component Synthesis of Novel 1,5-Benzodiazepine Derivatives and Their anti-BVDV (Bovine Viral Diarrhea Virus) Activity. Chin. J. Org. Chem. 2021, 41, 819–825. [Google Scholar] [CrossRef]
- Khalili, B.; Jajarmi, P.; Eftekhari-Sis, B.; Hashemi, M.M. Novel One-Pot, Three-Component Synthesis of New 2-Alkyl-5-aryl-(1H)-pyrrole-4-ol in Water. J. Org. Chem. 2008, 73, 2090–2095. [Google Scholar] [CrossRef]
- Kumar, D.; Kommi, D.N.; Bollineni, N.; Patel, A.R.; Chakraborti, A.K. Catalytic procedures for multicomponent synthesis of imidazoles: Selectivity control during the competitive formation of tri- and tetrasubstituted imidazoles. Green Chem. 2012, 14, 2038–2049. [Google Scholar] [CrossRef]
- Xiang, S.; Fan, W.; Zhang, W.; Li, Y.; Guo, S.; Huang, D. Aqueous CO2 fixation: Construction of pyridine skeletons in cooperation with ammonium cations. Green Chem. 2021, 23, 7950–7955. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; He, R.; Liu, J.; Liu, Y.; Chen, L.; Huang, Y.; Li, Y. Selective Synthesis of Substituted Pyridines and Pyrimidines through Cascade Annulation of Isopropene Derivatives. Org. Lett. 2022, 24, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Shukla, G.; Srivastava, A.; Singh, M.S. Metal- and Catalyst-Free, Formal [4 + 1] Annulation via Tandem C═O/C═S Functionalization: One-Pot Access to 3,5-Disubstituted/Annulated Isothiazoles. Org. Lett. 2016, 18, 2451–2454. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Sharma, H.; Saha, N.; Chakraborti, A.K. Domino synthesis of functionalized pyridine carboxylates under gallium catalysis: Unravelling the reaction pathway and the role of the nitrogen source counter anion. Chem. Asian J. 2022, 17, e202200304. [Google Scholar] [CrossRef]
- Yaremenko, I.A.; Belyakova, Y.Y.; Radulov, P.S.; Novikov, R.A.; Medvedev, M.G.; Krivoshchapov, N.V.; Korlyukov, A.A.; Alabugin, I.V.; Terent’ev, A.O. Inverse α-Effect as the Ariadne’s Thread on the Way to Tricyclic Aminoperoxides: Avoiding Thermodynamic Traps in the Labyrinth of Possibilities. J. Am. Chem. Soc. 2022, 144, 7264–7282. [Google Scholar] [CrossRef]
- Sasada, T.; Kobayashi, F.; Sakai, N.; Konakahara, T. An Unprecedented Approach to 4,5-Disubstituted Pyrimidine Derivatives by a ZnCl2-Catalyzed Three-Component Coupling Reaction. Org. Lett. 2009, 11, 2161–2164. [Google Scholar] [CrossRef]
- Chen, J.; Meng, H.; Zhang, F.; Xiao, F.; Deng, G.-J. Transition-metal-free selective pyrimidines and pyridines formation from aromatic ketones, aldehydes and ammonium salts. Green Chem. 2019, 21, 5201–5206. [Google Scholar] [CrossRef]
- Jiang, J.; Huang, H.; Deng, G.J. Four-component thiazole formation from simple chemicals under metal-free conditions. Green Chem. 2019, 21, 986–990. [Google Scholar] [CrossRef]
- Bai, Y.; Tang, L.; Huang, H.; Deng, G.-J. Synthesis of 2,4-diarylsubstituted-pyridines through a Ru-catalyzed four component reaction. Org. Biomol. Chem. 2015, 13, 4404–4407. [Google Scholar] [CrossRef]
- Chen, J.; Chang, D.; Xiao, F.; Deng, G.-J. Four-component quinazoline synthesis from simple anilines, aromatic aldehydes and ammonium iodide under metal-free conditions. Green Chem. 2018, 20, 5459–5463. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Z.; Xiao, F.; Deng, G.-J. Base-promoted aerobic oxidative synthesis of fused 1,3,5-triazines under metal-free conditions. Green Chem. 2020, 22, 6778–6782. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Liu, Y.; Chen, B. Synthesis of Pyrimidines with Ammonium Acetate as Nitrogen Source Under Solvent-Free Conditions. Asian J. Org. Chem. 2019, 8, 1122. [Google Scholar] [CrossRef]
- Duan, T.T.; Zhai, T.R.; Liu, H.H.; Yan, Z.L.; Zhao, Y.; Feng, L.; Ma, C. One-pot three-component synthesis of quinazolines via a copper-catalysed oxidative amination reaction. Org. Biomol. Chem. 2016, 14, 6561–6567. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Chen, J.X.; Liu, M.C.; Ding, J.C.; Gao, W.X.; Huang, X.B.; Wu, H.Y. Unexpected Copper-Catalyzed Cascade Synthesis of Quinazoline Derivatives. J. Org. Chem. 2013, 78, 11342–11348. [Google Scholar] [CrossRef]
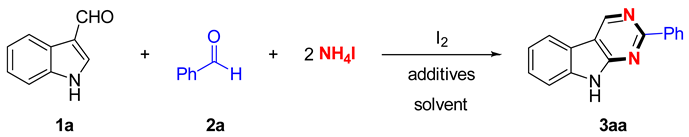 | ||||
|---|---|---|---|---|
| Entry | Additive | Oxidant | Solvent | Yield (%) 2 |
| 1 | KI | DMSO | o-DCB | 45 |
| 2 | KIO3 | DMSO | o-DCB | 68 |
| 3 | NaIO4 | DMSO | o-DCB | 76 |
| 4 | I2O5 | DMSO | o-DCB | trace |
| 5 | NIS | DMSO | o-DCB | trace |
| 6 | NaIO4 | DMSO | PhCl | 65 |
| 7 | NaIO4 | DMSO | toluene | 53 |
| 8 | NaIO4 | DMSO | mesitylene | 40 |
| 9 | NaIO4 | DMSO | PhCF3 | 39 |
| 10 | NaIO4 | DMSO | DMF | trace |
| 11 | NaIO4 | DMSO | CH3CN | trace |
| 12 | NaIO4 | DMSO | NMP | trace |
| 13 | NaIO4 | DMSO | Pyridine | trace |
| 14 | NaIO4 | H2O2 | o-DCB | 38 |
| 15 | NaIO4 | TBHP | o-DCB | 35 |
| 16 | NaIO4 | DTBP | o-DCB | 30 |
| 17 | NaIO4 | K2S2O8 | o-DCB | trace |
| 18 | NaIO4 | Na2S2O8 | o-DCB | 15 |
| 19 3 | NaIO4 | DMSO | o-DCB | 58 |
| 20 4 | NaIO4 | DMSO | o-DCB | 63 |
| 21 5 | NaIO4 | DMSO | o-DCB | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Yang, R.; Xiao, F.; Li, T.; Mao, G.; Deng, G.-J. Four-Component Synthesis of 9H-Pyrimido[4,5-b]indoles Using Ammonium Iodide as the Nitrogen Source. Catalysts 2023, 13, 623. https://doi.org/10.3390/catal13030623
Chen Y, Yang R, Xiao F, Li T, Mao G, Deng G-J. Four-Component Synthesis of 9H-Pyrimido[4,5-b]indoles Using Ammonium Iodide as the Nitrogen Source. Catalysts. 2023; 13(3):623. https://doi.org/10.3390/catal13030623
Chicago/Turabian StyleChen, Yufeng, Ruitong Yang, Fuhong Xiao, Tong Li, Guojiang Mao, and Guo-Jun Deng. 2023. "Four-Component Synthesis of 9H-Pyrimido[4,5-b]indoles Using Ammonium Iodide as the Nitrogen Source" Catalysts 13, no. 3: 623. https://doi.org/10.3390/catal13030623
APA StyleChen, Y., Yang, R., Xiao, F., Li, T., Mao, G., & Deng, G.-J. (2023). Four-Component Synthesis of 9H-Pyrimido[4,5-b]indoles Using Ammonium Iodide as the Nitrogen Source. Catalysts, 13(3), 623. https://doi.org/10.3390/catal13030623







